21 May 2023: Clinical Research
Value of IL-1β and IL-23 in Predicting 28-Day Mortality Due to Sepsis: A Retrospective Study
Jing Cao1ABE*, Wenguang Liu1CDE, Yong Li1ABF, Bin Chen1CDE, Tingfeng Yu1DEF, Zhengbing He1BCD, Yanke Hong1EFDOI: 10.12659/MSM.940163
Med Sci Monit 2023; 29:e940163
Abstract
BACKGROUND: This research aimed to explore the utility of Interleukin-1β (IL-1β) and IL-23 as potential biomarkers for the diagnosis and prognosis of sepsis.
MATERIAL AND METHODS: This study included 74 adult individuals with sepsis, 45 ICU controls, and 50 healthy individuals attending routine physical examinations. IL-1β and IL-23 levels were assessed and analyzed on the admission day. Univariate Cox regression analyses were utilized to explore the association of IL-1β and IL-23 with sepsis survival. Furthermore, receiver operating characteristic (ROC) analysis was employed to evaluate the value of IL-1β and IL-23 to predict 28-day mortality due to sepsis.
RESULTS: Serum concentrations of IL-1β and IL-23 were significantly higher in septic patients relative to healthy and ICU controls (P<0.001). IL-1β and IL-23 levels in non-survivors were significantly higher than in survivors (P<0.001). IL-1β (hazard ratio; HR=1.06, P<0.001) and IL-23 (HR=1.02, P=0.031) were independent risk variables for 28-day mortality in sepsis patients, which were strongly associated with the severity of sepsis. The area under the ROC curve for predicting 28-day fatality in sepsis was 0.66 for IL-1β (P=0.024, 95% confidence interval; CI: 0.54-0.76) and 0.77 for IL-23 (P<0.001, 95% CI: 0.65-0.86). Furthermore, compared with low serum IL-1β (<9.41 pg/mL) and IL-23 (<6.77 pg/mL) levels, septic patients with high serum IL-1β (≥9.41 pg/mL) and IL-23 (≥6.77 pg/mL) levels had poorer survival.
CONCLUSIONS: Serum IL-1β and IL-23 values were higher in patients with sepsis and are potential diagnostic and prognostic markers for sepsis, but this needs to be confirmed by prospective studies.
Keywords: Diagnosis, IL23A Protein, Human, Interleukin 1beta (193-195), Prognosis, Sepsis, Adult, Humans, Biomarkers, Intensive Care Units, Interleukin-23, Retrospective Studies, ROC Curve, Interleukin-1beta
Background
Sepsis is a life-threatening organ malfunction resulting from an abnormal host reaction to an infection [1], resulting in a high death rate among patients referred to the Intensive Care Unit (ICU) [2]. Globally, around 19–48.9 million people develop sepsis, among which nearly 11 million die [3]. According to a survey in China, the incidence of sepsis in the ICU was 20.6 for 100 ICU hospitalizations (95% confidence interval; CI=15.8–25.4), with a 90-day fatality rate of 35.5% [4]. Given the high morbidity and fatality of sepsis, diagnosing and managing sepsis poses a significant challenge in public health.
Due to a lack of a characteristic clinical presentation of sepsis, early prevention and treatment are difficult [5]. The single measurement of biomarkers has been extensively studied as a rapid and accurate method of infection diagnosis. Many novel biomarkers have been identified, such as CD5L [6], interleukin-26 (IL-26) [7], IL-17D [8], procalcitonin (PCT), and IL-10 [9], yet they have not been promoted and applied clinically due to low sensitivity and specificity. Hence, biomarkers with good diagnostic performance are crucial for early diagnosis, therapy, and prediction of sepsis.
IL-1β, a crucial pro-inflammatory cytokine, is involved in autoimmune inflammatory responses and multiple cellular activities, such as cell proliferation, differentiation, and apoptosis [10]. IL-1β dysregulation is associated with many pathological conditions, including sepsis [11], rheumatoid arthritis [12], and inflammatory bowel disease [13]. IL-23 is the central cytokine of autoimmunity and is an extremely promising therapeutic target for inflammatory diseases [14,15]. An imbalance in the IL-23/IL-17 axis is closely related to developing sepsis [16]. However, studies on the involvement of IL-1β and IL-23 in the development and progression of sepsis are still relatively scarce. Accordingly, we focused on assessing IL-1β and IL-23 for sepsis diagnosis and prediction.
Material and Methods
SEPTIC PATIENTS AND CONTROLS:
This retrospective, single-center investigation was conducted in a 2200-bed tertiary, comprehensive hospital in China. From January to December 2021, 74 individuals who fulfilled the clinical criteria for sepsis-3.0 [17] at Yiyang Central Hospital were enrolled as the sepsis group, consisting of 55 men and 19 women, with a median age of 56 years (inter-quartile range [IQR], 43–68). The control group consisted of 45 critically ill patients without sepsis but with critical illness (poly-trauma, cerebral trauma, or burns), who were hospitalized in the ICU, including 29 men and 16 women, with a median age of 61 years (IQR, 52.5–68). The healthy control group consisted of 50 healthy individuals from the Physical Examination Center of our hospital, including 29 males and 21 females, with a median age of 54.5 years (IQR 45.5–67). Septic patients were allocated into 2 groups based on 28-day survival: the survival group (n=43) included 33 males, and 10 females, with a median age of 53 years (IQR 43–70), and the non-survival group (n=31) consisted of 22 males and 9 females, with a median age of 56 years (IQR 44–66).
The exclusion criteria were: (1) age less than 18 years old; (2) pregnant, possibly pregnant, or lactating women; (3) patients with cancer, immunodeficiency disease, autoimmune disease, and using immunosuppressive medication in the past 8 weeks; (4) with acute cardiovascular and cerebrovascular disease; (5) missing key clinical information; and (6) refused to take part in the research. The Ethics Committee of Yiyang Central Hospital authorized this study (no. 2021-0065). Informed consent was waived as the study was retrospective.
