14 September 2022: Clinical Research
C-Reactive Protein/Albumin Ratio at Diagnosis of Pediatric Inflammatory Bowel Disease: A Retrospective Multi-Center Study
Aleksandra Glapa-NowakDOI: 10.12659/MSM.937842
Med Sci Monit 2022; 28:e937842
Abstract
BACKGROUND: This study aimed to evaluate the C-reactive protein-to-albumin (CRP/albumin) ratio at diagnosis of pediatric inflammatory bowel disease (IBD).
MATERIAL AND METHODS: Serum CRP/albumin ratio was calculated for patients with Crohn’s disease (CD; n=186) and ulcerative colitis (UC; n=159) aged 3-18 years.
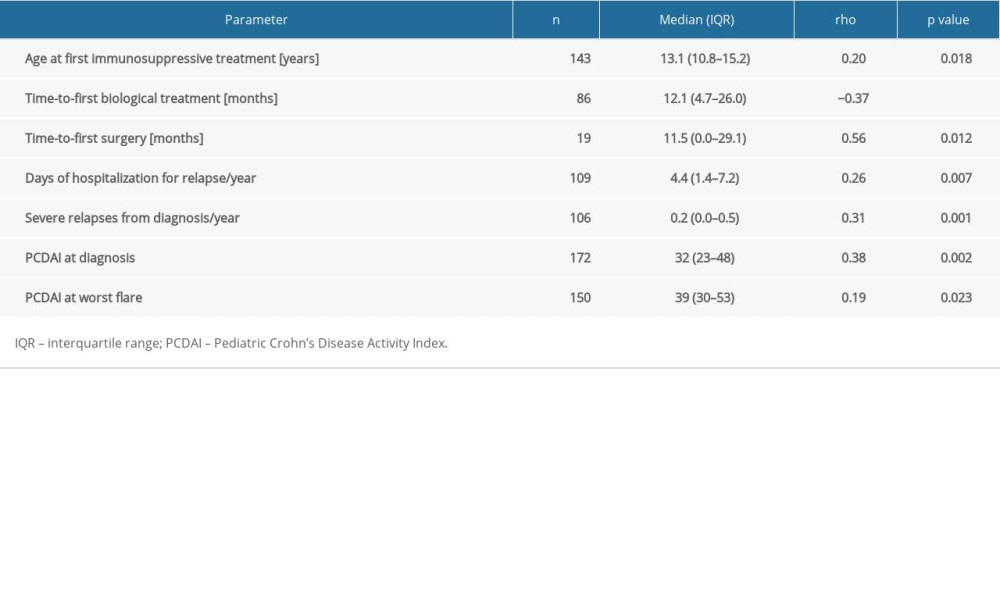
RESULTS: Patients with CD differed in CRP/albumin ratio at diagnosis in groups with quiescent, mild, moderate, and severe disease (P=0.011). CRP/albumin ratio at diagnosis was significant in differentiating patients with severe CD from quiescent disease at diagnosis (area under the curve (AUC)=0.94, odds ratio (OR)=63.4, 95% confidence interval (CI) 7.1-569.1, P<0.0001). CRP/albumin ratio at diagnosis could moderately differentiate penetrating from non-penetrating disease behavior in CD at diagnosis (AUC=0.73, OR=6.3, 95% CI 2.0-19.3, P<0.001). Furthermore, CRP/albumin ratio at diagnosis weakly differentiated IBD patients in need of biological treatment in a step-up procedure (AUC=0.58, OR=2.1, 95% CI 1.3-3.4, P=0.022) and in need of surgery (AUC=0.63, OR=3.1, 95% CI 1.4-7.2, P=0.006). For the IBD, CRP/albumin ratio at diagnosis was weakly correlated with age at first immunosuppressive treatment (rho=0.20, P=0.018), time from diagnosis to first biological treatment (rho=-0.37, P<0.001), days spent in hospital (rho=0.26, P=0.007), number of severe relapses (rho=0.31, P=0.001), and Pediatric Crohn’s Disease Activity Index (rho=0.38, P=0.002).
CONCLUSIONS: The present findings add to previous studies carried out in adult patients and show that the CRP/albumin ratio at diagnosis was not significantly associated with the course of either CD or UC in children. However, CRP/albumin ratio could differentiate patients with severe CD from those with quiescent disease.
