07 June 2023: Review Articles
Ultrasound-Guided Brachial Plexus Block by Costoclavicular Space Approach: A Narrative Review
Taotao Xing1BEF*, Lan Ge12ADDOI: 10.12659/MSM.939920
Med Sci Monit 2023; 29:e939920
Abstract
ABSTRACT: In recent years, ultrasound-guided costoclavicular brachial plexus block (CCB) has gained attention as a novel approach for brachial plexus nerve block. Human anatomy studies have identified the costoclavicular space as the area between the midpoint of the clavicle and the first rib. This space accommodates the brachial plexus, axillary arteries, and veins. Its superficial and fixed position makes it a promising option for infraclavicular brachial plexus blockage, providing a safe and reliable analgesic effect. CCB combines the benefits of real-time ultrasound visualization of the nerve block needle, avoidance of peripheral blood vessels, and targeted delivery of local anesthetics to the nerve. Consequently, it significantly reduces the associated complications of other classical approaches such as interscalene brachial plexus block (ISB), supraclavicular brachial plexus block (SCB), lateral sagittal infraclavicular brachial plexus block (LS-ICB), and axillary brachial plexus block. These complications include phrenic paralysis, incomplete brachial plexus block, and pneumothorax. This narrative review examines the literature on brachial plexus block in the costoclavicular space, discussing the anatomical position, the procedure, clinical indications, choice of local anesthetic concentration and volume, and continuous nerve block of CCB. The aim is to provide a basis for future clinical practice and enhanced safety.
Keywords: Brachial Plexus Block, Nerve Block, Ultrasonography, Humans, Ultrasonography, Interventional, Anesthetics, Local, Brachial Plexus
Background
Brachial plexus block is a well-established anesthetic and analgesic technique for upper-limb surgery and is widely used by clinicians [1]. However, the classical brachial plexus approach has advantages and disadvantages depending on individual patients and the surgical area involved. Therefore, selecting the most appropriate brachial plexus block method is crucial to avoid complications [2]. The costoclavicular space has gained recognition in human anatomy studies, and with advancements in ultrasound technology, more brachial plexus blocks are being performed under ultrasound guidance [3]. This approach has reduced puncture-related complications and improved the speed and safety of the brachial plexus block [4,5]. Costoclavicular brachial plexus block (CCB) is a new technique for infraclavicular brachial plexus block, providing anesthesia and postoperative analgesia. Ultrasound-guided CCB has gained maturity and has become a research focus in recent years. Compared to other approaches, CCB offers advantages such as clear visualization of the brachial plexus, simplicity of the procedure, rapid onset, and reliable analgesic effects. Additionally, it reduces complications such as phrenic paralysis, pneumothorax, and nerve block catheter-related issues, thus improving safety and patient comfort [6]. With the increasing number of anatomy and clinical studies conducted in recent years, our understanding of CCB has become more comprehensive.
Anatomy of the Brachial Plexus Nerve in the Costoclavicular Space
The brachial plexus consists of the anterior branch of the C4 nerve, the C5 to C8 nerves, and the anterior branch of the T1 nerve [7,8]. It primarily innervates the upper limbs and controls the motor and sensory functions of the hands and arms. In the infraclavicular approach [9], the brachial plexus nerve is divided into lateral, medial, and posterior bundles, which extend downward into the axilla through its upper region. The lateral cord of the brachial plexus originates from nerve fibers spanning from C5 to C7 and innervates the following muscles: the biceps brachii muscle, supplying motor innervation to the muscles responsible for elbow flexion; the brachialis muscle, involved in elbow flexion; the coracobrachialis muscle, assisting in shoulder flexion; the Pronator teres muscle, contributing to forearm pronation; the flexor carpi radialis muscle, responsible for wrist flexion; the palmaris longus muscle, involved in wrist flexion; and the radial wrist extensor muscles, supporting elbow, wrist, finger, and thumb extension. The medial cord of the brachial plexus receives nerve fibers from C8 to T1 and provides innervation to the following muscles: the flexor digitorum profundus muscle, controlling flexion of the thumb, index finger, and middle finger; The ulnar nerve supplies the deep flexor muscles of the little finger and ring finger, as well as the intrinsic muscles of the hand, and the pectoralis major muscle, specifically its clavicular portion. The posterior cord of the brachial plexus includes the following nerves: The axillary nerve, originating from C5 to C6 nerve fibers, innervates the deltoid and teres minor muscles; The radial nerve, receiving contributions from C5 to T1 nerve fibers, predominantly C7, controls muscles involved in elbow and wrist extension, finger extension, and thumb extension; The thoracodorsal nerve, derived from C6 to C8 nerve fibers, innervates the latissimus dorsi muscle. Upon the application of electrical stimulation to the brachial plexus bundles, the muscles innervated by these nerve bundles elicit a contraction response [7].
The costoclavicular space is situated behind the midpoint of the clavicle and is a narrow area between the lateral and medial aspects of the clavicle and the first rib. The distribution of blood vessels and the brachial plexus within the costoclavicular space is relatively fixed, with minimal variation [10]. Karmakar et al [11] previously showed that the lateral, medial, and posterior bundles of the brachial plexus in this region are compact and superficial. Under ultrasound guidance, a suitable puncture angle can avoid the initial and axillary veins, leading to an optimal brachial plexus block. By examining fresh autopsy specimens, Sala-Blanch et al [12] observed the brachial plexus, axillary arteries, and veins crossing within the costoclavicular space. The 3 bundles of the brachial plexus are positioned outside the axillary artery: the lateral bundle is the most superficial, the medial bundle is deeper, and the posterior bundle is the most external. Connective tissue envelopes the medial and posterior bundles, separating them from the lateral bundle, in accordance with the microscopic dissection results reported by Monzó et al [13]. Carles et al [14] further indicated that the costoclavicular space lies beneath the pectoralis major and subclavian muscle, and performing continuous nerve block in this area can enhance catheter stability and reduce the risk of catheter displacement. Moreover, the lack of fixed devices in the patient’s neck retains their mobility unaffected. Hence, the clinical application of CCB is supported by solid anatomical foundations.
Clinical Applications of CCB
Ultrasound-Guided CCB
ULTRASOUND-GUIDED CCB:
Ultrasound is a non-invasive, real-time, and dynamic visualization technique that allows anesthesiologists to directly visualize the relationship between nerves, blood vessels, and surrounding tissues during brachial plexus block [15]. This improves the success rate and reduces complications [4], serving as a valuable tool in the costoclavicular brachial plexus block procedure. Ultrasound provides clear visualization of the anatomical location, depth, and relationships of the brachial plexus bundle with surrounding muscles and blood vessels.