DATA COLLECTION:
Clinical data were obtained retrospectively from the hospital information system (HIS), including age, sex, underlying disease (urinary stones, cerebral vascular disease, diabetes mellitus, hypertension, hypoproteinemia), vital signs, length of hospital stay, and 28-day mortality. Within 24 h of admission, laboratory values were obtained retrospectively from the Laboratory Information System (LIS), including routine blood tests, creatinine (Cr; standard values: 44.0–136.0 μmol/L), blood urea nitrogen (BUN, standard values: 1.8–7.1 mmol/L), uric acid (UA; standard values: 150–420 μmol/L), total bilirubin (TBI; normal values: 5.1–19.0 μmol/L), lactate (standard values: 0.5–2.2 mmol), C-reactive protein (standard values: <10 mg/L), procalcitonin (standard values: <0.5 ng/mL), and SOFA (Sequential Organ Failure Assessment) scores.
SAMPLE MEASUREMENT METHODS:
Venous blood samples were collected at admission within 24 h and stored in a freezer at −80°C. Immunotag™ Human IL-23 ELISA was purchased from G-Biosciences (St. Louis, USA). IL-1β and IL-23 serum values were detected utilizing enzyme-linked immunosorbent assay (ELISA) kits.
STATISTICAL ANALYSES:
The statistical analysis was carried out using SPSS v25.0. (IBM, Chicago, IL, USA). Figures 4 and 5 were generated using GraphPad Prism 9.0 (GraphPad, Inc., San Diego, CA). Risk Factor Analysis (Figure 2) and Cox Models Forest (Figure 3) were performed by using Data Analysis tools in Hiplot Pro (https://hiplot.com.cn/), a comprehensive web service for biomedical data analysis and visualization. For data with non-normal distribution, non-parametric methods were used for statistical analyses. Continuous variables were presented as medians (interquartile range) and 3 groups were compared using the Kruskal-Wallis test. Two groups were assessed utilizing the Mann-Whitney U test. The χ2 or Fisher’s exact test, as relevant, was employed to compare the frequencies (percentage) of categorical variables. To evaluate the diagnostic and prognostic usefulness of IL-1β and IL-23 in sepsis, the receiver operating characteristics (ROC) curve was utilized. SOFA score was employed to evaluate the degree of sepsis. Afterward, risk factor analysis was conducted to elucidate the association of IL-1β, IL-23, and procalcitonin (PCT) with SOFA score. Furthermore, univariate Cox regression analysis was conducted to assess the risk variables for sepsis fatality at 28 days. Finally, we performed survival analysis (SA) utilizing the Kaplan-Meier (K-M) technique and a log-rank test according to the cutoff value of IL-1β (9.41 pg/mL) and IL-23 (6.77 pg/mL). P values <0.05 were judged statistically significant.
Results
INITIAL PARAMETERS:
Table 1 displays the basic demographics and laboratory data of participants. We enrolled 74 individuals with sepsis, 50 sex- and age-matched healthy controls, and 45 sex- and age-matched ICU controls. The 28-day mortality rate was 40.7% in the septic patients (n=31). Compared to healthy controls, the median levels of white blood cells (WBC), neutrophils, and monocytes were significantly higher in septic patients, whereas the median levels of lymphocytes, hematocrit, and platelets (PLT) were significantly lower in septic patients. Cr, BUN, total bilirubin (TBI), PCT, and SOFA ratings were all significantly higher in septic individuals contrasted with ICU controls (all P<0.05). IL-1β and IL-23 in the sera of 74 septic patients on the hospitalization day were significantly higher than those of ICU patients and healthy controls (P<0.05).
CLINICAL ROLES OF IL-1β AND IL-23 IN DIAGNOSING SEPSIS:
Figure 1 shows the ROC curves of IL-1β and IL-23 for diagnosing sepsis. When distinguishing between 74 septic patients and 45 non-sepsis patients (ICU controls), the AUC of IL-1β was 0.72 [P<0.001, 95% CI (0.64–0.80)], and the AUC of IL-23 was 0.80 [P<0.001, 95% CI (0.73–0.87)]. The ROC curves demonstrated that the diagnostic reliabilities of IL-1β and IL-23 were lower than the SOFA score [AUC=0.98, P<0.001, 95% CI (0.97–1.00)] but superior to PCT [AUC=0.71, P<0.001, 95% CI (0.61–0.80)].
SERUM VALUES OF IL-1β AND IL-23 WERE HIGHER IN THE NON-SURVIVAL GROUP:
Sepsis individuals were further stratified by 28-day mortality into survival and non-survival. Table 2 displays the distinct features of the survival and non-survival groups. There was no statistically significant difference between these indicators: age, sex, underlying disease, vital signs, and length of hospital stay (all P>0.05). There were no significant differences in these laboratory indicators: blood routine, Cr, BUN, UA, TBI, partial pressure of oxygen (PaO2), partial pressure of carbon dioxide (PaCO2), lactate, and C-reactive protein (CRP; all P>0.05). However, the median PCT, IL-1β, IL-23, and SOFA scores in the non-survival group were significantly higher than in the survival group. To elucidate the association of serum IL-1β and IL-23 levels with the severity of sepsis, we performed risk factor analysis, which showed that higher SOFA scores were associated with higher levels of IL-1β (r=0.480, P<0.001), IL-23 (r=0.476, P<0.001), and PCT (r=0.204, P=0.027) (Figure 2).
CLINICAL ROLE OF L-1β AND IL-23 IN PREDICTING 28-DAY MORTALITY IN SEPTIC INDIVIDUALS:
Univariate Cox regression analysis was conducted to assess the variables associated with 28-day mortality in septic individuals. PCT (hazard ratio; HR=1.02, P=0.001), IL-1β (HR=1.06, P<0.001), IL-23 (HR=1.02, P=0.031), and SOFA score (HR=1.15, P=0.004) were independent risk variables for 28-day mortality in septic individuals (Figure 3).
