22 June 2023: Clinical Research
Thiol/Disulfide Homeostasis and Ischemia-Modified Albumin Levels in 61 Patients with Hypercapnia: A Case-Control Study
Selahattin Gürü1ABCDEFG*, Gültekin KadıDOI: 10.12659/MSM.940674
Med Sci Monit 2023; 29:e940674
Abstract
BACKGROUND: Hypercapnia is abnormally high arterial partial pressure of carbon dioxide (CO₂). CO₂ can affect redox signaling mechanisms, leading to production of oxidative derivatives. Thiol is formed by attaching a sulfhydryl group to a carbon atom. Under oxidative stress, it forms covalent bonds called thiol disulphide bonds. Serum albumin is modified to ischemia-modified albumin (IMA) by exposure to free radicals. This case-control study aimed to evaluate thiol/disulphide homeostasis and IMA levels in 61 patients with hypercapnia.
MATERIAL AND METHODS: We studied 61 patients with hypercapnia and 61 normocapnic volunteers in the control group, between May 2018 and January 2019; 56 of these patients were diagnosed with chronic obstructive pulmonary disease and 5 of them were diagnosed with obstructive sleep apnea syndrome. Arterial blood samples analyzed by using the Ellman reagent for thiol/disulphide data. A colorometric assay was used for detection of IMA levels.
RESULTS: Native thiol and total thiol values in the hypercapnic group were significantly lower than in the control group (P=0.024, P=0.006 respectively), as IMA values were significantly higher (P<0.001). There was no statistically significant difference between the hypercapnic and control groups in terms of disulphide, disulphide/native thiol, disulphide/total thiol, and native thiol/total thiol values (P>0.05).
CONCLUSIONS: In hypercapnic patients, there are changes in thiol/disulphide homeostasis and IMA levels. All significant differences in this study support that changes in thiol disulphide homeostasis and IMA in hypercapnic patients are indicators of oxidative stress.
Keywords: Hypercapnia, Oxidative Stress, biomarkers, ischemia-modified albumin, Homeostasis, Humans, Serum Albumin, Sulfhydryl Compounds, Disulfides, Case-Control Studies, Carbon Dioxide
Background
Normal carbon dioxide (CO2) levels in arterial blood depend on a balance between the amount of CO2 that is produced and excreted from the lungs through alveolar ventilation [1]. Hypercapnia, also referred to as CO2 retention, is defined as abnormally high arterial partial pressure of CO2 (PaCO2) [2]. The criterion standard value for hypercapnia is PaCO2 >45 mmHg in arterial blood gas, and hypercapnic respiratory failure is a common medical emergency. Hypercapnia is caused either by metabolically increased CO2 production or by respiratory failure. It should be considered as a syndrome rather than a single disease etiology. Arterial or venous blood gas is the most valuable laboratory test since it provides the evaluation of serum CO2, pH, and bicarbonate [2]. The decrease in alveolar ventilation might be due to a decrease in the number of ventilations per minute, or it might occur with an increase in dead space in the lung [3]. CO2 and some gases can affect the redox signaling mechanisms that lead to the production of oxidative derivatives [4].
The thiol structure is formed by the bonding of the sulfhydryl group, which consists of a sulfur and a hydrogen atom, to a carbon atom [4]. As a result of an oxidation reaction, thiols form covalent bonds that are called disulphide bonds or SS-bonds [5]. Under oxidative stress, mixed disulphides are formed by the oxidation of cysteine residues [6]. The disulphide bonds that are formed can be reversibly reduced to thiol groups. Thus, dynamic thiol/disulphide homeostasis emerges [7]. This homeostasis plays important roles in antioxidant mechanisms, enzymatic activities, cellular signaling mechanisms, detoxification, and apoptosis [6,8]. Abnormalities of dynamic thiol/disulphide homeostasis have been indicated in the literature in various diseases such as diabetes mellitus, cardiovascular diseases, cancer, rheumatoid arthritis, chronic kidney disease, acquired immunodeficiency syndrome (AIDS), and liver disorders. On an examination defined by Erel and Neşelioğlu, serum total thiol and native thiol levels are measured and disulphide concentrations are routinely calculated. Thus, thiol/disulphide homeostasis among patients can be demonstrated via an automated method that is already available [6,9].
Ischemia-modified albumin (IMA) is an elevated biochemical marker in ischemia and oxidative stress-related diseases [7]. Human serum albumin is modified to IMA by exposure to free radicals; therefore, IMA levels are determined by measuring the changing exogenous cobalt binding capacity [10].