The ultrasound-guided CCB procedure can be performed as follows:
POSTURE: Based on existing studies, most patients had their affected upper limbs abducted at a 90° angle while tilting their head to the opposite side [16]. This position brings the costoclavicular space closer to the body surface, allowing for better stretching of the brachial plexus and axillary arteries and veins. This positioning ensures accurate relative positioning of nerves and vessels, producing a clear ultrasound image.
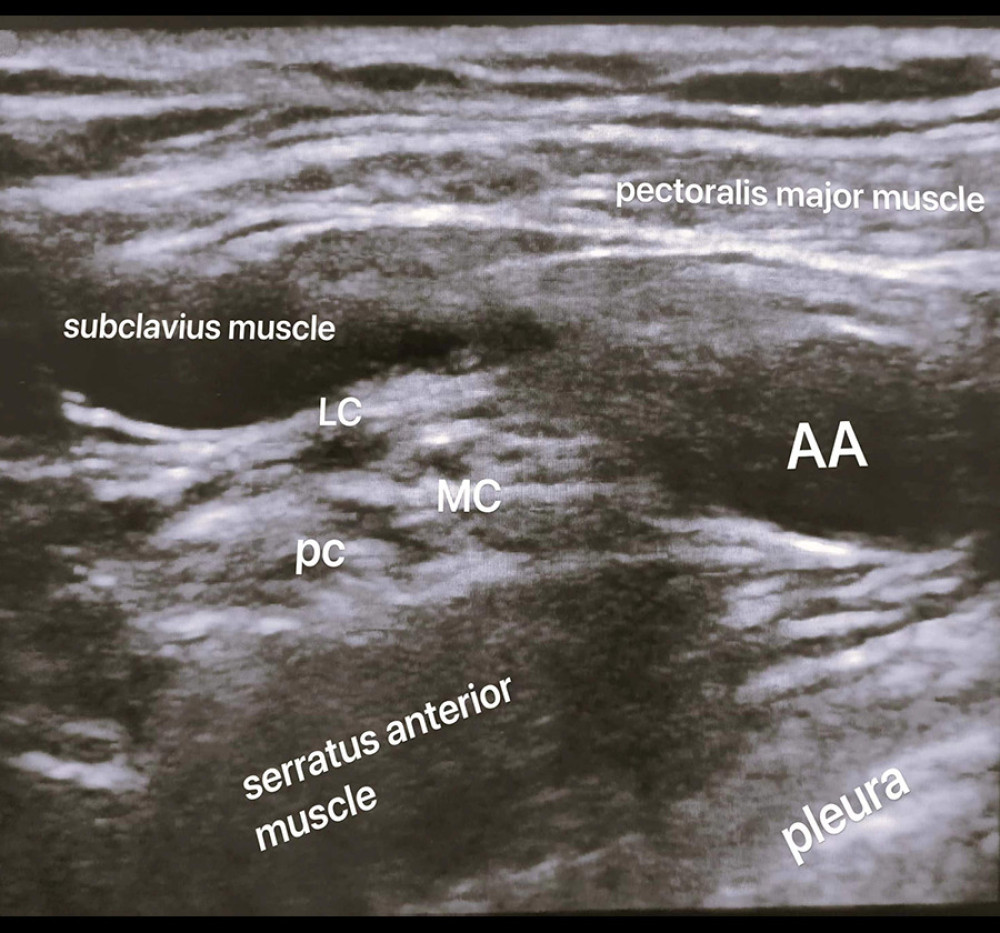
METHOD: Similar to the technique described by Li et al [17], a high-frequency linear array probe is placed at the midpoint of the clavicle. By sliding the probe downward, various structures such as the pectoralis major muscle, subclavius muscle, brachial plexus nerve, axillary artery, and vein in the costoclavicular space, as well as other tissue structures like the cephalic vein, thoracoacromial artery, and serratus anterior, can be identified. The probe should be adjusted slightly from a superficial to a deep angle, revealing the pectoralis major, brachial plexus, and pleura. Under the optimal ultrasound image [17,18] (Figure 1), the needle is inserted using an in-plane technique from the outside to the inside of the probe. Once the needle tip is visualized, it is inserted into the middle of the 3 bundles or between the lateral and medial bundles, and the medication is administered while ensuring there is no blood return. To prevent nerve injury, the injection pressure should be adjusted [19], keeping it below 15 psi, and the injection time should be longer than 2–3 min. By evaluating the sensory and motor blockade responses of the ulnar nerve, radial nerve, musculocutaneous nerve, and median nerve, the efficacy of the nerve blockade can be determined [18]. In cases where the tip of the coracoid process joint appears unclear, or the development of nerves and vessels is not well-defined due to the depth of the lateral subclavian fossa in certain patients, some researchers [20] have proposed an alternative puncture path using the opposite direction in-plane needle approach. The procedure is similar to the previously-described method. Once the best image is obtained, the needle is inserted inside the high-frequency linear array probe to avoid the surrounding blood vessels, yielding successful outcomes [21].
MEDICATION REGIMEN: In a prospective study involving 40 patients, Kewlani et al. [22] used 0.5% ropivacaine for the block and determined that the 95% effective volume was 18.9 mL. Other studies have also used a 20 mL volume with satisfactory results within 30 min. However, there is limited research on brachial plexus block through the costoclavicular space using an out-of-plane needle or solely relying on a nerve stimulator [23–25], warranting further investigation.
DURATION OF ANESTHESIA: The duration of anesthesia in the brachial plexus block varies depending on the type of local anesthetic used and the technique employed. Typically, anesthesia lasts 4–16 hours. Short-acting local anesthetics like lidocaine [28] provide anesthesia for 2–4 h, whereas longer-acting agents such as ropivacaine [22] can provide anesthesia for 8–16 h or even longer. Adding epinephrine to the local anesthetic solution can prolong the duration of the block by slowing absorption of the anesthetic agent.
CLINICAL INDICATIONS OF CCB:
In clinical practice, the CCB approach, specifically the lateral sagittal infraclavicular plexus block (LS-ICB) [26], is commonly used for distal upper-arm surgery. This is because the C5 to C7 nerve branches [9], including the suprascapular, subscapular, axillary, and lateral thoracic nerves, have already separated before reaching the subclavicle. Thus, the ICB approach typically covers anesthesia limited to the elbow, forearm, wrist, and hand. With its anatomical structure, the costoclavicular space is a suitable alternative for LS-ICB as a new infraclavicular brachial plexus block approach. Leurcharusmee et al analyzed 90 patients undergoing distal upper-limb surgery and found that CCB offers advantages over the traditional lateral sagittal process approach, including shorter operation time and faster fulfillment of surgical needs [27]. Other studies on CCB [11,17,27,28] have primarily focused on forearm surgery.
