23 May 2023: Epidemiology
Causes of Death Following Gastric Cancer Diagnosis: A Population-Based Analysis
Ting Lou12BCE, Xueqian Hu12DEF, Ning Lu12EF, Tingsu Zhang12AG*DOI: 10.12659/MSM.939848
Med Sci Monit 2023; 29:e939848
Abstract
BACKGROUND: Causes of death (CODs) in patients with gastric cancer (GC) need to be studied. We examined the cancer-specific and non-cancer deaths among patients diagnosed with GC from 1975 to 2019.
MATERIAL AND METHODS: We obtained medical records from the Surveillance, Epidemiology, and End Results (SEER) database. We used SEER*Stat software to calculate standardized mortality ratios (SMRs) for specific CODs and performed a competing risk analysis to evaluate the cumulative mortality of specific CODs.
RESULTS: The final study cohort included 42 813 patients with GC, with a mean age at diagnosis of 67.7 years. As the end of 2021, a total of 36 924 (86.2%) patients died. Of these deaths, 24 625 (66.7%) were from GC, 6513 (17.6%) were from other types of cancers, and 5786 (15.7%) were from non-cancer causes. The most prevalent non-cancer CODs were heart diseases (2104; 5.7%), cerebrovascular diseases (501; 1.4%), and pneumonia/influenza (335; 0.9%). Among patients who survived over 5 years, non-cancer causes surpassed GC as the main CODs. Patients with GC had a higher risk of dying from many non-cancer causes than expected in the general population, particularly from suicide (SMR, 3.03; 95% CI, 2.35-3.85) and septicemia (SMR, 2.93; 95% CI, 2.51-3.4). The competing risk analysis showed that the cumulative mortality of GC gradually declined with a more recent diagnosis.
CONCLUSIONS: Although GC was the leading COD among patients with GC, non-cancer CODs accounted for a substantial number of deaths. These findings provide useful guidance on potential death risks among patients with GC.
Keywords: Mortality, SEER Program, Stomach Neoplasms, Humans, Aged, Cause of Death, Heart Diseases, Risk Factors
Background
Gastric cancer (GC) is a prevalent type of gastrointestinal cancer in the United States, with around 26 500 new cases expected to be diagnosed in 2023. The disease is often diagnosed at an advanced stage and results in a high mortality rate, with an estimated 11 130 deaths in 2023 [1]. Despite this, the 5-year relative survival rates for GC have been increasing in recent decades, from 15% between 1975 and 1977 to 33% between 2012 and 2018. This is due in part to the advancements in surgical resection and the wide use of newer immunotherapy and targeted therapy [1–3]. As a result, an increasing number of patients are surviving long enough to experience the challenges of non-cancer-related comorbidities [4]. Some of these comorbidities are fatal and result in deaths that are not related to cancer, altering the typical prognostic pattern for GC. Therefore, it is crucial to comprehend the distribution of various causes of death (CODs) following a diagnosis of GC in order to establish effective intervention and surveillance strategies.
Several studies have been conducted to analyze the CODs in specific types of cancers. In the case of US patients with metastatic prostate cancer, 77.8% of total deaths were caused by prostate cancer, 5.5% were caused by other types of cancers, and 16.7% were caused by non-cancer causes [5]. Among lung cancer patients who survived for 5 to 10 years, 21.8% died from primary lung cancer, 10.2% died from other types of cancer, 6.8% died from cardiac disease, and 5.3% died from non-malignant pulmonary disease [6]. Previous research on this topic for GC has been limited, with only one study conducted in Sweden to date, which included 56 240 patients with GC diagnosed between 1970 and 2014 and found that non-gastric malignancies, ischemic heart diseases or cerebrovascular diseases, and respiratory diseases were the major non-cancer CODs [7]. However, it is unclear whether these findings from Europe can be applied to patients in the United States. Additionally, without comparing the risk of death from specific non-cancer causes to that of the general population, it is challenging to devise an accurate and suitable strategy for patients with GC.
In this study, we conducted the most comprehensive and up-to-date population-based analysis of CODs among GC patients in the United States over a 44-year follow-up period. Our aim was to identify any associations between demographic and tumor-related characteristics and the risk of specific CODs in patients with GC. Additionally, we provided a thorough evaluation of how the risk of death from each cause in patients with GC has shifted in comparison to that of the general population in the United States during the same time period.
Material and Methods
STUDY DESIGN AND DATA:
The data analyzed in this study were extracted from the Surveillance, Epidemiology, and End Results (SEER) Program. The SEER database has been collecting medical records from 8 cancer registries since 1975, covering approximately 28% of the US population (
Patients diagnosed with malignant GC between 1975 and 2019 were identified using the SEER*Stat software (accessed on November 21, 2021) [8]. Primary GC was identified using the codes of the International Classification of Disease for Oncology third edition (ICD-O-3) C16.0-C16.9. The following histology variables were selected: 8000/3, 8010/3, 8020–8022/3, 8140/3, 8142–8145/3, 8210–8211/3, 8255/3, 8260–8263/3, 8310/3, 8323/3, 8480–8481/3, and 8490/3. Patients who survived less than 1 day or for whom complete data were unavailable were excluded. The following demographic and tumor-related characteristics were extracted: age at diagnosis, sex, race, calendar period at diagnosis, cancer-directed surgery, chemotherapy, and radiotherapy. For the convenience of statistics, the variable of age at diagnosis was divided into 5 groups: <50, 50–59, 60–69, 70–79, and ≥80 years, and the variable of calendar period at diagnosis was divided into 5 groups: 1975–1980, 1981–1990, 1991–2000, 2001–2010, and 2011–2019. The SEER Cause of Death Recode was used to determine the COD for every patient with GC [9]. CODs were defined using the ICD-10 World Health Organization codes. The data on CODs were then stratified by variables such as sex, age at diagnosis, race, surgery, chemotherapy, and radiotherapy, each according to the latency period (<1 years, 1–5 years, and >5 years following GC diagnosis).