Within the scope of our study, patients with arterial hypercapnia, which was previously detected when they were admitted to the emergency department, were evaluated in terms of thiol and disulphide homeostasis by the automated assay proposed by Erel and Neşelioğlu. There are some studies reporting that the relationship between hypercapnia and thiol/disulphide homeostasis has been evaluated [3]. On the other hand, IMA levels have also been investigated in the same patient group. It has been pointed out in the literature that the relationship between respiratory distress and IMA levels has been examined [11,12]. However, there is no other study in the literature that only evaluated IMA levels in hypercapnic adult patients. This case-control study aimed to evaluate thiol/disulphide homeostasis and ischemia-modified albumin levels in 60 patients with hypercapnia.
Material and Methods
STUDY DESIGN AND POPULATION:
The local ethics committee has given a permission for the study (approve number: 26379996/93). All patients and all healthy volunteers or their legal guardians were informed about the study and they provided written consent. The study included 61 patients who were found to have hypercapnia by arterial blood gas measurement in the emergency room of a hospital, 56 of these patients were diagnosed with chronic obstructive pulmonary disease (COPD) and 5 of them were diagnosed with obstructive sleep apnea syndrome (OSAS). In addition, 61 volunteers without acute or chronic metabolic and respiratory diseases and who did not differ significantly from the patient group in terms of age, sex, and body mass index (BMI) were also included in the study as the control group. Hypercapnia was detected in arterial blood gas samples taken from patients who came to the emergency department with respiratory distress or any other problem, and blood samples were taken for thiol/disulphide parameters and IMA just before starting the treatment. Because it might affect thiol/disulphide homeostasis, we excluded individuals with diabetes mellitus, cardiovascular disease, cancer, pancreatitis, kidney diseases, rheumatoid arthritis, AIDS, liver disorder, any active infection, and active smokers. Although hypercapnia was detected, patients whose treatment was started before the blood sample was taken for the study were also excluded from the study, as their results could have been affected.
SAMPLE COLLECTION AND ANALYSIS:
Blood samples were centrifuged at 1200× g for 15 min. Afterwards, the samples were stored at −80°C. With the automated assay put forward by Erel and Neşelioğlu, serum native thiol and total thiol levels were quantified. After the serum extraction, the measurement of the parameters mentioned by the automated system took approximately 12 min for each sample. Through the measurement of half of the values between total thiol and native thiol, disulphide concentrations were collected. After this process, the ratios between disulphide and native thiol, disulphide and total thiol, and native thiol and total thiol wer detected [6]. IMA levels were measured with Bar-Or et al’s colorometric assay [10].
STATISTICAL ANALYSIS:
Obtained data were computerized and evaluated by using SPSS for Windows 22.0 (SPSS, Inc, Chicago, IL). Descriptive statistics are presented as median (range, 25–75%), frequency distribution, and percentage. The Pearson chi-square test was used to evaluate categorical variables. The conformity of the variables to normal distribution was examined by using visual (histogram and probability graphs) and analytical methods (Kolmogorov-Smirnov/Shapiro-Wilk Test). For the variables that were found not to fit the normal distribution, the Mann-Whitney U test was used as a statistical method between 2 independent groups. The Spearman correlation test was used to determine the relationship between the variables. The correlation coefficients between 0 and 0.25 were interpreted as weak, 0.26–0.50 as medium, 0.51–0.75 as strong, and 0.76–1.0 as very strong correlation. Statistical significance level was accepted as
Results
DESCRIPTIVE DATA:
Within the scope of the study, a total of 122 individuals, 61 of whom were in the patient group and 61 in the control group, were examined. Of the 61 participants in the hypercapnic patient group, 56 were diagnosed with COPD and 5 were diagnosed with OSAS. The median age of the patient group was 67 (25%: 57.5–75%: 77.0) years, while the median age of the control group was 62 (25%: 49–75%: 74.5) years. There was no statistically significant difference in age between the patient and control groups (P=0.079) (Table 1).
When the groups were compared by sex; 65.6% of the patient group was male and 34.4% were female, while 54.1% of the control group was male and 45.9% was female. There was no statistically significant difference between the patient and control groups in terms of sex (P>0.05) (Table 1).
The body mass index (BMI) of the patients and healthy volunteers included in the study was calculated. Accordingly, the median BMI value of the patient group was 24.1 (25%: 21.7–75%: 27.1) kg/m2, while it was 24.2 (25%: 22.0–75%: 29.2) kg/m2 in the control group. There was no statistically significant difference between the patient and control groups in terms of BMI (P>0.05) (Table 1).
