04 September 2023: Review Articles
A Systematic Review of Publications on the Associations Between Sleep Architecture and Arterial Hypertension
Justyna Kanclerska1ABE, Anna Szymańska-ChabowskaDOI: 10.12659/MSM.941066
Med Sci Monit 2023; 29:e941066
Abstract
ABSTRACT: Sleep research has garnered substantial interest among scientists owing to its correlation with various diseases, particularly elevated blood pressure observed in patients with obstructive sleep apnea. This systematic review aims to identify and analyze publications exploring the associations between sleep architecture and arterial hypertension. A comprehensive search of PubMed (MEDLINE), Scopus, and Embase databases yielded 111 reports, of which 7 manuscripts were included in the review. Four of the studies reported a significant reduction in the duration of the N3 phase of sleep in hypertensive patients, while 2 studies found a statistically significant reduction in the duration of the N2 and rapid eye movement (REM) stages of sleep. Three studies indicated increased sleep fragmentation in hypertensive patients. They showed a longer duration of the N1 stage of sleep, shorter duration of overall sleep time, and an increased apnea-hypopnea index in hypertensive patients. These findings underscore the association between the duration of non-REM/REM sleep stages and elevated BP, providing substantial evidence. Moreover, a notable increase in sleep fragmentation was observed among patients with hypertension. However, further research is warranted to expand and deepen our understanding of this intricate relationship. This systematic review serves as a valuable resource, guiding future investigations and contributing to advancements in the field of sleep and arterial hypertension.
Keywords: Arousal, Blood Pressure, Hypertension, Sleep Deprivation, Sleep Stages, Humans, Sleep, Sleep apnea, obstructive, Databases, Factual
Background
In recent years, sleep research has gained immense interest among scientists owing to its increasing prevalence and its correlation with various diseases, particularly in the cardiovascular system. The association between cardiovascular diseases and sleep disorders has been observed, which is possibly due to shared pathogenesis factors. Among these diseases, hypertension is a prominent condition, often undetected in its early stages. The contributing factors of hypertension development are the overactivation of the autonomic nervous system [1] and the inappropriate activation of the renin-angiotensin-aldosterone system [2]. The definition of sleep presented by Carskadon is as follows: “Sleep is a recurring, reversible neuro-behavioral state of relative perceptual disengagement from and unresponsiveness to the environment. Sleep is typically accompanied (in humans) by postural recumbence, behavioral quiescence, and closed eyes.” [3]. Scientists have observed that sleep can be categorized into stages of rapid eye movement (REM), in which humans spend around 75% of sleep, and non-rapid eye movement (NREM), with cycles of these stages occurring multiple times during the sleep period [4]. The NREM phase is further divided into the N1, N2, and N3 stages, presenting a progressively deeper sleep with each phase. The phases are selected with different sleep parameters that are recorded during a polysomnography examination. There is evidence that sleep deprivation leads to elevations in blood pressure and heart rate values [5]. The short sleep duration that appears especially during middle age is commonly associated with arterial hypertension, as well as with the higher blood pressure values present among patients with obstructive sleep apnea [6]. Moreover, disruptions in the timing and duration of sleep can contribute toward greater variability in blood pressure values and to the appearance of a non-dipping blood pressure pattern [7,8]. There is also a proven association between high blood pressure and sleep apnea [9]. Sympathetic, humoral, and cellular responses [10] to sleep apnea over time are responsible for vascular dysfunction and the consequent development of arterial hypertension [11]. These manifestations can be exacerbated by sleep deprivation [12,13], which commonly occurs in patients with sleep apnea because of their poor sleep architecture [14]. The apnea-hypopnea index has been found to have an association with blood pressure values and the arousal index. An elevated apnea-hypopnea index has been closely linked to higher blood pressure values [15]. Additionally, an elevation in blood pressure is seen not only among patients with obstructive sleep apnea but also in patients with restless leg syndrome [16], insomnia [17], or sleep bruxism [18].
Polysomnographic examination is the criterion standard tool for analyzing sleep architecture. It has shown that with the progressive deepening of the NREM phase of sleep, there is a decrease in sympathetic nervous system activity [19]. In young healthy normotensive individuals, nocturnal blood pressure dipping has been observed during the shift to the deep slow wave sleep stage (NREM sleep stage N3) [20]. The association between slow wave sleep in the modulation of blood pressure was investigated in 2 community-based cohort studies. These studies showed that the percentage of slow wave sleep was inversely associated with the incidence of hypertension [21,22]. A link between sleep architecture and hypertension has also been uncovered. However, there is still a necessity to conduct further research in this context.
Therefore, this systematic review aims to identify and analyze publications on the association between sleep architecture and arterial hypertension, with a particular focus on sleep fragmentation and the duration of the non-REM/REM phases of sleep, to address the existing research gap.
Material and Methods
ELIGIBILITY CRITERIA:
The inclusion criteria for this review included English-language research articles focusing on sleep architecture in the adult hypertensive population. Exclusion criteria consisted of pediatric patients, studies involving co-existing obstructive sleep apnea, animal research, genetic studies, hypertension in pregnancy, and studies lacking polysomnography data. Additionally, studies with sample sizes of less than 10 patients, case reports, reviews, letters to the editor, and those not reporting on sleep architecture and its association with hypertension were excluded.