STATISTICAL ANALYSIS:
To compare the risk of each specific COD among GC patients with that among the general US population, we calculated standardized mortality ratios (SMRs) with exact 95% confidence intervals (CIs) using the SEER*Stat software [8]. As previously mentioned, SMR was defined as the ratio of the observed to the expected number of deaths [10]. “Observed” was defined as the actual total death of a given COD in a specific time frame, and “expected” was defined as the expected number of deaths due to the same COD in a demographically similar population (regarding sex, race, and age) within the same time frame [11]. SMR is a useful multiplicative measure for determining excess mortality relative to the background mortality [12]. The criterion for a statistically significant increase in the risk of dying from a specific COD was a 2-sided P<0.05. The SMRs were calculated for the whole cohort and stratified groups.
Furthermore, to evaluate the cumulative mortality as a function of years since diagnosis for specific CODs after GC diagnosis, we used R software [13]. CODs other than those analyzed were considered competing risks. According to a previous study, we conducted a competing risk analysis to assess the probability of mortality caused by GC, other cancers, cardiovascular diseases, and respiratory diseases [11]. This analysis estimates the probability of death from a specific cause over time, taking into account the possibility of death from other causes.
Results
PATIENT CHARACTERISTICS:
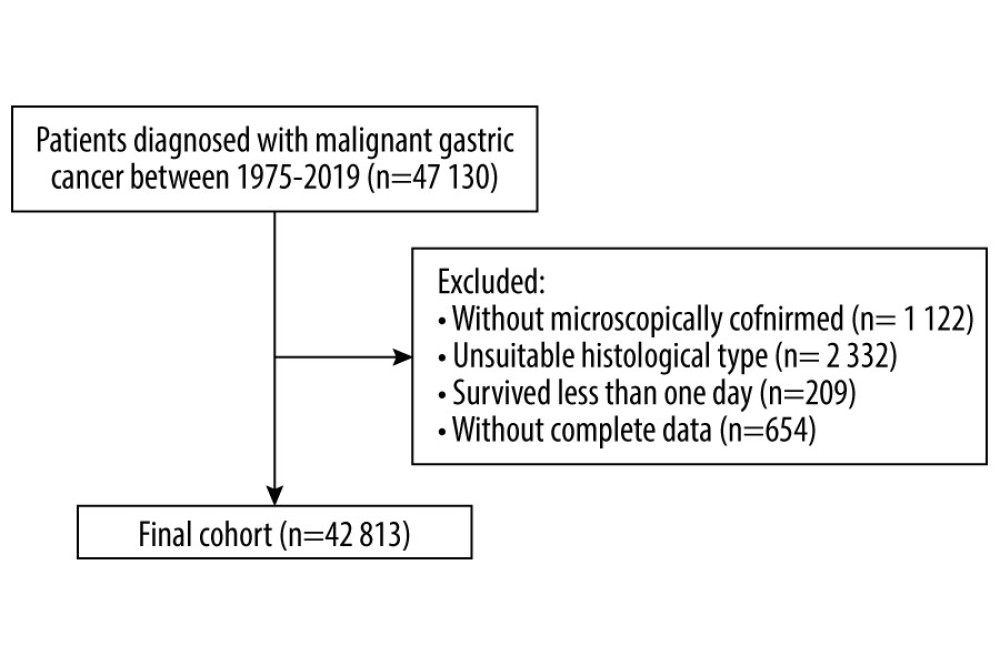
In total, 42 813 patients diagnosed with GC from 1975 to 2019 were eligible for the present study (Figure 1). The mean age at diagnosis was 67.7 years. Most patients were men (63.3%, 27 088), aged from 70 to 79 years at diagnosis (27.9%, 11 938), white (67.8%, 29 048), and diagnosed from 2011 to 2019 (25.2%, 10 785). By the end of the follow-up period (2021), 36 924 (86.2%) deaths occurred. The highest proportion of deaths (59.2%, 21 851) occurred within 1 year of GC diagnosis. On the other hand, 30.2% of deaths (11 157) happened within 1 to 5 years following GC diagnosis, and 10.6% of deaths (3 916) happened after more than 5 years after diagnosis (Table 1). Of the total deaths, 24 625 (66.7%) were due to GC, 6513 (17.6%) were due to other cancers, and 5786 (15.7%) were due to non-cancer causes. The major COD from cancers other than GC was esophageal cancer (4019). The most common non-cancer CODs were diseases of the heart (2104, 5.7%), cerebrovascular diseases (501, 1.4%), and pneumonia and influenza (121, 0.3%; Table 2).
