25 September 2023: Review Articles
Pharmacological Effects of Cinobufagin
Hao Zhang1E, Baiyu Jian2G*, Haixue Kuang1ADGDOI: 10.12659/MSM.940889
Med Sci Monit 2023; 29:e940889
Abstract
ABSTRACT: Cinobufagin (CBF) is a bufadienolide, which is a major active ingredient of toad venom. In recent years, CBF has attracted increasing attention due to its highly potent and multiple pharmacological activities. To better understand the status of research on CBF, we collated recent studies on CBF to provide a valuable reference for clinical researchers and practitioners. According to reports, CBF exhibits extensive pharmacological properties, including antitumor, analgesic, cardioprotection, immunomodulatory, antifibrotic, antiviral, and antiprotozoal effects. Studies on the pharmacological activity of CBF have mainly focused on its anticancer activity. It has been demonstrated that CBF has a therapeutic effect on liver cancer, osteosarcoma, melanoma, colorectal cancer, acute promyelocytic leukemia, nasopharyngeal carcinoma, multiple myeloma, gastric cancer, and breast cancer. However, the direct molecular targets of CBF are currently unknown. In addition, there are few reports on toxicological and pharmacokinetic of CBF. Subsequent studies focusing on these aspects will help promote the development and application of CBF in clinical practice.
Keywords: Nasopharyngeal Neoplasms, Bone Neoplasms, Bufanolides, Humans, cinobufagin, Biotransformation, Amphibian Venoms
Background
Toad venom (Bufonis Venenum, known as ‘Chansu’ in Chinese) has been used as a traditional Chinese medicine for more than a thousand years and is listed in the Chinese Pharmacopoeia (2020 edition) [1,2]. It is prepared from the dried white secretion of the auricular glands and the skin glands of
Cinobufagin (CBF), a bufadienolide derived from toad venom, was first isolated by Jensen et al from Chansu in 1932 [11]. Multiple studies have focused on its pharmacological effects and molecular mechanisms. This suggests that CBF has broad prospects in therapeutic applications. However, at present there is no systematic review on CBF. This article reviews the pharmacological properties of CBF, including its antitumor, analgesic, cardioprotection, immunomodulatory, antifibrotic, antiviral, and antiprotozoal effects, and its physicochemical and pharmacokinetic properties. This article aims to review in vitro and preclinical studies on cinobufagin, a bufadienolide derived from toad venom.