SEARCH STRATEGY AND SOURCES REVIEWED:
A comprehensive search strategy was conducted, incorporating the following search terms: (sleep architecture, sleep stages, sleep structure) and/or (hypertension, and high blood pressure) in combination between the first and second term. These terms were searched within titles and keywords. The literature review was independently conducted by JK and HM, who reviewed PubMed (MEDLINE), Scopus, and Embase databases (access date January 20, 2022, 6: 45 p.m.). The search was limited to English-language publications going as far back as the year 2000. No other filter was applied during the search, but later on, the manual selection of the articles was performed. In addition to electronic searches, reference lists of relevant articles were manually searched to identify additional studies.
DATA COLLECTION PROCESS:
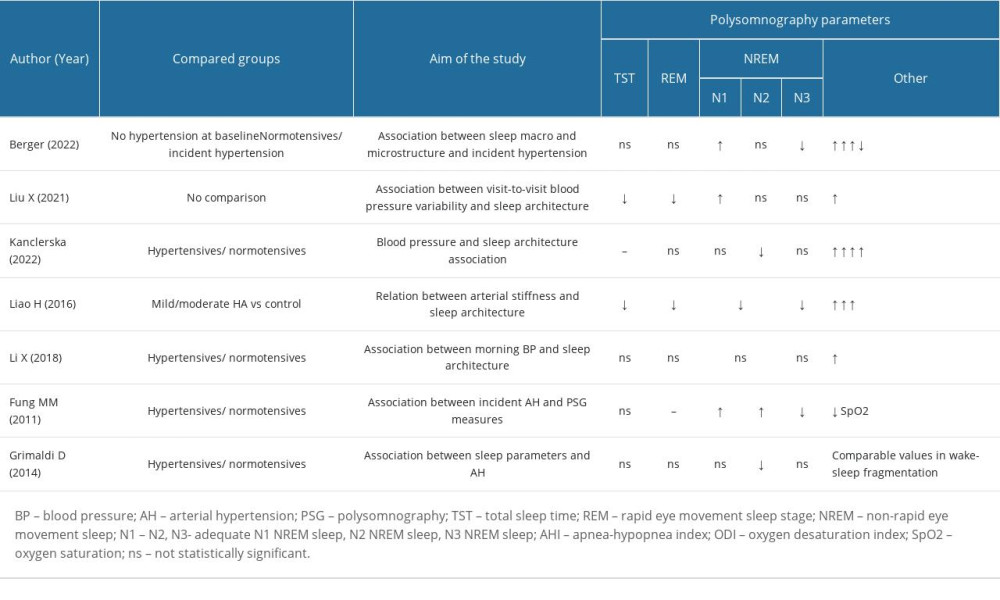
Data collection from the selected reports was performed independently by 2 researchers. The articles were manually selected based on the title and abstract to ensure their relevance to this systematic review. No automation tools were used during the selection process. The data were extracted into Excel to perform further analysis. The characteristics of the considered studies, including author, publication year, study design, sample size, age and sex of participants, study setting, sleep and blood pressure measurement methods, and the main findings are shown in Table 1. In case of discrepancies or disagreements between the 2 researchers, a third researcher was consulted to ensure accuracy and completeness of the data collection process. The data extraction process was carefully monitored to minimize errors or omissions, and the Excel spreadsheet was thoroughly checked for completeness and accuracy before proceeding with the analysis.
RISK OF BIAS:
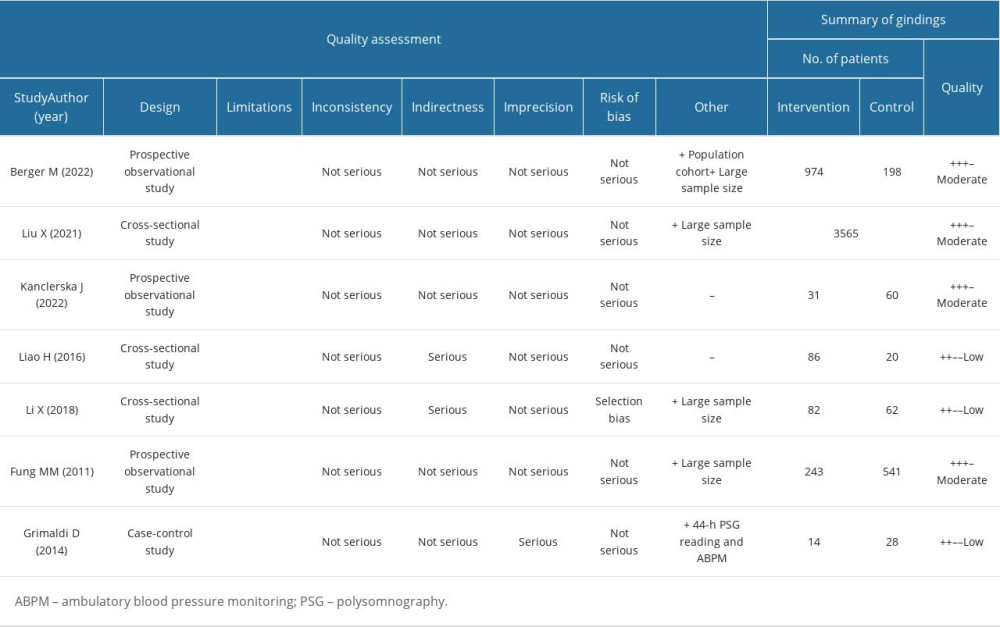
The risk of bias in the included studies was assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system [23], which considers various factors, including study design, risk of bias, inconsistency, indirectness, imprecision, and publication bias, to evaluate the quality of evidence-based research. Specific criteria were used to assess the risk of bias in the included studies, such as selection bias, performance bias, detection bias, attrition bias, and reporting bias. Each study was independently analyzed by 2 researchers, with consensus reached through discussion. The results were assessed independently using the Risk of Bias-2 (RoB-2) tool for randomized studies [24]or the Risk of Bias in Non-Randomized Studies – of Interventions (ROBINS-I) tool for non-randomized studies [25]. The results of the assessment are shown in Table 2. All 7 studies included in this systematic review demonstrated a statistically significant relationship between sleep architecture (at least 1 polysomnography parameter) and arterial hypertension.