The risks of all-cause mortality and non-gastric malignant cancer mortality in patients with GC were 7.84 (95% CI, 7.76–7.9) and 5.42 times (95% CI, 5.15–5.69) higher than that expected from the general population, respectively (Table 2). In terms of non-cancer deaths, the risks of death from common causes were significantly higher than that expected form the general population (suicide, 3.03 times; septicemia, 2.91 times; chronic liver disease and cirrhosis, 2.58 times; pneumonia and influenza, 1.97 times; diseases of the heart, 1.38 times; cerebrovascular diseases, 1.41 times; and chronic obstructive pulmonary disease [COPD], 1.33 times; Table 2)
Subgroup analysis showed that the SMR of cardiac death was lowest for individuals diagnosed with GC from 1975 to 1980 (1.17; 95% CI, 1.03–1.32), and increased to 1.77 (95% CI, 1.55–2.01) among those diagnosed from 2011 to 2019. The SMR of septicemia was highest for patients younger than 50 years at diagnosis (7.79; 95% CI, 3.73–14.32), and decreased to 2.21 (1.51–2.90) among those older than 79 years at diagnosis.
CODS WITHIN 1 YEAR AFTER GC DIAGNOSIS:
A total of 21 851 deaths occurred within 1 year after diagnosis of GC: 16 377 (74.9%) deaths were due to GC, 3487 (15.9%) deaths were due to other cancers, and 1987 (9.2%) deaths were due to non-cancer causes. The most common non-cancer CODs in this period was diseases of the heart (784; 3.6%), followed by cerebrovascular diseases (170; 0.8%), and pneumonia and influenza (97; 0.4%; Table 2).
The overall risk of death among patients with GC within 1 year after diagnosis was higher than that in the general US population (SMR, 23.00; 95% CI, 22.70–23.31), as was the risk of death from septicemia (SMR, 7.3; 95% CI, 5.84–9.19), suicide (SMR 7.12; 95% CI, 5.06–9.73), pneumonia and influenza (SMR, 2.94; 95% CI, 2.38–3.58), nephritis nephrotic syndrome and nephrosis (SMR 2.67; 95% CI, 1.91–3.62), diseases of the heart (SMR, 2.45; 95% CI, 2.28–2.63), cardiovascular disease (SMR, 2.34; 95% CI, 2.00–2.72), and COPD (SMR, 1.99; 95% CI 1.61–2.44; Table 2).
During this period, the CODs in certain subgroups based on demographic and tumor-related characteristics were similar to those observed in the general US population, with diseases of the heart being the most common COD.
CODS WITHIN 1 TO 5 YEARS AFTER GC DIAGNOSIS:
In total, 11 157 patients died within 1 to 5 years after cancer diagnosis, of which, 7444 (66.7%) died from GC, 2266 (20.3%) died from other cancers, and 1447 (13.0%) died from non-cancer causes, including diseases of the heart (516, 4.6%), cerebrovascular diseases (131, 1.2%), and pneumonia and influenza (95, 0.9%).
When compared with the general population, patients with GC had a higher risk of death from other malignant cancers (SMR, 7.20; 95% CI, 6.90–7.50), tuberculosis (SMR, 4.04; 95% CI, 1.31–9.42), other infectious and parasitic diseases (SMR, 2.91; 95% CI, 1.90–4.27), suicide and self-inflicted injury (SMR, 2.68; 95% CI, 1.66–4.09), chronic liver disease and cirrhosis (SMR, 2.16; 95% CI, 1.41–3.17), symptoms, signs, and ill-defined conditions (SMR, 1.93; 95% CI, 1.29–2.80), pneumonia and influenza (SMR, 1.82; 95% CI, 1.47–2.22), septicemia (SMR, 1.70; 95% CI, 1.15–2.43), and accidents and adverse effects (SMR, 1.64; 95% CI, 1.25–2.12; Table 2).
The risk of death from diseases of the heart was significantly higher among patients who were 50 to 69 years of age when they were diagnosed (SMR, 2.06; 95% CI, 1.47–2.81), diagnosed in 2011 to 2019 (SMR, 1.43; 95% CI, 1.16–1.75), received chemotherapy (SMR, 1.25; 95% CI, 1.03–1.50), received radiation (SMR, 1.40; 95% CI, 1.13–1.72), and diagnosed with cardia adenocarcinoma (SMR, 1.40; 95% CI, 1.16–1.66).
CODS MORE THAN 5 YEARS AFTER GC DIAGNOSIS:
Approximately 3916 patients with GC died after more than 5 years of diagnosis, of which, most patients died from non-cancer causes (2352 deaths, 60.1%). On the other hand, 804 (20.5%) died from GC, and 760 (19.4%) died from other cancers. The most common non-cancer causes of death in this period were diseases of the heart (804, 20.5%), followed by cerebrovascular diseases (200, 5.1%) and COPD (146, 3.7%; Table 2).
In this period, the overall risk of death among patients with GC was moderately higher than that in the general US population (SMR, 1.72; 95% CI, 1.66–1.77). With regard to non-cancer CODs, risk of death from other infectious and parasitic diseases (SMR, 2.21; 95% CI, 1.48–3.17), septicemia (SMR, 2.06; 95% CI, 1.57–2.64), chronic liver disease and cirrhosis (SMR, 1.92; 95% CI, 1.24–2.83), pneumonia and influenza (SMR, 1.68; 95% CI, 1.42–1.98), nephritis nephrotic syndrome and nephrosis (SMR, 1.63; 95% CI, 1.28–2.05), accidents and adverse effects (SMR, 1.53; 95% CI, 1.22–1.90), hypertension without heart diseases (SMR, 1.44; 95% CI, 1.02–1.97), Alzheimer disease (SMR, 1.41; 95% CI, 1.16–1.70), COPD (SMR, 1.21; 95% CI, 1.03–1.43), cerebrovascular diseases (SMR, 1.16; 95% CI, 1.00–1.33), and diseases of the heart (SMR, 1.12; 95% CI, 1.04–1.20) were significantly higher in patients with GC than in the general US population (Table 2).
