08 December 2022: Clinical Research
Predicting Chemotherapy Resistance in Gestational Trophoblastic Neoplasia: Ratio of Neutrophils, Lymphocytes, Monocytes, and Platelets
Gatot Nyarumenteng Adhipurnawan Winarno1ABCDEF*, Ayu Insafi Mulyantari1ABCDEF, Andi Kurniadi1BD, Dodi Suardi1BD, Zulvayanti Zulvayanti1BD, Nurvita Trianasari2CDOI: 10.12659/MSM.938499
Med Sci Monit 2022; 28:e938499
Abstract
BACKGROUND: Neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and platelet-to-lymphocyte ratio (PLR) have recently been used as inflammatory biomarkers for cancer patients. This study aims to determine the role of pretreatment NLR, LMR, and PLR in predicting chemoresistance in gestational trophoblastic neoplasia (GTN) patients.
MATERIAL AND METHODS: A total of 129 low-risk and high-risk GTN patients who had received first-line chemotherapy were enrolled in this historical cohort study. The pretreatment NLR, LMR, and PLR values were analyzed to predict the resistance to first-line chemotherapy in low-risk and high-risk GTN patients.
RESULTS: Chemoresistant patients had significantly higher NLR than chemosensitive patients in low-risk and high-risk GTN patients (P<0.05). In high-risk GTN, patients with lower LMR and higher PLR tended to have chemoresistance to first-line chemotherapy (P=0.008, P=0.001). Univariate analysis revealed that the NLR, LMR, and PLR cut-off points of 2.654, 3.8, and 192.174, respectively, were associated with chemoresistance in high-risk GTN (P=0.0001, P=0.011, P=0.0001). The combination of NLR, PLR, and FIGO score in high-risk GTN was the best combination among other combinations with cut-off value >17 (P=0.001).
CONCLUSIONS: Higher NLR, lower LMR, and higher PLR were associated with chemoresistance for high-risk GTN patients. Furthermore, NLR, LMR, and PLR can improve the accuracy of predicting resistance to first-line chemotherapy in high-risk GTN.
Keywords: Antineoplastic Combined Chemotherapy Protocols, Biomarkers, Pharmacological, gestational trophoblastic disease, Humans, Pregnancy, Female, Neutrophils, Monocytes, Cohort Studies, Lymphocytes
Background
The incidence of gestational trophoblastic neoplasia (GTN) is higher in developing countries, with a mortality rate of as high as 50% for ultra-high-risk GTN. Control of this condition is still unsatisfactory since the tumor can affect women at any reproductive age who need to preserve their reproductive functions. However, GTN tumors are generally highly responsive to chemotherapy, compared with many other malignant tumors, with a 75–100% cure rate [1,2].
The International Federation of Gynecology and Obstetrics (FIGO) scoring system classifies GTN into low-risk and high-risk. This scoring system is widely used to determine whether the patient will need single or multi-agent chemotherapy. GTN patients with scores of 6 or less are at low-risk and are treated with single-agent chemotherapy, while GTN patients with scores of 7 or greater are at high-risk and require multi-agent chemotherapy [1]. The risk for premature menopause was reported to be higher when women were treated with multi-agent chemotherapy [3].
The FIGO scoring system considers various risk factors: age, antecedent pregnancy, pretreatment serum hCG, size of the tumor, site of metastases, number of metastases, and previously failed chemotherapy. For some risk factors, tumor size may not independently act as a predictor of chemotherapy response in GTN patients. Lack of radiologic equipment can interfere with optimal diagnosis of metastases in some developing countries [4]. In addition, despite an excellent response from chemotherapy based on the scoring system, approximately 25% of GTN tumors developed resistance to first-line chemotherapy or relapsed after completion of initial therapy [5].
The chemoresistance occurs mainly in patients with multiple metastases and sites of metastases other than the lung and vagina due to inadequate initial therapy. Salvage therapy or surgery may be considered for patients with those risk factors [6]. However, previous clinical reviews have shown that the state of inflammation within the tumor can impact the tumor’s response to the anticancer agent. Accumulating evidence has shown that some inflammatory biomarkers, such as the absolute count of neutrophils, lymphocytes, and monocytes, are associated with carcinogenesis and cancer progression. In addition, neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and platelet-to-lymphocyte ratio (PLR) are associated with prognosis of several types of cancer [7,8].
Therefore, we proposed using routine laboratory testing to predict chemotherapy response in GTN patients. This study aimed to examine the role of the pretreatment systemic inflammatory index NLR, LMR, and PLR in predicting chemotherapy resistance and improving therapeutics strategy by surgery or salvaging chemotherapy in GTN patients.
Material and Methods
STUDY DESIGN AND PATIENTS:
All GTN patients diagnosed from January 2017 to March 2022 at Dr. Hasan Sadikin Central General Hospital, Bandung, Indonesia, were identified for this historical cohort study. Additional inclusion criteria were: patients newly diagnosed with low-risk and high-risk GTN according to the FIGO 2000, receiving first-line chemotherapy according to the FIGO scoring system, and having at least 6 months of follow-up after completing chemotherapy for the chemosensitive group. The exclusion criteria were GTN patients with chronic disease, had another primary cancer, incomplete data, and lost to follow-up. This study was conducted after obtaining ethical clearance issued by the Ethics Committee of Dr. Hasan Sadikin Central General Hospital Bandung (LB.02.01/X.6.5/59/2022). Since this study was retrospective, informed consent was not required.
TREATMENT PROTOCOL:
Methotrexate (MTX) was used as single-agent chemotherapy for the low-risk group. The MTX dose was 0.4 mg/Kg intravenous (i.v.) or intramuscular (i.m.) for 5 days every 14 days, with a maximum dose of 25 mg. When the normalization hCG level occurred, an additional 2–3 cycles of consolidation therapy were performed to prevent recurrence.