Results
SELECTION OF STUDIES:
The study selection process involved a total of 111 initially identified publications. After removing duplicates (n=7) and excluding papers that did not meet the inclusion criteria, such as animal studies (n=4), studies involving patients with obstructive sleep apnea (n=53), studies focusing on pediatric populations or pregnant women, as well as case reports and medication effect descriptions, a 2-step process was followed. First, 2 reviewers independently screened the titles and abstracts of the retrieved studies to select appropriate publications. Of the remaining 20 full-text records that passed this stage, each was independently analyzed for eligibility by the researchers. No automation tools were used during this process. The same 2 reviewers examined the full texts of the research that had passed screening during the full-text evaluation stage. Discussion between the 2 reviewers helped to settle the differences in their assessments of the title/abstract and entire text. Finally, a total of 7 manuscripts were included in the systematic review (Table 1).
QUALITY OF STUDIES:
The risk of bias in each included study was assessed and is presented in Table 2. Most of the studies demonstrated no major issues with the comparability of participants among the examined patients. However, selection bias was detected in 1 of the analyzed papers by Li X et al. [26]. No detection bias, or problems with the measurement or classification of outcomes, were found in any of the studies. The statistical analysis provided in each study was deemed to have high methodological quality. Notably, among the 4 studies [21,26–28], a substantial improvement in the GRADE score was applied, as indicated in Table 2.
STUDY DESCRIPTION:
In 3 out of 7 studies, there were statistically significant results showing a reduction in the N3 sleep stage [21,27–29], while in 2 others [30,31], there was statistical significance in the reduction of the duration of the N2 sleep stage. The duration of REM sleep was also observed to be reduced in 2 studies [26,28,29]. Furthermore, 2 papers reported statistical significance in the reduction of total sleep time [28,29], and 3 papers noted more frequent arousals among the group of patients with hypertension [27,29,30].
One of the analyzed studies focused on microstructural and macrostructural parameters of sleep [27] that can be used to predict the development of hypertension. Berger et al investigated potential risk factors associated with sleep that increased the probability of developing arterial hypertension. The study followed 1172 participants who did not have hypertension prior to the study for an average of 5.2 years. Home-based polysomnography results and in-office measurements of blood pressure were analyzed. Among the microstructural changes observed during sleep, this study found that patients with diminished absolute delta and sigma power had a statistically significant probability of developing hypertension [27]. Furthermore, the study also explored macrostructural changes associated with sleep and demonstrated an inverse association between slow wave activity (N3 sleep stage) and the risk of developing incident hypertension. The N1 phase of sleep was observed to account for a greater percentage of sleep. Interestingly, Berger et al also noticed a statistically significant difference in the objective sleep parameters. During follow-up, it was observed that patients who had developed incident hypertension during the study had changes in sleep efficiency (P=0.024). Additionally, these patients were also found to have higher arousal and apnea-hypopnea indices.
A similar study design was conducted by Fung et al [21], focusing on 784 elderly men (age ≥65 years) with no hypertension at the time of in-home polysomnography screening. The study had a follow-up period of 3.4 years. Blood pressure was recorded (2 measurements on the right arm) at the beginning of the examination and at the time of follow-up. The results of the study were consistent with those of the Berger study, with higher percentages of the N1 and N2 stages of sleep, a higher apnea-hypopnea index, and a lower percentage of the N3 phase of sleep being observed. After adjusting for various factors, statistical significance was found for the N3 sleep stage (P=0.002) [21].