Generally, the CODs more than 5 years of GC diagnosis in certain subgroups based on demographic and tumor-related characteristics were similar to those observed in the overall population. The risk of dying from pneumonia and influenza was higher among patients who were diagnosed from 1975 to 2000, received surgery and system therapy, and were diagnosed with non-cardia adenocarcinoma. The risk of dying from COPD was higher among male patients.
CUMULATIVE MORTALITY:
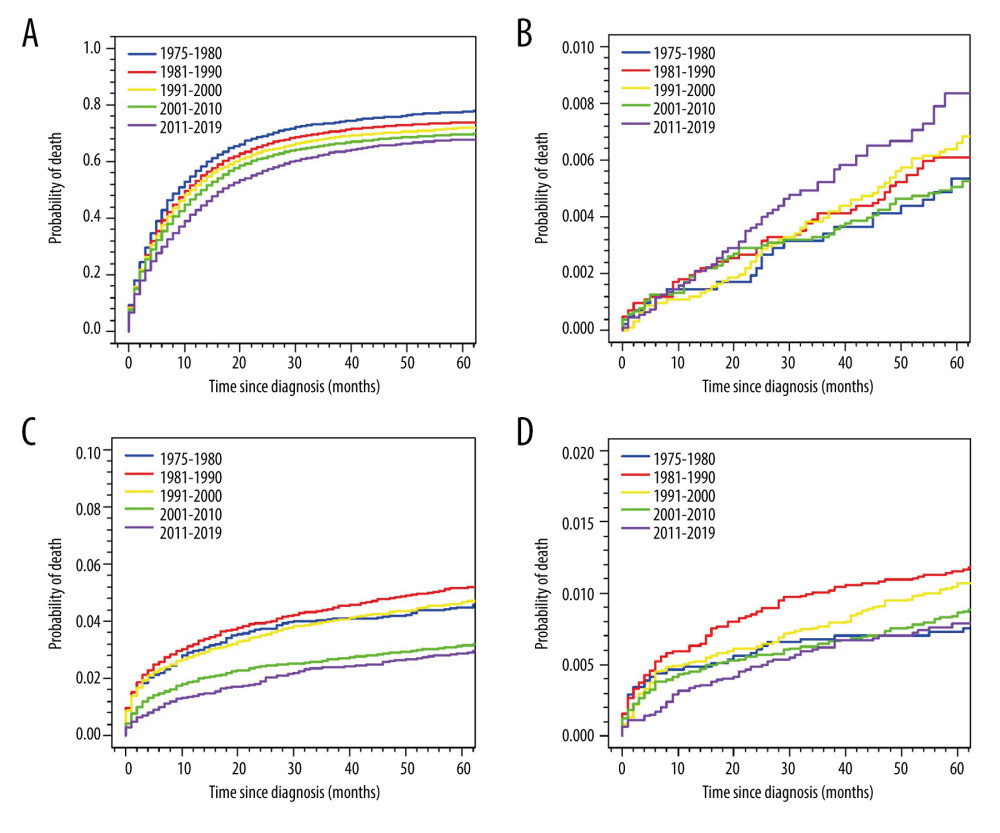
Figure 2 depicts the cumulative mortality of specific CODs for the entire cohort. The cumulative mortality of GC gradually decreased with a more recent diagnosis. On the other hand, the cumulative mortality of cardiac and cerebrovascular diseases has significantly decreased after the 21st century, while the cumulative mortality of respiratory diseases, such as COPD and pneumonia/influenza, was notably higher among GC patients diagnosed from 1981 to 1990 (Figure 2).
Discussion
By using the SEER database, we were the first to analyze the CODs of 42 813 patients diagnosed with GC in the United States during 4 decades from 1975 to 2019. We present the proportion of deaths that occurred within different time periods following GC diagnosis and the distribution of CODs among the study population. We also provide an overall comparison of the risk of specific CODs among GC patients with that of the general population. Lastly, we provide insights from subgroup analysis of how demographic and tumor-related characteristics, as well as treatment modalities, impact the risk of specific CODs. Our findings show that, although most deaths in the 5 years following a diagnosis of GC were attributed to GC, the frequency of these deaths decreased over time and was eventually surpassed by non-cancer CODs. After 5 years of diagnosis, the most common non-GC CODs were heart disease, other cancers, cerebrovascular disease, and COPD. Overall, patients with GC experienced 7.84 times higher risk of dying than that expected from the general population. Even over 5 years after diagnosis, the risk was still significantly higher (SMR, 1.72; 95% CI, 1.66–1.77). Additionally, following initial GC diagnosis, patients with GC had a greater risk of death from many non-GC CODs than did the general US population. Therefore, it is important to prioritize surveillance of both GC and other potential causes of death in patients with GC. This will enable the timely and effective provision of supportive care.