For shoulder joint and proximal upper-limb operations, where the suprascapular nerve innervates 70% of sensory function [29] and the remaining by the axillary nerve, the brachial plexus nerve block via the subclavian approach often results in incomplete anesthesia. Interscalene brachial plexus block (ISB) or supraclavicular brachial plexus block (SCB) are usually employed to achieve satisfactory blockade [30–32]. In 2017, Carles García-Vitoria proposed that the costoclavicular space can serve as a channel connecting the supraclavicular and subclavian areas. Placing a catheter in the infraclavicular space allows for continuous nerve block in the supraclavicular area, providing effective analgesia and benefits from the protection offered by the pectoralis major and subclavian muscles. This significantly reduces the likelihood of catheter displacement and prolapse after brachial plexus catheterization via the subclavian approach [33]. In 2020, Koyyalamudi et al [34] injected a cadaver with 20 mL of 0.1% methylene blue solution 5 times and dissected the brachial plexus and its branches from the C4 level to the lower armpit, documenting the spread of dye and staining of nerve trunks, bundles, and branches, including the suprascapular nerve. Aliste [23] also confirmed that the costoclavicular space could be used for anesthesia in patients undergoing shoulder surgery by comparing ISB and CCB in a study involving 44 patients. Luo et al [16] evaluated the non-inferiority of brachial plexus block via CCB and ISB in 212 patients in 2023, demonstrating that CCB could provide a nerve block method with fewer complications for high-risk patients undergoing shoulder surgery. Furthermore, several case reports [35–37] have shown that brachial plexus block via the costoclavicular space approach allows for drug spread to the supraclavicular area, meeting the needs of shoulder and upper-arm humerus surgery. Therefore, CCB surpasses the block range of the traditional subclavian approach and fulfills surgical requirements for the forearm and proximal upper arm, including the humerus and shoulder.
CONCENTRATION AND VOLUME OF CCB LOCAL ANESTHETIC:
Determining the optimal concentration and volume of local anesthetics for brachial plexus block has been a significant research focus in recent years. Lidocaine, a classic choice for nerve block, has been favored by researchers. Studies by Tran et al [38,39] and González et al [40] investigated brachial plexus block via SCB, ICB, and axillary approaches using sequential lidocaine administration. The effective volume of 1.5% lidocaine ranged from 23.5 mL to 35 mL. In a study conducted by Sotthisopha et a. [28] in 2017, sequential CCB was performed on 57 patients using 1.5% lidocaine, with a 90% effective volume (ED90) of 34 mL.
Ropivacaine, known for its unique sensorimotor separation effect at low concentrations [41], has gained popularity among surgeons, especially orthopedic surgeons, and patients [42,43]. The concentration of ropivacaine is often determined based on its pharmacological properties, with intraoperative concentrations ranging from 0.5% to 0.75% [41]. Recent studies [23–25] have utilized a 20 mL dose of 0.35–0.5% ropivacaine, achieving excellent analgesic effects. In a study by Kew et al [22] involving 40 patients undergoing distal upper-limb surgery, the half-effective dose (ED50) of 0.5% ropivacaine was found to be 13.5 mL, while the 95% effective dose (ED95) was 18.9 mL. Wong et al [44] also reported similar findings in a study of 48 patients undergoing hand surgery, where the minimum effective volume of 0.5% ropivacaine in 90% of cases was comparable to results of Kew et al.
For postoperative analgesia, a lower concentration of 0.2% ropivacaine can selectively block sensory nerves while preserving limb motor function [45,46].
MULTIPOINT INJECTION OF CCB:
Numerous studies [40,47,48] have examined brachial plexus block through interscalene, supraclavicular, axillary, and lateral sagittal infraclavicular approaches. Most of these studies indicate that multipoint injection offers advantages such as lower total drug volume and faster onset of effectiveness than single injections. However, it also presents disadvantages, such as increased needle punctures for nerve blocks and a higher risk of vascular puncture complications. In the case of CCB, both anatomical and clinical studies have identified a tissue septum [13] between the medial, lateral, and posterior bundles of the brachial plexus within the costoclavicular space. This septum can impact the diffusion of local anesthetics and the onset time between nerve bundles. Li et al [17] observed that while the success rate of CCB is nearly 97%, the effectiveness of each bundle block differs. The medial and posterior bundles exhibit faster motion block speeds than the lateral bundle. This discrepancy may be attributed to the fact that the posterior and medial bundles are uniformly wrapped within the same diaphragm, while connective tissue separates the lateral bundle as a whole. Building upon previous research, Monzó et al [49] found that 92.5% of patients had a rebound diaphragm within the costoclavicular space, leading to an illusion of drug diffusion upon injection. It was further proposed that the best ultrasound-guided image could be obtained for multipoint injection. Local anesthetics (5 and 10 mL) were administered to the lateral and posterior bundles to counteract the diaphragm effect and achieve a more effective nerve block. Layera et al [50] also demonstrated that the success rate of 2-point injection is similar to that of Monzó. Using 2 injections enables faster anesthesia onset compared to a single injection while achieving similar success rates in terms of surgical anesthesia and analgesia. However, 2 injections increase the number of punctures and the incidence of complications such as Horner’s syndrome. Whether a 3-point injection for the brachial plexus 3-bundle block can yield a faster and more effective anesthetic effect and whether it leads to other complications remains unclear. Further research is required to address these questions.
CONTINUOUS BRACHIAL PLEXUS BLOCK OF CCB:
Continuous nerve block [51] involves placement of a nerve block catheter near the target nerve, providing long-term and effective analgesia. It is commonly used for treatment of chronic pain conditions such as complex regional pain syndrome [52], refractory phantom limb pain [53], advanced cancer [54], trigeminal neuralgia [55], and patients requiring prolonged postoperative analgesia for rehabilitation [56]. Continuous brachial plexus block is frequently employed for postoperative pain management and upper-limb rehabilitation exercises [37,58,59]. Due to the long block time, it can significantly reduce postoperative rebound pain [57] and promote recovery of postoperative function, thus improving patient satisfaction [43]. Traditional approaches include interscalene, supraclavicular, and infraclavicular techniques [60]. However, due to the superficial positioning of most cervical catheters, weak muscle support, and frequent neck movement, there is a high risk of catheter displacement. Studies have shown that the catheter displacement rate for the interscalene approach is 35% [61]. Therefore, clinical anesthesiologists often prefer the lateral sagittal infraclavicular approach for continuous nerve block [62]. This approach provides greater stability and lower catheter detachment rates due to the presence of the muscle tunnel formed by the pectoralis major and subclavian muscles [63].