Keywords: Albumins, Colitis, Ulcerative, Crohn Disease, CRP Protein, Human, Hospitalization, Adult, C-Reactive Protein, Child, Humans, Inflammatory Bowel Diseases, Neoplasm Recurrence, Local
Background
Inflammatory bowel diseases (IBD) are chronic conditions with globally rising burden and complex, still unknown etiology [1,2]. The 2 main pathophysiological entities – Crohn’s disease (CD) and ulcerative colitis (UC) – are conditions with variable clinical course, including frequent flares, requiring surgery and hospitalizations, and generating psychological problems [3]. The accurate diagnosis of IBD requires a combination of disease history, physical and laboratory examination, imaging procedures such as enterography, and histology [4]. Although several diagnostic indicators have been developed to date, the search for inexpensive and quick markers of disease outcomes in IBD is ongoing.
Despite multiple advantages of calprotectin, this marker still has some limitations (eg, costs and sample availability) [5–7]. Combined measurements of erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and albumin, along with fecal calprotectin, have been shown to have diagnostic utility for CD [8]. Other inflammatory markers in adults include neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), and lymphocyte/monocyte ratio (LMR) [9]. Of these, combined NLR and PLR can predict active endoscopic disease in adults with UC [10] and differentiate adults with CD from non-CD controls [11]. Some promising results for this group of markers were also found in children [12,13].
Serum CRP and albumin measurements are relatively cheap and available. CRP is an acute-phase protein and is more frequently elevated in children with CD than in those with UC [14]. Serum CRP response seem to differ individually, sometimes with unexpectedly low concentrations despite clinical history [15]. This may be caused by differences in CRP genotypes and other extraintestinal inflammatory processes. Three single-nucleotide polymorphisms (rs1205, rs1130864, and rs1417938) are associated with higher CRP concentrations at diagnosis, but the genotype was not linked with CD phenotype and remission after anti-tumor necrosis factor-alpha (anti-TNF) therapy [14]. In adults, lower CRP concentrations at diagnosis (in approximately 30% of patients with CD) led to milder disease phenotype [3], which suggests these patients require only basic therapy. This in turn would avoid overtreatment and unnecessary costs and risks [3]. In adults with UC, CRP above 45 mg/L and more than 8 stools a day at the third day of intensive treatment is an indicator for colectomy [16]. Similarly, in pediatric IBD, high inflammatory burden at diagnosis suggests poor prognosis, and high CRP is linked with higher risk of immunosuppression intake and surgery [14].
Albumin concentrations can also reflect systemic inflammatory burden, nutritional status, and poor prognosis in multiple disease entities. Hypoalbuminemia is associated with infliximab failure in adult UC [17–19], whereas higher concentrations of albumin predicts remission 8 weeks after infliximab induction [20]. Together, low albumin and high CRP concentrations are linked with disease severity [21,22].
CRP/albumin ratio has shown high value in acute pancreatitis [23], colorectal cancer [24], and sepsis [25]. In IBD, CRP/albumin ratio has high discriminative power for active disease in adults [26,27] and correlates with moderate and severe endoscopic activity in UC [28]. CRP/albumin ratio measured after infliximab salvage can potentially predict colectomy in acute severe UC [29], but this depends on timing of the test [17]. Serum CRP/albumin ratio measured on the third day after infliximab intake can reach 79% sensitivity and 80% specificity to predict colectomy, with more efficiency than that of Mayo scores [17]. Predicting unresponsiveness to anti-TNF therapies is an important aspect of modern precision medicine and can help avoid unnecessary costs and risks [17,30].
Available reports on CRP/albumin ratio in IBD concern adult patients [26–28]. Therefore, this retrospective study aimed to evaluate the CRP/albumin ratio at diagnosis of IBD in 345 pediatric patients aged between 3 and 18 years in relation with IBD severity described with different clinical parameters. The measurement of disease severity is notoriously difficult to define, which has been reported previously [31]. It is based on clinical data, endoscopic picture, and extraintestinal features. In our study these included: Paris classification in terms of extent and behavior, past surgeries, disease activity assessment, and patient’s nutritional status, which can also inform about disease severity to some extent [31]. The severity is also described by number of exacerbations per year, days spent in hospital, and necessary treatment, because it suggests no response to previous therapy.
