05 September 2023: Review Articles
The Impact of Mechanical Energy Assessment on Mechanical Ventilation: A Comprehensive Review and Practical Application
Filip BuršaDOI: 10.12659/MSM.941287
Med Sci Monit 2023; 29:e941287
Abstract
ABSTRACT: Mechanical ventilation (MV) provides basic organ support for patients who have acute hypoxemic respiratory failure, with acute respiratory distress syndrome as the most severe form. The use of excessive ventilation forces can exacerbate the lung condition and lead to ventilator-induced lung injury (VILI); mechanical energy (ME) or power can characterize such forces applied during MV. The ME metric combines all MV parameters affecting the respiratory system (ie, lungs, chest, and airways) into a single value. Besides evaluating the overall ME, this parameter can be also related to patient-specific characteristics, such as lung compliance or patient weight, which can further improve the value of ME for characterizing the aggressiveness of lung ventilation. High ME is associated with poor outcomes and could be used as a prognostic parameter and indicator of the risk of VILI. ME is rarely determined in everyday practice because the calculations are complicated and based on multiple equations. Although low ME does not conclusively prevent the possibility of VILI (eg, due to the lung inhomogeneity and preexisting damage), individualization of MV settings considering ME appears to improve outcomes. This article aims to review the roles of bedside assessment of mechanical power, its relevance in mechanical ventilation, and its associations with treatment outcomes. In addition, we discuss methods for ME determination, aiming to propose the most suitable method for bedside application of the ME concept in everyday practice.
Keywords: Respiration, Artificial, Humans, Respiration, Aggression, Respiratory Distress Syndrome, Thorax, ventilator-induced lung injury
Background
Mechanical ventilation (MV) is used to provide basic organ support to patients who have acute hypoxemic respiratory failure and acute respiratory distress syndrome (ARDS) [1]. Gas exchange usually improves under the influence of positive pressure ventilation (PPV); however, the already-damaged lung parenchyma is affected by mechanical forces (energies) generated by PPV. These mechanical forces can worsen the underlying lung injury and cause ventilator-induced lung injury (VILI) [2]. Ventilator settings can, through forces exerted on the lungs by the ventilator, directly influence the development of VILI. For example, a 2000 study by the ARDS Network concluded that a reduction of tidal volume (Vt) to 6 ml/PBW (predicted body weight) had a significant effect on mortality in worldwide critical care [3]. Driving pressure (DP), which is the difference between positive end-expiratory pressure (PEEP) and plateau pressure), is relatively simple to set up in clinical practice, and its association with mortality has been demonstrated previously [4]. However, reducing DP as well as ventilation with a Vt of 6 ml/kg or less usually leads to hypercapnia. To reduce hypercapnia, most clinicians resort to increasing the respiratory rate (RR) [5]; this is, however, generally associated with an increase in mechanical energy (ME). The effect of additional parameters, such as Vt, DP, PEEP, plateau pressure, or flow, on the ventilator’s contribution to lung injury is currently an important topic of research in the field of mechanical ventilation. Many parameters contribute to ventilator-induced lung injury (VILI) – there is growing evidence grows that these include RR [6], air flow[7], and strain [8,9]. The combined effect of these parameters represents the ME of ventilation. The effect of the ME generated by a PPV device on the respiratory system depends not only on the ventilatory settings, but also on the lung properties (eg, lung inhomogeneity, and lung size) [10]. Historically, ME measurements have been possible since the 1960s [11], but their use has not been implemented in everyday practice so far. ME seems to be a good prognostic marker, and high ME may indicate an increased risk of VILI development, but no randomized controlled trials (RCT) have defined clear cut-off limits of ME associated with VILI or mortality. Although associations between high ME and poor patient outcomes have been reported in many studies (see below), the fundamental question remains – is ME only a prognostic marker, or could it be used as a tool for the optimization and individualization of treatment?
Two characteristics are used to describe the energy applied to the respiratory system MV to exchange the alveolar gas. Mechanical power (MP) is the energy applied to the respiratory system of the patient by the ventilator per unit of time, typically a minute (ie, the cumulative inspiratory energy in joules per minute), while ME is the energy transferred to the lungs per individual breath by the ventilator [12]. It can be derived by integrating the curve characterizing the dependence of tidal volume on transpulmonary pressure [13].
