23 December 2022: Clinical Research
Factors for In-Hospital Mortality in 145 Male Patients with Fournier’s Gangrene: A 10-Year Observational Study from a Single Tertiary Referral Center in Indonesia
Yufi Aulia Azmi12ABCDEF, Firas Farisi AlkaffDOI: 10.12659/MSM.938578
Med Sci Monit 2022; 28:e938578
Abstract
BACKGROUND: Fournier’s gangrene (FG) is a potentially fatal necrotizing infection. Due to the rapid progression of the disease, the fatality rate remains high despite advances in therapy. This 10-year observational study from a single tertiary referral center in Indonesia aimed to identify the risk factors for in-hospital mortality from 145 male patients diagnosed with FG.
MATERIAL AND METHODS: This retrospective cohort study was conducted at one of Indonesia’s largest tertiary referral hospitals. The risk factors of in-hospital mortality were analysed using data collected through hospital medical records. All patients diagnosed with FG from January 2012 until December 2021 were included. Outcome measured was sociodemographic factors, comorbidities, laboratory findings, length of stay, culture results, and disease outcome. The microbiological culture was performed on FG lesions isolates. The statistical analysis was conducted using SPSS version 26.0.
RESULTS: The analysis included 145 male patients with a median age of 52 (IQR, 43-61) years. Of them, 38 (26.20%) patients died. There were more patients with diabetes mellitus (DM) in non-survivor groups compared to survivor groups (76.3% vs 57%, p=0.035). On multivariate analysis, DM and Clostridium perfringens infection were found to be independent factors of in-hospital mortality [adjusted odds ratio (aOR)2.583, 95% confidence interval (CI)=1.061-6.289, aOR 5.982,95% CI=1.241-28.828, respectively].
CONCLUSIONS: The mortality rate for FG was considerably high. DM and Clostridium perfringens infection were shown to be independent risk factors for mortality among men.
Keywords: Adolescent Health, Gangrene, Infectious Disease Medicine, Mortality, Risk Factors, Humans, Male, Adult, Middle Aged, Fournier Gangrene, Tertiary Care Centers, Hospital Mortality, Indonesia, Diabetes Mellitus, Clostridium Infections
Background
Fournier’s gangrene (FG) is a sporadic disease that rapidly spreads. It involves a potentially fatal necrotizing infection of soft tissues that most often affects the external genitalia and perineum but may also affect the abdominal wall and thighs [1]. Although the main etiology of FG remains unclear, the probable underlying causes are anorectal illnesses, urogenital anomalies, and trauma [1]. Infectious diseases potentially provoke high mortality among FG patients. Most bacteria identified as causes of FG are
The diagnosis of Fournier’s gangrene can be assisted by a combination of blood and imaging studies, although the primary diagnosis is clinical. Clinicians should maintain a high suspicion for any inflammatory or infectious process involving the perineum or genitals, especially in older diabetic men and others at high risk [5]. Fournier’s gangrene is managed with surgical intervention and medical resuscitation, as the patient is often septic and in shock [6].
Due to the rapid progression of the disease, the fatality rate remains high despite early surgical interventions, advances in critical care, and new medications [7,8]. In the latest review, the mortality in high-income countries rates between 20% and 40% (Sorensen and Krieger 2016). In developing countries, the mortality rate was between 17% and 28% [9,10]. Several variables have been identified as risk factors for mortality in patients with FG, including older age, congestive heart failure, renal failure, and coagulopathy [7]. Additionally, laboratory indicators such as hematocrit, serum sodium, and serum potassium are also significantly associated with mortality [11–13].
Considering that men are more likely to have FG and that the mortality rates of FG is high, we believe that it is necessary to evaluate the mortality rates and to explore the risk factors associated with in-hospital mortality among male FG patients. This 10-year observational study from a single tertiary referral center in Indonesia aimed to identify the risk factors for in-hospital mortality from 145 male patients diagnosed with FG.
Material and Methods
DATA COLLECTION:
Sociodemographic factors, comorbidities, laboratory findings, length of stay, culture results, and the outcome were collected from the patient medical record. Microbiological culture was performed on FG lesions isolates. FG severity index (FGSI) was manually scored based on the data from the medical records [14]. The diagnosis of FG was based on the presence of pain, erythema, ulcers, swelling, crepitus, necrosis, and purulent discharge found in the emergency room and confirmed by tissue inspection in the operating room. Mortality was defined as death during the hospital stay.