Material and Methods
PATIENTS:
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Bioethical Committee from the Poznań University of Medical Sciences on 5 November 2015 (protocol number 960/15). All parents/legal guardians consented to participation of their children in the study. Dual consent of the patient and parent was collected in case of children over 16 years old. A retrospective multi-center cohort study (the Polish Pediatric Crohn’s and Colitis Cohort, POCOCO) was conducted between April 2016 and March 2019 in 7 centers in Poland (Poznań, Warsaw (2 centers), Wrocław, Zabrze, Katowice and Bydgoszcz) [32,33]. The inclusion criteria were diagnosis of UC or CD and age 3–18 years. We excluded patients with life-threatening and severe general conditions. The diagnosis of IBD was based on European diagnostic criteria and confirmed by experienced gastroenterologists [4,34,35]. The histologic examination were according to European criteria [36].
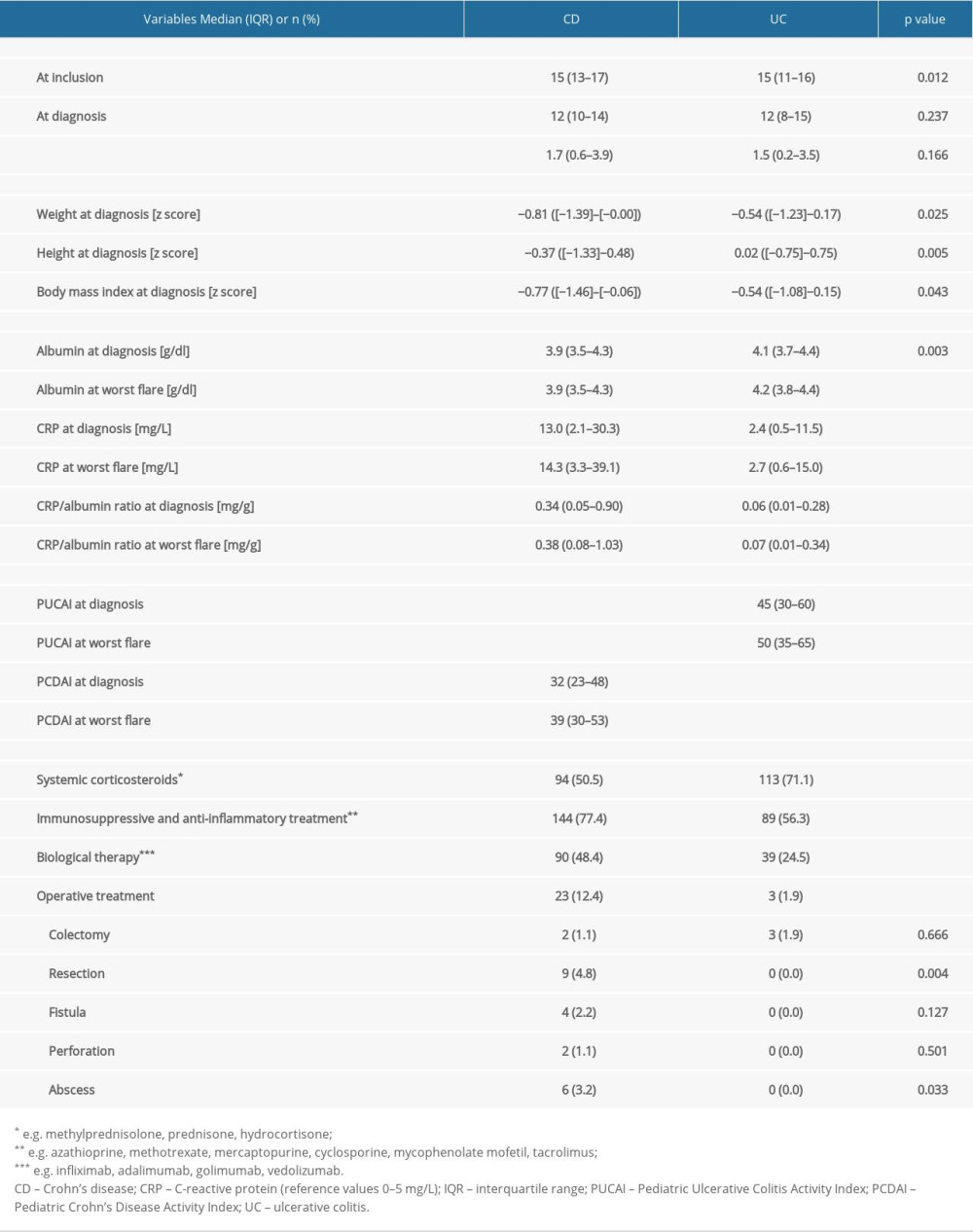
The data on CRP [mg/L] and albumin concentrations [g/dl] at diagnosis were collected retrospectively. The ratio for 345 patients aged 3–18 years was calculated (CD n=186 and UC n=159; F=149, M=196). The lowest and highest concentrations of CRP/albumin ratio at diagnosis were defined with 1st and 3rd quartiles, respectively, independently for UC and CD.