Multi-agent chemotherapy was performed in the high-risk group. The combination used was Etoposide 100 mg/m2/day i.v. infusion for day 1 and day 2, Actinomycin-D 0.5 mg i.v. bolus for day 1 and day 2, MTX 300 mg/m2 i.v. infusion for 12 h, Cyclophosphamide 600 mg/m2 for day 8, i.v. infusion, Vincristine 0.8 mg/m2 i.v. bolus for day 8. Additional 15 mg i.m. folinic acid rescue was given on day 2. The cycle was repeated after14 days, and the chemotherapy cycle depended on the hCG level. An additional 2–3 cycles of consolidation were needed after normalization of hCG [1].
DATA COLLECTION:
Data on demographics and clinical features were collected from patient’s medical records, including patient’s age, disease state (GTN stage, and FIGO score), chemotherapy agent defined as single-agent (methotrexate) and multi-agent chemotherapy (Etoposide, Methotrexate, Actinomycin-D, Cyclophosphamide, and Vincristine, known EMA/CO), and chemotherapy response (chemoresistance, chemosensitive, relapse). We determined GTN classification according to FIGO score: 6 and less as low-risk, and 7 and greater as high-risk. hCG level <5 IU/L was set as the cut-off for a normal value. Chemosensitive was defined as normalization of hCG levels for at least 3 consecutive weeks. Chemoresistance was defined as plateauing hCG (<10% change) over 3 cycles of therapy or at least 1 increasing hCG (>10%) over 2 cycles or if significant toxicity has occurred [1,6,9].
We obtained the laboratory data from the hospital database, including platelet (Plt), Absolute Neutrophil Count (ANC), Absolute Lymphocyte Count (ALC), and Absolute Monocyte Count (AMC), which were collected from blood examinations performed routinely on every patient before starting treatment. NLR was calculated by dividing ANC by ALC, LMR by dividing ALC with AMC, and PLR by dividing Plt with ALC.
STATISTICAL ANALYSIS:
Demographic and clinical features (age, GTN stage, chemotherapy agent, laboratory findings) were compared to chemotherapy response using the
Results
DEMOGRAPHIC AND CLINICAL FEATURES:
A total of 277 patients were diagnosed with GTN from January 2017 until March 2022. The excluded cases were as follows: 3 (0.3%) GTN patients with chronic disease, 125 (45%) with incomplete data, and 20 (7.2%) lost to follow-up. The remaining 129 patients were included for final analysis, both for low-risk (N=66) and high-risk GTN (N=63). The median age of the patients was 35 years. Most of the patients were diagnosed in stage I (79.8%), followed by stage III (15.5%). There was no significant difference in the demographic and clinical features of the patients with the patient’s response to chemotherapy in this study (Table 1).
LOW-RISK GTN:
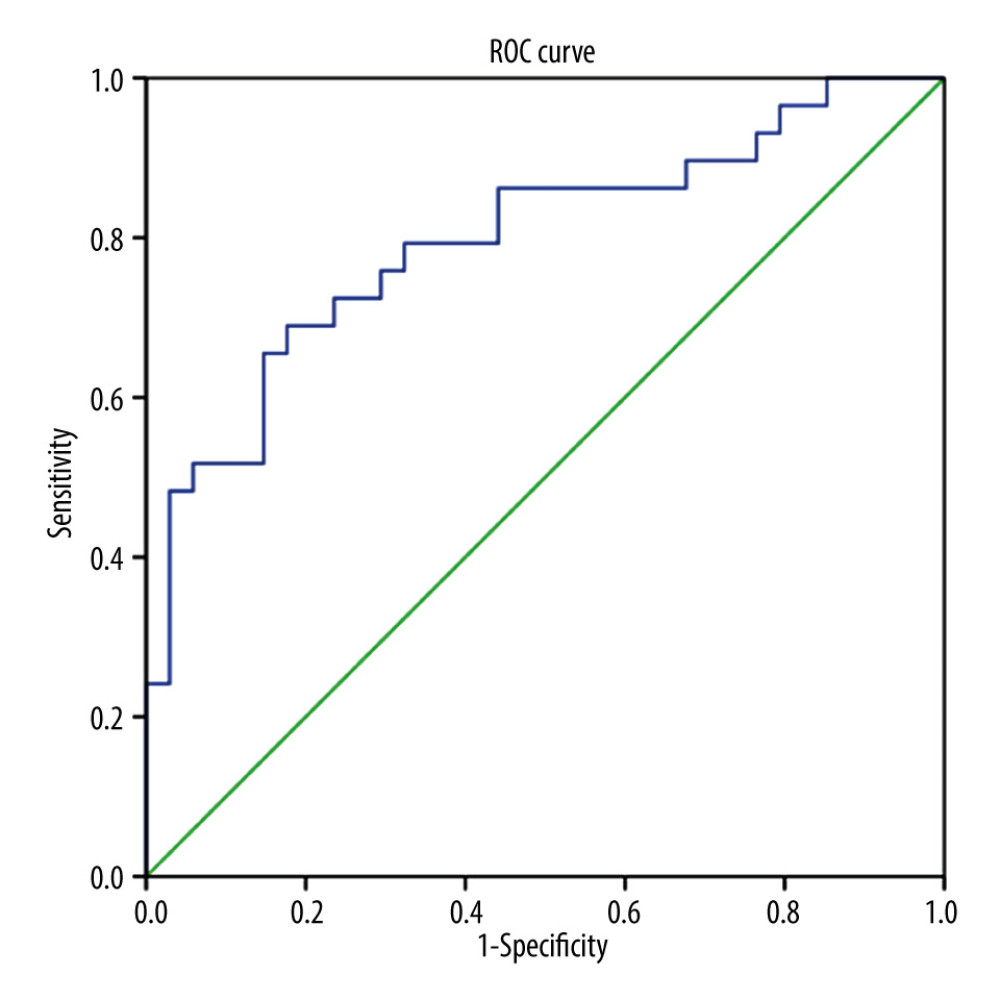
For low-risk GTN, the pretreatment NLR, LMR, and PLR were analyzed with the chemotherapy response. NLR was found to be significantly higher in chemoresistant (N=32) compared to chemosensitive (N=34) patients (4.06±5.268 vs 2.11±0.671, P=0.001), but we found no significant differences for LMR and PLR between these 2 groups (P>0.05) (Table 2). We also found that chemoresistant patients had significantly higher FIGO scores. ROC curve analysis (Figure 1) showed NLR was a predictor for chemoresistance in GTN, with 68.8% sensitivity, 70% specificity, 68.8% PPV, 70.6% NPV, and 74.5% AUC. The optimal cut-off point for NLR was 2.434 (Table 3).