Physicochemical Properties
As shown in Figure 1, CBF is a bufadienolide with properties such as molecular structure[(1R,2S,4R,5R,6R,7R,10S,11S,14S,16R)-14-hydroxy-7,11-dimethyl-6-(6-oxopyran-3-yl)-3-oxapentacyclo [8.8.0.02,4.02,7.011,16] octadecan-5-yl] acetate, molecular formula: C26H34O6, molecular weight of 442.54 g/mol, and melting point of 222–223°C [12–14].
Pharmacological Activities of CBF
ANTITUMOR EFFECTS OF CBF:
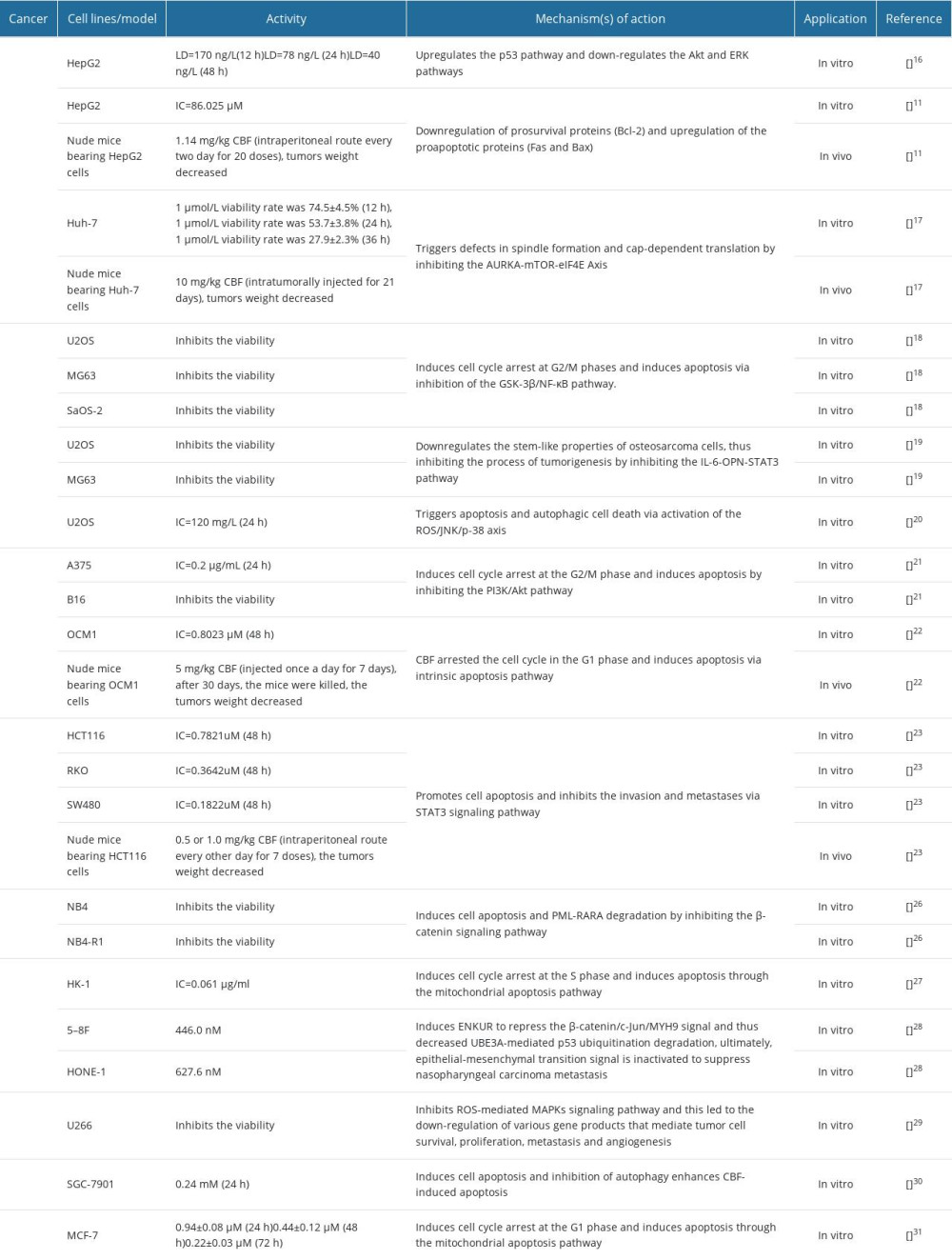
Cancer is a common disease globally that seriously affects human health. Recent studies have illuminated that CBF suppressed the growth of a wide variety of tumor cells, including liver cancer, osteosarcoma, melanoma, colorectal cancer, acute promyelocytic leukemia, nasopharyngeal carcinoma, multiple myeloma, breast cancer, and gastric carcinoma [15]. The anticancer activities and related mechanisms of CBF are listed in Table 1.
LIVER CANCER:
Liver cancer is a common digestive malignant cancer worldwide with a high mortality rate. Several studies have shown that CBF can inhibit proliferation of hepatocellular carcinoma cells. Wendong Feng et al used CCK-8 assay to examine the growth of cells treated with CBF [16]. The results showed that when CBF concentration increased from 0 to 320 ng/l, the inhibition of HepG2 cells increased from 0% to 65% at 12 h, to 87% at 24 h, and to 99% at 48 h, and the LD50 of CBF was estimated to be 170, 78, and 40 ng/L, respectively. These data indicated that CBF inhibits the proliferation of HepG2 cells in a concentration- and time-dependent manner and has cytotoxicity to HepG2 cells even at very low concentrations. CBF has been found to induce HepG2 cells apoptosis. The apoptotic rate of HepG2 cells treated with CBF (100 ng/L) for 12 h, and 24 h were 13.6% and 25.5%, respectively, whereas the apoptotic rate of HepG2 cells in the control group was 1.6% and 3.2%, respectively. Moreover, CBF reduced the migration ability of HepG2 cells. Liang et al reported that CBF could inhibit HepG2 cell proliferation in vitro (IC50 values were 86.025 μM) and tumor growth in vivo [11]. Xiaohan Jin et al demonstrated that viability rates of Huh-7 cells treated with 1 μmol/L CBF for 12, 24, and 36 h were 74.5±4.5%, 53.7±3.8%, and 27.9±2.3%, respectively, in vitro and CBF induced cell cycle G2/M phase arrest in Huh-7 cells. CBF also suppressed the growth of Huh-7 tumors in vivo when CBF was intratumorally injected at a 10 mg/kg dose for 21 days [17].