We have demonstrated that heart disease was the most common non-cancer COD in patients with GC, which is in line with the reports from other cancers [14–17]. It could be partly explained by the primacy of cardiovascular mortality in the United States [18]. In addition, an increase in the risk of cardiac death for patients with GC was observed when compared with that of the general population, regardless of age, sex, and race. This was associated with the high incidence of cardiotoxicity among patients who received HER2-targeted therapies and fluorouracil-based chemotherapy [19–21]. Furthermore, radiation has been shown to induce heart disease [22]. Therefore, the higher risk of cardiac death was shown when evaluating the subgroups of patients who received radiation. We suggest that clinicians should regularly monitor the heart function of patients with GC and intervene as early as possible.
Cerebrovascular disease-related death was among the most important non-cancer CODs in our study, and the corresponding risk was significantly greater than that in the general US population. The association between cerebrovascular disease and GC is still ambiguous. A previous analysis showed a substantial proportion of GC patients experienced subsyndromal delirium after curative resection [23]. Another study reported a case of Trousseau syndrome complication for advanced GC [24]. Also, the high incidence of hypercoagulability and thromboembolisms among cancer patients might be an explanation [25,26].
It is worth noting that our study showed that the cumulative mortality of cardiovascular disease deaths significantly decreased after the start of the 21st century. This finding seems to be in contrast with the contemporary tendency in the general US population, where the age-adjusted decline in cardiovascular disease mortality has slowed in recent years [18,27]. This discrepancy might be explained by the advancement in consensus statements on recommended definitions and practices in the field of cardio-oncology over the past 2 decades [28–33]. These advancements in the field have likely led to improved management and prevention of cardiovascular disease in patients with GC.
Additionally, we found that COPD was one of the most prevalent comorbidities among patients with GC. This is consistent with previous studies, such as a study conducted with Brazilian patients with GC who underwent cancer-directed resection [4]. In a Swedish study, a larger proportion of patients with GC died from COPD than in our study [7]. This finding can be partly explained by regional differences in the incidence of COPD among patients with GC. A study reported that the United Kingdom (8.4%) had the lowest COPD prevalence in Europe, while Germany (13.4%) and Spain (16.8%) had the highest prevalence [34]. In addition, we found that patients with GC experienced a greater risk of dying from COPD than that expected in the general US population. The gut-lung axis in carcinogenesis might be a potential explanation [35]. Therefore, it is important for healthcare providers to closely monitor and manage pulmonary function in patients with GC.
In our study, we found that other cancers became a more frequent COD as time passed since the initial diagnosis of GC, increasing from 15.9% within 1 year to 20.5% more than 5 years after diagnosis. Additionally, patients with GC were found to have a higher risk of death from other malignant cancers than were the general population. These findings suggest that proper screening for other cancers after a diagnosis of GC may be crucial for these patients. A previous study from the Swedish Cancer Registry, conducted from 1990 to 2015, also revealed that patients with GC have a significantly increased risk of developing secondary primary cancers, such as small intestinal, esophageal, and kidney cancers [36]. Thus, it is important for patients with GC to be aware of their increased risk for other cancers and to undergo appropriate follow-up and screenings.
Our study found that the risk of death from septicemia increased mostly within the first years following a diagnosis of GC, specifically in patients who underwent surgery, chemotherapy, and radiotherapy. A previous study showed that postoperative infections adversely affect overall survival and relapse-free survival [37]. However, opposing results were also reported in a study that did not support an association between sepsis and the final oncologic outcome after resection [38]. Additionally, our study showed that the risk of death from pneumonia and influenza was higher among patients with GC, which is consistent with the results for patients who underwent gastrectomy [39,40]. Treatment with nab-paclitaxel and trastuzumab has also been reported to be associated with an increased risk of pneumonia among patients with GC [41,42]. Nutritional status has been confirmed as an independent predictor of infections in patients with GC. Low nutritional status combined with infections can have a synergistic effect, leading to worse overall survival [43]. Therefore, it seems necessary to provide nutrition supplementation for patients with GC.
Furthermore, our study showed an increased risk of suicide within 5 years of GC diagnosis, which is consistent with previous findings [44]. Therefore, a psychiatric evaluation and support should be an essential part of the treatment plan for patients with GC.
The present study has some limitations. Firstly, the SEER database relies on CODs recorded on death certificates, which can be subject to potential reporting biases and inaccuracies [45,46]. Secondly, it should be noted that the database utilized in our study lacks certain specific clinical information, including therapeutic procedures, comorbidities, imaging data, and nutritional status. This information could potentially impact the risk of death and the underlying CODs. Additionally, it is important to note that this study is based on a US population; therefore, the results may not be generalizable to other countries or populations.