Nevertheless, the catheter transposition rate for the infraclavicular approach was found to be 22% [64]. Carles’s anatomical study of the costoclavicular space in 2017 [14] laid the groundwork for subsequent continuous nerve blocks in this region, located between the pectoralis major, subclavian, and anterior serratus muscles. During ultrasound-guided continuous brachial plexus block in the costoclavicular space, the nerve block needle passes through the pectoralis major and subclavian muscles, and the catheter is secured within the muscle tunnels, resulting in low catheter transposition rates postoperatively. There have been no reported cases of catheter displacement, dislodgment, or catheter-induced nerve injuries associated with continuous nerve blocks in the costoclavicular space [65,66]. Moreover, the variability of the position of the brachial plexus within the costoclavicular space is minimal, with the nerve bundles and blood vessels being relatively concentrated and fixed. Anesthesiologists with knowledge of the relevant anatomy can perform brachial plexus blocks more efficiently compared to other nerve blocks [66]. In addition, compared to the interscalene or supraclavicular approaches, continuous nerve block in the costoclavicular space requires a lower puncture position, has less impact on neck movement, and offers a better overall experience. This approach has also been proven safe and effective for single-dose nerve blocks in pediatric patients [65]. However, the precise efficacy of continuous nerve blocks [66–68] in the costoclavicular space is yet to be determined. In the past, continuous nerve blocks via the interscalene approach were prone to catheter prolapse due to the extensive range of neck motion. The effectiveness of continuous nerve blocks via the costoclavicular approach has been demonstrated [37,69], but further randomized controlled trials are needed to establish its safety.
INCIDENCE OF HEMI-DIAPHRAGMATIC PARALYSIS AT CCB:
The diaphragm is the primary inspiratory muscle in the human body, accounting for two-thirds of total ventilation [70]. Hemi-diaphragmatic paralysis (HDP) is a common complication of brachial plexus block [29], mainly when local anesthetic drugs spread to block the phrenic nerve, resulting in a 30% reduction in vital capacity and a 20% decrease in ventilation [71]. While most patients are asymptomatic due to compensation from the contralateral diaphragm, individuals with respiratory diseases, severe heart conditions, or obesity may experience chest tightness and even respiratory failure [72]. The phrenic nerve originates from C3 to C5, runs adjacent to the sternocleidomastoid muscle, descends laterally to the anterior scalene muscle, and is located 0.18 cm from the brachial plexus. Studies have shown that the interscalene approach for brachial plexus block can lead to 100% HDP [73]. Experts suggest that reducing the volume of local anesthesia and minimizing drug spread to the septal nerve can help decrease the incidence of HDP. However, even when the total amount of local anesthetic in the interscalene approach is reduced to 5 mL, a 27% incidence of HDP remains [74].
Furthermore, the phrenic nerve separates from the brachial plexus nerve at a distance of 3 mm/cm as it descends from the anterior scalene muscle. Some researchers propose that blocking the brachial plexus away from the phrenic nerve can achieve adequate analgesia while reducing complications such as phrenic paralysis. Although effective in reducing postoperative pain, the supraclavicular approach still results in a 45% incidence of HDP [75,76]. Therefore, careful consideration is necessary when performing brachial plexus blocks in patients with lung disease. In recent years, the costoclavicular space has gained recognition due to its anatomical distance from the diaphragm and the low rate of phrenic nerve block. A randomized controlled trial by Boohwi Hong involving 80 patients who received an injection of 25 mL of 0.375% ropivacaine demonstrated that costoclavicular space block reduced the incidence of diaphragm paralysis to 3% [25].
Similarly, Aliste et al reported a 0% incidence of phrenic nerve block using 20 mL of 0.5% ropivacaine for CCB injection in 2019 [23]. Luo et al revealed that in shoulder arthroscopic surgery, the incidence of HDP in CCB was 7.55%, significantly lower than 92.45% for the interscalene approach. The incidences of shortness of breath and Horner syndrome were also lower in CCB, at 0% and 10.38%, respectively, compared to 18.87% and 10.38%, respectively, in the interscalene approach4[16]. The clinical applications of CCB have recently expanded from the distal to the proximal upper limb and shoulder region [23,77], providing effective anesthesia and analgesia. Its low incidence of diaphragmatic paralysis offers a reliable and safe nerve block method for patients with lung diseases undergoing upper-limb surgeries such as shoulder and humerus procedures. However, for continuous nerve block in the costoclavicular space, there is no available information on the potential accumulation of local anesthetic drugs during long-term infusion and its relation to HDP. Nonetheless, some studies have suggested that diluting the concentration of local anesthetic drugs with normal saline during infusion can alleviate symptoms of limb numbness [78,79]. Therefore, this approach could potentially be used as a solution if the accumulation of local anesthetics leads to HDP.
Limitations of CCB
LIMITATIONS IN VISUALIZING CCB:
While the costoclavicular space can be easily identified, performing a nerve block in thin or obese patients presents challenges [80]. Thin patients often have a deep paraclavicular fossa behind the coracoid process, resulting in a large ultrasound shadow area and unclear tissue development. Moreover, some patients may have a protruding coracoid process, making it difficult to visualize the needle tip clearly [20]. In obese patients, the costoclavicular space is situated within a layer of fat, the pectoralis major, and the subclavian muscle, which can impede proper needle visualization. These patients are at a higher risk of multiple needle attempts, puncturing the cephalic, axillary veins, and even the acromial artery [81]. To overcome these challenges, some researchers suggest approaching the subclavicular region from medial to lateral to enhance needle visualization by avoiding interference from the coracoid process. However, this technique may result in slower blockade of the medial bundle due to the distribution of nerves within the costoclavicular space, where the medial bundle is located more superficially than the lateral bundle.
THORACIC OUTLET SYNDROME:
Thoracic outlet syndrome [82] is a challenging clinical condition to diagnose, characterized by the compression of neurovascular bundles in the thoracic outlet, leading to pain and numbness in the shoulder and upper limbs. Possible causes of thoracic outlet syndrome include clavicle trauma, malunion of clavicle fractures, and hemorrhage in the costoclavicular and subclavicular space. In the case of CCB, the neurovascular structures within the costoclavicular space are closely situated and immobile. However, during the nerve block procedure, there is a risk of inadvertently puncturing blood vessels due to the unclear visualization of tissue vessels or needle tips [81]. This can result in bleeding and subsequent compression of the brachial plexus. Therefore, patients who experience prolonged numbness in the upper limbs following CCB should consider the potential for nerve injury during the procedure and the possibility of developing thoracic outlet syndrome.
HEMODIALYSIS PATIENTS USING A DOUBLE-LUMEN CATHETER:
The number of patients with end-stage renal disease (ESRD) requiring kidney replacement therapy has increased recently, and hemodialysis has become a widely used method for managing acute and chronic kidney failure [83]. In clinical practice, hemodialysis access can be achieved through an arteriovenous fistula or the placement of a double-lumen catheter. Among these options, the placement of a double-lumen catheter is preferred by patients due to its minimal impact on blood circulation, effectiveness in preventing infections, and convenience of use [84]. The common sites for inserting a double-lumen catheter in hemodialysis patients include the internal jugular, subclavian, and femoral veins [85]. However, when a double-lumen catheter is placed in the subclavian vein, it can occupy the costoclavicular space, increasing the risk of nerve injury or displacement by interfering with the insertion and angle of the nerve block needle.