Oxygenation and decarboxylation of hemoglobin and blood depend on factors such as alveolar perfusion and the ability to exchange the gases with blood. If the alveoli and the interstitium are severely damaged, extracorporeal membrane oxygenation (ECMO) should be considered instead of mechanical ventilation with high MP [14], and MP seems to be useful parameter for indication of ECMO [15]. The modern ME concept was first described by Gattinoni et al [16], who constructed an equation using individual ventilatory parameters for determining the total mechanical energy value (Eq. 2, presented in detail below). In addition, this equation also allows us to evaluate the effects of individual parameters on MP, showing that the Vt, DP, and inspiration flow increase ME exponentially with a growth factor of 2, while an increase in the positive end-expiratory pressure (PEEP) leads to a roughly linear increase in ME. ME also grows exponentially with the RR (growth factor of 1.4). Although this equation is relatively complicated for everyday use, it can be perceived as a guide for clinicians, telling them the degree to which individual parameters are associated with greater ME elevation, rather than as a tool for exact ME calculation [10].
No high-quality interventional trial showing that bedside measurement of ME improves patients’ outcomes has been performed so far and further investigation is needed. This article aims to review the roles of bedside assessment of mechanical power and its relevance in mechanical ventilation. It also aims to provide clinicians with information necessary to introduce the ME concept into everyday clinical practice and presents current ME calculations facilitating bedside use. It summarizes the current algorithms for ME/MP calculation and discusses their clinical relevance and future directions of MP in critically ill patients.
It should be noted that various authors use various abbreviations for the same variables in their papers. For clarity, we unified these abbreviations in this paper and use the same abbreviations throughout the text.
MP as a Marker of Outcome
MP AS A PREDICTOR OF OUTCOME IN ANIMAL AND PRECLINICAL STUDIES:
An animal study by Araos et al indicated that histological lung injury and fibro-proliferation scores were positively correlated with MP [17]. Xie et al reported correlations of MP with serum transforming growth factor β1 (TGF-β1) and connective tissue growth factor (CTGF) (markers of fibrosis); the correlation coefficients were 0.424 and 0.581, respectively [18]. In a retrospective analysis of patients undergoing open lung biopsy, Hsin-Hsien Li demonstrated that MP at ARDS diagnosis and ARDS duration before lung biopsy were independently associated with histological fibrosis [19]. MP was also associated with markers of VILI (C-X-C motif chemokine ligand 10/C-X-C Motif Chemokine Receptor 3) in an animal study by Xie Y et al [20].
ME AS A PREDICTOR OF OUTCOME IN RETROSPECTIVE CLINICAL STUDIES:
Fuller et al performed a retrospective analysis of 1705 mechanically ventilated patients in their Lung-Protective Ventilation Initiated in the Emergency Department trial (LOV-ED trial) [21]. Their study assessed clinical outcomes associated with the implementation of the early protocol of lung-protective mechanical ventilation. Although higher MP was shown to be independently significantly associated with a higher risk for ARDS development, the results were not too convincing (adjusted odds ratio (OR), 1.03 [1.00–1.06]).
In a study by Parhar et al, who analyzed a group of 731 ARDS patients, MP higher than 22 J/min in ARDS patients was associated with increased 28-day hospital mortality [22].
A retrospective analysis of a cohort of 306 children with ARDS from 55 pediatric intensive care units was performed by Bhalla et al [23], who found that higher MP was associated with a lower likelihood of being ventilator-free on day 28. Similarly, higher MP was associated with increased mortality in pediatric [24] and adult [25–31] ARDS patients, as well as in patients without ARDS [32,33].
A pooled database of 4549 patients with ARDS from 6 randomized clinical trials of protective mechanical ventilation and 1 large observational cohort of patients with ARDS was processed by Costa et al [34]. In their adjusted analyses, the DP, RR, and MP were significant predictors of mortality.