Mechanistic studies indicated that CBF could inhibit proliferation and induce apoptosis of HepG2 cells by upregulating the p53 pathway and downregulating the Akt and ERK pathways [16]. In addition, CBF triggers defects in spindle formation and cap-dependent translation in liver cancer cells by inhibiting the AURKA-mTOR-eIF4E axis [17].
OSTEOSARCOMA:
Osteosarcoma is the most common primary malignant bone tumor. CBF has been reported to inhibit cell proliferation in osteosarcoma cells, including U2OS, MG63, and SaOS-2, in a time- and dose-dependent manner [18]. Researchers found that CBF induced cell cycle arrest at G2/M phases and apoptosis in U2OS, MG63, and SaOS-2 cells. Interestingly, toxicity studies have suggested that CBF has less toxicity or no toxicity to the human osteoblast cell line hFOB 1.19.
Researchers have conducted several studies on the potential mechanism of CBF for osteosarcoma therapy. Junqiang Yin et al found CBF-induced apoptosis via inhibition of the GSK-3β/NF-κB pathway [18]. Chuan Zhang et al demonstrated that CBF suppresses the characteristics of osteosarcoma cancer cells by inhibiting the IL-6-OPN-STAT3 pathway [19]. Kun Ma et al proved that CBF triggered apoptosis and autophagic cell death via activation of the ROS/JNK/p-38 axis [20].
MELANOMA:
Malignant melanoma is the deadliest type of skin cancer. CBF inhibited human malignant melanoma A375 cells proliferation (IC50=0.2 μg/mL, 24h) and cell colony formation. Additional studies demonstrated that CBF markedly increased the levels of ATM serine/threonine kinase (ATM) and checkpoint kinase 2 (Chk2) and decreased the levels of cell division cycle 25C (CDC25C), cyclin-dependent kinase 1 (CDK1), and cyclin B, subsequently inducing G2/M cell cycle arrest in A375 cells [21]. Moreover, CBF induced A375 cells apoptosis by inhibiting the PI3K/Akt pathway [21]. CBF also induced cell cycle arrest at the G2/M phase and cell apoptosis in B16 cells [21]. In addition, CBF exerted cytotoxicity against human uveal melanoma OCM1 cells (IC50= 0.8023 μM, 48 h) and inhibited the growth of OCM1 xenograft tumors in nude mice [22]. Researcher found that CBF arrested the cell cycle in the G1 phase in a concentration-dependent manner in OCM1 cells and induced cell apoptosis of OCM1 by activating the mitochondrial apoptotic signal pathway [22].
COLORECTAL CANCER:
Colorectal cancer is a common malignant tumor of the digestive system. CBF has been reported to inhibit the proliferation, migration, and invasion and also promote apoptosis of colorectal cancer cell lines (HCT116, RKO, and SW480 cells) [23]. In vivo, assays confirmed CBF inhibited the growth of HCT116 xenograft tumors in nude mice [23]. CBF also promoted apoptosis and inhibited the invasion, metastases, and angiogenesis of colorectal cancer cells [23, 24]. In addition, CBF demonstrated a strong reversal effect of multidrug resistance in P-gp-overexpressing LoVo/ADR, HCT116/L, and Caco-2/ADR cells, but did not affect their parental cells [25]. It also enhanced the effect of DOX against P-gp-overexpressing LoVo/ADR cell xenografts in nude mice [25].
Molecular mechanistic studies have illustrated CBF suppressed colorectal cancer growth via STAT3 pathway inhibition [23]. CBF also suppressed tumor neovascularization by disrupting the endothelial mTOR/HIF-1α pathway to trigger ROS- mediated vascular endothelial cell apoptosis [24]. The specific mechanism behind the reversal of drug resistance by CBF was also investigated. The results showed that CBF inhibited the activity of the P-gp ATPase but without altering the expression of P-gp [25].
ACUTE PROMYELOCYTIC LEUKEMIA:
Acute promyelocytic leukemia is a hematopoietic malignancy characterized by the accumulation of large amounts of immature blood cells in hematopoietic tissues. Studies have revealed that CBG inhibited the viability of NB4 and NB4-R1 cells in a time-and dose-dependent manner [26], and it induced NB4 and NB4-R1 cell apoptosis and PML-RARA degradation in a caspase-dependent manner by inhibiting the β-catenin signaling pathway.