OUTCOME DATA:
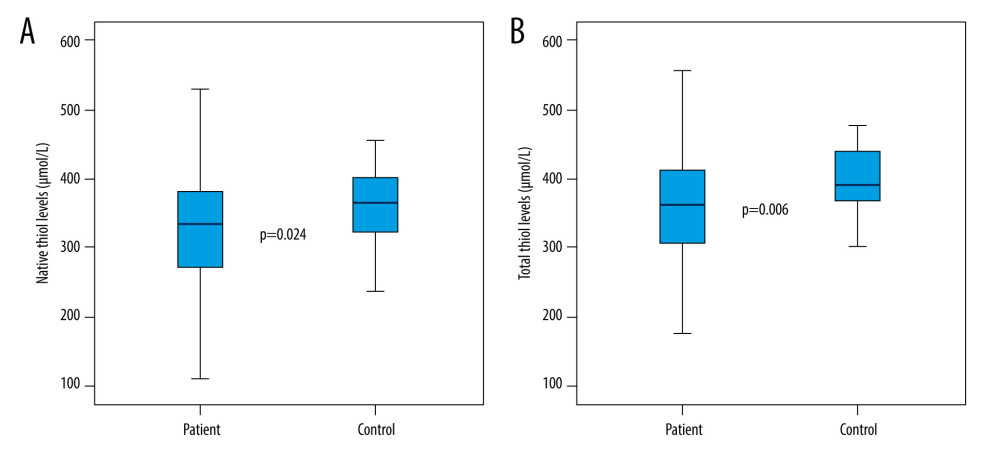
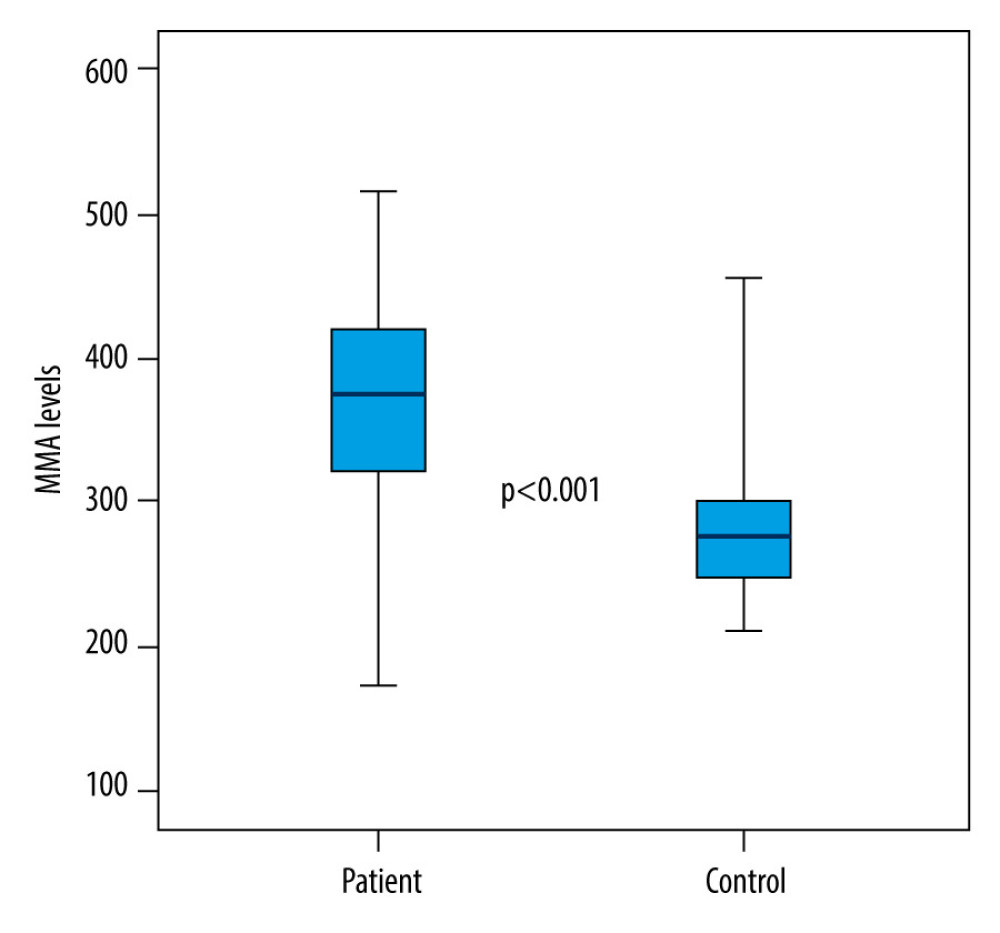
Between the patient and control groups examined within the scope of the study, statistically significant differences were found in terms of pH, PaCO2, native thiol, total thiol, and IMA values (respectively; P<0.001; P<0.001; P=0.024; P=0.006; P<0.001). While the pH, native thiol, and total thiol values of the patients in the patient group were significantly lower than in the control group, PaCO2 and IMA values were significantly higher (Table 2; Figures 1, 2). There was no statistically significant difference between the patient and control groups in terms of disulphide, disulphide/native thiol, disulphide/total thiol, or native thiol/total thiol values (P>0.05) (Table 2).