Conclusions
In conclusion, the present study provides a comprehensive analysis of the CODs among patients with GC in the United States, using the SEER database. Our findings indicate that a significant proportion of deaths among patients with GC are due to non-cancer causes, particularly heart diseases, cerebrovascular diseases, and pneumonia/influenza, especially among long-term survivors. These results suggest that patients with GC should be counseled regarding future health risks and that multidisciplinary care should be emphasized in the management of these patients.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A, Cancer statistics, 2022: Cancer J Clin, 2022; 72(1); 7-33
2. Rawla P, Barsouk A, Epidemiology of gastric cancer: Global trends, risk factors and prevention: Przeglad Gastroenterologiczny, 2019; 14(1); 26-38
3. Leja M, Grinberga-Derica I, Bilgilier C, Steininger C: Helicobacter, 2019; 24(Suppl 1); e12635
4. Coimbra FJF, de Jesus VHF, Franco CP, Predicting overall and major postoperative morbidity in gastric cancer patients: J Surg Oncol, 2019; 120(8); 1371-78
5. Elmehrath AO, Afifi AM, Al-Husseini MJ, Causes of death among patients with metastatic prostate cancer in the US from 2000 to 2016: JAMA Netw Open, 2021; 4(8); e2119568
6. Abdel-Rahman O, Causes of death in long-term lung cancer survivors: A SEER database analysis: Curr Med Res Opin, 2017; 33(7); 1343-48
7. Xie SH, Chen H, Lagergren J, Causes of death in patients diagnosed with gastric adenocarcinoma in Sweden, 1970–2014: A population-based study: Cancer Sci, 2020; 111(7); 2451-59
8. Surveillance Research Program, National Cancer Institute SEER*Stat software version 8.4.0.1. National Cancer Institute: Surveillance, Epidemiology, and End Results Program Web site Available at https:www.seer.cancer.gov/seerstat
9. , Surveillance Epidemiology, and End Results cause of death recode 1969+ (03/01/2018) - SEER Data Reporting Tools: National Cancer Institute, Surveillance, Epidemiology, and End Results Program Web site Available at https://seer.cancer.gov/codrecode/1969_d03012018/index.html
10. Fidler MM, Reulen RC, Winter DL, Long term cause specific mortality among 34 489 five year survivors of childhood cancer in Great Britain: population based cohort study: Br Med J (Clin Res Ed), 2016; 354; i4351
11. Afifi AM, Elmehrath AO, Ruhban IA, Causes of death following nonmetastatic colorectal cancer diagnosis in the U.S.: A population-based analysis: Oncologist, 2021; 26(9); 733-39
12. Fidler MM, Reulen RC, Henson K, Population-based long-term cardiac-specific mortality among 34 489 five-year survivors of childhood cancer in Great Britain: Circulation, 2017; 135(10); 951-63
13. Scrucca L, Santucci A, Aversa F, Regression modeling of competing risk using R: An in depth guide for clinicians: Bone Marrow Transplant, 2010; 45(9); 1388-95
14. Wu XQ, Li JY, Du WJ, Causes of death following small cell lung cancer diagnosis: A population-based analysis: BMC Pulm Med, 2022; 22(1); 262
15. Lu L, Ma L, Zhang X, Analyzing non-cancer causes of death of colorectal carcinoma patients in the US population for the years 2000–2016: Cancer Med, 2021; 10(8); 2740-51
16. Afifi AM, Saad AM, Al-Husseini MJ, Causes of death after breast cancer diagnosis: A US population-based analysis: Cancer, 2020; 126(7); 1559-67
17. Kanitkar AA, Schwartz AG, George J, Soubani AO, Causes of death in long-term survivors of non-small cell lung cancer: A regional Surveillance, Epidemiology, and End Results study: Ann Thorac Med, 2018; 13(2); 76-81
18. McClellan M, Brown N, Califf RM, Warner JJ, Call to action: Urgent challenges in cardiovascular disease: A presidential advisory from the American Heart Association: Circulation, 2019; 139(9); e44-e54
19. Santoni M, Guerra F, Conti A, Incidence and risk of cardiotoxicity in cancer patients treated with targeted therapies: Cancer Treat Rev, 2017; 59; 123-31
20. Barish R, Gates E, Barac A, Trastuzumab-induced cardiomyopathy: Cardiology Clin, 2019; 37(4); 407-18
21. Jin X, Bai Y, Gao L, Wu S, Incidence of and risk factors for cardiotoxicity after fluorouracil-based chemotherapy in locally advanced or metastatic gastric cancer patients: Cancer Chemother Pharmacol, 2019; 84(3); 599-607
22. Koutroumpakis E, Deswal A, Yusuf SW, Radiation-induced cardiovascular disease: Mechanisms, prevention, and treatment: Curr Oncol Rep, 2022; 24(5); 543-53