Furthermore, we constructed ROC curves to predict 28-day mortality by using serum IL-1β and IL-23 values (Figure 4). For the performance of variables in predicting 28-day mortality, the AUC of IL-23 was 0.77 [P<0.001, 95% CI (0.65–0.86)], with 87.10% sensitivity and 67.44% specificity, which was higher than PCT [AUC=0.67, P=0.008, 95% CI (0.55–0.77)] and IL-1β [AUC=0.66, P=0.024, 95% CI (0.54–0.76)], but lower than SOFA score [AUC=0.78, P<0.001, 95% CI (0.61–0.87)].
According to the threshold values of IL-1β (9.41 pg/mL) and IL-23 (6.77 pg/mL) for the estimated 28-day mortality of septic individuals, K-M survival curves were created (Figure 4). Patients with higher serum IL-1β (Figure 5A) and IL-23 (Figure 5B) concentrations had worse survival than patients with lower serum IL-1β and IL-23 concentrations.
Discussion
LIMITATIONS:
Due to the limited experimental settings, this research has some flaws. First, because no fundamental experiments were undertaken, it is difficult to assess if the hypothesized mechanisms of action of IL-1β and IL-23 in sepsis agree with those proposed here. We hope that domestic and international investigators will perform trials to verify our claims. Second, our investigation was a single-center retrospective study with few participants, which may have resulted in selection bias and somewhat unreliable results. Consequently, the trial outcomes could be contingent. We shall perform a more thorough and in-depth prospective investigation to acquire the best possible trial findings. Third, an ICU control group was set up in this study, but non-septic patients with underlying diseases of poly-trauma, cerebral trauma, or burns were in critical condition when they were admitted, and required care and treatment in the ICU. We analyzed only the laboratory indicators within 24 h of admission in the ICU controls. The reliability of the results was further reduced by the delayed organismal stress response, which resulted in large differences between the ICU controls and septic patients. Fourth, there was no dynamic monitoring of serum IL-1β and IL-23 levels in patients with sepsis; therefore, it was difficult to assess the temporal correlation between the evolution of sepsis and their value. Overall, these results need to be further confirmed by additional studies.
Conclusions
Levels of IL-1β and IL-23 were significantly higher in patients. Early monitoring of IL-1β and IL-23 levels in individuals can be potential diagnostic and predictive biomarkers for sepsis since they are independent risk factors for predicting mortality within 28 days of sepsis. However, this needs to be confirmed by further prospective studies.
Figures
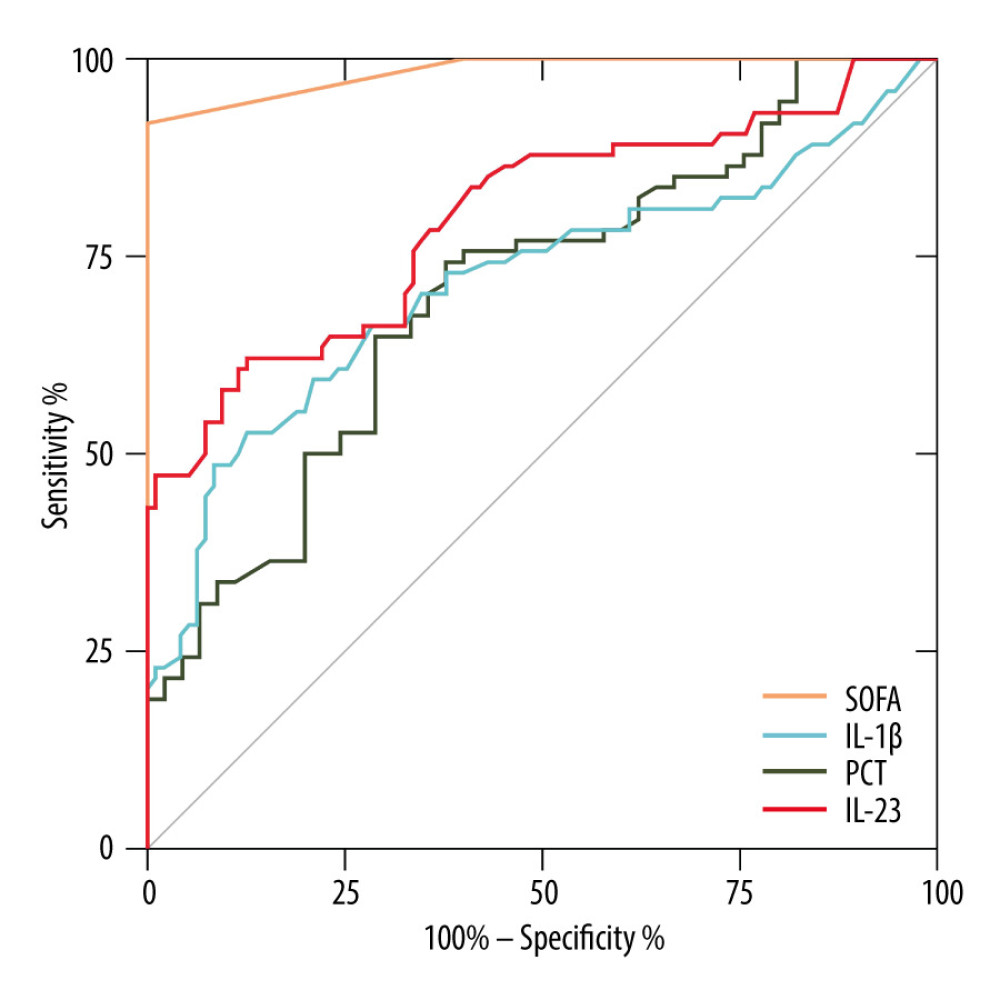 Figure 1. Receiver operating characteristic curve (ROC) of IL-1β, IL-23, SOFA score, and PCT for diagnosis of sepsis. Area under the ROC curve, 0.984 (P<0.001) for SOFA score, 0.798 (P<0.001) for IL-23, 0.717 (P<0.001) for IL-1β, and 0.705 (P<0.001) for PCT. GraphPad Prism 9.0, GraphPad Software, Inc.