HIGH-RISK GTN:
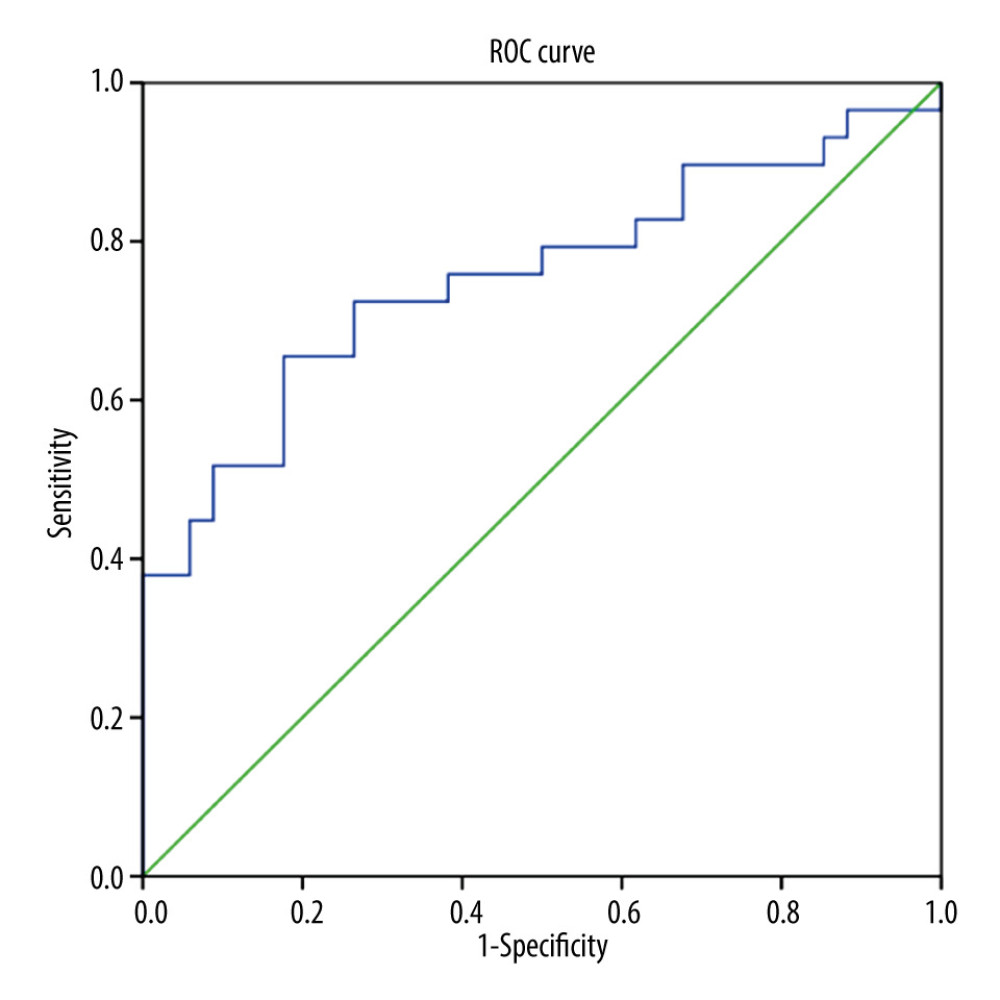
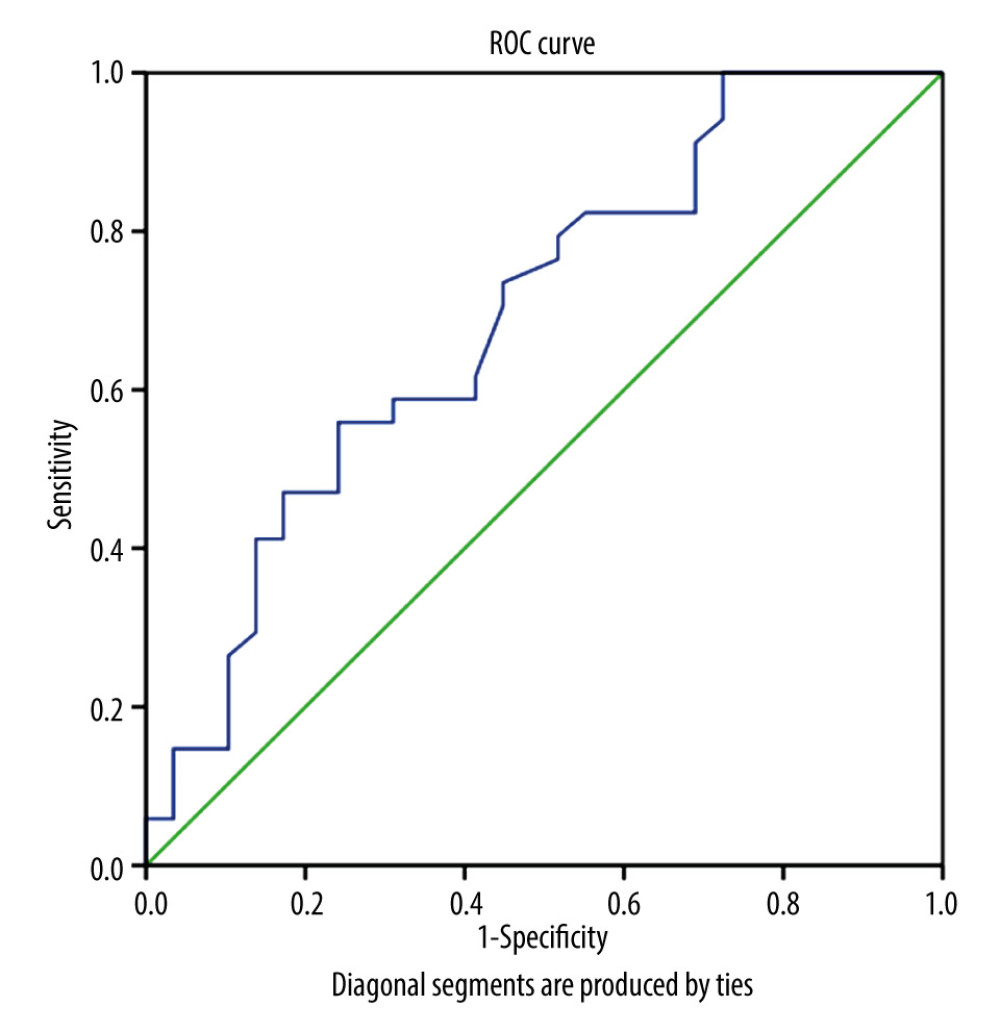
In high-risk GTN, the NLR, LMR, and PLR were significantly different between the chemoresistant and chemosensitive groups. The mean value of NLR and PLR were higher in the chemoresistant group (N=29) compared to the chemosensitive group (N=34) (4.96±3.403 vs 2.24±1.138 and 264.27±135.549 vs 164.44±57.064, respectively), in contrast with mean LMR value, which was lower in the chemoresistant group than in chemosensitive group (4.16±1.649 vs 3.03±1.576) (Table 2). ROC curve analysis showed that NLR, LMR, and PLR were predictors of chemoresistance in high-risk GTN patents (Figures 1–3). NLR had the highest sensitivity, specificity, and AUC, among other relevant ratios (Table 3).