STATISTICAL ANALYSIS:
The statistical analysis was conducted using the SPSS version 26.0 (IBM Corp., Armonk, N.Y., USA). Data normality was determined using one-sample Kolmogorov-Smirnov test. Data was presented as mean±standard deviation (SD) for normally distributed data, as median [interquartile range (IQR)] for skewed data, and as frequency (percentage) for nominal data. Independent t-test, Mann-Whitney test, chi-square test, and Fisher’s exact test were used as appropriate. Two step logistic regression analyses were performed with in-hospital mortality as the outcome for the risk factor analysis. In the first step, univariate logistic regression analysis was performed for the clinical characteristics and the culture results. In this step, crude odds ratio (cOR) was obtained. In the second step, backward multivariate logistic regression analysis was performed, by including all variables with p-values <0.05 from the univariate analysis. In this step, an adjusted odds ratio (aOR) was obtained. Variables with a p-value <0.05 from the multivariate logistic regression analysis were considered the independent risk factor for in-hospital mortality.
Results
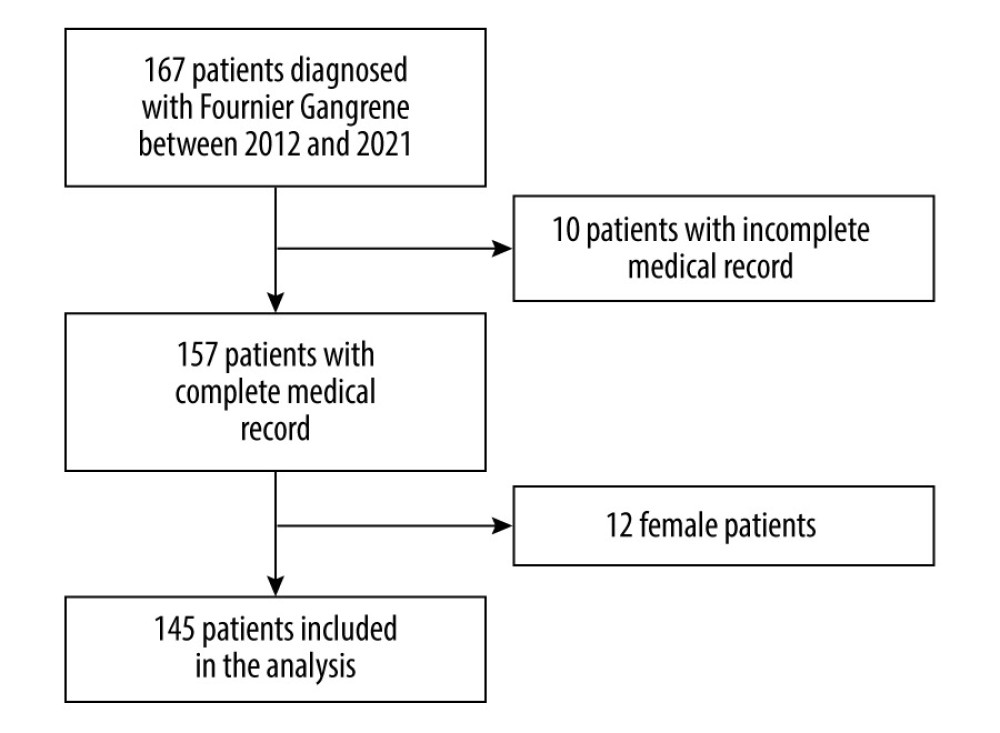
One hundred sixty-seven patients with FG were admitted to the hospital between January 2012 and December 2021. Of them, 145 were included in the analysis (Figure 1). The median age of the study population was 52 (43–61) years. Over half of the FG was in the scrotum. The prevalence rate for in-hospital mortality was 26.2%. The median length of stay in the hospital was 12 (5–23) days. The length of stay was longer in survivor than in non-survivor patients (12 (7–23) days vs 8 (4–19) days,
In the univariate regression analysis, patients with DM as comorbidity (cOR=2.430, 95% CI=1.049 to 5.629, p=0.038) and patients with
Discussion
In this study, we analysed the risk factors of in-hospital mortality using data collected through hospital medical records. It was found that there were more patients with diabetes mellitus (DM) in non-survivor groups compared to survivor groups (76.3% vs 57%,
We estimated that the in-hospital mortality rate of FG patients in our study population was 26.2%. In Indonesia, it has previously been identified that the mortality rate of FG ranged from 17 to 28% [9,10]. Ergo, our findings are in line with such previous findings. Further, we identified that DM and
In our study, the median age was 52 (43–61) years, which was in line with previous report [15]. While older age is a substantial and well-known independent predictor of death for patients with FG [4,16–18], we were unable to demonstrate the association between mortality and age. Similarly, previous study from another tertiary hospital in Indonesia also showed no significant difference in age between survivors and non-survivors [9]. It is questionable whether increasing age may increase the risk of mortality in individuals with FG. Age is an independent predictor of death in population-based studies when combined with other risk factors such as renal insufficiency or surgical delay [19]. In addition, it has been noted that despite advancements in treatment techniques, antimicrobial agents, and intensive care procedures, FG still has a mortality risk up to 50% in specific regions in Indonesia or globally [4,20,21].