Figure 1. Receiver operating characteristic curve (ROC) of IL-1β, IL-23, SOFA score, and PCT for diagnosis of sepsis. Area under the ROC curve, 0.984 (P<0.001) for SOFA score, 0.798 (P<0.001) for IL-23, 0.717 (P<0.001) for IL-1β, and 0.705 (P<0.001) for PCT. GraphPad Prism 9.0, GraphPad Software, Inc. 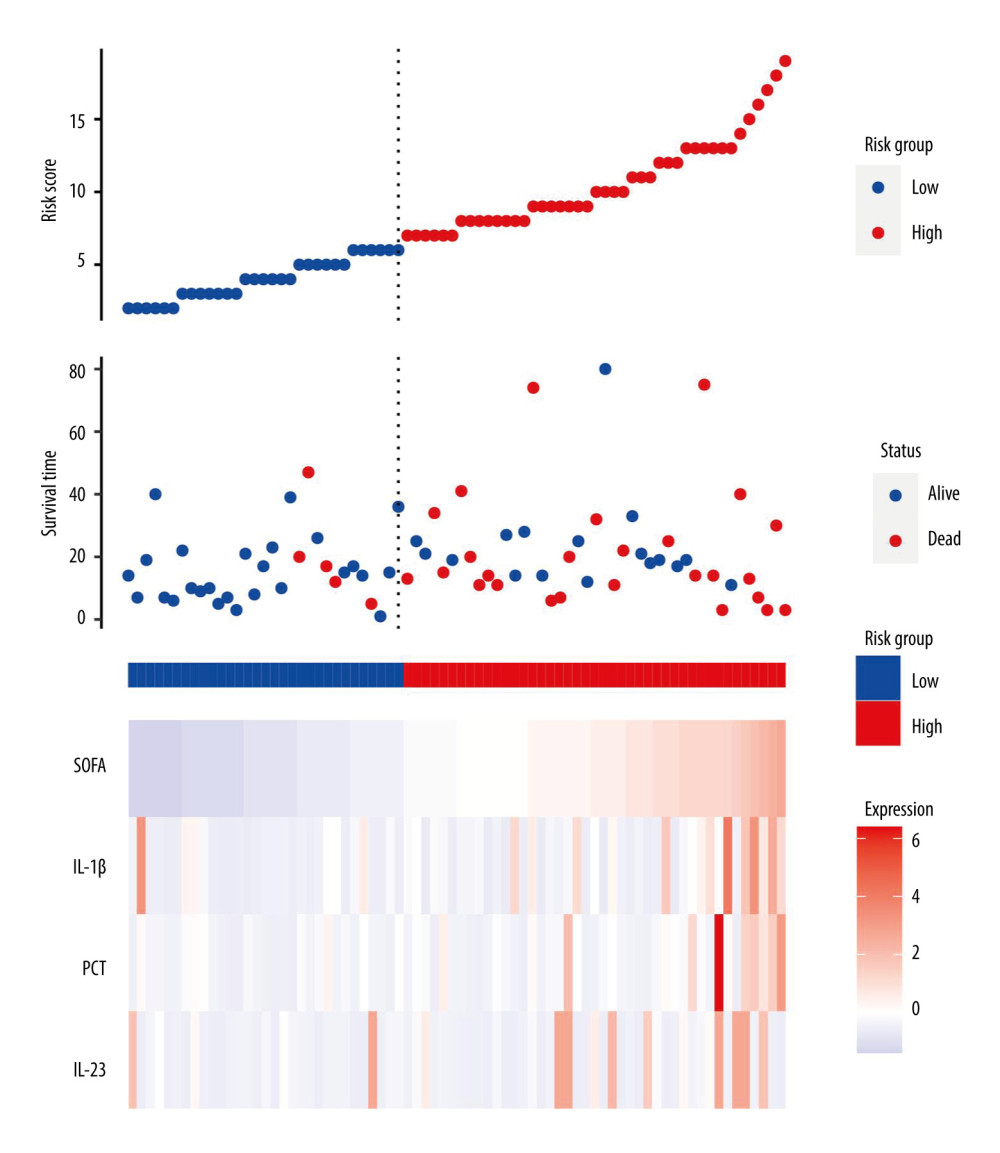 Figure 2. Serum IL-23, IL-1β, and PCT levels at admission correlated with disease severity (SOFA score) in septic patients. Hiplot Pro 2.0, Shanghai Tengyun Biotechnology Co., Ltd., https://hiplot.com.cn/.