Furthermore, the insertion site of the double-lumen catheter is deeper and may involve deeper tissues [86], raising the risk of nerve injury. Hemodialysis patients who receive double-lumen catheters also face complications such as catheter-related infections [87], subcutaneous tunnel infections [88], and catheter dysfunction, with catheter-related bacteremia being the main reason for catheter removal [89]. Therefore, caution should be exercised when performing CCB procedures to minimize the risk of skin puncture, catheter-related infections, and systemic complications.
ANTITHROMBOTIC THERAPY:
With the increasing incidence of cardiovascular diseases, more patients are being prescribed antithrombotic medications to prevent thrombosis. However, when these patients undergo regional anesthesia, their abnormal coagulation function poses an increased risk. While studies have shown that bleeding complications following peripheral nerve block (PNB) in such patients are rare, with an estimated incidence rate of 0.67%, the occurrence of a hematoma can lead to serious adverse consequences [90–92]. ESAIC/ESRA and ASRA guidelines classify peripheral nerve blocks based on their potential risk of severe bleeding complications in patients on antithrombotic therapy. Superficial nerve blocks, such as routine interscalene, supraclavicular, axillary, sciatic, and femoral nerve blocks, are considered low-risk, allowing for broader use of perioperative anticoagulant drugs, including high-dose low-molecular-weight heparin. Deep nerve blocks like the lateral sagittal infraclavicular brachial plexus block (ICB) are classified as high-risk, and antithrombotic drugs should be managed according to recommendations for neuraxial procedures [93–95].
While there have been reports of bleeding-related complications associated with superficial nerve blocks, such as nerve damage and significant bleeding, studies have also shown that bleeding complications following peripheral nerve block are rare in patients receiving antithrombotic therapy [91,96–103]. In contrast, complications associated with deep nerve blocks can be more severe, including bleeding and even death [92,104]. CCB has been considered a shallower and safer approach than ICB, with vascular injuries mainly involving the puncture of the axillary vein and artery. Studies have demonstrated that these vessels can be promptly compressed in case of a puncture, suggesting that CCB may be a low-risk superficial nerve block with compressible vessels and a lower risk of bleeding than ICB [20,105,106]. However, there is limited research on the use of antithrombotic drugs in patients undergoing CCB, as most studies exclude patients using these medications. Some studies have reported successful cases of CCB in patients receiving anticoagulant therapy, highlighting its potential as a viable option with good outcomes and no perioperative bleeding complications [35].
Further research is needed to explore the clinical application of CCB in the presence of anticoagulant therapy. Selecting the best imaging method under ultrasound guidance is essential to avoid blood vessels, ensure clear visualization of the needle tip position, and achieve a safe and effective block. Additionally, in the costoclavicular space thoracic outlet syndrome can be caused by bleeding, hematoma, or nerve compression. Therefore, when performing CCB on patients on anticoagulant therapy, it is crucial to use ultrasound guidance to select the optimal image, ensuring avoidance of blood vessels, clear visualization of the needle tip position, and successful and safe nerve blocking.
Conclusions
The increasing number of cadaver specimens and clinical studies on CCB has enhanced our understanding of various aspects, including ultrasound manipulation, clinical indications, drug concentration and dose, continuous nerve block, and hemidiaphragm paralysis. CCB has expanded its clinical indications to include shoulder joints and humerus, offering a valuable anesthetic option for patients with pulmonary conditions and contraindications for brachial plexus block. However, further research is required to establish its safety profile. Additionally, there is a lack of clinical data regarding the safety and effectiveness of CCB for continuous nerve block, highlighting the need for future investigation in this area.
References
1. Hsu AC, Tai YT, Lin KH, Infraclavicular brachial plexus block in adults: A comprehensive review based on a unified nomenclature system: J Anesth, 2019; 33(3); 463-77
2. Neumeister EL, Beason AM, Thayer JA, El Bitar Y, Perioperative pain management in hand and upper extremity surgery: Clin Plast Surg, 2020; 47(2); 323-34
3. Gamble SG, Costo-clavicular syndrome: Arch Phys Med Rehabil, 1951; 32(8); 516-22
4. Gu L, An H, Zhang X, Jiang W, Clinical application of ultrasound microscopy-guided pediatric brachial plexus nerve block anesthesia: Contrast Media Mol Imaging, 2022; 2022 3383898
5. Zadrazil M, Opfermann P, Marhofer P, Brachial plexus block with ultrasound guidance for upper-limb trauma surgery in children: A retrospective cohort study of 565 cases: Br J Anaesth, 2020; 125(1); 104-9
6. Kumari P, Kumar A, Sinha C, Kumar A, Ultrasound-guided continuous costoclavicular brachial plexus block: Indian J Anaesth, 2020; 64(7); 637
7. Rhone TB, Brachial plexus block anaesthesia: Ann Surg, 1935; 101(5); 1153-70
8. Soeding P, Eizenberg N, Review article: Anatomical considerations for ultrasound guidance for regional anesthesia of the neck and upper limb: Can J Anaesth J Can Anesth, 2009; 56(7); 518-33
9. Chin KJ, Singh M, Velayutham V, Chee V, Infraclavicular brachial plexus block for regional anaesthesia of the lower arm: Cochrane Database Syst Rev, 2010(2); CD005487
10. Dahlstrom KA, Olinger AB, Descriptive anatomy of the interscalene triangle and the costoclavicular space and their relationship to thoracic outlet syndrome: A study of 60 cadavers: J Manipulative Physiol Ther, 2012; 35(5); 396-401
11. Karmakar MK, Sala-Blanch X, Songthamwat B, Tsui BCH, Benefits of the costoclavicular space for ultrasound-guided infraclavicular brachial plexus block: Description of a costoclavicular approach: Reg Anesth Pain Med, 2015; 40(3); 287-88