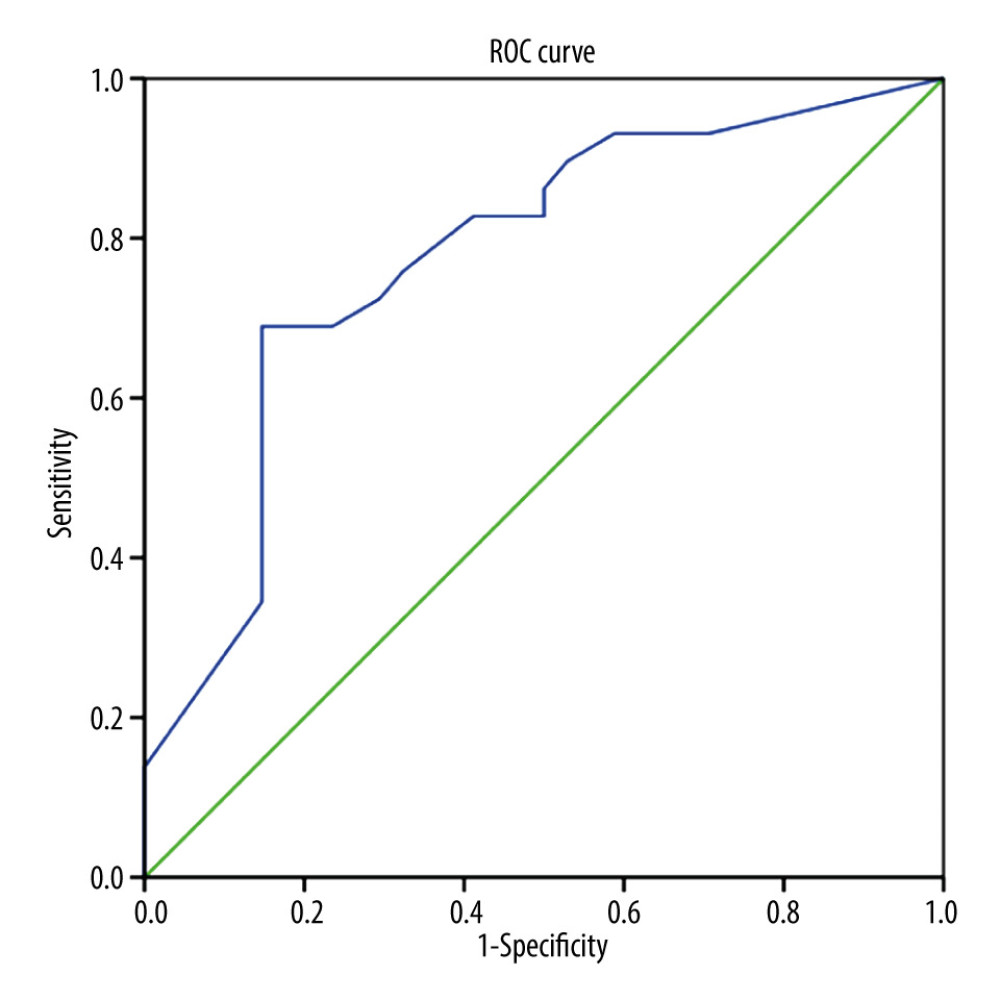
For high-risk GTN, the combination of the pre-chemotherapy NLR, LMR, PLR, and FIGO score were analyzed. We found that only the combination of FIGO score, NLR, and PLR was significantly different (P<0.05). We found no significant differences in the combination of FIGO score, NLR, and LMR, or FIGO score, LMR, and PLR (P>0.05) (Table 4). Furthermore, we found that the combination of FIGO score, NLR, and PLR was associated with chemoresistance (P=0.0001) and the cut-off point of 17 had 72.4% sensitivity, 70.6% specificity, and AUC value 77.6% in predicting chemoresistance (Figure 4).
Discussion
To the best of our knowledge, this is the first study that evaluates the utility of NLR, PLR, and LMR in predicting the chemoresistance of GTN patients. Our study successfully collected data from 129 GTN patients from 2017 to 2022. Unfortunately, we lost a large amount of laboratory data in the first 2 years because the system of laboratory results was not yet computer-based. From 129 patients, about 48% of patients were diagnosed as high-risk GTN according to FIGO score. This result differed from another study conducted in Japan, which found that the GTN incidence for low-risk GTN was 3 times higher than for high-risk GTN. We suspect this is because almost all cancer patients in Indonesia came to the hospital at an advanced stage. In addition, for GTN cases, poor follow-up compliance after molar evacuation also might be related to this finding [10].