DISEASE SEVERITY:
Pediatric Ulcerative Colitis Activity Index (PUCAI) or Pediatric Crohn’s Disease Activity Index (PCDAI) were used to define disease activity at diagnosis and the worst flare [37]. In CD, quiescence was defined as PCDAI ≤10. A PCDAI score of 11–30 points was considered as a mild activity, 31–49 as moderate, and ≥50 as severe [38–40]. In UC, PUCAI ≤10 was defined as quiescent, 10–34 as a mild activity, 35–64 as moderate activity, and ≥65 as severe disease [37,41]. The worst flare was defined when PUCAI/PCDAI was the highest in the medical history. Approximately 30.1% of patients had their worst flare at diagnosis, while 64 patients (18.5%) received their first immunosuppressive drug at diagnosis (CD n=47; UC n=17). Patients were treated with azathioprine, methotrexate, mercaptopurine, cyclosporine, mycophenolate mofetil, or tacrolimus according to standard guidelines [42,43].
Localization and behavior of disease was defined using the Paris classification [44]. The criteria to initiate the biological treatment were as per the regulations of the drug programs specified by the Polish Ministry of Health, uniform for all centers [45]. Corticosteroids were administered according to contemporary European guidelines [43]. Serum albumin concentration was determined by the spectrophotometric bromocresol green method (Abbott Alinity, Santa Clara, USA). Serum CRP concentration was measured with the Alinity CRP Vario Reagent Kit 7P5620 (Abbott Alinity, Santa Clara, USA).
STATISTICAL ANALYSIS:
Two-tailed Fisher’s exact test was used for the comparison of categorical parameters. The normality of data was determined with Shapiro-Wilk test. Continuous variables were presented as medians and 1st–3rd quartiles. The comparisons between 2 groups were performed with the Mann-Whitney U test and comparisons among multiple groups were performed with the Kruskal-Wallis test. To determine the relationships between CRP/albumin ratio and other parameters associated with disease severity, Spearman’s correlation analysis was used. Receiver operating characteristic (ROC) curve analysis was used to obtain the optimal cut-off values of the CRP/albumin ratio by Youden index with standard errors (SE). Maximum sensitivity and specificity were calculated. Statistical significance was set at
Results
CRP was elevated (>5 mg/L) in 65.9% of patients with CD and in 39.6% of patients with UC. CRP/albumin ratio at diagnosis and at worst flare were higher in patients with CD compared to those of patients with UC (Table 1). In 25.3% of patients with CD, the first dose of immunosuppressant drug was taken directly after diagnosis. CRP/albumin ratio at diagnosis did not differ between patients with CD with and without immunosuppressive medications (
We found that 6.3% of patients with CD had quiescent disease at the date of diagnosis, 42.5% had mild activity, 27.0% had moderate activity, and 24.1% had severe activity, whereas 2.7% of patients with UC had quiescent disease at diagnosis, 25.7% had mild activity, 52.0% had moderate activity, and 19.6% were severe.