Blood pressure variability has been recognized as an independent risk factor for cardiovascular disease [29–33]. Sleep disturbances can also predict the onset of this group of diseases. In the third study we reviewed, conducted by Liu et al [28], the focus was on the association between blood pressure variability and sleep architecture. The study included a large sample of patients (n=3565) who underwent in-lab polysomnography and had at least 3 in-office blood pressure measurements taken 12 months before the polysomnography examination. Having regard for possible confounders, a statistically significant inverse correlation was seen between systolic blood pressure variability and duration of the REM stage of sleep. The results indicated that patients with higher systolic blood pressure variability had shorter durations of REM sleep (P=0.001), as well as reduced durations of N2 and N3 sleep stages. Conversely, the N1 sleep phase was longer in patients with greater systolic blood pressure variability (P=0.001) than in those with normal blood pressure variability.
Kanclerska et al conducted a study that provided evidence for the differences in sleep structures between hypertensive and normotensive patients [30]. The study involved a 1-night polysomnography conducted in the sleep laboratory on 91 patients, along with ambulatory blood pressure monitoring. It was observed that the N2 phase of sleep was prolonged among hypertensive patients, which was a statistically significant finding consistent with the results of Liu et al [28]. Moreover, statistically significant differences in the apnea-hypopnea index and oxygen desaturation index were observed between the experimental and control groups. The study also revealed increased sleep fragmentation (measured according to the arousal index) in hypertensive patients, compared with normotensive patients.
A different approach to the analysis of the sleep architecture among patients with hypertension was presented by Liao et al [29]. The authors focused on pulse wave velocity, which is a key index for estimating arterial elasticity. Pulse wave velocity is recognized as a predictor for the development of cardiovascular diseases, including hypertension. In their study, a total of 86 hypertensive patients underwent ambulatory blood pressure monitoring and in-lab polysomnography and were compared with 20 normotensive individuals from the control group. The results of this study were consistent with the previously discussed papers, demonstrating that hypertensive patients had a longer sleep latency (P<0.032) and shorter duration of total sleep time (P=0.001). Additionally, the N3 phase of sleep was significantly shorter in the hypertensive group (19.93% in the control group vs 8.58% in the hypertensive group, P<0.001). This finding aligns with the previous research discussed. Interestingly, Liao et al also observed longer durations of the N1 and N2 phases of sleep, as well as higher apnea-hypopnea and microarousal indices in the experimental group. This result corresponds with the study outcomes of Kanclerska et al.
Li et al [26] conducted a study to investigate sleep architecture in relation to 24-h ambulatory blood pressure monitoring, with a focus on morning blood pressure values, among 144 hypertensive patients. A decrease in sleep efficiency, shorter slow wave sleep, and increases in the proportion of light sleep and the arousal index were observed. These results were consistent with the previously analyzed studies.
However, a study by Grimaldi et al [31] presented contrasting results to the aforementioned studies. The study included 14 patients with recently diagnosed untreated hypertension (<1 year since the diagnosis), according to established guidelines [34]. These patients were compared with a normotensive control group consisting of 28 participants. Both groups underwent a 2-night polysomnography examination as well as 44-h ambulatory blood pressure monitoring. They found no statistically significant differences in total sleep time, slow wave sleep, or REM sleep. This might be attributed to the small sample size of patients or other individual factors, such as weight and family predisposition for developing hypertension. Interestingly, in contrast to previous studies, Grimaldi et al observed a statistically significant reduction in the percentage of N2 sleep among hypertensive patients (P=0.03). Furthermore, it appeared that hypertensive patients had particularly low systolic blood pressure values during the N1, N2, and REM phases of sleep, as opposed to the control group.
Indeed, the findings from the studies reviewed confirm that there is an existing association between changes in sleep architecture and blood pressure concerning REM and non-REM sleep phases. The evidence supports that hypertensive individuals exhibit alterations in their sleep patterns, compared with normotensive individuals. Increased sleep fragmentation, as indicated by a higher arousal index, was consistently observed in hypertensive patients across multiple studies. Additionally, several studies reported increased sleep latency and decreased total sleep time duration among hypertensive individuals. Furthermore, hypertensive patients exhibited higher apnea-hypopnea index values, indicating increased sleep-disordered breathing, and lower oxygen saturation levels than normotensive individuals. These findings collectively emphasize the relationship between sleep architecture and blood pressure regulation in the context of hypertension.
Discussion
SLEEP DISORDERS AND CARDIOVASCULAR RISK:
Sleep architecture disorders have become increasingly apparent in recent years. Obstructive sleep apnea, insomnia, and circadian rhythm disorders have been linked to an increased cardiovascular risk [7,8]. Researchers are recognizing the importance of identifying and treating sleep disorders as a way to reduce the risk of developing cardiovascular diseases. Screening for sleep disorders may become a routine part of the cardiovascular risk assessment. The reviewed papers investigated the relationship between changes in sleep architecture and blood pressure. Sleep architecture was analyzed using either at-home or sleep laboratory polysomnography, which is the criterion standard examination tool for the diagnosis of sleep disorders. Blood pressure measurements were recorded either by 24-h ambulatory blood pressure monitoring or were measured manually at the doctor’s office. It is important to note that many studies on sleep disorders and hypertension have been conducted using respiratory polygraphy, which is a recognized, widely available, and inexpensive method used in the diagnosis of obstructive sleep apnea. However, it does not provide the capability to analyze sleep architecture, as it lacks the recording of electroencephalography. Consequently, publications that relied solely on polygraphy were excluded from this review, as the assessment of parameters such as total sleep time, sleep latency, and arousals cannot be accurately estimated with this method.