We found that higher NLR was associated with chemoresistance in patients with low-risk and high-risk FIGO scores. Therefore, the NLR might be a promising predictor of chemoresistance in GTN patients. However, in our study, low LMR and high PLR were only predictive of chemoresistance in high-risk GTN patients.
Recent studies have reported a correlation between the systemic inflammatory response and cancer progression, including response to chemotherapy. However, the underlying mechanism remains unclear. Some internal factors that may affect the efficacy of chemotherapy are the immune state, as well as the pro-tumor and anti-tumor state, of the host. Moreover, when chronic inflammation occurs, the production of cytokines and other inflammatory factors can impact the tumor’s response to chemotherapy [11,12]. Remodeling of the extracellular matrix due to inflammatory signals leads to stromal matrix deposition. Increased tissue stiffness is the consequence of tissue thickness alteration. These changes can interfere with the effectiveness of delivering therapeutic agents [7].
Components of systemic inflammatory response such as lymphocytes, neutrophils, monocytes, and platelet have both pro-tumor and anti-tumor effects. As a pro-tumor agent, monocytes promote angiogenesis and suppress T cell function. However, monocytes also act as an anti-tumor agent by generating tumor cytotoxicity and preventing metastasis [13,14]. Lymphocytes are essential for the inflammation process within the tumor, tumor progression, and tumor growth. Conversely, a study in China showed that lymphocytes also had a protective role by inhibiting the development of thyroid tumors [15,16].
Metabolic stress caused by inflammation or malignancy tends to increased ANC. Increased numbers of neutrophils would physiologically inhibit lymphocyte activity, causing a higher NLR [14,17]. This theory is in line with the results of the present study, which found the NLR value is higher in chemoresistant patients in both high-risk and low-risk GTN. In our study, higher NLR occurred because of high neutrophil counts and low lymphocyte counts in both low-risk and high-risk GTN.
In addition to being associated with poorer prognosis in patients with a solid tumor, increased NLR is also related to poor chemotherapy response rates in prostate cancer [18]. Ayako et al evaluated ALC and NLR value in patients with metastatic breast cancer and revealed that a high NLR and ALC is correlated with poor survival. Our study found that NLR was significantly higher in chemoresistant patients than in chemosensitive patients. NLR alone also can be used as a chemoresistance predictor since it has good sensitivity and specificity [19]. This result is in line with a study conducted by Ali et al, which showed that higher NLR can be used as a biomarker of invasion in gestational trophoblastic disease (GTD) [20].
Low LMR can be presented as a low lymphocyte or high monocyte value. Lymphocytes play a role in leading inflammatory processes in tumor and cytotoxic cell death. Recent studies reported that ALC is associated with the prognosis of various solid tumors, such as ovarian and cervical cancer. Conversely, since monocytes can differentiate into tumor-associated macrophages (TAM), accumulation of monocytes can lead to metastasis. Higher monocytes counts are associated with poorer survival for some cancers [20,21].
Our study found that low LMR was presented as low lymphocyte counts and high monocyte counts in high-risk GTN, and it was associated with chemoresistance. In low-risk GTN, the lymphocyte count in chemoresistant patients was not significantly lower than in chemosensitive patients, and the monocyte count in chemoresistant patients was not significantly higher than in chemosensitive patients. Thus, we found that LMR in low-risk GTN was not significantly lower in chemoresistant patients.
Low LMR can also be used as a chemoresistance predictor, even though it has lower sensitivity and specificity than NLR. This finding is in line with other studies and with accepted theory.
Previous studies have demonstrated increased platelet count as an indicator of enhanced tumor activity and angiogenesis [22]. Our study showed that higher PLR is associated with chemoresistance in high-risk GTN. PLR also acts as a predictor of chemoresistance occurrence in high-risk GTN. This finding is in line with other studies. A meta-analysis by Ma et al showed that increased PLR was associated with poor prognosis in ovarian cancer patients [23]. Poor overall survival and disease-free survival are also correlated with high PLR in gastric cancer [24].
In addition, our study also evaluated use of the FIGO score to determine chemotherapy options for the patients. We found that a higher FIGO score was associated with chemoresistance in low-risk GTN. This is an exciting finding since the oncologist used the FIGO score to select use of single- or multi-agent chemotherapy for GTN patients. Future studies with larger samples are needed to evaluate or refine the cut-off value for low-risk and high-risk GTN.
This is the first study to evaluate the combination of NLR, LMR, PLR, and FIGO score as predictors for chemoresistance in high-risk GTN patients, with good sensitivity and specificity. These biomarkers can be measured from simple routine blood examinations. Moreover, identifying these biomarkers might contribute to improving the therapeutic strategy in GTN patients. Surgery or salvage chemotherapy can be an option when clinicians manage patients with poor biomarkers.
The limitations of this study are the nature of the retrospective study and the small number of patients due to lost to follow-up and incomplete data, and the missing data might have biased our results. Also, patients with other inflammatory conditions needed to be excluded since NLR, LMR, and PLR are non-specific inflammatory biomarkers.
Despite its limitations, this study can be used as a first step for other researchers to improve the therapeutic strategy for GTN patients. In addition, NLR, LMR, and PLR individually appear to have utility as predictors in high-risk GTN patients, and the combination of NLR, PLR, and FIGO score can be used as a predictor as well.
Conclusions
In conclusion, the pretreatment NLR, LMR, and PLR values were associated with chemoresistance in high-risk GTN patients.