12. Sala-Blanch X, Reina MA, Pangthipampai P, Karmakar MK, Anatomic basis for brachial plexus block at the costoclavicular space: A cadaver anatomic study: Reg Anesth Pain Med, 2016; 41(3); 387-91
13. Monzó E, Boezaart AP, Tubbs RS, A reliable septum exists between the lateral cord and medial and posterior cords in the costoclavicular region: Clinical and microanatomical considerations in brachial plexus anesthetic blockade: Clin Anat, 2021; 34(3); 411-19
14. García-Vitoria C, Bosch M, A reliable gate for continuous regional anesthesia catheter insertion: Anesthesiology, 2017; 127(4); 712
15. McCartney CJL, Lin L, Shastri U, Evidence basis for the use of ultrasound for upper-extremity blocks: Reg Anesth Pain Med, 2010; 35(2 Suppl); S10-15
16. Luo Q, Yang C, Wei W, Effects of costoclavicular block versus interscalene block in patients undergoing arthroscopic shoulder surgery under monitored anaesthesia care: A randomized, prospective, non-inferiority study: Korean J Anesthesiol, 2023 [Online ahead of print]
17. Li JW, Songthamwat B, Samy W, Ultrasound-guided costoclavicular brachial plexus block: Sonoanatomy, technique, and block dynamics: Reg Anesth Pain Med, 2017; 42(2); 233-40
18. : NYSORA – world leader in anesthesiology education, NYSORA
19. Gadsden J, Latmore M, Levine DM, Robinson A, High opening injection pressure is associated with needle-nerve and needle-fascia contact during femoral nerve block: Reg Anesth Pain Med, 2016; 41(1); 50-55
20. Murata H, Hida K, Ogami-Takamura K, Hara T, Importance of careful identification of the axillary vessels during ultrasound-guided costoclavicular brachial plexus block: Reg Anesth Pain Med, 2019; 44(1); 138-40
21. Tulgar S, Ugutmen E, A modified technique for the application of ultrasound-guided costoclavicular brachial plexus block for elbow surgery leading to differential block: J Clin Anesth, 2018; 47; 65-66
22. Kewlani A, Bhatia N, Makkar JK, Kumar V, Median effective volume of 0.5% ropivacaine for ultrasound-guided costoclavicular block: Anesthesiology, 2021; 134(4); 617-25
23. Aliste J, Bravo D, Layera S, Randomized comparison between interscalene and costoclavicular blocks for arthroscopic shoulder surgery: Reg Anesth Pain Med, 2019; 44(4); 472-77
24. Dost B, Kaya C, Ustun YB, Lateral sagittal versus costoclavicular approaches for ultrasound-guided infraclavicular brachial plexus block: A comparison of block dynamics through a randomized clinical trial: Cureus, 2021; 13(3); e14129
25. Hong B, Lee S, Oh C, Hemidiaphragmatic paralysis following costoclavicular versus supraclavicular brachial plexus block: A randomized controlled trial: Sci Rep, 2021; 11(1); 18749
26. Aliste J, Layera S, Bravo D, Randomized comparison between perineural dexamethasone and combined perineural dexamethasone-dexmedetomidine for ultrasound-guided infraclavicular block: Reg Anesth Pain Med, 2022 [Online ahead of print]
27. Leurcharusmee P, Elgueta MF, Tiyaprasertkul W, A randomized comparison between costoclavicular and paracoracoid ultrasound-guided infraclavicular block for upper limb surgery: Can J Anesth Can Anesth, 2017; 64(6); 617-25
28. Sotthisopha T, Elgueta MF, Samerchua A, Minimum effective volume of lidocaine for ultrasound-guided costoclavicular block: Reg Anesth Pain Med, 2017; 42(5); 571-74
29. El-Boghdadly K, Chin KJ, Chan VWS, Phrenic nerve palsy and regional anesthesia for shoulder surgery: Anatomical, physiologic, and clinical considerations: Anesthesiology, 2017; 127(1); 173-91
30. Mariano ER, Afra R, Loland VJ, Continuous interscalene brachial plexus block via an ultrasound-guided posterior approach: A randomized, triple-masked, placebo-controlled study: Anesth Analg, 2009; 108(5); 1688-94
31. Kim H, Kim HJ, Lee ES, Postoperative pain control after arthroscopic rotator cuff repair: arthroscopy-guided continuous suprascapular nerve block versus ultrasound-guided continuous interscalene block: Arthrosc J Arthrosc Relat Surg, 2021; 37(11); 3229-37
32. Karaman T, Karaman S, Aşçı M, Comparison of ultrasound-guided supraclavicular and interscalene brachial plexus blocks in postoperative pain management after arthroscopic shoulder surgery: Pain Pract, 2019; 19(2); 196-203
33. Marhofer D, Marhofer P, Triffterer L, Dislocation rates of perineural catheters: A volunteer study: Br J Anaesth, 2013; 111(5); 800-6
34. Koyyalamudi V, Langley NR, Harbell MW, Evaluating the spread of costoclavicular brachial plexus block: An anatomical study: Reg Anesth Pain Med, 2021; 46(1); 31-34
35. Beh ZY, Hasan MS, Ultrasound-guided costoclavicular approach infraclavicular brachial plexus block for vascular access surgery: J Vasc Access, 2017; 18(5); e57-e61
36. Kwon W, Lee SM, Bang S, Costoclavicular block for shoulder surgery in a patient with tracheobronchopathia osteochondroplastica and COPD: J Clin Anesth, 2019; 55; 13-14
37. Regufe R, Artilheiro V, Dias MB, Continuous costoclavicular brachial plexus block in a pediatric patient for postfracture rehabilitation: Pediatr Anesth, 2020; 30(6); 720-21
38. Tran DQH, Dugani S, Dyachenko A, Minimum effective volume of lidocaine for ultrasound-guided infraclavicular block: Reg Anesth Pain Med, 2011; 36(2); 190-94
39. Tran DQH, Dugani S, Correa JA, Minimum effective volume of lidocaine for ultrasound-guided supraclavicular block: Reg Anesth Pain Med, 2011; 36(5); 466-69
40. González AP, Bernucci F, Pham K, Minimum effective volume of lidocaine for double-injection ultrasound-guided axillary block: Reg Anesth Pain Med, 2013; 38(1); 16-20
41. Simpson D, Curran MP, Oldfield V, Keating GM, Ropivacaine: A review of its use in regional anaesthesia and acute pain management: Drugs, 2005; 65(18); 2675-717
42. Cappelleri G, Ambrosoli AL, Gemma M, Intraneural ultrasound-guided sciatic nerve block: Minimum effective volume and electrophysiologic effects: Anesthesiology, 2018; 129(2); 241-48
43. Zhang P, Chang H, Yang T, Study on MEV90 of 0.5% ropivacaine for US-guided caudal epidural block in anorectal surgery: Front Med, 2022; 9; 1077478
44. Wong MH, Karmakar MK, Mok LYH, Minimum effective volume of 0.5% ropivacaine for ultrasound-guided costoclavicular brachial plexus block: A dose finding study: Eur J Anaesthesiol, 2020; 37(9); 780-86