23. Hwang H, Lee KM, Son KL, Incidence and risk factors of subsyndromal delirium after curative resection of gastric cancer: BMC Cancer, 2018; 18(1); 765
24. Hariu T, Furutani A, Yamasaki SA case of trousseau syndrome complication during chemotherapy for advanced gastric cancer: Gan To Kagaku Ryoho, 2019; 46(13); 2243-45 [in Japanese]
25. Liew NC, Alemany GV, Angchaisuksiri P, Asian venous thromboembolism guidelines: Updated recommendations for the prevention of venous thromboembolism: Int Angiol, 2017; 36(1); 1-20
26. Labianca A, Bosetti T, Indini A, Risk prediction and new prophylaxis strategies for thromboembolism in cancer: Cancers, 2020; 12(8); 2070
27. Goff DC, Khan SS, Lloyd-Jones D, Bending the curve in cardiovascular disease mortality: Bethesda + 40 and beyond: Circulation, 2021; 143(8); 837-51
28. Leong DP, Lenihan DJ, Clinical practice guidelines in cardio-oncology: Heart Fail Clin, 2022; 18(3); 489-501
29. Muhandiramge J, Zalcberg JR, van Londen GJ, Cardiovascular disease in adult cancer survivors: A review of current evidence, strategies for prevention and management, and future directions for cardio-oncology: Curr Oncol Rep, 2022; 24(11); 1579-92
30. Cheng KH, Wu YW, Hou CJ, Hung CM, An overview of cardio-oncology, a new frontier to be explored: Acta Cardiologica Sinica, 2021; 37(5); 457-63
31. Fradley MG, Beckie TM, Brown SA, Recognition, prevention, and management of arrhythmias and autonomic disorders in cardio-oncology: A scientific statement from the American Heart Association: Circulation, 2021; 144(3); e41-e55
32. Herrmann J, López-Fernández T, Lyon AR, Year in cardiovascular medicine: Cardio-oncology 2020–21: Eur Heart J, 2022 [Online ahead of print]
33. Beavers CJ, Rodgers JE, Bagnola AJ, Cardio-oncology drug interactions: A scientific statement from the American Heart Association: Circulation, 2022; 145(15); e811-e38
34. Loosen SH, Labuhn S, Jördens MS, Prevalence of chronic obstructive pulmonary disease among 48,061 digestive tract cancer patients in Europe: Ann Palliat Med, 2022; 11(9); 2813-18
35. Ante Z, Ernst P, Brassard P, Risk of gastric cancer in chronic obstructive pulmonary disease: Eur J Cancer Prev, 2022; 31(4); 326-32
36. Zheng G, Sundquist K, Sundquist J, Second primary cancers after gastric cancer, and gastric cancer as second primary cancer: Clin Epidemiol, 2021; 13; 515-25
37. Tokunaga M, Tanizawa Y, Bando E, Poor survival rate in patients with postoperative intra-abdominal infectious complications following curative gastrectomy for gastric cancer: Ann Surg Oncol, 2013; 20(5); 1575-83
38. Climent M, Hidalgo N, Vidal Ó, Postoperative complications do not impact on recurrence and survival after curative resection of gastric cancer: Eur J Surg Oncol, 2016; 42(1); 132-39
39. Kamiya A, Hayashi T, Sakon R, Long-term postoperative pneumonia in elderly patients with early gastric cancer: BMC Surg, 2022; 22(1); 220
40. Miki Y, Makuuchi R, Tokunaga M, Risk factors for postoperative pneumonia after gastrectomy for gastric cancer: Surg Today, 2016; 46(5); 552-56
41. Shitara K, Takashima A, Fujitani K, Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): An open-label, randomised, non-inferiority, phase 3 trial: Lancet Gastroenterol Hepatol, 2017; 2(4); 277-87
42. Shitara K, Bang YJ, Iwasa S, Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer: N Engl J Med, 2020; 382(25); 2419-30
43. Xiao Y, Wei G, Ma M, Association among prognostic nutritional index, post-operative infection and prognosis of stage II/III gastric cancer patients following radical gastrectomy: Eur J Clin Nutr, 2022; 76(10); 1449-56
44. Bowden MB, Walsh NJ, Jones AJ, Demographic and clinical factors associated with suicide in gastric cancer in the United States: J Gastrointest Oncol, 2017; 8(5); 897-901
45. Sun M, Trinh QD, A Surveillance, Epidemiology and End Results (SEER) database malfunction: Perceptions, pitfalls and verities: BJU Int, 2016; 117(4); 551-52
46. German RR, Fink AK, Heron M, The accuracy of cancer mortality statistics based on death certificates in the United States: Cancer Epidemiol, 2011; 35(2); 126-31
Figures
Tables
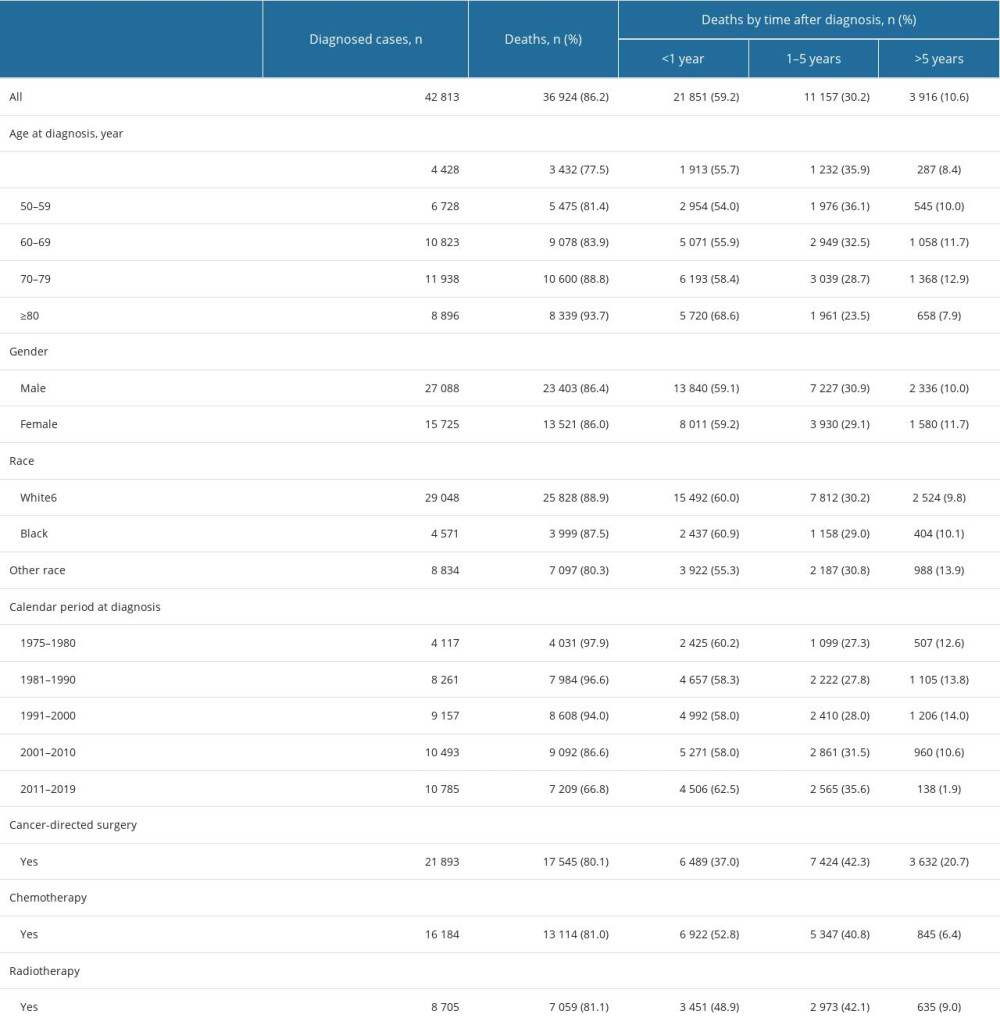 Table 1. Baseline characteristics of patients with gastric adenocarcinoma and of those who died according to the time of death after diagnosis.