Discussion
The thiol molecule is known as an indicator of oxidative stress [13]. In this study, the relationship between hypercapnia and thiol/disulphide homeostasis, which is an indicator of oxidative stress, was assessed. We compared 61 patients with hypercapnia with 61 healthy volunteers. We showed that thiol levels were significantly lower and IMA levels were significantly higher in hypercapnic patients. Ergin et al found thiol and native thiol values were significantly lower in 43 patients with carbon monoxide (CO) intoxication and similarly associated this result with oxidative stress [13]. The fact that thiol levels were significantly lower in the patient group in this study indicates that thiols might be a indicator of oxidative stress in this patient group as well. In patients diagnosed with OSAS, Şengören et al found a decrease in native thiol and total thiol levels, similar to the results of our study, and an increase in IMA levels, which indicates oxidative stress, was also observed [3]. Şener et al reported that native thiol and total thiol in patients with acquired pneumonia were lower as a result of oxidative stress [14]. In our study, no significant increase was found in the disulphide value in the individuals in the patient group. In the literature, disulphide value has been found to be positively correlated with oxidative stress in many studies. Furthermore, Ergin et al found disulphide values were high in patients with CO intoxication [15]. Inthe patient group in our study, the median PaCO2 value was 58 mmHg, which was higher than the normal values. The disulphide values might be much higher in patients with even more severe hypercapnia compared to the patients in our study. In this study, no significant difference was found in the disulphide/native thiol and disulphide/total thiol ratios in the patient group compared to the control group.
Although the native thiol and total thiol values in the denominator of the ratios were low in the patient group, in accordance with the hypothesis of this study, the fact that the disulphide values were not significantly lower in the patient group than the control group seems to have led to similar ratios in the patient and the control group.
Various scientific studies have been reported in the literature on IMA and oxidative stress. It has been reported that disulfide and IMA increase as a marker of increased oxidative stress in inflammatory diseases. Kalkan et al reported that IMA and other oxidative stress markers are increased in autoimmune gastritis patients [4]. Similar findings in respiratory diseases are also available in the literature. For instance, Öztekin et al found significantly higher IMA values in the group consisting of 47 patients with a diagnosis of newborn transient tachypnea, some of whom were hypercapnic, compared to the control group without respiratory distress [12]. Kahveci et al also reported significantly higher IMA values in preterm babies with respiratory distress syndrome [11]. Furthermore, Doğru et al obtained IMA values significantly higher in children with asthma attack and stable asthma compared to the control group, and IMA values were significantly higher in the patient group than the control group [16]. Özyurt et al reported a significant correlation between both thiol and IMA and bronchiectasis patients [17]. In our study, it is considered important that the patients were only hypercapnic, and they were not required to be in severe respiratory distress. However, the high IMA value might indicate that the IMA value can increase significantly in hypercapnic patients even before they are in severe respiratory distress.
The present study has some limitations. First of all, the thiol/disulphide parameters of the patients were not compared with the values after hypercapnia treatment of the same patients. Some patients were able to achieve normal PaCO2 levels in the clinic where they were admitted from the emergency room. In addition, the post-treatment levels of the patients could not be assessed due to the crowded emergency room. For this reason, thiol/disulphide parameters and IMA values were compared with a control group of healthy volunteers. The thiol/disulphide and IMA values before and after the treatment in hypercapnic patients could add a different interpretation in future studies. Additionally, in this study, the sample size was small. More valid data can be obtained from groups with a larger number of patients.