Tables
Table 1. Comparison of characteristics and clinical features of study patients with chemotherapy outcome in GTN patients at Hasan Sadikin Hospital.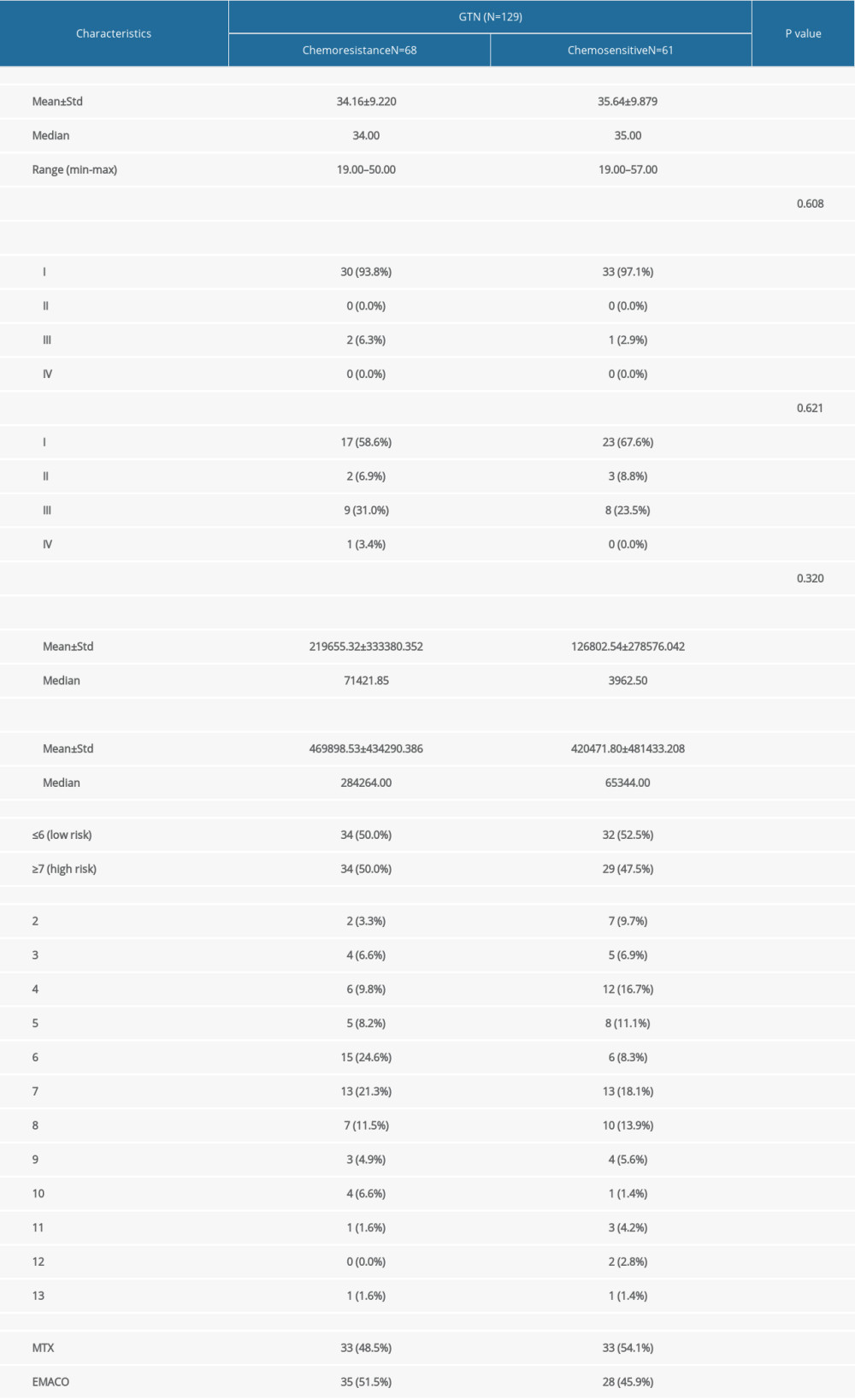 Table 2. Comparison of pre-chemotherapy NLR, LMR, PLR, and FIGO score with chemotherapy outcome in low-risk and high-risk GTN at Hasan Sadikin Hospital.
Table 2. Comparison of pre-chemotherapy NLR, LMR, PLR, and FIGO score with chemotherapy outcome in low-risk and high-risk GTN at Hasan Sadikin Hospital.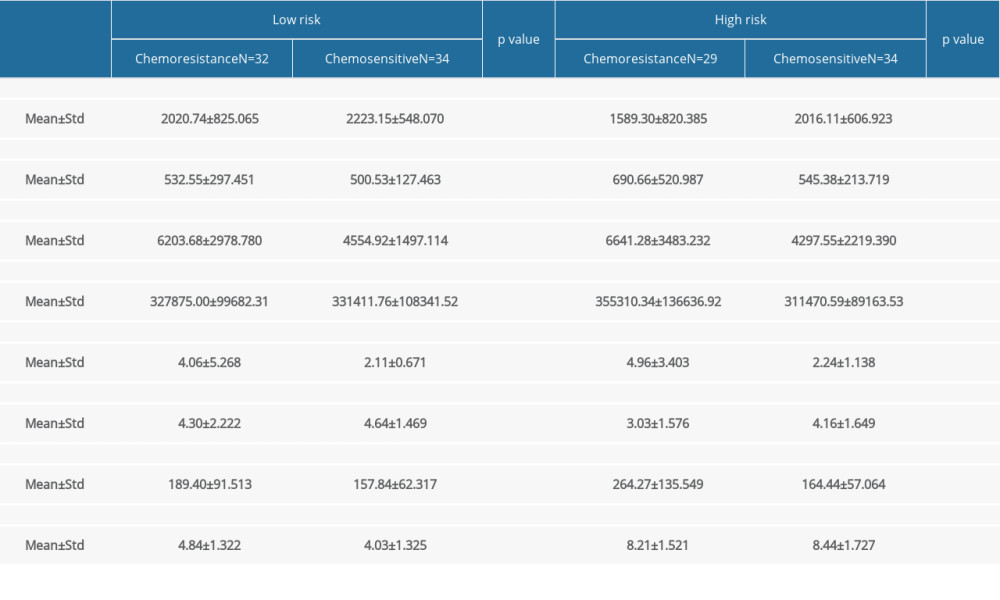 Table 3. Relationship between pre-chemotherapy ALC, AMC, ANC, NLR, LMR, PLR, FIGO score and chemotherapy outcome in high-risk GTN at Hasan Sadikin Hospital.