ARTERIAL HYPERTENSION:
In 2019, the American College of Cardiology and American Heart Association [35] published new guidelines that updated the categories of arterial hypertension. The guidelines are as follows: (1) normal (<120 systolic and <80 mmHg diastolic), (2) elevated (120–129 systolic and <80 mmHg diastolic), (3) stage 1 hypertension (130–139 systolic or 80–89 mmHg diastolic), and (4) stage 2 hypertension (≥140 systolic or ≥90 mmHg diastolic). These measurements should be based on averaged measurements on at least 2 separate in-office visits, and should be correlated with at-home measurements. Alternatively, 24-h ambulatory blood pressure monitoring can be used. The studies that were analyzed in this review had either made use of in-office blood pressure measurements, as per the above mentioned guidelines, or ambulatory blood pressure monitoring.
Arterial hypertension is a disease encountered worldwide. In 2023, the WHO estimated that 1.28 billion adults aged 30–79 years had hypertension, with the majority living in middle to low income countries. Shockingly, approximately 46% of adults are oblivious to their condition [36]. Arterial hypertension presents a serious risk of developing end organ damage, which can affect the heart, brain, eyes, and kidneys [37,38]. These data convince us that hypertension is a global health problem that should be screened for more carefully. To avoid the development of these complications, treatment should be started immediately after diagnosis.
The underlying pathomechanisms of hypertension involve the dysregulation of the renin-angiotensin-aldosterone system [2] leading to oxidative stress and, consequently, to renal tubular sodium reabsorption, vasoconstriction, vascular smooth muscle remodeling, and inflammation, ultimately resulting in endothelial dysfunction [39]. Moreover, overactivation of the autonomic nervous system (particularly the sympathetic nervous system) is also one of the proven pathomechanisms behind the development of arterial hypertension [1,40]. Its inappropriate activity can result in an increase in cardiovascular morbidity and mortality, as well as changes in various stages of sleep, ultimately affecting the quality of sleep [41–43].
There is a subtype of hypertension called resistant hypertension, which is associated with a particularly high cardiovascular risk. This subtype is often related to sleep disorders, most frequently, obstructive sleep apnea [44]. The importance of acknowledging the above-mentioned pathomechanisms is necessary to create new methods of treatment, as the effectiveness of current antihypertensive treatment is low [36].
SLEEP ARCHITECTURE:
It has been postulated that sleep is essential for many vital functions, including energy conservation, modulation of immune responses, cognition, performance, vigilance, and the psychological state [45,46]. Human sleep architecture is primarily divided into 2 phases, namely the NREM and REM sleep phases. The non-REM phase of sleep is further subdivided into the N1, N2, and N3 stages. These 3 stages are associated with progressively deeper levels of sleep [47,48]. Researchers have emphasized the importance of sleep continuity for adaptive mental health functions [49]. Disturbances in sleep continuity have been associated with several negative health outcomes, including depression [50], activation of the sympathetic nervous system, elevation in heart rate [12], and an increase in the prevalence of hypertension [6]. Therefore, there is a known correlation between sleep and hypertension. In summary, the relationship between sleep and hypertension is well-established. Sleep disturbances, including sleep deprivation, sleep disorders (such as obstructive sleep apnea), and circadian rhythm disorders, can contribute to elevated blood pressure and increase the risk of developing hypertension. Maintaining healthy sleep patterns and addressing sleep disorders can have a significant impact on blood pressure control and overall cardiovascular health.
SLEEP DISORDERS AND HYPERTENSION:
Moreover, a strong connection between hypertension and obstructive disorders has been observed during sleep. Several studies have linked the presence of sleep disorders and hypertension, focusing primarily on the correlation between obstructive sleep apnea and hypertension [51]. It has been hypothesized that the increase in blood pressure among patients with obstructive sleep apnea is due to hypoxia and its subsequent activation of the sympathetic nervous system [52]. While obstructive sleep apnea has been well-established as a risk factor for hypertension, in this review, we specifically excluded obstructive sleep apnea to focus on the relationship between essential hypertension (hypertension without obstructive sleep apnea) and specific sleep architecture disorders. There is also a large amount of research published on obstructive sleep apnea that was beyond the scope of this review. Even after the exclusion of obstructive sleep apnea, there appears to be strong evidence suggesting that hypoxia and respiratory events favor the appearance of hypertension. Interestingly, there is no safe level of respiratory obstruction. Even when the degree of respiratory obstruction is well within the normal range, there is a strong correlation with elevations in blood pressure. In addition, hypertension shares the same pathomechanism as other sleep disorders, namely sleep bruxism [30]. The increased variability of blood pressure values in sleep bruxism is accompanied by inflammation; therefore, sleep bruxism has recently been regarded as one of the risk factors behind the development of hypertension and other cardiovascular events [53]. It has also been proven that patients with sleep bruxism have a higher arousal index, consequently resulting in sleep fragmentation [54].