Conclusions
In conclusion, our study revealed that thiol/disulphide homeostasis changed in the direction of oxidative stress and IMA levels increased in hypercapnic individuals compared to normocapnic controls. All significant differences in hypercapnic patients obtained in this study support that hypercapnia is an oxidative stress factor. Different results can be obtained with more comprehensive and large-participant studies on oxidative stress in hypercapnic patients.
References
1. Rawat D, Modi P, Sharma S, Hypercapnea: StatPearls, 2023, Treasure Island (FL), StatPearls Publishing Copyright© 2023, StatPearls Publishing LLC
2. Tobin MJ, Laghi F, Jubran A, Ventilatory failure, ventilator support, and ventilator weaning: Compr Physiol, 2012; 2(4); 2871-921
3. Sengoren Dikis O, Acat M, Casim H, The relationship of thiol/disulfide homeostasis in the etiology of patients with obstructive sleep apnea: A case-control study: Aging Male, 2020; 23(5); 679-86
4. Asfuroğlu Kalkan E, Boz S, Erel Ö, Thiol/disulfide homeostasis and ischemia modified albumin levels in autoimmune gastritis and their relations with gastric emptying: Turk J Med Sci, 2020; 50(1); 163-70
5. Cremers CM, Jakob U, Oxidant sensing by reversible disulfide bond formation: J Biol Chem, 2013; 288(37); 26489-96
6. Circu ML, Aw TY, Reactive oxygen species, cellular redox systems, and apoptosis: Free Radic Biol Med, 2010; 48(6); 749-62
7. Reddy VS, Sethi S, Agrawal P, Ischemia modified albumin (IMA) and albumin adjusted-IMA (AAIMA) as biomarkers for diabetic retinopathy: Nepal J Ophthalmol, 2015; 7(14); 117-23
8. Biswas S, Chida AS, Rahman I, Redox modifications of protein-thiols: Emerging roles in cell signaling: Biochem Pharmacol, 2006; 71(5); 551-64
9. Erel O, Neselioglu S, A novel and automated assay for thiol/disulphide homeostasis: Clin Biochem, 2014; 47(18); 326-32
10. Bar-Or D, Lau E, Winkler JV, A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia – a preliminary report: J Emerg Med, 2000; 19(4); 311-15
11. Kahveci H, Tayman C, Laoğlu F, Serum ischemia-modified albumin in preterm babies with respiratory distress syndrome: Indian J Clin Biochem, 2016; 31(1); 38-42
12. Oztekın O, Kalay S, Tayman C, Levels of ischemia-modified albumin in transient tachypnea of the newborn: Am J Perinatol, 2015; 30(2); 193-98
13. Patsoukis N, Georgiou C, Patsoukis N, Determination of the thiol redox state of organisms: new oxidative stress indicators: Anal Bioanal Chem, 2004; 378; 1783-92
14. Şener A, Kurtoğlu Çelik G, Özhasenekler A, Evaluation of dynamic thiol/disulfide homeostasis in adult patients with community-acquired pneumonia: Hong Kong Journal of Emergency Medicine, 2018; 26; 343-50
15. Ergin M, Caliskanturk M, Senat A, Disulfide stress in carbon monoxide poisoning: Clin Biochem, 2016; 49(16–17); 1243-47
16. Dogru M, Akoglu H, Kilinckaya MF, Ischemia-modified albumin levels in children with asthma: A pilot study: Arch Argent Pediatr, 2018; 116(4); e522-e28
17. Ozyurt S, Ozcelik N, Kara BY, Evaluation of thiol/disulfide homeostasis in bronchiectasis: Can Respir J, 2022; 2022; 8340450
Figures
Tables
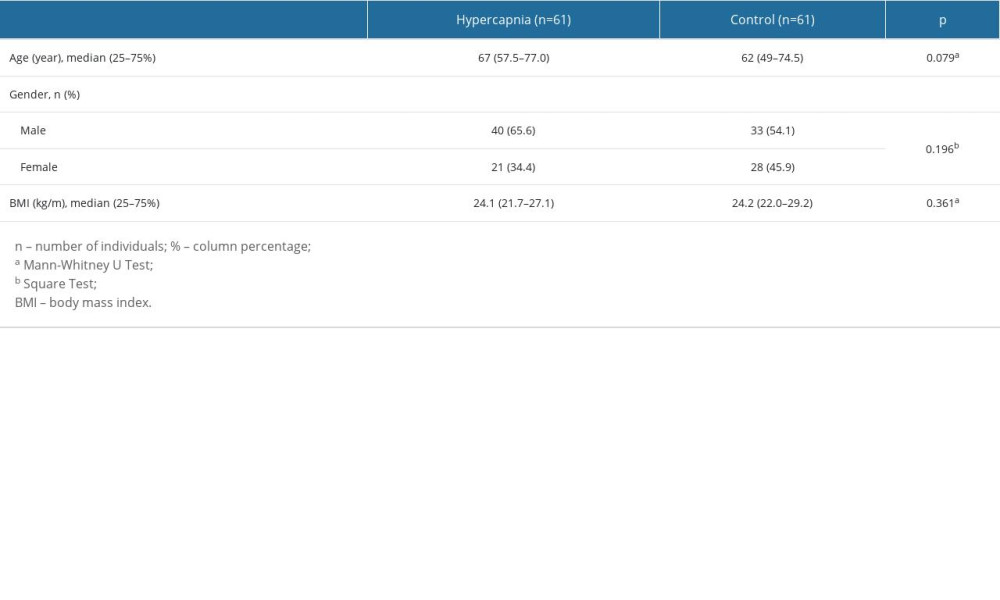 Table 1. Comparison of demographic characteristics in hypercapnic and control groups.