Figure 2. Serum IL-23, IL-1β, and PCT levels at admission correlated with disease severity (SOFA score) in septic patients. Hiplot Pro 2.0, Shanghai Tengyun Biotechnology Co., Ltd., https://hiplot.com.cn/. 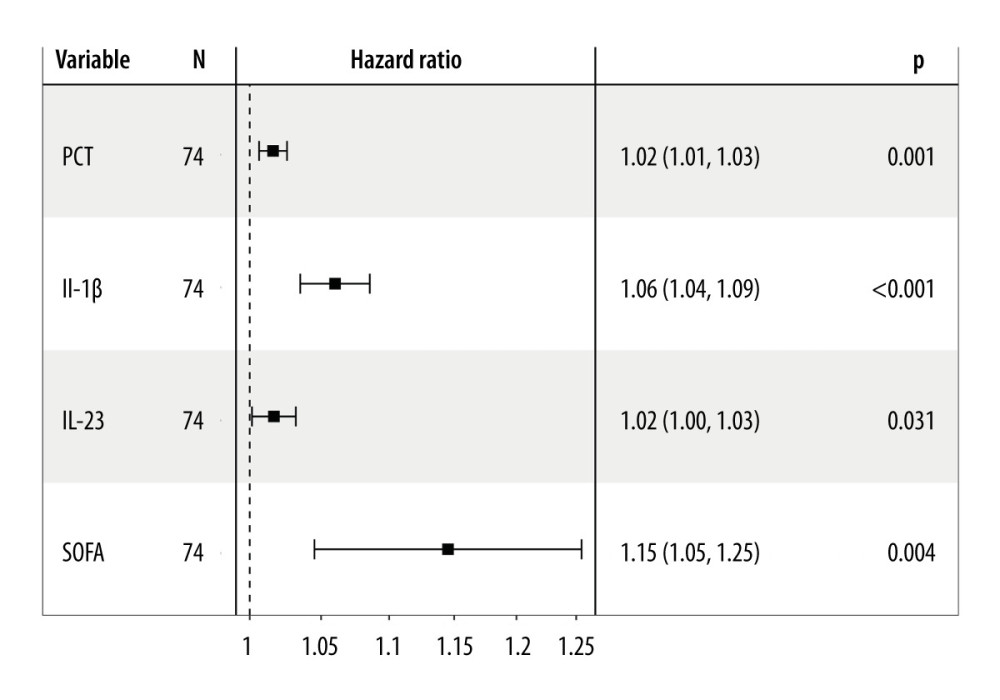 Figure 3. Independent factors predicting 28-day mortality in septic patients. Hiplot Pro 2.0, Shanghai Tengyun Biotechnology Co., Ltd, https://hiplot.com.cn/.
Figure 3. Independent factors predicting 28-day mortality in septic patients. Hiplot Pro 2.0, Shanghai Tengyun Biotechnology Co., Ltd, https://hiplot.com.cn/. 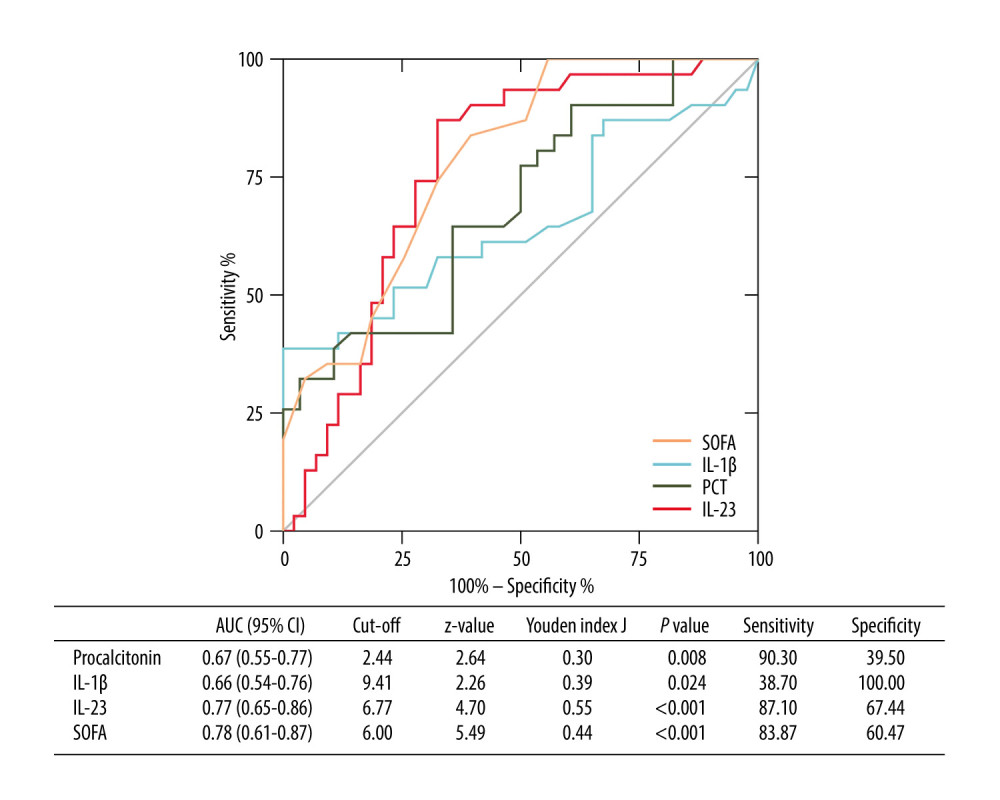 Figure 4. Receiving operating characteristic (ROC) curve of IL-1β, IL-23, SOFA score, and PCT at admission for predicting 28-day mortality in septic patients. GraphPad Prism 9.0, GraphPad Software, Inc.
Figure 4. Receiving operating characteristic (ROC) curve of IL-1β, IL-23, SOFA score, and PCT at admission for predicting 28-day mortality in septic patients. GraphPad Prism 9.0, GraphPad Software, Inc. 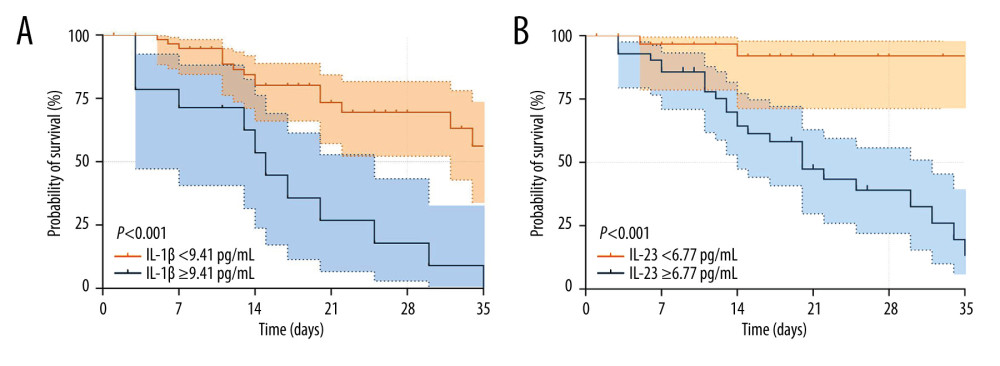 Figure 5. Kaplan-Meier survival curves of 74 adult patients with sepsis based on the IL-1β (A) cutoff value (9.41 pg/mL) and IL-23 (B) cutoff value (6.77 pg/mL) on day of ICU admission. GraphPad Prism 9.0, GraphPad Software, Inc.