One of the risk factors of FG is chronic diseases such as diabetes, substance abuse, and others [22]. In this study, 62.1% of the patients had DM as comorbidity. Patients with DM had a 2.5 times higher risk of in-hospital mortality than patients without DM. Previous studies have shown the correlation between DM and a poor prognosis in FG patients, aligned with our findings [23–25]. When blood sugars are not properly controlled, diabetes is known to harm the immune system, thus increasing mortality in FG patients [25,26].
In previous study, Tenório et al. developed a Simplified Fournier Gangrene Severe Index (SFGSI) scoring system that identified extension of the lesion to the abdomen, hematocrit, serum potassium levels, and creatinine levels as independent risk factors for mortality [28].
In our investigation, the prevalence of
The most reliable clinical indicators for predicting a poor prognosis in persons with FG continue to be contested and vary across studies. Although the FGSI score system is often used to predict mortality in FG patients, it has a low sensitivity and specificity [9,33]. Throughout the last two decades, this score’s validity was assessed in several case series in an effort to establish its predictive validity; nevertheless, the results were inconsistent [34,35]. According to a preliminary study, people with the higher FGSI score are more likely than the general population to have more surgical procedures, remain in the hospital longer, develop sepsis, develop complications, and die [12]. In our study, no statistically significant difference between FGSI scores of survivors and non-survivors was observed. There was no correlation with any of its constituting components except for white blood cells count. Thus, our finding supports the notion that FGSI is not a good predictor of mortality.
This study has certain limitations. Both the retrospective nature and single-center observation in this study reflect significant constraints. Despite these limits, we conducted the first epidemiological research on FG and its related mortality risk factor in Indonesia, using high sample numbers and lengthy study duration. We note that our work logically builds on and advances previous research on FG.
Conclusions
We found a considerable mortality rate for FG in male patients. DM as comorbidity and the presence of
Tables
Table 1. Clinical characteristics of patients with Fournier’s gangrene.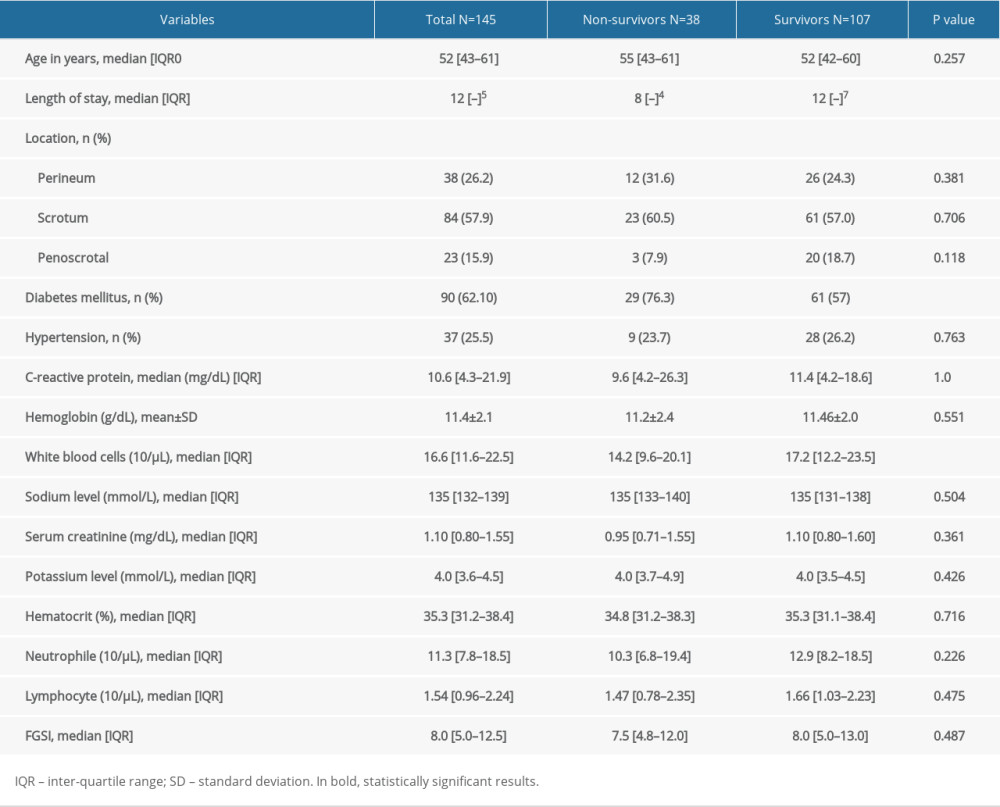 Table 2. Bacterial culture results from the Fournier’s gangrene patients.