Table 1. Comparison of demographic characteristics in hypercapnic and control groups.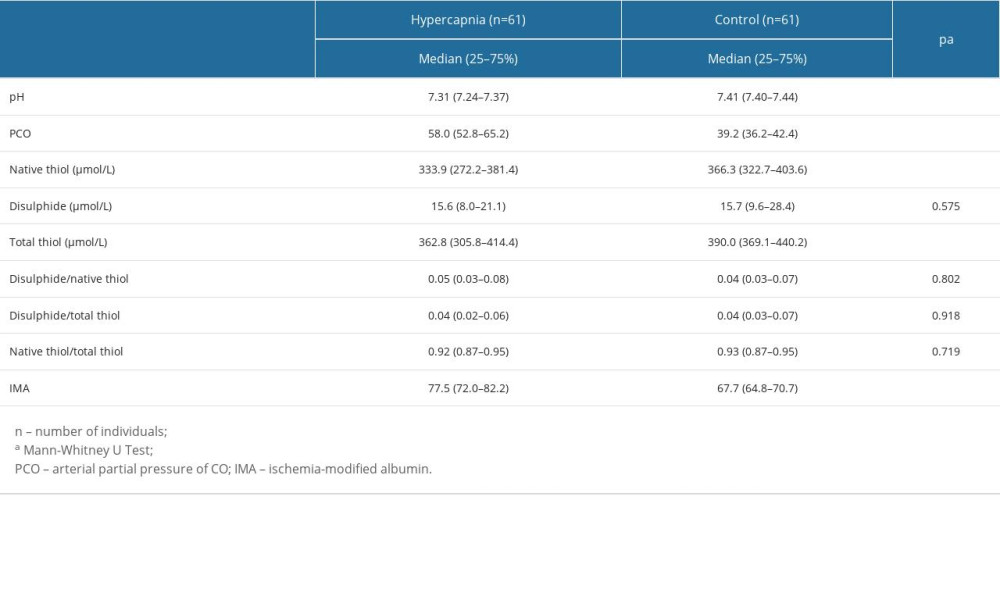 Table 2. The distribution of blood gas and oxidative stress parameters between the hypercapnic and the control groups.
Table 2. The distribution of blood gas and oxidative stress parameters between the hypercapnic and the control groups. Table 1. Comparison of demographic characteristics in hypercapnic and control groups.
Table 1. Comparison of demographic characteristics in hypercapnic and control groups. Table 2. The distribution of blood gas and oxidative stress parameters between the hypercapnic and the control groups.
Table 2. The distribution of blood gas and oxidative stress parameters between the hypercapnic and the control groups. In Press
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
12 Mar 2024 : Clinical Research
Comparing Neuromuscular Blockade Measurement Between Upper Arm (TOF Cuff®) and Eyelid (TOF Scan®) Using Miv...Med Sci Monit In Press; DOI: 10.12659/MSM.943630
11 Mar 2024 : Clinical Research
Enhancement of Frozen-Thawed Human Sperm Quality with Zinc as a Cryoprotective AdditiveMed Sci Monit In Press; DOI: 10.12659/MSM.942946
12 Mar 2024 : Database Analysis
Risk Factors of Age-Related Macular Degeneration in a Population-Based Study: Results from SHIP-TREND-1 (St...Med Sci Monit In Press; DOI: 10.12659/MSM.943140
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952