NASOPHARYNGEAL CARCINOMA:
Nasopharyngeal carcinoma is an epithelial carcinoma arising from the nasopharyngeal mucosal lining. CBF significantly inhibited the proliferation of HK-1 cells [27]. Further analyses demonstrated that CBF induces cell cycle arrest at the S phase in HK-1 cells by downregulating the levels of CDK2 and cyclin E. Moreover, CBF downregulated the protein level of Bcl-2 and upregulated the levels of Bax, subsequently increasing the levels of cytoplasmic cytochrome c, Apaf-1, cleaved PARP1, cleaved caspase-3, and cleaved caspase-9, leading to HK-1 apoptosis. Furthermore, CBF increased ROS levels and decreased the mitochondrial membrane potential in HK-1 cells.
In addition, CBF inhibited 5–8F and HONE-1 cells proliferation and decreased the metastasis of 5–8F cells in vivo [28]. Molecular analysis revealed that CBF induced ENKUR to repress the β-catenin/c-Jun/MYH9 signal and thus decreased UBE3A-mediated p53 ubiquitination degradation. As a result, the epithelial-mesenchymal transition signal was inactivated to suppress nasopharyngeal carcinoma metastasis [28].
MULTIPLE MYELOMA:
Multiple myeloma is a clonal B-cell neoplasia. Compared to peripheral blood mononuclear cells, CBF showed much higher cytotoxicity against multiple myeloma U266 cells [29]. Mechanistic studies have demonstrated that CBF suppressed multiple myeloma growth through modulation of the ROS-mediated MAPKs signaling pathway.
OTHER CANCERS:
Some scholars have studied other antitumor activities of CBF. Xuanxuan Xiong et al found that CBF inhibited gastric cancer SGC-7901 cell proliferation and induced caspase-mediated apoptosis [30]. The IC50 value of CBF treatment at 24 h was 0.24 mM. Ling Zhu et al determined the inhibitory effect of CBF on MCF-7 cells [31], showing that CBF inhibited the growth of breast cancer cell lines MCF-7 in a time- and dose-dependent manner, and the IC50 values at 24, 48, and 72 h after treatment were 0.94±0.08, 0.44±0.12, and 0.22±0.03 μM, respectively.
Analgesic Effects of CBF
CBF had been widely used in the treatment of carcinoma and played an important role in the relief of cancer pain. It has been reported that CBF was better than morphine in the treatment of cancer pain. Although morphine works immediately, CBF has an effect on peripheral opioid receptors, has no adverse effects such as addiction like morphine and other opioids, and can be used as a substitute for morphine in the treatment of patients with cancer pain. The underlying mechanism responsible for the analgesic action of CBF was associated with an improved level of peripheral β-END and the role of peripheral opioid receptors [32]. Tao Chen et al found that CBF relieved cancer pain in mice and the CBF-induced local analgesic effect might be associated with increased activity of the POMC/β-END/μ-OR pathway released from invaded CD3/4/8 lymphocytes in cancer tissues [33]. Longsheng Xu demonstrated that CBG exerted significant antinociceptive effects in thermal and chemical pain models, possibly via activating 7nAChR, thereby triggering inhibition of the NF-κB signaling pathway [34].
Cardiotonic Effects of CBF
The effect of CBF on experimentally induced heart failure caused by acute local ischemia was examined through ligation of the left anterior descending coronary artery in a perfused guinea pig heart. The results demonstrated that CBF (3×10−7 M) restored coronary flow in the perfused guinea-pig heart to 90% of the pre-ligation level. Cardiac output and left ventricular pressure in the perfused heart increased to levels prior to the occurrence of acute local ischemia, and left ventricular work was enhanced by CBF (3×10−7 M) to 108% of the pre-ligation level. These findings suggest that CBF exhibits potent cardiotonic action in experimentally induced heart failure resulting from acute local ischemia [35]. Furthermore, researchers have discovered that CBF can inhibit Na+-K+-ATPase activity, shorten the action potential duration, and increase cardiac contractility in guinea pig hearts [36].
Immunomodulatory Effects of CBF
Scholars have found that CBF had potential immune system regulatory effects. They found that CBF stimulated cell proliferation of splenocytes and peritoneal macrophages and markedly enhanced the phagocytic activation of macrophages. CBF also significantly increased CD4+CD8+ double-positive T-cell populations and the percentage of S-phase cells of splenic lymphocytes and increased the ratio of Th1/Th2 [37,38]. Another study showed that CBF enhanced the protective efficacy of formalin-inactivated
Antifibrotic Effects of CBF
CBF was reported to suppress fibroblast activation and differentiation, epithelial-mesenchymal transition, and, eventually, the extracellular matrix deposition by inhibiting the TGF-β1/Smad3 signaling. In vivo, experiments also proved that CBF attenuated bleomycin-induced pulmonary fibrosis in mice [40].