Table 2. Bacterial culture results from the Fournier’s gangrene patients.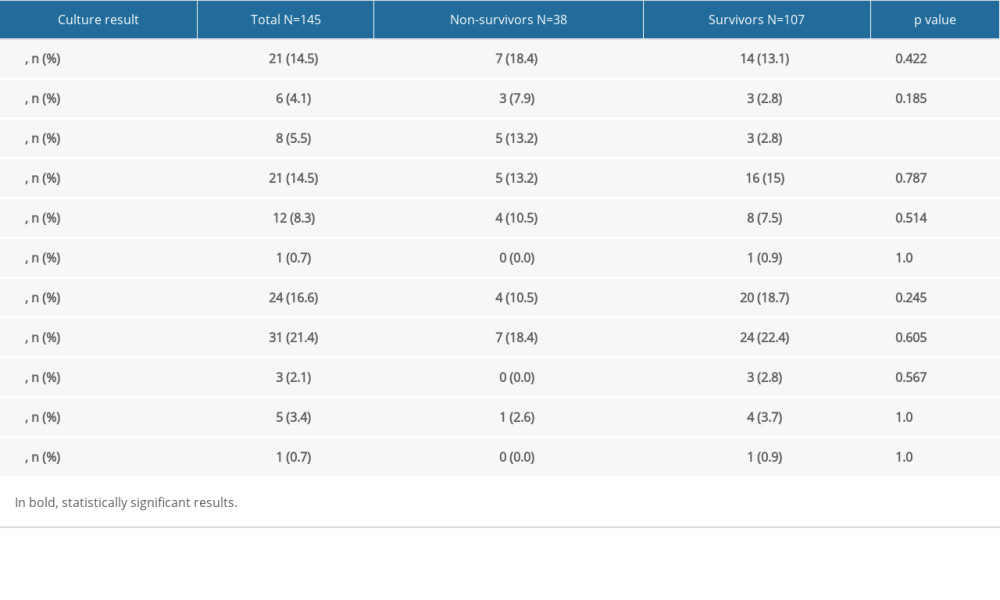 Table 3. Independent risk factors for in-hospital mortality in patients with Fournier’s gangrene.