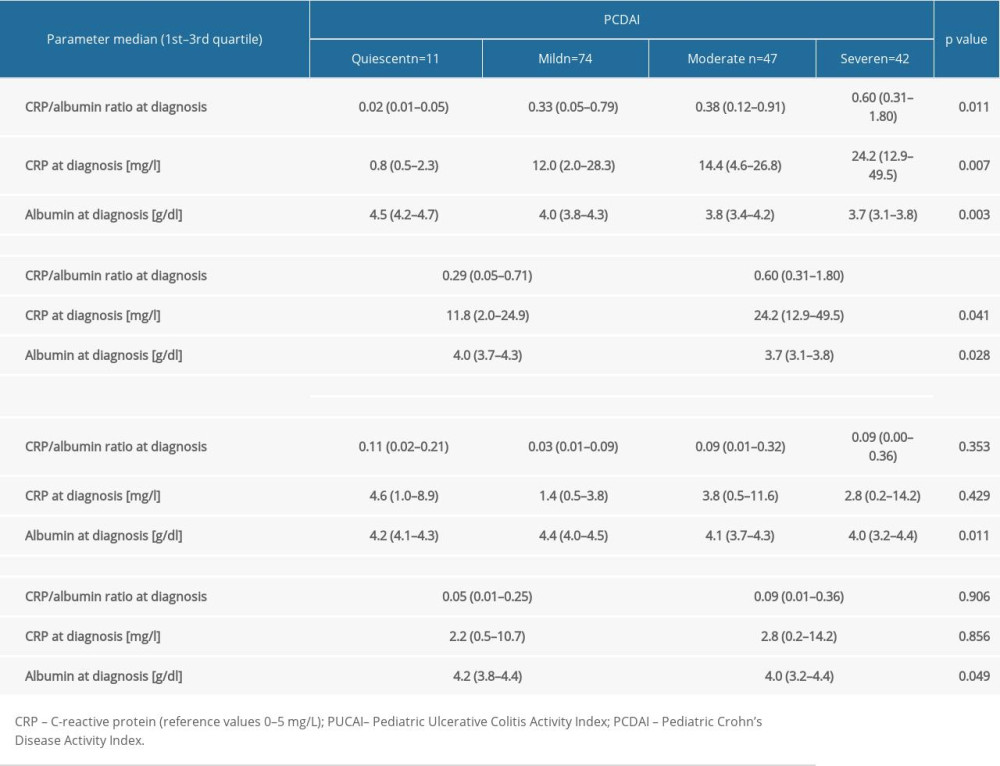
CRP/albumin ratio at diagnosis differed depending on PCDAI severity groups at diagnosis (
At worst flare, CRP/albumin ratio concentrations differed significantly across PCDAI severity groups (
CRP/albumin ratio at diagnosis in patients with CD was correlated weakly with age at first immunosuppressive drug, time from diagnosis to first biological treatment, days spent in hospital, number of severe relapses, and PCDAI (Table 3). No such correlations were found in patients with UC. CRP/albumin ratio at diagnosis was poorly correlated with PUCAI at diagnosis (rho=0.16;
Patients with lowest (<1st quartile) values of CRP/albumin ratio at diagnosis were significantly younger at induction of first biological treatment than those with highest CRP/albumin ratio – age 11 (7–13) vs 13 (10–15) (
CRP/albumin ratio was not useful in identifying the need for biological treatment or surgery (area under the ROC curve (AUC) for both <0.65). In patients with IBD who took immunosuppressive agents at diagnosis (n=64), CRP/albumin ratio poorly differentiated patients in need of biological treatment, with an AUC=0.664 (SE 0.068,
Serum CRP/albumin ratio at diagnosis showed clinical utility in differentiating patients with CD who have severe disease from those with quiescent disease AUC=0.94 (SE=0.041, OR=63.4, 95% CI 7.1–569.1,
Serum CRP/albumin ratio at diagnosis was poor/moderate in differentiating patients with various disease localizations and behaviors defined by the Paris classification. The highest sensitivity and AUC value for CRP/albumin ratio at diagnosis were obtained for differentiating penetrating from non-penetrating disease behavior in CD at diagnosis (AUC=0.73, OR=6.3, 95% CI 2.0–19.3,
Discussion
Finding a reliable and inexpensive indicator and predictor of IBD severity remains a challenge. This retrospective study is the first report on the relationships of CRP/albumin ratio with IBD characteristics in children. When patients were grouped into quiescent, mild, moderate, and severe disease according to the PCDAI, there was a difference in CRP/albumin ratio at diagnosis. However, the indicator seems to be useful only in discriminating between severe CD vs quiescent disease at diagnosis, showing 92.3% sensitivity and 84.1% specificity (AUC 0.94), whereas CRP/albumin ratio at diagnosis weakly differentiated patients with IBD in need of treatment escalation (biologics and surgery). In CD, CRP/albumin ratio at diagnosis was correlated weakly with age at first immunosuppressive treatment, time from diagnosis to first biological treatment, days spent in hospital, number of severe relapses, and PCDAI. Although these results were statistically significant, their clinical utility is limited. Serum CRP/albumin ratio at diagnosis was poor/moderate in differentiating patients with various disease localizations and behaviors, with the highest AUC for differentiating penetrating from non-penetrating disease behavior in CD.