Urner et al analyzed the association between the intensity of dynamic MP and mortality [31]. They evaluated outcomes in patients with and without ARDS classified according to their DP (greater or lower than 15 cmH20) and MP (cut-off 17 J/minute). However, they only took the readings once a day at 8 AM. Despite this limitation, the authors concluded that both driving pressure and MP at the baseline were associated with a proportionally increased risk for Intensive Care Unit (ICU) mortality.
In the recent analysis by Senturk et al, the MP was assessed retrospectively from electronic recording systems [35]. In the 3042 patients, mortality in MP<11.3 J/min was 35.4%, and 49.1% in MP>11.3J/min. Mechanical ventilation days and ICU-length of stay were also significantly longer in the MP>11.3 J/min group. They used only a simple equation (No. 3 below) and concluded that the MP from the first 24 h was predictive for the ICU patients’ prognosis.
PARAMETERS OF MV AND ME AS A PREDICTOR OF MORTALITY:
The effect of lower and higher PEEP in ARDS patients with similar DP was described by Calvanti et al [36]. The 28-day mortality (all-cause mortality) was higher in the group with high PEEP, possibly due to the increased ME. In their retrospective analysis including 8207 patients, Serpa et al showed that higher ME was independently associated with higher in-hospital mortality in mechanically ventilated patients [37]. This was true even at low tidal volumes – MP higher than 17.0 J/min was independently associated with in-hospital mortality and increased risk of death. A similar study evaluating Vt based on ideal body weight found that even after normalization to the predicted body weight, the same Vt can generate different lung strains and different lung damage based on individual lung conditions [38]. A retrospective analysis of MP in COVID-19 (coronavirus disease 2019) and non-COVID-19 ARDS patients was performed in 1737 patients with almost 30% mortality [39]. The median MP during the first 24 h of ventilation was 19.3 [14.6–24.0] J/min in patients with and 13.2 [10.2–18.0] J/min in patients without COVID-19, and higher MP was associated with increased mortality independent of COVID-19. The authors considered only the first 24 h of MV to calculate the average MP.
INDEXED ME (NORMALIZED TO BODY WEIGHT, ETC.) FOR MORTALITY PREDICTION:
Zhang et al investigated the reliability of ME for mortality prediction; the best results (especially in severe ARDS) were achieved with ME normalized to predicted body weight [40]. Some other authors also demonstrated an association of ME normalized to predicted body weight [41,42], even to lung capacity (specific power) [43] with mortality.
Methods of ME Calculation and its Bedside Use
ME AND ITS BEDSIDE USE:
However, because of the calculation’s complexity and specific limitations (see individual equations), MP calculation is nowadays rarely provided directly by ventilators, and bedside estimation of MP needs to be done manually based on ventilator-provided values supported by specifically measured parameters [44]. The complexity of lung disease and ventilator settings obviously do not allow ME to be used as the sole parameter. It appears that the best approach lies in the individualization of mechanical ventilation, taking ME into account as a summary parameter [45–57].
Unfortunately, the complexity of ME calculations and measurements limits their widespread use. The geometrical method has become the criterion standard in ME determination, but its bedside use is not practical [58]. Other methods using transpulmonary pressure (Ptp), which is difficult to measure accurately and is not widely monitored, have been proposed for both volume- and pressure-controlled ventilation. However, simplified equations have been derived from the original one to enable bedside calculations; individual methods along with their accuracy and limitations will be discussed below.