Table 3. Independent risk factors for in-hospital mortality in patients with Fournier’s gangrene.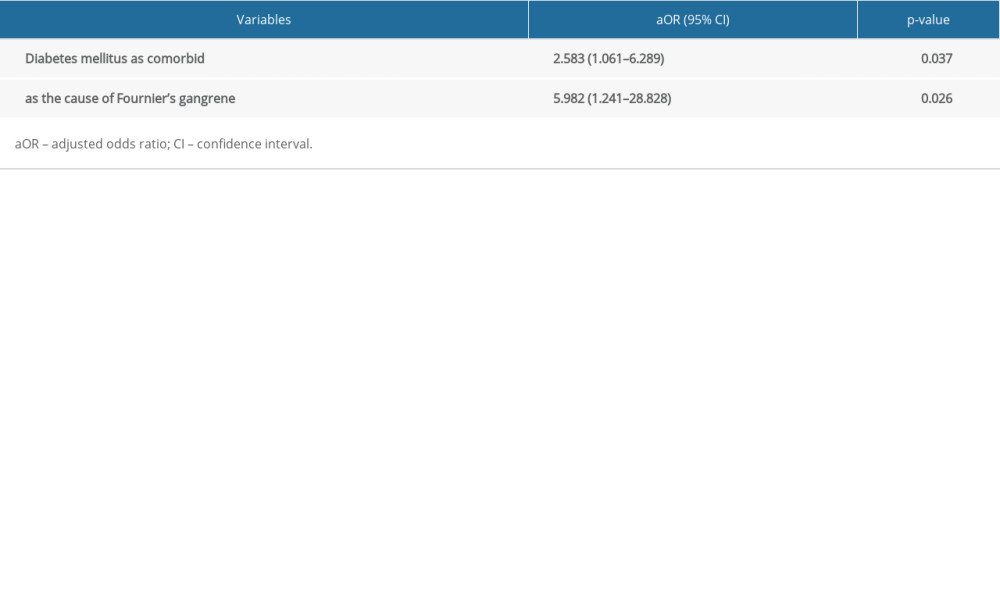 Supplementary Table 1. Univariate regression analysis for in-hospital mortality in patients with Fournier’s gangrene.
Supplementary Table 1. Univariate regression analysis for in-hospital mortality in patients with Fournier’s gangrene.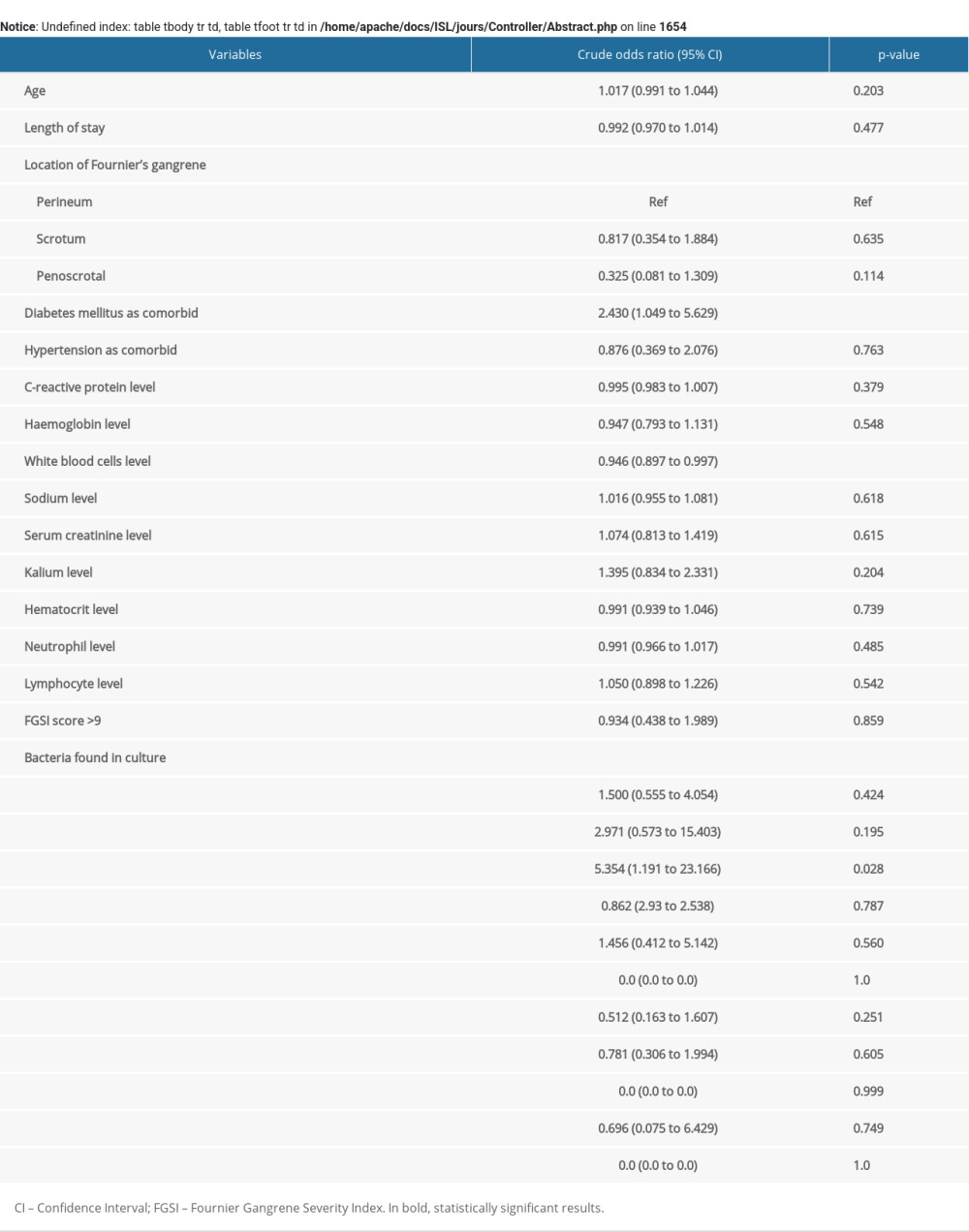
References
1. Taken K, Oncu MR, Ergun M, Fournier’s gangrene: Causes, presentation and survival of sixty-five patients: Pak J Med Sci, 2016; 32; 746-50
2. Peetermans M, de Prost N, Eckmann C, Necrotizing skin and soft-tissue infections in the intensive care unit: Clin Microbiol Infect, 2020; 26; 8-17
3. Bonne SL, Kadri SS, Evaluation and management of necrotizing soft tissue infections: Infect Dis Clin North Am, 2017; 31; 497-511
4. Sorensen MD, Krieger JN, Fournier’s gangrene: Epidemiology and outcomes in the general US population: Urol Int, 2016; 97; 249-59
5. Leslie SW, Rad J, Foreman J: Fournier gangrene, 2022, Treasure Island (FL)
6. Chernyadyev SA, Ufimtseva MA, Vishnevskaya IF, Fournier’s gangrene: Literature review and clinical cases: Urol Int, 2018; 101; 91-97
7. Radcliffe RS, Khan MA, Mortality associated with Fournier’s gangrene remains unchanged over 25 years: BJU Int, 2020; 125; 610-16
8. Wongwaisayawan S, Krishna S, Haroon M, Fournier gangrene: pictorial review: Abdom Radiol (NY), 2020; 45; 3838-48
9. Noegroho BS, Siregar S, Mustafa A, Rivaldi MA, Validation of FGSI scores in predicting Fournier gangrene in tertiary hospital: Res Rep Urol, 2021; 13; 341-46
10. Wirjopranoto S, Azmi YA, Outcome management of Fournier’s gangrene cases at tertiary hospital: 7 Years experience: Urologia, 2021; 89; 104-7
11. Tenório CEL, Lima SVC, deAlbuquerque AV, Risk factors for mortality in Fournier’s gangrene in a general hospital: Use of simplified founier gangrene severe index score (SFGSI): Int Braz J Urol, 2018; 44; 95-101
12. Sparenborg JD, Brems JA, Wood AM, Fournier’s gangrene: A modern analysis of predictors of outcomes: Transl Androl Urol, 2019; 8; 374-78
13. Sallami S, Chelif M, Ben Rhouma S, MP-4.13: Apical periprostatic nerve blockade before transrectal ultrasound-guided prostate biopsy: Are there any failure factors? Our 254 patients: Urology, 2008; 72; S88