Figure 5. Kaplan-Meier survival curves of 74 adult patients with sepsis based on the IL-1β (A) cutoff value (9.41 pg/mL) and IL-23 (B) cutoff value (6.77 pg/mL) on day of ICU admission. GraphPad Prism 9.0, GraphPad Software, Inc. References
1. Fortini A, Faraone A, Meini S, Validity of “Sepsis-3” criteria in identifying patients with community-onset sepsis in Internal Medicine wards; A prospective, multicenter study: Eur J Intern Med, 2021; 85; 92-97
2. Qiu Z, Shen K, Shu MValue of Hepcidin as a diagnostic biomarker of sepsis in critically ill adults: Zhonghua Wei Zhong Bing Ji Jiu Yi Xue, 2018; 30(7); 652-57 [in Chinese]
3. Chiu C, Legrand M, Epidemiology of sepsis and septic shock: Curr Opin Anaesthesiol, 2021; 34(2); 71-76
4. Xie J, Wang H, Kang Y, The epidemiology of sepsis in Chinese ICUs: A national cross-sectional survey: Crit Care Med, 2020; 48(3); e209-e18
5. Bracht H, Hafner S, Weiss MSepsis update: Definition and epidemiology: Anasthesiol Intensivmed Notfallmed Schmerzther, 2019; 54(1); 10-20 [in German]
6. Gao X, Liu Y, Xu F, Assessment of apoptosis inhibitor of macrophage/CD5L as a biomarker to predict mortality in the critically ill with sepsis: Chest, 2019; 156(4); 696-705
7. Tu H, Lai X, Li J, Interleukin-26 is overexpressed in human sepsis and contributes to inflammation, organ injury, and mortality in murine sepsis: Crit Care, 2019; 23(1); 290
8. Yan X, Tu H, Liu Y, Interleukin-17D aggravates sepsis by inhibiting macrophage phagocytosis: Crit Care Med, 2020; 48(1); e58-e65
9. Zhang W, Wang W, Hou W, The diagnostic utility of IL-10, IL-17, and PCT in patients with sepsis infection: Front Public Health, 2022; 10; 923457
10. Dinarello C, Arend W, Sims J, IL-1 family nomenclature: Nat Immunol, 2010; 11(11); 973
11. Kimura A, Kitajima M, Nishida K, NQO1 inhibits the TLR-dependent production of selective cytokines by promoting IkappaB-zeta degradation: J Exp Med, 2018; 215(8); 2197-209
12. Yang J, Wang J, Liang X, IL-1beta increases the expression of inflammatory factors in synovial fluid-derived fibroblast-like synoviocytes via activation of the NF-kappaB-mediated ERK-STAT1 signaling pathway: Mol Med Rep, 2019; 20(6); 4993-5001
13. Luong P, Hedl M, Yan J, INAVA-ARNO complexes bridge mucosal barrier function with inflammatory signaling: Elife, 2018; 7; e38539
14. Moschen AR, Tilg H, Raine T, IL-12, IL-23 and IL-17 in IBD: Immunobiology and therapeutic targeting: Nat Rev Gastroenterol Hepatol, 2019; 16(3); 185-96
15. Bridgewood C, Newton D, Bragazzi N, Unexpected connections of the IL-23/IL-17 and IL-4/IL-13 cytokine axes in inflammatory arthritis and enthesitis: Semin Immunol, 2021; 58; 101520
16. Sun JK, Zhang WH, Chen WX, Effects of early enteral nutrition on Th17/Treg cells and IL-23/IL-17 in septic patients: World J Gastroenterol, 2019; 25(22); 2799-808
17. Singer M, Deutschman CS, Seymour CW, The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3): JAMA, 2016; 315(8); 801-10
18. von Cube M, Schumacher M, Timsit JF, Sepsis: Lancet, 2020; 396(10265); 1804
19. Gaini S, Relster MM, Pedersen C, Johansen IS, Prediction of 28-days mortality with sequential organ failure assessment (SOFA), quick SOFA (qSOFA) and systemic inflammatory response syndrome (SIRS) – a retrospective study of medical patients with acute infectious disease: Int J Infect Dis, 2019; 78; 1-7
20. Pierrakos C, Velissaris D, Bisdorff M, Biomarkers of sepsis: Time for a reappraisal: Crit Care, 2020; 24(1); 287
21. Prescott HC, Angus DC, Enhancing recovery from sepsis: A review: JAMA, 2018; 319(1); 62-75
22. Gupta DL, Bhoi S, Mohan T, Coexistence of Th1/Th2 and Th17/Treg imbalances in patients with post traumatic sepsis: Cytokine, 2016; 88; 214-21
23. Deng P, Tang N, Li L, Diagnostic value of combined detection of IL-1beta, IL-6, and TNF-alpha for sepsis-induced cardiomyopathy: Med Clin (Barc), 2022; 158(9); 413-17
24. Evavold CL, Kagan JC, Inflammasomes: Threat-assessment organelles of the innate immune system: Immunity, 2019; 51(4); 609-24
25. Liu X, Nemeth DP, McKim DB, Cell-type-specific interleukin 1 receptor 1 signaling in the brain regulates distinct neuroimmune activities: Immunity, 2019; 50(2); 317-33e316
26. Xiong S, Hong Z, Huang LS, IL-1beta suppression of VE-cadherin transcription underlies sepsis-induced inflammatory lung injury: J Clin Invest, 2020; 130(7); 3684-98
27. Frieder J, Kivelevitch D, Haugh I, Anti-IL-23 and anti-IL-17 biologic agents for the treatment of immune-mediated inflammatory conditions: Clin Pharmacol Ther, 2018; 103(1); 88-101
Figures
 Figure 1. Receiver operating characteristic curve (ROC) of IL-1β, IL-23, SOFA score, and PCT for diagnosis of sepsis. Area under the ROC curve, 0.984 (P<0.001) for SOFA score, 0.798 (P<0.001) for IL-23, 0.717 (P<0.001) for IL-1β, and 0.705 (P<0.001) for PCT. GraphPad Prism 9.0, GraphPad Software, Inc.