Biomarkers that are easily available, simple to calculate, and objective are needed to rapidly describe the changing nature of pediatric IBD. Serum CRP/albumin ratio has been proposed as such a marker, but available reports come from adult cohorts. Previous studies conducted in adult patients cannot be directly compared with the results of the present study. In a single-center study from China (601 adults) [26], disease activity in CD was strongly correlated (rho=0.76) with CRP/albumin ratio, whereas in the present study, CRP/albumin ratio at diagnosis was only weakly (rho=0.38) correlated with the PCDAI at diagnosis. In patients who took immunosuppressants at diagnosis, this relationship was stronger but still moderate (rho=0.46). Sayar et al reported that AUC values of 0.941 (cut-off 0.6 and sensitivity 88.9%, specificity 90.3%) determined severe disease in adults [21]. In our study, the AUC for CRP/albumin ratio to differentiate severe disease from less severe at diagnosis was 0.697 (cut-off 0.3 and sensitivity 50%, specificity 76%) and this significant, but weak relationship was found only for patients with CD. This could be explained by disease duration. In a recent study of adults with UC from Japan, CRP/albumin ratio was a useful serum marker for disease activity only in patients who had the disease for more than 7 years [28]. Disease duration in our cohort was approximately 2 years (range 0–14 years).
Recent reports in adult UC showed promising results for using routinely obtained CRP/albumin ratio marker in therapy optimization and hence precision medicine in IBD. A CRP/albumin ratio cut-off of 0.37 after infliximab has accurately predicted need for colectomy, with AUC of 0.73, but the optimal timing of the test requires further research [27,29]. In adults, CRP/albumin ratio measurements are useful particularly in UC, but high CRP alone is associated with the disease phenotype and better response to biological treatment [46–49]. In children, CRP response can differ between individuals even in overt clinical disease. Further research is needed to determine whether this is dependent on CRP genotype.
One of the study limitations is the retrospective data collection. One must always consider the bias caused during data collection and by different approaches of attending clinicians. The measurement of high-sensitivity CRP would also increase the precision of analyses [50]. Calprotectin measurement would further help defining the severity of disease. Nevertheless, this is the first large study to show relationships between CRP/albumin ratio and IBD phenotype in children. We also included analysis for patients who used immunosuppressive agents at diagnosis, a fact rarely considered previously, which may affect the level of inflammatory markers. Despite robustness of the sample, prospective studies are required to confirm the role of this biomarker in children.
Conclusions
These findings add to previous studies carried out in adult patients and show that the CRP/albumin ratio at diagnosis was not significantly associated with the course of either CD or UC in children. However, CRP/albumin ratio could help to differentiate patients with severe CD from those with quiescent disease.
References
1. GBD 2017 Inflammatory Bowel Disease Collaborators, The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017: Lancet Gastroenterol Hepatol, 2020; 5(1); 17-30
2. Ng SC, Shi HY, Hamidi N: Lancet, 2017; 390; 2769-78
3. Kruis W, Katalinic A, Klugmann T, Predictive factors for an uncomplicated long-term course of Crohn’s disease: A retrospective analysis: J Crohns Colitis, 2013; 7; e263-70
4. Levine A, Koletzko S, Turner D, ESPGHAN revised Porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents: J Pediatr Gastroenterol Nutr, 2014; 58; 795-806
5. Henderson P, Casey A, Lawrence SJ, The diagnostic accuracy of fecal calprotectin during the investigation of suspected pediatric inflammatory bowel disease: Am J Gastroenterol, 2012; 107; 941-49