14. Laor E, Palmer LS, Tolia BM, Outcome prediction in patients with Fournier’s gangrene: J Urol, 1995; 154; 89-92
15. Singh A, Ahmed K, Aydin A, Fournier’s gangrene. A clinical review: Arch Ital Urol Androl, 2016; 88; 157-64
16. Luján Marco S, Budía A, Di Capua C, Evaluation of a severity score to predict the prognosis of Fournier’s gangrene: BJU Int, 2009; 106; 373-76
17. Ansari Djafari A, Rahavian A, Javanmard B, Factors related to mortality in patients with Fournier’s gangrene or necrotising fasciitis; A 10-year cross-sectional study: Arch Acad Emerg Med, 2021; 9; e33
18. Benjelloun EB, Souiki T, Yakla N, Fournier’s gangrene: Our experience with 50 patients and analysis of factors affecting mortality: World J Emerg Surg, 2013; 8; 13
19. Montrief T, Long B, Koyfman A, Auerbach J, Fournier gangrene: A review for emergency clinicians: J Emerg Med, 2019; 57; 488-500
20. Corcoran AT, Smaldone MC, Gibbons EP, Validation of the Fournier’s gangrene severity index in a large contemporary series: J Urol, 2008; 180; 944-48
21. Altarac S, Katušin D, Crnica S, Fournier’s gangrene: Etiology and outcome analysis of 41 patients: Urol Int, 2012; 88; 289-93
22. Lewis GD, Majeed M, Olang CA, Fournier’s gangrene diagnosis and treatment: A systematic review: Cureus, 2021; 13(10); e18948
23. Bensardi FZ, Hajri A, Kabura S, Fournier’s gangrene: Seven years of experience in the emergencies service of visceral surgery at Ibn Rochd University Hospital Center: Ann Med Surg (Lond), 2021; 71; 102821
24. Shyam DC, Rapsang AG, Fournier’s gangrene: Surgeon, 2013; 11; 222-32
25. El-Qushayri AE, Khalaf KM, Dahy A, Fournier’s gangrene mortality: A 17-year systematic review and meta-analysis: Int J Infect Dis, 2020; 92; 218-25
26. Chernyadyev SA, Ufimtseva MA, Vishnevskaya IF, Fournier’s gangrene: Literature review and clinical cases: Urol Int, 2018; 101; 91-97
27. Sorensen MD, Krieger JN, Rivara FP, Fournier’s Gangrene: Population based epidemiology and outcomes: J Urol, 2009; 181; 2120-26
28. Tenório CEL, Lima SVC, de Albuquerque AV, Risk factors for mortality in Fournier’s gangrene in a general hospital: Use of simplified founier gangrene severe index score (SFGSI): International Braz J Urol, 2018; 44; 95-101
29. Stevens DL, Aldape MJ, Bryant AE, Life-threatening clostridial infections: Anaerobe, 2012; 18; 254-59