Fragmented sleep is less efficient than consolidated sleep and can often result in daytime sleepiness, fatigue, and a lack of concentration [55]. Frequent arousals during sleep are associated with longer wake after sleep onset, reduced sleep efficiency, and more frequent sleep phase shifts [56,57]. Ineffective sleep can lead to the development of hypertension as well as increased cardiovascular risk [15]. Most of the analyzed research correlates the fragmentation of sleep with arterial hypertension.
It has also been broadly discussed that there is a relationship between hypertension and the apnea-to-hypopnea index, even when the index is within the normal range [58], which we found in this systematic review [27–30]. Past studies have illustrated a linear association between the apnea-hypopnea index and blood pressure values and the prevalence of cardiovascular disease [59,60]. It appears that the apnea-hypopnea index may be a predictive parameter in the assessment of potential future cardiovascular disease development.
As stated in analyzed papers, a shorter N3 sleep stage can result in insufficient regeneration, a slower anabolic process, negligible cellular regeneration, impairment of immunological function, and an increase in oxidative stress [61]. Oxidative stress is one of the fundamental mechanisms responsible for the development of hypertension. Reactive oxygen species play an important role in the homeostasis of the vascular wall. Overproduction of reactive oxygen species has been linked to the development of arterial hypertension [62]. The mechanism of activation of the sympathetic nervous system, the cytokine response, and the magnitude and extent of the proinflammatory immune response have been well documented in patients with hypertension [63].
Additionally, the N1 stage of sleep has been shown to be prolonged in individuals with sleep fragmentation and frequent arousals. The N2 sleep stage, on the other hand, appears to have the greatest discrepancies in the results of the analyzed studies in this review. We see a necessity for further research to be conducted on the N2 sleep phase among the hypertensive group of patients.
Although the REM stage of sleep is important for memory [64] and emotional processing [65], the relationship between REM and hypertension has been poorly described. Research suggests that patients with hypertension can have impaired emotional regulation, a greater risk of depression and anxiety, and difficulty regulating stress and emotions [66].
Among the analyzed research, sleep latency was increased and total sleep time was decreased in 2 studies. Studies have proven that there is a strong association between the reduced duration of sleep and hypertension [67]. It has been documented that a higher risk of unsatisfactory blood pressure regulation appears in adults habitually sleeping for less than 6 h a day [68].
Overall, while more research is needed to fully understand the relationship between sleep architecture and hypertension, as the existing evidence suggests that there is a connection between the two. It is important for individuals with hypertension, or at risk of developing hypertension, to prioritize good sleep habits and address any sleep disorders that are present.
There is also a great need for future research on the prevention of cardiovascular disease or the management of treatable sleep disorders. Early detection and treatment of sleep disorders can be a key strategy for reducing the risk of developing cardiovascular diseases and improving overall health and quality of life. Further research is needed to identify effective interventions for the prevention and management of cardiovascular diseases in people with sleep disorders.
The study had several limitations that need to be acknowledged. First, there was a limited number of studies available that specifically examined the association between hypertension and sleep architecture disorders in patients without sleep apnea. This scarcity of research in the field highlights the need for further investigation in this area. Additionally, some of the reviewed studies had a moderate risk of bias, which may have influenced the validity of their findings. Furthermore, in some cases, the examined groups consisted of a small number of patients, which could limit the generalizability of the results. These limitations emphasize the need for larger scale, well-designed studies to provide more robust evidence on the relationship between hypertension and sleep architecture disorders in individuals without sleep apnea.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
1. Esler M, The sympathetic system and hypertension: Am J Hypertens, 2000; 13(6 Pt 2); 99S-105S
2. Moon JY, Recent update of renin-angiotensin-aldosterone system in the pathogenesis of hypertension: Electrolyte Blood Press, 2013; 11(2); 41-45
3. Carskadon WC, Dement MA, 2011 Normal human sleep: An overview: Principles and practice of sleep medicine, 2011; 16-26, St. Louis, Elsevier Saunders Available at: https://doi.org/10.1016/B978-1-4160-6645-3.00002-5