THE GEOMETRICAL METHOD OF CALCULATION:
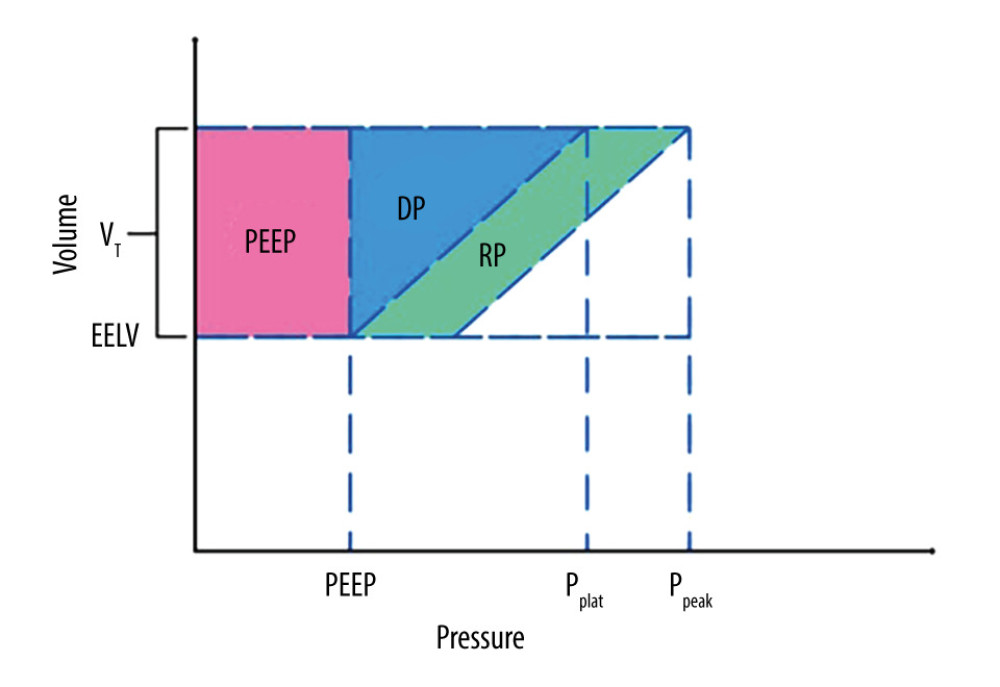
The geometrical method represents the criterion standard for determining ME as far as a physical measurement is concerned. It is presented in detail in Figure 1 [58]. The energy of a single breath is described as the area under the pressure–volume ventilation curve [59]. The method assumes constant inspiration flow (square inspiration flow), the absence of spontaneous breathing, and a linear relationship between inspiration time and tidal volume, which increases during inspiration. In other words, the equation assumes constant values for respiratory system compliance and resistance during ventilation. If compliance changes during inspiration due to tidal recruitment, the method cannot yield accurate results. These relations were derived from the equation of motion (Eq. 1).
where Ppeak is the peak airway pressure, DP is the elastic pressure or driving pressure that overcomes the elastic recoil of alveoli, PR is the resistive pressure that overcomes airway resistance, and PEEP is the positive end-expiratory pressure.
The geometrical method has been used in the experimental animal model of VILI using Ptp by Cressoni et al [60]. In their study, both controlled and assisted ventilation regimens were used. Unit conversion to J/min was achieved by multiplying the area under the curve by a conversion constant of 0.098 and the respiration frequency (RR). In their experiment, various combinations of MV settings were tested. MP above approximately 12 J/min (MP applied to the lung expressed using Ptp) led to the development of whole lung edema. The authors reported a significant relationship between ME and lung elastance (r2=0.33, P<0.01) as well as a negative relationship between PaO2/FIO2 (Partial Pressure of Oxygen/Fraction of Inspired Oxygen) (r2=0.40, P<0.001).
ME IN VOLUME-CONTROLLED VENTILATION:
The original equation for ME in volume-controlled ventilation (Eq. (2), derived by Gatinnoni et al, implements Vt, RR, resistance, elastance, and inspiration time [16].
Where RR is the respiratory rate, ELrs is the elastance of the respiratory system, Vt is the tidal volume, PEEP is the positive end-expiratory pressure, and I: E is the inspiratory-to-expiratory ratio.
To acquire the numerical values, it is necessary to perform an inspiration-hold maneuver. The equation takes into account both the flow resistance and the elasticity of the respiratory system, but it assumes a linear change in compliance during a breath (in a given range of pressures/volumes). Constant resistance and inspiration flow were also assumed during inspiration. Overall, this formula leads to an underestimation of ME compared to the geometrical method (albeit clinically negligible, below 10%) [61]. Moreover, the equation complexity limits its bedside use.
Wu et al compared the Gattinoni’s equation (Eq. 2) with MP measured by integration of the areas within the pressure–volume loops [62] in 25 invasively ventilated patients, arguing that Gattinoni’s equation is influenced by PEEP (which theoretically does not contribute to MP) and, therefore, is not accurate. The correlation btween MP obtained using their method and Eq 2 was relatively low, with R2 of 0.75 and 0.66 at PEEP values of 5 and 10 cmH2O, respectively. Hence, the authors concluded that PEEP does not produce net displacement or contribute to MP, which is in opposition to many authors [16,63]. Their conclusion is, however, questionable – if excluding PEEP from their geometrical method, it is logical that the correlation with an equation agreeing well with the geometrical method that includes PEEP will be low.