30. Sureka SK, Agarwal V, Agnihotri S, Is en-bloc transurethral resection of bladder tumor for non-muscle invasive bladder carcinoma better than conventional technique in terms of recurrence and progression? : A prospective study: Indian J Urol, 2014; 30; 144-49
31. Buboltz JB, Murphy-Lavoie HM: Gas Gangrene, 2022, Treasure Island (FL)
32. Srivastava I, Aldape MJ, Bryant AE, Septicum gas gangrene: A literature review: Anaerobe, 2017; 48; 165-71
33. Üreyen O, Acar A, Gökçelli U, Predictive value of FGSI and UFGSI scoring systems used in the prediction of mortality in patients with Fournier’s gangrene: A multi-center study: Ulus Travma Acil Cerrahi Derg, 2017; 23(5); 389-94
34. Bozkurt O, Sen V, Demir O, Esen A, Evaluation of the utility of different scoring systems (FGSI, LRINEC and NLR) in the management of Fournier’s gangrene: Int Urol Nephrol, 2014; 47; 243-48
35. Wetterauer C, Ebbing J, Halla A, A contemporary case series of Fournier’s gangrene at a Swiss tertiary care center-can scoring systems accurately predict mortality and morbidity?: World J Emerg Surg, 2018; 13; 25
Tables
 Table 1. Clinical characteristics of patients with Fournier’s gangrene.
Table 1. Clinical characteristics of patients with Fournier’s gangrene. Table 2. Bacterial culture results from the Fournier’s gangrene patients.
Table 2. Bacterial culture results from the Fournier’s gangrene patients. Table 3. Independent risk factors for in-hospital mortality in patients with Fournier’s gangrene.
Table 3. Independent risk factors for in-hospital mortality in patients with Fournier’s gangrene. Table 1. Clinical characteristics of patients with Fournier’s gangrene.
Table 1. Clinical characteristics of patients with Fournier’s gangrene. Table 2. Bacterial culture results from the Fournier’s gangrene patients.
Table 2. Bacterial culture results from the Fournier’s gangrene patients. Table 3. Independent risk factors for in-hospital mortality in patients with Fournier’s gangrene.
Table 3. Independent risk factors for in-hospital mortality in patients with Fournier’s gangrene. Supplementary Table 1. Univariate regression analysis for in-hospital mortality in patients with Fournier’s gangrene.
Supplementary Table 1. Univariate regression analysis for in-hospital mortality in patients with Fournier’s gangrene. In Press
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952