Table 1. Baseline characteristics of patients with gastric adenocarcinoma and of those who died according to the time of death after diagnosis.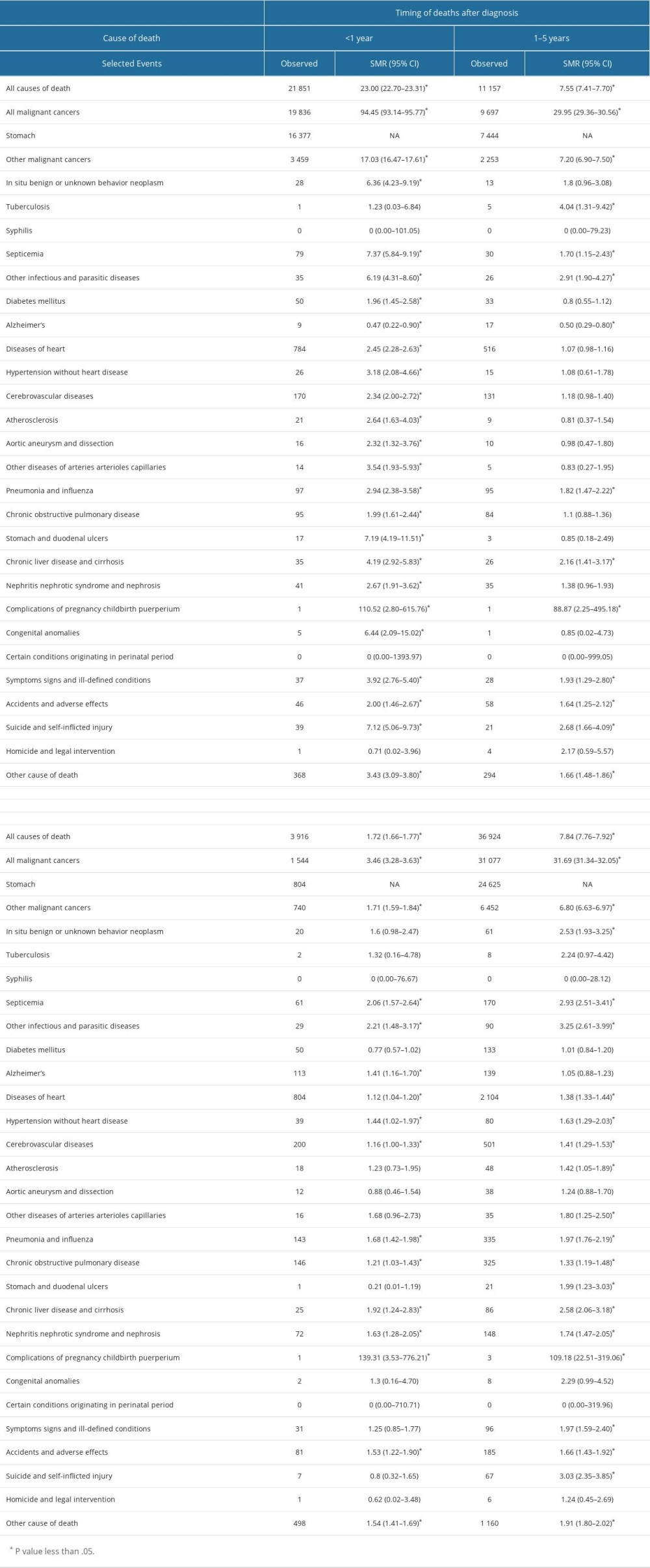 Table 2. Standardized-mortality ratios (SMRs) for each cause of death following gastric cancer diagnosis.
Table 2. Standardized-mortality ratios (SMRs) for each cause of death following gastric cancer diagnosis. Table 1. Baseline characteristics of patients with gastric adenocarcinoma and of those who died according to the time of death after diagnosis.
Table 1. Baseline characteristics of patients with gastric adenocarcinoma and of those who died according to the time of death after diagnosis. Table 2. Standardized-mortality ratios (SMRs) for each cause of death following gastric cancer diagnosis.
Table 2. Standardized-mortality ratios (SMRs) for each cause of death following gastric cancer diagnosis. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952