4. Patel AK, Reddy V, Shumway KR, Araujo JF, Physiology, sleep stages: StatPearls [Internet] Sep 7, 2022, Treasure Island (FL), StatPearls Publishing Available at: https://www.ncbi.nlm.nih.gov/books/NBK526132/
5. Knutson KL, Van Cauter E, Rathouz PJ, Association between sleep and blood pressure in midlife: The CARDIA sleep study: Arch Intern Med, 2009; 169(11); 1055-61
6. Calhoun DA, Harding SM, Sleep and hypertension: Chest, 2010; 138(2); 434-43
7. Lanfranchi PA, Pennestri MH, Fradette L, Nighttime blood pressure in normotensive subjects with chronic insomnia: Implications for cardiovascular risk: Sleep, 2009; 32(6); 760-66
8. Folkow B, Mental stress and its importance for cardiovascular disorders; Physiological aspects, “from-mice-to-man”: Scand Cardiovasc J, 2001; 35(3); 163-72
9. Peppard PE, Young T, Palta M, Skatrud J, Prospective study of the association between sleep-disordered breathing and hypertension: N Engl J Med, 2000; 342(19); 1378-84
10. Chin K, Nakamura T, Shimizu K, Effects of nasal continuous positive airway pressure on soluble cell adhesion molecules in patients with obstructive sleep apnea syndrome: Am J Med, 2000; 109(7); 562-67
11. Panza JA, Quyyumi AA, Brush JE, Epstein SE, Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension: N Engl J Med, 1990; 323(1); 22-27
12. Kato M, Phillips BG, Sigurdsson G, Effects of sleep deprivation on neural circulatory control: Hypertension, 2000; 35(5); 1173-75
13. Lusardi P, Effects of insufficient sleep on blood pressure in hypertensive patients a 24-h study: Am J Hypertens, 1999; 12(1); 63-68
14. Davies CWH, Case-control study of 24 hour ambulatory blood pressure in patients with obstructive sleep apnoea and normal matched control subjects: Thorax, 2000; 55(9); 736-40
15. Nieto FJ, Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study: JAMA, 2000; 283(14); 1829
16. Sieminski M, Partinen M, Nocturnal systolic blood pressure is increased in restless legs syndrome: Sleep Breath, 2016; 20(3); 1013-19
17. Jarrin DC, Alvaro PK, Bouchard MA, Insomnia and hypertension: A systematic review: Sleep Med Rev, 2018; 41; 3-38
18. Michalek-Zrabkowska M, Wieckiewicz M, Gac P, Effect of sleep bruxism intensity on blood pressure in normotensives: J Clin Med, 2021; 10(6); 1304
19. Hornyak M, Cejnar M, Elam M, Sympathetic muscle nerve activity during sleep in man: Brain, 1991; 114(Pt 3); 1281-95
20. Sayk F, Teckentrup C, Becker C, Effects of selective slow-wave sleep deprivation on nocturnal blood pressure dipping and daytime blood pressure regulation: Am J Physiol Regul Integr Comp Physiol, 2010; 298(1); R191-97
21. Fung MM, Peters K, Redline S, Decreased slow wave sleep increases risk of developing hypertension in elderly men: Hypertension, 2011; 58(4); 596-603
22. Javaheri S, Zhao YY, Punjabi NM, Slow-wave sleep is associated with incident hypertension: The sleep heart health study: Sleep, 2018; 41(1); zsx179
23. Guyatt GH, Oxman AD, Vist GE, GRADE: An emerging consensus on rating quality of evidence and strength of recommendations: BMJ, 2008; 336(7650); 924-26
24. Sterne JAC, Savović J, Page MJ, RoB 2: A revised tool for assessing risk of bias in randomised trials: BMJ, 2019; 366; l4898
25. Sterne JA, Hernán MA, Reeves BC, ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions: BMJ, 2016; 355; i4919
26. Li X, Li J, Liu K, Association between sleep disorders and morning blood pressure in hypertensive patients: Clin Exp Hypertens, 2018; 40(4); 337-43
27. Berger M, Vakulin A, Hirotsu C, Association between sleep microstructure and incident hypertension in a population-based sample: The HypnoLaus study: J Am Heart Assoc, 2022; 11(14); e025828
28. Liu X, Logan J, Kwon Y, Visit-to-visit blood pressure variability and sleep architecture: J Clin Hypertens (Greenwich), 2021; 23(2); 323-30
29. Liao H, Zhao L, Liu K, Chen X, Investigation of the relationship between arterial stiffness and sleep architecture in patients with essential hypertension: Clin Exp Hypertens, 2016; 38(1); 113-18
30. Kanclerska J, Wieckiewicz M, Poreba R, Polysomnographic evaluation of sleep bruxism intensity and sleep architecture in nonapneic hypertensives: A prospective, observational study: J Clin Med, 2022; 11(11); 3113
31. Grimaldi D, Provini F, Calandra-Buonaura G, Cardiovascular-sleep interaction in drug-naïve patients with essential grade I hypertension [published correction appears in Chronobiol Int. 2014;31(2):300[: Chronobiol Int, 2013; 30(1-2); 31-42
32. Clark D, Nicholls SJ, St John J, Visit-to-visit blood pressure variability, coronary atheroma progression, and clinical outcomes: JAMA Cardiol, 2019; 4(5); 437
33. Parati G, Ochoa JE, Lombardi C, Bilo G, Assessment and management of blood-pressure variability [published correction appears in Nat Rev Cardiol. 2014;11(6): 314]: Nat Rev Cardiol, 2013; 10(3); 143-55
34. Chobanian AV, Bakris GL, Black HR, Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: Hypertension, 2003; 42(6); 1206-52