Figure 1. Receiver operating characteristic curve (ROC) of IL-1β, IL-23, SOFA score, and PCT for diagnosis of sepsis. Area under the ROC curve, 0.984 (P<0.001) for SOFA score, 0.798 (P<0.001) for IL-23, 0.717 (P<0.001) for IL-1β, and 0.705 (P<0.001) for PCT. GraphPad Prism 9.0, GraphPad Software, Inc. Figure 2. Serum IL-23, IL-1β, and PCT levels at admission correlated with disease severity (SOFA score) in septic patients. Hiplot Pro 2.0, Shanghai Tengyun Biotechnology Co., Ltd., https://hiplot.com.cn/.
Figure 2. Serum IL-23, IL-1β, and PCT levels at admission correlated with disease severity (SOFA score) in septic patients. Hiplot Pro 2.0, Shanghai Tengyun Biotechnology Co., Ltd., https://hiplot.com.cn/. Figure 3. Independent factors predicting 28-day mortality in septic patients. Hiplot Pro 2.0, Shanghai Tengyun Biotechnology Co., Ltd, https://hiplot.com.cn/.
Figure 3. Independent factors predicting 28-day mortality in septic patients. Hiplot Pro 2.0, Shanghai Tengyun Biotechnology Co., Ltd, https://hiplot.com.cn/. Figure 4. Receiving operating characteristic (ROC) curve of IL-1β, IL-23, SOFA score, and PCT at admission for predicting 28-day mortality in septic patients. GraphPad Prism 9.0, GraphPad Software, Inc.
Figure 4. Receiving operating characteristic (ROC) curve of IL-1β, IL-23, SOFA score, and PCT at admission for predicting 28-day mortality in septic patients. GraphPad Prism 9.0, GraphPad Software, Inc. Figure 5. Kaplan-Meier survival curves of 74 adult patients with sepsis based on the IL-1β (A) cutoff value (9.41 pg/mL) and IL-23 (B) cutoff value (6.77 pg/mL) on day of ICU admission. GraphPad Prism 9.0, GraphPad Software, Inc.
Figure 5. Kaplan-Meier survival curves of 74 adult patients with sepsis based on the IL-1β (A) cutoff value (9.41 pg/mL) and IL-23 (B) cutoff value (6.77 pg/mL) on day of ICU admission. GraphPad Prism 9.0, GraphPad Software, Inc. Tables
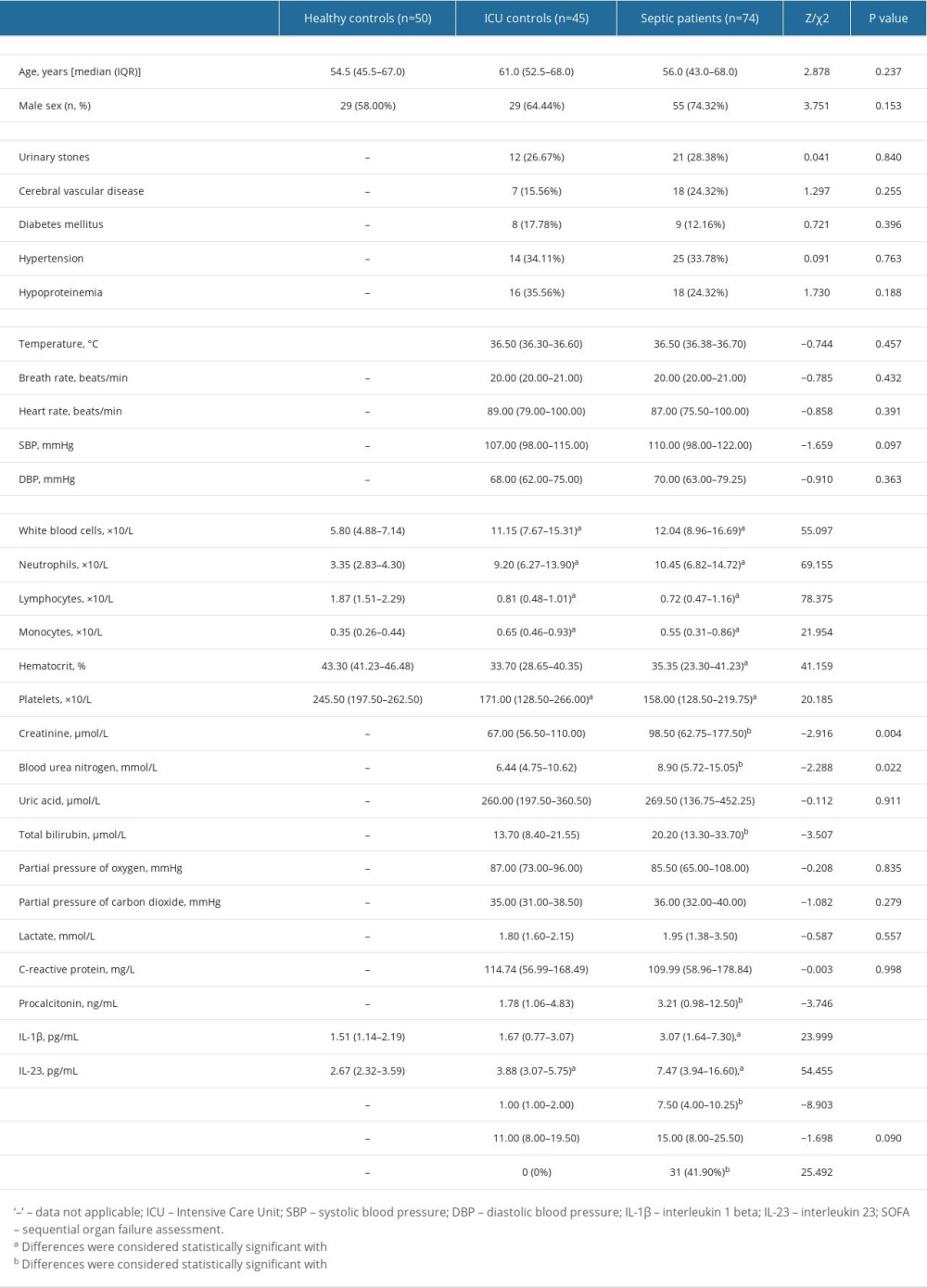 Table 1. Comparison of clinical indicators in the septic patients, ICU controls, and healthy controls.