Antiviral Effects of CBF
The emergence of severe acute respiratory infectious diseases is often attributed to zoonotic beta coronaviruses such as SARS-CoV, MERS-CoV, and SARS-CoV-2 [41]. As reported by Jin et al, CBF showed high anti-MERS-CoV activities (IC50, 0.017 μM) and the selectivity indices (SI, CC50/IC50) were >564.7 for CBF [42]. CBF could be a promising bufadienolide for therapeutic use against emerging coronavirus infections such as COVID-19.
CBF was found to inhibit enterovirus 71(EV71) infection in vitro in cell viability and plaque reduction assays. The IC50 of CBF was (10.94±2.36) nmol/L, the 50% cytotoxic concentration (CC50) of CBF was (1277±223) nmol/L, and the anti-EV71 selectivity index (SI50) of CBF was 116.7, suggesting promise for drug development. Researchers found that CBF disrupted the synthesis of EV71 protein, but did not affect EV71 RNA replication. Hence, CBF may be a promising candidate for the treatment of EV71-caused disease [43].
Antiprotozoal Effects of CBF
Bio-guided studies have revealed the notable antimicrobial and antiparasitic properties of bufadienolides [44]. The antitrypanosomal activity of CBF was tested in vitro against
The Pharmacokinetics of CBF
Pharmacokinetic studies are an important aspect of drug evaluation. Currently, there are limited pharmacokinetic data available on CBF. Wenlong Wei et al reported the pharmacokinetics of CBF in rats [46]. A method was established for rapidly and accurately determining the concentration of CBF in plasma after intragastric administration in a dose of 20 mg/kg in rats, and its specificity, accuracy, precision, recovery, matrix effect, and stability were studied. The Cmax, the time to Cmax (Tmax), and the t1/2 of CBF were 45.83±4.56 ng/mL, 0.083±0 h, and 2.79±0.93 h, respectively. This result indicated that CBF was absorbed and eliminated quickly. The main metabolite was desacetylcinobufagin. The Cmax and area under the curve (AUC0-t) of desacetylcinobufagin were much higher than CBF in plasma samples. However, there are few studies about the pharmacological actions of desacetylcinobufagin.
Future Directions
In this review, we provide a summary of the physicochemical, pharmacodynamic, and pharmacokinetic characteristics of CBF to establish a basis for clinical research. According to reports, CBF has been found to possess multiple pharmacological activities, including antitumor, analgesic, cardiotonic, immunomodulatory, antifibrotic, antiviral, and antiprotozoal effects. While there have been numerous studies on its anticancer activity, there is a relative scarcity of research regarding its other pharmacological effects. CBF has demonstrated therapeutic effects on various types of cancer, such as liver cancer, osteosarcoma, melanoma, colorectal cancer, acute promyelocytic leukemia, nasopharyngeal carcinoma, multiple myeloma, gastric cancer, and breast cancer. However, most of the research focusing on its antitumor activity has been limited to the cellular level, necessitating the need for more in vivo and clinical trial investigations. Additionally, the direct targets and mechanism of action of CBF have yet to be fully elucidated. A comprehensive understanding of the targets will aid in guiding clinical treatment strategies involving CBF. Combining CBF with traditional chemotherapy drugs is considered an optimal option for reducing drug resistance and improving cancer treatment outcomes. Nevertheless, there are few reports on the combined treatment of cancers with CBF and traditional chemotherapy drugs. The toxicity of CBF has raised significant concerns regarding its safety as a drug candidate. However, there is a dearth of studies investigating the toxicological and pharmacokinetic aspects of CBF.
Despite possessing numerous pharmacological activities, CBF exhibits poor aqueous solubility, leading to low bioavailability, thus limiting its clinical application [7]. Structural modification is a potential approach to improve CBF’s solubility. However, such modifications may also impact its bioactivity. Developing new pharmaceutical formulations is another way to enhance the bioavailability and solubility of CBF. Various techniques, including solid dispersion, microemulsion, submicroemulsion, cyclodextrin inclusion, and nanodrug delivery systems, have been employed to improve solubility [47,48]. Nevertheless, these methods still face limitations, such as the unresolved instability issues of liposomes, such as aggregation, fusion, and drug leakage during storage. Moreover, the safety concerns associated with these materials hinder their progress in clinical application.
Conclusions
CBF holds great potential as a versatile drug with various pharmacological activities. Further research encompassing in-depth analytical studies, identification of direct binding targets, pharmacokinetic and toxicological investigations, and development of new strategies to overcome its poor solubility are necessary to advance the development and clinical application of CBF.