6. Czub E, Nowak JK, Moczko J, Fecal pyruvate kinase is not suitable for discrimination between inflammatory bowel disease exacerbation and acute gastroenteritis: Dev Period Med, 2015; 19; 167-73
7. Czub E, Nowak JK, Szaflarska-Poplawska A, Comparison of fecal pyruvate kinase isoform M2 and calprotectin in assessment of pediatric inflammatory bowel disease severity and activity: Acta Biochim Pol, 2014; 61; 99-102
8. Daniluk U, Daniluk J, Krasnodebska M, The combination of fecal calprotectin with ESR, CRP and albumin discriminates more accurately children with Crohn’s disease: Adv Med Sci, 2019; 64; 9-14
9. Cherfane CE, Gessel L, Cirillo D, Monocytosis and a low lymphocyte to monocyte ratio are effective biomarkers of ulcerative colitis disease activity: Inflamm Bowel Dis, 2015; 21; 1769-75
10. Akpinar MY, Ozin YO, Kaplan M, Platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio predict mucosal disease severity in ulcerative colitis: J Med Biochem, 2018; 37; 155-62
11. Feng J-R, Qiu X, Wang F, Diagnostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in Crohn’s disease: Gastroenterol Res Pract, 2017; 2017; 3526460
12. Şimşek-Onat P, Hizarcioglu-Gulsen H, Ergen YM, Neutrophil-to-lymphocyte ratio: An easy marker for the diagnosis and monitoring of inflammatory bowel disease in children: Dig Dis Sci, 2022 [Online ahead of print]
13. Chen R, Li L, Chao K, Platelet-to-lymphocyte percentage ratio index: A simple non-invasive index to monitor the endoscopic activity in Crohn’s disease: Therap Adv Gastroenterol, 2020; 13; 175628482097944
14. Henderson P, Kennedy NA, Van Limbergen JE, Serum C-reactive protein and CRP genotype in pediatric inflammatory bowel disease: influence on phenotype, natural history, and response to therapy: Inflamm Bowel Dis, 2015; 21; 596-605
15. Turner D, Mack DR, Hyams J, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) or both? A systematic evaluation in pediatric ulcerative colitis: J Crohns Colitis, 2011; 5; 423-29
16. Travis SP, Farrant JM, Ricketts C, Predicting outcome in severe ulcerative colitis: Gut, 1996; 38; 905-10
17. Con D, Andrew B, Nicolaides S, Biomarker dynamics during infliximab salvage for acute severe ulcerative colitis: C-reactive protein (CRP)-lymphocyte ratio and CRP-albumin ratio are useful in predicting colectomy: Intest Res, 2022; 20; 101-13
18. Fasanmade AA, Adedokun OJ, Olson A, Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis: Int J Clin Pharmacol Ther, 2010; 48; 297-308
19. Syal G, Robbins L, Kashani A, Hypoalbuminemia and bandemia predict failure of infliximab rescue therapy in acute severe ulcerative colitis: Dig Dis Sci, 2021; 66; 199-205
20. García-Bosch O, Aceituno M, Ordás I, Long-term follow-up of patients treated with infliximab for ulcerative colitis: Predictive factors of response-an observational study: Dig Dis Sci, 2016; 61; 2051-59
21. Sayar S, Kurbuz K, Kahraman R, A practical marker to determining acute severe ulcerative colitis: CRP/albumin ratio: North Clin Istanb, 2020; 7; 49-55
22. Tilakaratne S, Lemberg DA, Leach ST, C-reactive protein and disease activity in children with Crohn’s disease: Dig Dis Sci, 2010; 55; 131-36
23. Kaplan M, Ates I, Akpinar MY, Predictive value of C-reactive protein/albumin ratio in acute pancreatitis: Hepatobiliary Pancreat Dis Int, 2017; 16; 424-30
24. Tominaga T, Nonaka T, Sumida Y, The C-reactive protein to albumin ratio as a predictor of severe side effects of adjuvant chemotherapy in stage III colorectal cancer patients: PLoS One, 2016; 11; e0167967
25. Ranzani OT, Zampieri FG, Forte DN, C-reactive protein/albumin ratio predicts 90-day mortality of septic patients: PLoS One, 2013; 8; e59321
26. Chen Y-H, Wang L, Feng S-Y, The relationship between C-reactive protein/albumin ratio and disease activity in patients with inflammatory bowel disease: Gastroenterol Res Pract, 2020; 2020; 3467419
27. Qin G, Tu J, Liu L, Serum albumin and C-reactive protein/albumin ratio are useful biomarkers of Crohn’s disease activity: Med Sci Monit, 2016; 22; 4393-400