35. Whelton PK, Carey RM, Aronow WS, 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and e136–e39] [published correction appears in Hypertension. 2018; 72(3):e33]: Hypertension, 2018; 71(6); 1269-324
36. World Health Organization: Guideline for the pharmacological treatment of hypertension in adults, 2021, Geneva, World Health Organization
37. Jordan J, Kurschat C, Reuter H, Arterial hypertension: Dtsch Arztebl Int, 2018; 115(33–34); 557-68
38. Fuchs FD, Whelton PK, High blood pressure and cardiovascular disease: Hypertension, 2020; 75(2); 285-92
39. Manrique C, Lastra G, Gardner M, Sowers JR, The renin angiotensin aldosterone system in hypertension: Roles of insulin resistance and oxidative stress: Med Clin North Am, 2009; 93(3); 569-82
40. Manolis AJ, Poulimenos LE, Kallistratos MS, Sympathetic overactivity in hypertension and cardiovascular disease: Curr Vasc Pharmacol, 2014; 12(1); 4-15
41. Rozanski A, Blumenthal JA, Kaplan J, Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy: Circulation, 1999; 99(16); 2192-217
42. Kaye DM, Lefkovits J, Jennings GL, Adverse consequences of high sympathetic nervous activity in the failing human heart: J Am Coll Cardiol, 1995; 26(5); 1257-63
43. Hou H, Zhao Y, Yu W, Association of obstructive sleep apnea with hypertension: A systematic review and meta-analysis: J Glob Health, 2018; 8(1); 010405
44. Carnethon MR, Johnson DA, Sleep and resistant hypertension: Curr Hypertens Rep, 2019; 21(5); 34
45. Zielinski MR, McKenna JT, McCarley WR, Functions and mechanisms of sleep: AIMS Neurosci, 2016; 3(1); 67-104
46. Zielinski MR, Krueger JM, Sleep and innate immunity: Front Biosci (Schol Ed), 2011; 3(2); 632-42
47. Kishi A, Yasuda H, Matsumoto T, NREM sleep stage transitions control ultradian REM sleep rhythm: Sleep, 2011; 34(10); 1423-32
48. Watson NF, Badr MS, Belenky G, Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: Methodology and discussion: Sleep, 2015; 38(8); 1161-83
49. Finan PH, Quartana PJ, Smith MT, The effects of sleep continuity disruption on positive mood and sleep architecture in healthy adults: Sleep, 2015; 38(11); 1735-42
50. Baglioni C, Battagliese G, Feige B, Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies: J Affect Disord, 2011; 135(1–3); 10-19
51. Van Ryswyk E, Mukherjee S, Chai-Coetzer CL, Sleep disorders, including sleep apnea and hypertension: Am J Hypertens, 2018; 31(8); 857-64
52. Henderson LA, Macefield VG, Obstructive sleep apnoea and hypertension: The role of the central nervous system: Curr Hypertens Rep, 2016; 18(7); 59
53. Salman LA, Shulman R, Cohen JB, Obstructive sleep apnea, hypertension, and cardiovascular risk: Epidemiology, pathophysiology, and management: Curr Cardiol Rep, 2020; 22(2); 6
54. Macaluso GM, Guerra P, Di Giovanni G, Sleep bruxism is a disorder related to periodic arousals during sleep: J Dent Res, 1998; 77(4); 565-73
55. Stepanski EJ, The effect of sleep fragmentation on daytime function: Sleep, 2002; 25(3); 268-76
56. Trindade M, de O, Rodriguez AG, Polysomnographic analysis of bruxism: Gen Dent, 2014; 62(1); 56-60
57. Wieczorek T, Wieckiewicz M, Smardz J, Sleep structure in sleep bruxism: A polysomnographic study including bruxism activity phenotypes across sleep stages: J Sleep Res, 2020; 29(6); e13028
58. Gać P, Urbanik D, Macek P, Martynowicz H, Coexistence of cardiovascular risk factors and obstructive sleep apnoea in polysomnography: Respir Physiol Neurobiol, 2022; 295; 103782
59. Punjabi NM, Caffo BS, Goodwin JL, Sleep-disordered breathing and mortality: A prospective cohort study: PLoS Med, 2009; 6(8); e1000132
60. Wang L, Cai A, Zhang J, Association of obstructive sleep apnea plus hypertension and prevalent cardiovascular diseases: A cross-sectional study: Medicine (Baltimore), 2016; 95(39); e4691
61. Sharma S, Kavuru M, Sleep and metabolism: An overview: Int J Endocrinol, 2010; 2010; 270832
62. González J, Essential hypertension and oxidative stress: New insights: World J Cardiol, 2014; 6(6); 353
63. Singh MV, Chapleau MW, Harwani SC, Abboud FM, The immune system and hypertension: Immunol Res, 2014; 59(1–3); 243-53
64. Rasch B, Born J, About sleep’s role in memory: Physiol Rev, 2013; 93(2); 681-766
65. Werner GG, Schabus M, Blechert J, Wilhelm FH, Differential effects of REM sleep on emotional processing: initial evidence for increased short-term emotional responses and reduced long-term intrusive memories: Behav Sleep Med, 2021; 19(1); 83-98
66. Wiener A, Rohr CS, Naor N, Villringer A, Okon-Singer H, Emotion regulation in essential hypertension: Roles of anxiety, stress, and the pulvinar: Front Behav Neurosci, 2020; 14; 80
67. Grandner MA, Chakravorty S, Perlis ML, Habitual sleep duration associated with self-reported and objectively determined cardiometabolic risk factors: Sleep Med, 2014; 15(1); 42-50
68. Li H, Ren Y, Wu Y, Zhao X, Correlation between sleep duration and hypertension: A dose-response meta-analysis: J Hum Hypertens, 2019; 33(3); 218-28
In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952