Figures
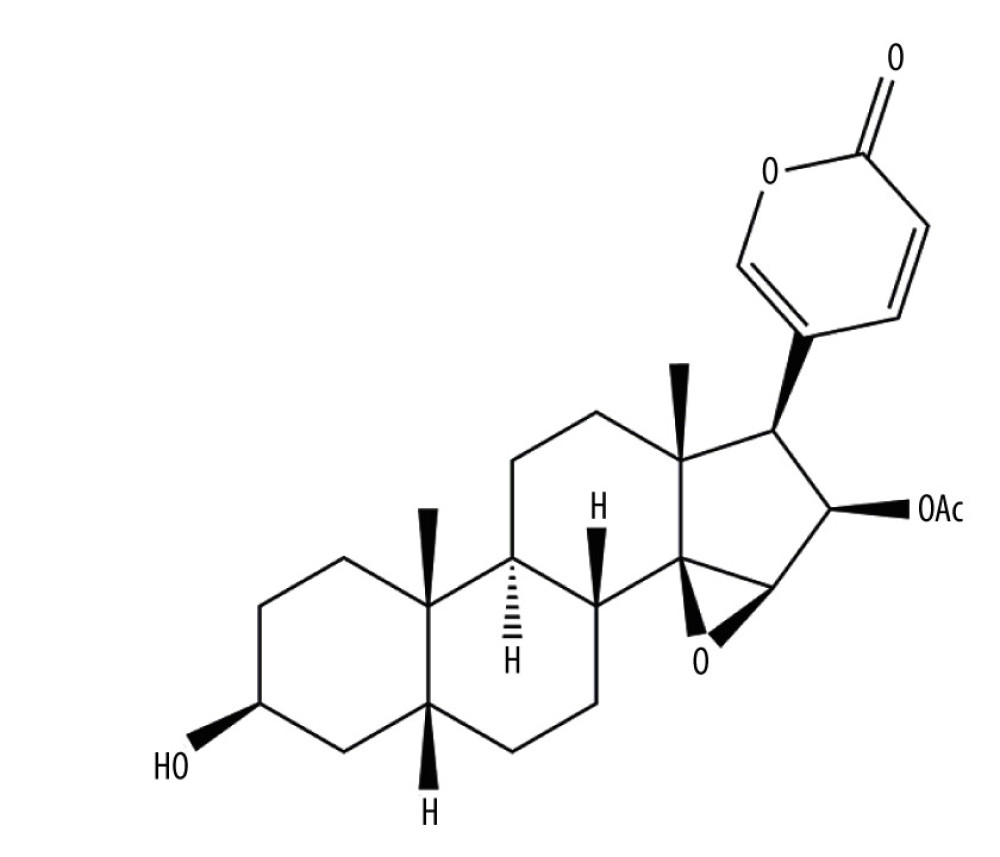 Figure 1. Cinobufagin.
Figure 1. Cinobufagin. Reference
1. Chan CO, Jing J, Xiao W, Enhanced intestinal permeability of bufalin by a novel bufalin-peptide-dendrimer inclusion through Caco-2 cell monolayer: Molecules, 2017; 22(12); 2088
2. Li FJ, Hu JH, Ren X, Toad venom: A comprehensive review of chemical constituents, anticancer activities, and mechanisms: Arch Pharm (Weinheim), 2021; 354(7); e2100060
3. Apryani E, Ali U, Wang ZY, The spinal microglial IL-10/beta-endorphin pathway accounts for cinobufagin-induced mechanical antiallodynia in bone cancer pain following activation of alpha7-nicotinic acetylcholine receptors: J Neuroinflammation, 2020; 17(1); 75
4. Ye Q, Zhou X, Han F, Zheng C, Toad venom-derived bufadienolides and their therapeutic application in prostate cancers: Current status and future directions: Front Chem, 2023; 11; 1137547
5. Li C, Hashimi SM, Cao S, The mechanisms of chansu in inducing efficient apoptosis in colon cancer cells: Evid Based Complement Alternat Med, 2013; 2013; 849054
6. Qi J, Zulfiker AHM, Li C, Good D, Wei MQ, The development of toad toxins as potential therapeutic agents: Toxins (Basel), 2018; 10(8); 336
7. Shao H, Li B, Li H, Novel strategies for solubility and bioavailability enhancement of bufadienolides: Molecules, 2021; 27(1); 51
8. Kolodziejczyk-Czepas J, Stochmal A, Bufadienolides of Kalanchoe species: An overview of chemical structure, biological activity and prospects for pharmacological use: Phytochem Rev, 2017; 16(6); 1155-71
9. Wei W, Yu Y, Wang X, Simultaneous determination of bufalin and its nine metabolites in rat plasma for characterization of metabolic profiles and pharmacokinetic study by LC(−)MS/MS: Molecules, 2019; 24(9); 1662
10. Lim WJ, Yap AT, Mangudi M, Gambir, “Gambir Sarawak” and toad venom: Drug Test Anal, 2017; 9(3); 491-99
11. Liang P, Ma Y, Yang L, Uncovering the mechanisms of active components from toad venom against hepatocellular carcinoma using untargeted metabolomics: Molecules, 2022; 27(22); 7758
12. Li C, Hashimi SM, Cao S, Chansu inhibits the expression of cortactin in colon cancer cell lines in vitro and in vivo: BMC Complement Altern Med, 2015; 15; 207
13. Jensen H, Chemical studies on toad poisons, iv, bufagin and cinobufagin: Science, 1932; 75(1932); 53-54
14. Hofer P, Linde H, Meyer KThe structure of cinobufagin: Experientia, 1959; 15; 297-98
15. Jia J, Li J, Zheng Q, Li D, A research update on the antitumor effects of active components of Chinese medicine ChanSu: Front Oncol, 2022; 12; 1014637
16. Feng W, Zhao X, Yao Q, Li D, Cinobufagin inhibits proliferation and induces apoptosis of hepatocarcinoma cells by activating apoptosis, AKT, and ERK pathways: Acta Biochim Pol, 2022; 69(4); 831-37
17. Jin X, Wang J, Zou S, Cinobufagin triggers defects in spindle formation and cap-dependent translation in liver cancer cells by inhibiting the AURKA-mTOR-eIF4E axis: Am J Chin Med, 2020; 48(3); 651-78