Table 1. Comparison of clinical indicators in the septic patients, ICU controls, and healthy controls.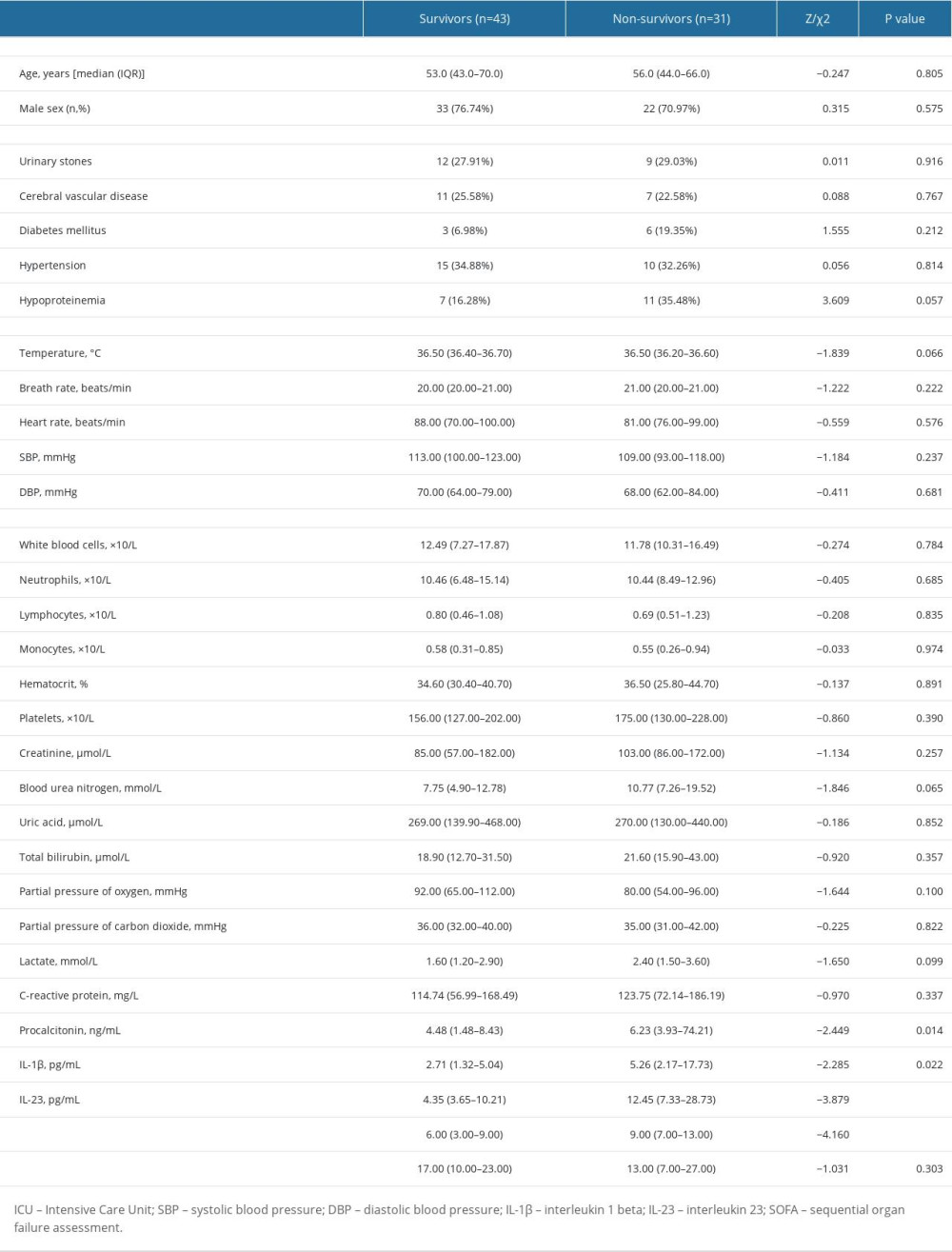 Table 2. Comparison of general data and laboratory indicators of patients with different prognosis.
Table 2. Comparison of general data and laboratory indicators of patients with different prognosis. Table 1. Comparison of clinical indicators in the septic patients, ICU controls, and healthy controls.
Table 1. Comparison of clinical indicators in the septic patients, ICU controls, and healthy controls. Table 2. Comparison of general data and laboratory indicators of patients with different prognosis.
Table 2. Comparison of general data and laboratory indicators of patients with different prognosis. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