Table 3. Relationship between pre-chemotherapy ALC, AMC, ANC, NLR, LMR, PLR, FIGO score and chemotherapy outcome in high-risk GTN at Hasan Sadikin Hospital.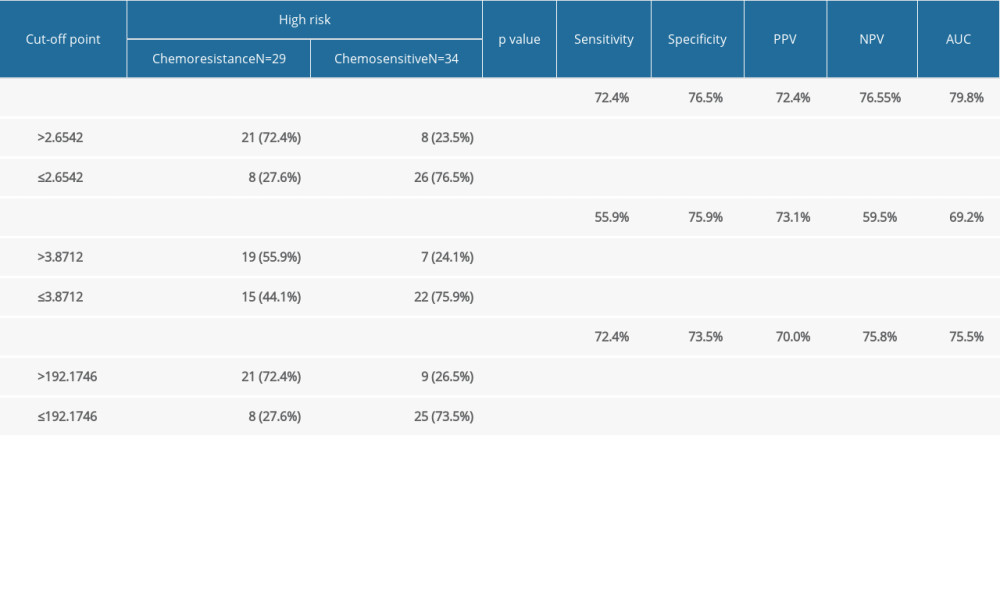 Table 4. Comparison of combination of FIGO score, NLR, LMR, and PLR with chemotherapy outcome in high-risk GTN at Hasan Sadikin Hospital.
Table 4. Comparison of combination of FIGO score, NLR, LMR, and PLR with chemotherapy outcome in high-risk GTN at Hasan Sadikin Hospital.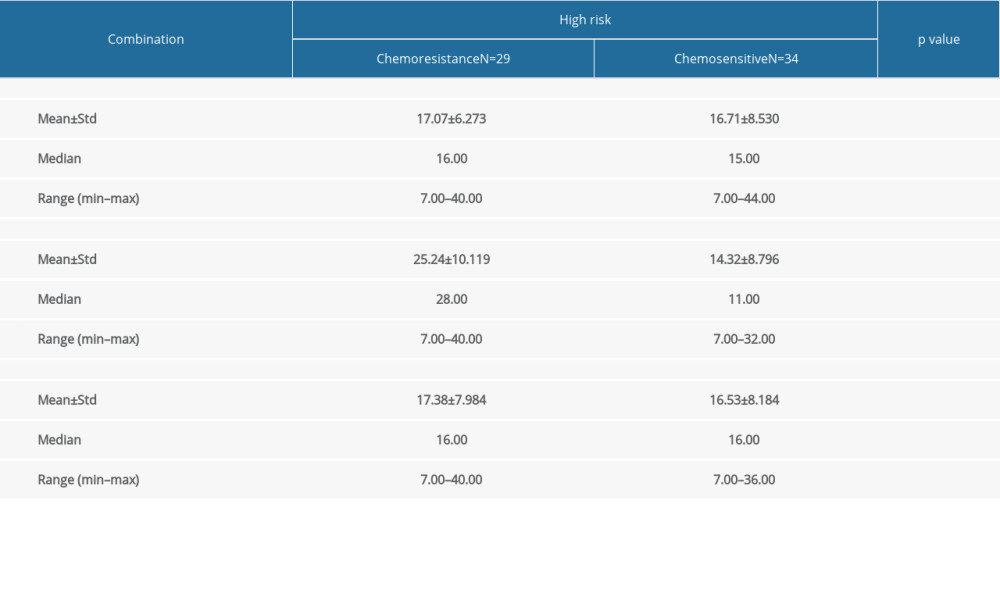
References
1. Ngan HYS, Seckl MJ, Berkowitz RS, Update on the diagnosis and management of gestational trophoblastic disease: Int J Gynecol Obstet, 2018; 143; 79-85
2. Choi MC, Joneborg U, Lee C, Kim SJ, Epidemiology: Gestational trophoblastic disease; 22
3. , Management of Gestational Trophoblastic Disease: Green-top Guideline No. 38 – June 2020: BJOG: Int J Obstet Gy, 2021; 128(3); e1-e27 Available from: https://onlinelibrary.wiley.com/doi/10.1111/1471-0528.16266
4. Eysbouts YK, Ottevanger PB, Massuger LFAG, Can the FIGO 2000 scoring system for gestational trophoblastic neoplasia be simplified? A new retrospective analysis from a nationwide dataset: Ann Oncol, 2017; 28(8); 1856-61
5. Alazzam M, Tidy J, Osborne R, Chemotherapy for resistant or recurrent gestational trophoblastic neoplasia: Cochrane Database Syst Rev, 2012; 12; CD008891
6. David M: NCCN Clinical practice guidelines in oncology: Gestational trophoblastic neoplasia, 2022, National Comprehensive Cancer Network
7. Olive KP, Fanning the flames of cancer chemoresistance: Inflammation and anticancer therapy: J Oncol Pract, 2017; 13(3); 181-83
8. Lu C, Zhou L, Ouyang J, Yang H, Prognostic value of lymphocyte-to-monocyte ratio in ovarian cancer: A meta-analysis: Medicine, 2019; 98(24); e15876
9. Lok C, van Trommel N, Massuger L, Practical clinical guidelines of the EOTTD for treatment and referral of gestational trophoblastic disease: Eur J Cancer, 2020; 130; 228-40
10. Yamamoto E, Nishino K, Niimi K, Ino K, Epidemiologic study on gestational trophoblastic diseases in Japan: J Gynecol Oncol, 2022; 33; e72
11. Balkwill F, Mantovani A, Inflammation and cancer: Back to Virchow?: Lancet, 2001; 357(9255); 539-45
12. Bracci L, Schiavoni G, Sistigu A, Belardelli F, Immune-based mechanisms of cytotoxic chemotherapy: Implications for the design of novel and rationale-based combined treatments against cancer: Cell Death Differ, 2014; 21(1); 15-25
13. Olingy CE, Dinh HQ, Hedrick CC, Monocyte heterogeneity and functions in cancer: J Leukoc Biol, 2019; 106(2); 309-22
14. Ocana A, Nieto-Jiménez C, Pandiella A, Templeton AJ, Neutrophils in cancer: Prognostic role and therapeutic strategies: Mol Cancer, 2017; 16(1); 137
15. Chen L, Kong X, Wang Z, Pretreatment systemic inflammation response index in patients with breast cancer treated with neoadjuvant chemotherapy as a useful prognostic indicator: Cancer Manag Res, 2020; 12; 1543-67
16. Feng J, Wang Y, Shan G, Gao L, Clinical and prognostic value of neutrophil-lymphocyte ratio for patients with thyroid cancer: A meta-analysis: Medicine, 2020; 99(20); e19686
17. Yoon NB, Son C, Um SJ, Role of the neutrophil-lymphocyte count ratio in the differential diagnosis between pulmonary tuberculosis and bacterial community-acquired pneumonia: Ann Lab Med, 2013; 33(2); 105-10
18. Templeton AJ, Pezaro C, Omlin A, Simple prognostic score for metastatic castration-resistant prostate cancer with incorporation of neutrophil-to-lymphocyte ratio: Prognostic Score with NLR in CRPC: Cancer, 2014; 120(21); 3346-52