18. Yin JQ, Wen L, Wu LC, The glycogen synthase kinase-3beta/nuclear factor-kappa B pathway is involved in cinobufagin-induced apoptosis in cultured osteosarcoma cells: Toxicol Lett, 2013; 218(2); 129-36
19. Zhang C, Ma K, Li WY, Cinobufagin suppresses the characteristics of osteosarcoma cancer cells by inhibiting the IL-6-OPN-STAT3 pathway: Drug Des Devel Ther, 2019; 13; 4075-90
20. Ma K, Zhang C, Huang MY, Cinobufagin induces autophagy-mediated cell death in human osteosarcoma U2OS cells through the ROS/JNK/p38 signaling pathway: Oncol Rep, 2016; 36(1); 90-98
21. Pan Z, Zhang X, Yu P, Cinobufagin induces cell cycle arrest at the G2/M phase and promotes apoptosis in malignant melanoma cells: Front Oncol, 2019; 9; 853
22. Zhang L, Huang X, Guo T, Study of cinobufagin as a promising anticancer agent in uveal melanoma through intrinsic apoptosis pathway: Front Oncol, 2020; 10; 325
23. Bai Y, Wang X, Cai M, Cinobufagin suppresses colorectal cancer growth via STAT3 pathway inhibition: Am J Cancer Res, 2021; 11(1); 200-14
24. Li X, Chen C, Dai Y, Cinobufagin suppresses colorectal cancer angiogenesis by disrupting the endothelial mammalian target of rapamycin/hypoxia-inducible factor 1alpha axis: Cancer Sci, 2019; 110(5); 1724-34
25. Yuan Z, Shi X, Qiu Y, Reversal of P-gp-mediated multidrug resistance in colon cancer by cinobufagin: Oncol Rep, 2017; 37(3); 1815-25
26. Bian Y, Xue M, Guo X, Cinobufagin induces acute promyelocytic leukaemia cell apoptosis and PML-RARA degradation in a caspase-dependent manner by inhibiting the beta-catenin signalling pathway: Pharm Biol, 2022; 60(1); 1801-11
27. Pan Z, Luo Y, Xia Y, Cinobufagin induces cell cycle arrest at the S phase and promotes apoptosis in nasopharyngeal carcinoma cells: Biomed Pharmacother, 2020; 122; 109763
28. Hou R, Liu X, Yang H, Chemically synthesized cinobufagin suppresses nasopharyngeal carcinoma metastasis by inducing ENKUR to stabilize p53 expression: Cancer Lett, 2022; 531; 57-70
29. Baek SH, Kim C, Lee JH, Cinobufagin exerts anti-proliferative and pro-apoptotic effects through the modulation ROS-mediated MAPKs signaling pathway: Immunopharmacol Immunotoxicol, 2015; 37(3); 265-73
30. Xiong X, Lu B, Tian Q, Inhibition of autophagy enhances cinobufagin-induced apoptosis in gastric cancer: Oncol Rep, 2019; 41(1); 492-500
31. Zhu L, Chen Y, Wei C, Anti-proliferative and pro-apoptotic effects of cinobufagin on human breast cancer MCF-7 cells and its molecular mechanism: Nat Prod Res, 2018; 32(4); 493-97
32. Chen T, Hu W, He H, A study on the mechanism of cinobufagin in the treatment of paw cancer pain by modulating local beta -endorphin expression in vivo: Evid Based Complement Alternat Med, 2013; 2013; 851256
33. Chen T, Yuan S, Wan XN, Chinese herb cinobufagin-reduced cancer pain is associated with increased peripheral opioids by invaded CD3/4/8 lymphocytes: Oncotarget, 2017; 8(7); 11425-41