45. Eledjam JJ, Cuvillon P, Capdevila X, Postoperative analgesia by femoral nerve block with ropivacaine 0.2% after major knee surgery: Continuous versus patient-controlled techniques: Reg Anesth Pain Med, 2002; 27(6); 604-11
46. Novello-Siegenthaler A, Hamdani M, Iselin-Chaves I, Fournier R, Ultrasound-guided continuous femoral nerve block: A randomized trial on the influence of femoral nerve catheter orifice configuration (six-hole versus end-hole) on post-operative analgesia after total knee arthroplasty: BMC Anesthesiol, 2018; 18(1); 191
47. Desgagnés MC, Lévesque S, Dion N, A comparison of a single or triple injection technique for ultrasound-guided infraclavicular block: A prospective randomized controlled study: Anesth Analg, 2009; 109(2); 668-72
48. Roy M, Nadeau MJ, Côté D, Comparison of a single- or double-injection technique for ultrasound-guided supraclavicular block: A prospective, randomized, blinded controlled study: Reg Anesth Pain Med, 2012; 37(1); 55-59
49. Monzó E, Hadzic A, Costoclavicular approach to the brachial plexus block: Simple or double injection?: Reg Anesth Pain Med, 2019 [Online ahead of print]
50. Layera S, Aliste J, Bravo D, Single- versus double-injection costoclavicular block: A randomized comparison: Reg Anesth Pain Med, 2020; 45(3); 209-13
51. Ilfeld BM, Continuous peripheral nerve blocks: A review of the published evidence: Anesth Analg, 2011; 113(4); 904-25
52. Dadure C, Motais F, Ricard C, Continuous peripheral nerve blocks at home for treatment of recurrent complex regional pain syndrome I in children: Anesthesiology, 2005; 102(2); 387-91
53. Lierz P, Schroegendorfer K, Choi S, Continuous blockade of both brachial plexus with ropivacaine in phantom pain: A case report: Pain, 1998; 78(2); 135-37
54. Fischer HB, Peters TM, Fleming IM, Else TA, Peripheral nerve catheterization in the management of terminal cancer pain: Reg Anesth, 1996; 21(5); 482-85
55. Umino M, Kohase H, Ideguchi S, Sakurai N, Long-term pain control in trigeminal neuralgia with local anesthetics using an indwelling catheter in the mandibular nerve: Clin J Pain, 2002; 18(3); 196-99
56. Lanitis S, Mimigianni C, Raptis D, The impact of educational status on the postoperative perception of pain: Korean J Pain, 2015; 28(4); 265-74
57. Holmberg A, Sauter AR, Klaastad Ø, Pre-operative brachial plexus block compared with an identical block performed at the end of surgery: A prospective, double-blind, randomised clinical trial: Anaesthesia, 2017; 72(8); 967-77
58. Megaw K, Johnston DF, Continuous infraclavicular brachial plexus block using retroclavicular approach for total elbow replacement: J Clin Anesth, 2020; 60; 111-12
59. Ilfeld BM, Continuous peripheral nerve blocks: An update of the published evidence and comparison with novel, alternative analgesic modalities: Anesth Analg, 2017; 124(1); 308-35
60. Hauritz RW, Hannig KE, Balocco AL, Peripheral nerve catheters: A critical review of the efficacy: Best Pract Res Clin Anaesthesiol, 2019; 33(3); 325-39
61. Auyong DB, Cantor DA, Green C, Hanson NA, The effect of fixation technique on continuous interscalene nerve block catheter success: A randomized, double-blind trial: Anesth Analg, 2017; 124(3); 959-65
62. Dhir S, Ganapathy S, Use of ultrasound guidance and contrast enhancement: A study of continuous infraclavicular brachial plexus approach: Acta Anaesthesiol Scand, 2008; 52(3); 338-42
63. Ponde VC, Continuous infraclavicular brachial plexus block: A modified technique to better secure catheter position in infants and children: Anesth Analg, 2008; 106(1); 94-96 table of contents
64. Ahsan ZS, Carvalho B, Yao J, Incidence of failure of continuous peripheral nerve catheters for postoperative analgesia in upper extremity surgery: J Hand Surg, 2014; 39(2); 324-29
65. Carioca F, Silva M, Bispo C, Costoclavicular brachial plexus block in paediatric anaesthesia: A retrospective pilot study: J Clin Anesth, 2021; 69; 110113
66. Krane EJ, Polaner D, The safety and effectiveness of continuous peripheral nerve blockade in children: Anesth Analg, 2014; 118(3); 499-500
67. Byun S, Pather N, Pediatric regional anesthesia: A review of the relevance of surface anatomy and landmarks used for peripheral nerve blockades in infants and children: Clin Anat N Y N, 2019; 32(6); 803-23
68. Heydinger G, Tobias J, Veneziano G, Fundamentals and innovations in regional anaesthesia for infants and children: Anaesthesia, 2021; 76(Suppl 1); 74-88
69. Tanaka N, Ida M, Nishiwada T, Kawaguchi M, Anesthetic management using costoclavicular brachial plexus block with patient-controlled analgesia in Pediatrics: A case report: Rev Esp Anestesiol Reanim, 2022; 69(10); 705-7
70. Bolton CF, Grand’Maison F, Parkes A, Shkrum M, Needle electromyography of the diaphragm: Muscle Nerve, 1992; 15(6); 678-81
71. Ricoy J, Rodríguez-Núñez N, Álvarez-Dobaño JM, Diaphragmatic dysfunction: Pulmonology, 2019; 25(4); 223-35
72. Guirguis M, Karroum R, Abd-Elsayed AA, Mounir-Soliman L, Acute respiratory distress following ultrasound-guided supraclavicular block: Ochsner J, 2012; 12(2); 159-62
73. Urmey WF, Talts KH, Sharrock NE, One hundred percent incidence of hemidiaphragmatic paresis associated with interscalene brachial plexus anesthesia as diagnosed by ultrasonography: Anesth Analg, 1991; 72(4); 498-503
74. Stundner O, Meissnitzer M, Brummett CM, Comparison of tissue distribution, phrenic nerve involvement, and epidural spread in standard- vs low-volume ultrasound-guided interscalene plexus block using contrast magnetic resonance imaging: A randomized, controlled trial: Br J Anaesth, 2016; 116(3); 405-12
75. Sivashanmugam T, Maurya I, Kumar N, Karmakar MK, Ipsilateral hemidiaphragmatic paresis after a supraclavicular and costoclavicular brachial plexus block: A randomised observer blinded study: Eur J Anaesthesiol, 2019; 36(10); 787-95
76. Renes SH, van Geffen GJ, Rettig HC, Minimum effective volume of local anesthetic for shoulder analgesia by ultrasound-guided block at root C7 with assessment of pulmonary function: Reg Anesth Pain Med, 2010; 35(6); 529-34
77. Jo Y, Oh C, Lee WY, Randomised comparison between superior trunk and costoclavicular blocks for arthroscopic shoulder surgery: A noninferiority study: Eur J Anaesthesiol, 2022; 39(10); 810-17