28. Furukawa S, Yagi S, Shiraishi K, Effect of disease duration on the association between C-reactive protein-albumin ratio and endoscopic activity in ulcerative colitis: BMC Gastroenterol, 2022; 22; 39
29. Choy MC, Seah D, Gorelik A, Predicting response after infliximab salvage in acute severe ulcerative colitis: J Gastroenterol Hepatol, 2018; 33; 1347-52
30. Denson LA, Curran M, McGovern DPB, Challenges in IBD research: Precision medicine: Inflamm Bowel Dis, 2019; 25; S31-39
31. Peyrin-Biroulet L, Defining severity in inflammatory bowel disease: Gastroenterol Hepatol (NY), 2015; 11; 474-76
32. Glapa-Nowak A, Szczepanik M, Kwiecień J, Insolation and disease severity in paediatric inflammatory bowel disease – a multi-centre cross-sectional study: J Clin Med, 2020; 9; 3957
33. Glapa-Nowak A, Bukowska-Posadzy A, Szczepanik M, Subjective psychophysical experiences in the course of inflammatory bowel disease – a comparative analysis based on the Polish Pediatric Crohn’s and Colitis Cohort (POCOCO): Int J Environ Res Public Health, 2021; 18; 784
34. IBD Working Group of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition, Inflammatory bowel disease in children and adolescents: recommendations for diagnosis – the Porto criteria: J Pediatr Gastroenterol Nutr, 2005; 41; 1-7
35. Van Assche G, Dignass A, Panes J, The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis: J Crohns Colitis, 2010; 4; 7-27
36. Magro F, Langner C, Driessen A, European consensus on the histopathology of inflammatory bowel disease: J Crohns Colitis, 2013; 7; 827-51
37. Turner D, Otley AR, Mack D, Development, validation, and evaluation of a pediatric ulcerative colitis activity index: A prospective multicenter study: Gastroenterology, 2007; 133; 423-32
38. Hyams JS, Ferry GD, Mandel FS, Development and validation of a pediatric Crohn’s disease activity index: J Pediatr Gastroenterol Nutr, 1991; 12; 439-47
39. Hyams J, Markowitz J, Otley A, Evaluation of the pediatric Crohn disease activity index: A prospective multicenter experience: J Pediatr Gastroenterol Nutr, 2005; 41; 416-21
40. Loonen HJ, Griffiths AM, Merkus MP, A critical assessment of items on the pediatric Crohn’s disease activity index: J Pediatr Gastroenterol Nutr, 2003; 36; 90-95
41. Turner D, Hyams J, Markowitz J, Appraisal of the pediatric ulcerative colitis activity index (PUCAI): Inflamm Bowel Dis, 2009; 15; 1218-23
42. Turner D, Levine A, Escher JC, Management of pediatric ulcerative colitis: Joint ECCO and ESPGHAN evidence-based consensus guidelines: J Pediatr Gastroenterol Nutr, 2012; 55; 340-61
43. Ruemmele FM, Veres G, Kolho KL, Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease: J Crohns Colitis, 2014; 8; 1179-207
44. Assa A, Rinawi F, Shamir R, The long-term predictive properties of the Paris Classification in paediatric inflammatory bowel disease patients: J Crohns Colitis, 2018; 12; 39-47
45. Jahnz-Różyk K, Kawalec P, Malinowski K, Drug policy in Poland: Value Health Reg Issues, 2017; 13; 23-26
46. Henriksen M, Jahnsen J, Lygren I, C-reactive protein: A predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study: Gut, 2008; 57; 1518-23
47. Thalmaier D, Dambacher J, Seiderer J, The +1059G/C polymorphism in the C-reactive protein (CRP) gene is associated with involvement of the terminal ileum and decreased serum CRP levels in patients with Crohn’s disease: Aliment Pharmacol Ther, 2006; 24; 1105-15
48. Reinisch W, Wang Y, Oddens BJ, C-reactive protein, an indicator for maintained response or remission to infliximab in patients with Crohn’s disease: A post-hoc analysis from ACCENT I: Aliment Pharmacol Ther, 2012; 35; 568-76
49. Jürgens M, Mahachie John JM, Cleynen I, Levels of C-reactive protein are associated with response to infliximab therapy in patients with Crohn’s disease: Clin Gastroenterol Hepatol, 2011; 9; 421-427e1
50. Kiss LS, Papp M, Lovasz BD, High-sensitivity C-reactive protein for identification of disease phenotype, active disease, and clinical relapses in Crohn’s disease: A marker for patient classification?: Inflamm Bowel Dis, 2012; 18; 1647-54
In Press
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952