34. Xu L, Zhang X, Feng Q, Alpha-7 nicotinic receptor-targeted cinobufagin induces antinociception and inhibits NF-kappaB signaling pathway in DRG neurons: ACS Chem Neurosci, 2019; 10(1); 497-506
35. Yamahara J, Tanaka S, Matsuda HThe mode of cardiac action of cardiotonic steroids isolated from Toad Cake in perfused working guinea-pig heart and effect of cinobufagin on experimental heart failure: Nihon Yakurigaku Zasshi, 1986; 88(6); 413-23 [in Japanese]
36. Hirai Y, Morishita S, Ito C, Sakanashi MEffects of bufadienolides and some kinds of cardiotonics on guinea pig hearts: Nihon Yakurigaku Zasshi, 1992; 100(2); 127-35 [in Japanese]
37. Wang XL, Zhao GH, Zhang J, Immunomodulatory effects of cinobufagin isolated from Chan Su on activation and cytokines secretion of immunocyte in vitro: J Asian Nat Prod Res, 2011; 13(5); 383-92
38. Yu Y, Wang H, Meng X, Immunomodulatory effects of cinobufagin on murine lymphocytes and macrophages: Evid Based Complement Alternat Med, 2015; 2015; 835263
39. Wu SC, Yi PF, Guo X: Microb Pathog, 2016; 99; 264-70
40. Li X, Bi Z, Liu S, Antifibrotic mechanism of cinobufagin in bleomycin-induced pulmonary fibrosis in mice: Front Pharmacol, 2019; 10; 1021
41. de Wit E, van Doremalen N, Falzarano D, Munster VJ, SARS and MERS: Recent insights into emerging coronaviruses: Nat Rev Microbiol, 2016; 14(8); 523-34
42. Jin YH, Jeon S, Lee J, Broad spectrum antiviral properties of cardiotonic steroids used as potential therapeutics for emerging coronavirus infections: Pharmaceutics, 2021; 13(11); 1839
43. Chen J, Xu L, Sun S, Identification of cinobufagin and resibufogenin as inhibitors of enterovirus 71 infection: Chemical Research in Chinese Universities, 2014; 30; 953-58
44. Cunha Filho GA, Schwartz CA, Resck IS, Antimicrobial activity of the bufadienolides marinobufagin and telocinobufagin isolated as major components from skin secretion of the toad Bufo rubescens: Toxicon, 2005; 45(6); 777-82
45. Rodriguez C, Ibanez R, Mojica L: Molecules, 2021; 26(14); 4217
46. Wei WL, Li H, Li ZW, Simultaneous determination of cinobufagin and its five metabolites in rat plasma by LC-MS/MS for characterization of metabolic profiles and pharmacokinetic study: Anal Methods-Uk, 2019; 11(42); 5464-71
47. Guan Q, Sun S, Li X, Preparation, in vitro and in vivo evaluation of mPEG-PLGA nanoparticles co-loaded with syringopicroside and hydroxytyrosol: J Mater Sci Mater Med, 2016; 27(2); 24
48. Sun S, Guan Q, Shang E, Hyaluronic acid-coated nanostructured lipid carriers for loading multiple traditional Chinese medicine components for liver cancer treatment: Pak J Pharm Sci, 2020; 33(1); 109-19
Figures
In Press
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
12 Mar 2024 : Clinical Research
Comparing Neuromuscular Blockade Measurement Between Upper Arm (TOF Cuff®) and Eyelid (TOF Scan®) Using Miv...Med Sci Monit In Press; DOI: 10.12659/MSM.943630
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952