78. Chung J, Bang S, Lee Y, Novel saline injection technique for the reversal of the continuous costoclavicular block: Chin Med J (Engl), 2021; 134(24); 3023-24
79. Park EY, Kil HK, Park WS, Effect of epidural saline washout on regression of sensory and motor block after epidural anaesthesia with 2% lidocaine and fentanyl in elderly patients: Anaesthesia, 2009; 64(3); 273-76
80. Williams LM, Singh K, Dua A, Infraclavicular nerve block: StatPearls, 2022, StatPearls Publishing
81. Luo Q, Yao W, Chai Y, Comparison of ultrasound-guided supraclavicular and costoclavicular brachial plexus block using a modified double-injection technique: a randomized non-inferiority trial: Biosci Rep, 2020; 40(6) BSR20200084
82. Laulan J, Fouquet B, Rodaix C, Outlet thoracic syndrome: Definition, aetiological factors, diagnosis, management and occupational impact: J Occup Rehabil, 2011; 21(3); 366-73
83. Stegmayr B, Willems C, Groth T, Arteriovenous access in hemodialysis: A multidisciplinary perspective for future solutions: Int J Artif Organs, 2021; 44(1); 3-16
84. Trivedi HS, Twardowski ZJ, Use of double-lumen dialysis catheters. Loading with locked heparin: ASAIO J, 1997; 43(6); 900-3
85. Gołębiowski T, Kusztal M, Letachowicz K, Difficulties with tunneling of the cuffed catheter: A single-centre experience: Sci Rep, 2018; 8(1); 3314
86. Pereira GM, Souza Alvarenga A, Almeida Felipe CR, Use of ultrasound to confirm guidewire position in hemodialysis catheter implantation: J Nephrol, 2022; 35(5); 1515-19
87. Tal MG, Yevzlin AS: J Vasc Access, 2023; 24(2); 232-37
88. Ferreira V, Neto MM, Cardeal da Costa JA, Association of Infections with the use of a temporary double-lumen catheter for hemodialysis: Nephrol Nurs J, 2018; 45(3); 261-67
89. Qureshi R, Salman B, Imtiaz S, Reasons for removal of non-tunneled double lumen catheters in incident dialysis patients: J Coll Physicians Surg Pak, 2018; 28(4); 284-87
90. Poredoš P, Peripheral nerve blocks in patients on antithrombotic drugs – a rescue or an unnecessary risk?: Acta Clin Croat, 2022; 61(Suppl 2); 67-77
91. Singh SK, Katyal S, Kumar A, Kumar P, Massive hemothorax: A rare complication after supraclavicular brachial plexus block: Anesth Essays Res, 2014; 8(3); 410-12
92. O’connor WR, Preston FW, Theis FV, Retroperitoneal hemorrhage following lumbar sympathetic block during treatment with dicumarol: Report of a fatality: Ann Surg, 1950; 131(4); 575-80
93. Kietaibl S, Ferrandis R, Godier A, Regional anaesthesia in patients on antithrombotic drugs: Joint ESAIC/ESRA guidelines: Eur J Anaesthesiol, 2022; 39(2); 100-32
94. Horlocker TT, Vandermeuelen E, Kopp SL, Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine evidence-based guidelines (fourth edition): Reg Anesth Pain Med, 2018; 43(3); 263-309
95. Kubulus C, Gürtesch CA, Wagenpfeil G, Sessler DI, Volk T, Antithrombotic drugs and the risk of bloody punctures in regional anesthesia – a retrospective registry analysis: Reg Anesth Pain Med, 2022 [Online ahead of print]
96. Godier A, Bloc S, How to deal with peripheral regional anaesthesia while antithrombotics on board?: Anaesth Crit Care Pain Med, 2019; 38(5); 457-59
97. Gleeton D, Levesque S, Trépanier CA, Symptomatic axillary hematoma after ultrasound-guided infraclavicular block in a patient with undiagnosed upper extremity mycotic aneurysms: Anesth Analg, 2010; 111(4); 1069-71
98. Clendenen SR, Robards CB, Wang RD, Greengrass RA, Case report: Continuous interscalene block associated with neck hematoma and postoperative sepsis: Anesth Analg, 2010; 110(4); 1236-38
99. Toscano A, Capuano P, Galatà M, Safety of ultrasound-guided serratus anterior and erector spinae fascial plane blocks: A retrospective analysis in patients undergoing cardiac surgery while receiving anticoagulant and antiplatelet drugs: J Cardiothorac Vasc Anesth, 2022; 36(2); 483-88
100. Wardhan R, Michel R, Vasilopoulos T, Yen E, Are the placement, maintenance, and removal of femoral and sciatic catheters associated with bleeding complications in vascular patients on antithrombotics? A single-center, retrospective cohort study: Anesth Analg, 2022; 134(1); 188-93
101. Joubert F, Gillois P, Bouaziz H, Bleeding complications following peripheral regional anaesthesia in patients treated with anticoagulants or antiplatelet agents: A systematic review: Anaesth Crit Care Pain Med, 2019; 38(5); 507-16
102. Plunkett AR, Buckenmaier CC, Safety of multiple, simultaneous continuous peripheral nerve block catheters in a patient receiving therapeutic low-molecular-weight heparin: Pain Med, 2008; 9(5); 624-27
103. Bigeleisen PE, Ultrasound-guided infraclavicular block in an anticoagulated and anesthetized patient: Anesth Analg, 2007; 104(5); 1285-87 tables of contents
104. Warner NS, Duncan CM, Kopp SL, Acute retroperitoneal hematoma after psoas catheter placement in a patient with myeloproliferative thrombocytosis and aspirin therapy: A A Case Rep, 2016; 6(2); 28-30
105. Chen YB, Bao HS, Hu TT, Comparison of comfort and complications of Implantable Venous Access Port (IVAP) with ultrasound-guided Internal Jugular Vein (IJV) and Axillary Vein/Subclavian Vein (AxV/SCV) puncture in breast cancer patients: A randomized controlled study: BMC Cancer, 2022; 22(1); 248
106. Farina A, Coppola G, Bassanelli G, Ultrasound-guided central venous catheter placement through the axillary vein in cardiac critical care patients: Safety and feasibility of a novel technique in a prospective observational study: Minerva Anestesiol, 2020; 86(2); 157-64
In Press
12 Mar 2024 : Clinical Research
Preoperative Blood Transfusion Requirements for Hemorrhoidal Severe Anemia: A Retrospective Study of 128 Pa...Med Sci Monit In Press; DOI: 10.12659/MSM.943126
12 Mar 2024 : Clinical Research
Tissue Inhibitors of Metalloproteinase 1 (TIMP-1) and 3 (TIMP-3) as New Markers of Acute Kidney Injury Afte...Med Sci Monit In Press; DOI: 10.12659/MSM.943500
12 Mar 2024 : Review article
Optimizing Behçet Uveitis Management: A Review of Personalized Immunosuppressive StrategiesMed Sci Monit In Press; DOI: 10.12659/MSM.943240
12 Mar 2024 : Clinical Research
Metabolomic Alterations in Methotrexate Treatment of Moderate-to-Severe PsoriasisMed Sci Monit In Press; DOI: 10.12659/MSM.943360
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952