19. Ueno A, Maeda R, Kin T, Utility of the absolute lymphocyte count and neutrophil/lymphocyte ratio for predicting survival in patients with metastatic breast cancer on eribulin: A real-world observational study: Chemotherapy, 2019; 64(5–6); 259-69
20. Guzel AI, Kokanali MK, Erkilinc S, Predictive role of the neutrophil lymphocyte ratio for invasion with gestational trophoblastic disease: Asian Pac J Cancer Prev, 2014; 15(10); 4203-6
21. Kwon BS, Jeong DH, Byun JM, Prognostic value of preoperative lymphocyte-monocyte ratio in patients with ovarian clear cell carcinoma: J Cancer, 2018; 9(7); 1127-34
22. Jiang L, Luan Y, Miao X, Platelet releasate promotes breast cancer growth and angiogenesis via VEGF-integrin cooperative signalling: Br J Cancer, 2017; 117(5); 695-703
23. Ma XM, Sun X, Yang GW, The platelet-to-lymphocyte ratio as a predictor of patient outcomes in ovarian cancer: A meta-analysis: Climacteric, 2017; 20(5); 448-55
24. Zhang X, Zhao W, Yu Y, Clinicopathological and prognostic significance of platelet-lymphocyte ratio (PLR) in gastric cancer: An updated meta-analysis: World J Surg Oncol, 2020; 18(1); 191
Figures
Tables
 Table 1. Comparison of characteristics and clinical features of study patients with chemotherapy outcome in GTN patients at Hasan Sadikin Hospital.
Table 1. Comparison of characteristics and clinical features of study patients with chemotherapy outcome in GTN patients at Hasan Sadikin Hospital. Table 2. Comparison of pre-chemotherapy NLR, LMR, PLR, and FIGO score with chemotherapy outcome in low-risk and high-risk GTN at Hasan Sadikin Hospital.
Table 2. Comparison of pre-chemotherapy NLR, LMR, PLR, and FIGO score with chemotherapy outcome in low-risk and high-risk GTN at Hasan Sadikin Hospital. Table 3. Relationship between pre-chemotherapy ALC, AMC, ANC, NLR, LMR, PLR, FIGO score and chemotherapy outcome in high-risk GTN at Hasan Sadikin Hospital.
Table 3. Relationship between pre-chemotherapy ALC, AMC, ANC, NLR, LMR, PLR, FIGO score and chemotherapy outcome in high-risk GTN at Hasan Sadikin Hospital. Table 4. Comparison of combination of FIGO score, NLR, LMR, and PLR with chemotherapy outcome in high-risk GTN at Hasan Sadikin Hospital.
Table 4. Comparison of combination of FIGO score, NLR, LMR, and PLR with chemotherapy outcome in high-risk GTN at Hasan Sadikin Hospital. Table 1. Comparison of characteristics and clinical features of study patients with chemotherapy outcome in GTN patients at Hasan Sadikin Hospital.
Table 1. Comparison of characteristics and clinical features of study patients with chemotherapy outcome in GTN patients at Hasan Sadikin Hospital. Table 2. Comparison of pre-chemotherapy NLR, LMR, PLR, and FIGO score with chemotherapy outcome in low-risk and high-risk GTN at Hasan Sadikin Hospital.
Table 2. Comparison of pre-chemotherapy NLR, LMR, PLR, and FIGO score with chemotherapy outcome in low-risk and high-risk GTN at Hasan Sadikin Hospital. Table 3. Relationship between pre-chemotherapy ALC, AMC, ANC, NLR, LMR, PLR, FIGO score and chemotherapy outcome in high-risk GTN at Hasan Sadikin Hospital.
Table 3. Relationship between pre-chemotherapy ALC, AMC, ANC, NLR, LMR, PLR, FIGO score and chemotherapy outcome in high-risk GTN at Hasan Sadikin Hospital. Table 4. Comparison of combination of FIGO score, NLR, LMR, and PLR with chemotherapy outcome in high-risk GTN at Hasan Sadikin Hospital.
Table 4. Comparison of combination of FIGO score, NLR, LMR, and PLR with chemotherapy outcome in high-risk GTN at Hasan Sadikin Hospital. In Press
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952