The same team who derived the Eq. (2) simplified it and derived Eq. (3) using Ppeak (peak inspiratory pressure, maximum end-expiratory pressure), Pplat (plateau pressure), Vt, RR, and PEEP.
where RR is the respiratory rate, Vt is the tidal volume, Ppeak is the maximum inspiration pressure, PEEP is the positive end-expiratory pressure and Pplat is plateau pressure.
The derived equation and geometrical method correlated well, with the bias between the geometric method and Eq. 3 being 0.053 (−0.81; 0.77) J/min and −0.4 (−1.50; 0.70) J/min at the inspiration flows of 30 L/min and 60 L/min, respectively (r2=0.96 and 0.97, P<0.01) [64]. At higher flows, however, the tissue resistance should be considered in addition to the physiological increase in the respiratory tract resistance. Still, it is necessary to keep in mind that if the high flow is caused by increased resistance of the endotracheal tube, the increase in ME is used to overcome the endotracheal tube resistance and, in effect, does not affect the lung and the incidence of VILI, respectively. On the other hand, impairment of lung mechanics leads to an increase in ME, which definitely contributes to VILI development [8].
Essentially the same equation (Eq. 4; originally without a constant 0.098 for correction to J/min) was proposed by Louis et al [61]
where RR is the respiratory rate, Vt is the tidal volume, Ppeak is the maximum inspiration pressure, Pplat is the plateau pressure, and PEEP is the positive end-expiratory pressure.
The geometrical method and both the original and simplified equations were compared in supinated and pronated patients with ARDS by Louis et al [61]. A good correlation was demonstrated with the absolute value of difference not exceeding 8.3%, which corresponds to a difference of approximately 2 J/min.
Giosa et al proposed another simplified equation not necessitating the inspiration-hold maneuver (Eq. 5) [65]. This equation is more suitable for bedside use; unfortunately, this comes at the cost of a decrease in calculation accuracy. Resistance in the equation is replaced by a constant value of 10 cmH2O/L/s, which well-matches most intubated patients. However, the more the actual resistance exceeds the value of 10, the more inaccurate the calculation will be. The equation underestimates ME in higher inspiration flows, where the respiratory tract resistance can be significantly increased by the occurrence of turbulent flow. Nevertheless, the calculated error is clinically negligible up to a value of 15 cmH2O/L/s, and the mean difference between the 2 methods of calculation at resistances of 20 cmH2O/L/s is approx.1.3 J/min [65]. The deviation associated with the use of Equation 5 from the geometrical method, ie, −0.0074 (−0.93;0.91) and −1.0 (−2.52;0.45) J/min for flows of 30 and 60 l/min, respectively, was acceptable [64].
where Vt is the tidal volume, Ppeak is the maximum inspiration pressure, PEEP is the positive end-expiratory pressure, and F is the inspiratory flow.
All equations shown above assume volume-controlled ventilation, absence of spontaneous breathing, and constant inspiration flow. Also, the respiratory system was considered homogeneous, but in clinical practice, lungs are usually heterogeneous due to lung inhomogeneities (especially in ARDS patients) [66], which means that even in the same patient, the same ME could be safe or damaging in different lung regions. However, despite the described simplifications, the equations correlate well with the geometrical method, the maximum calculation difference being 2 J/min. From this perspective, it would be logical to use methods of calculation not necessitating the inspiration-hold maneuver in clinical practice (Eq. 5).
Aşar et al describe a method using the Work of Breathing ventilator (WOBv) parameter [67] measured by some ventilators. WOBv is the amount of energy consumed to ventilate 1 liter of gas, and the unit is expressed as J/L. By multiplying WOBv with RR, we get work per unit of time (i.e., the power) [68]. Taking PEEP into account, Aşar et al derived an equation of dynamical mechanical power (MPdyn) (Eq. 6).
where MVe is the expiratory minute volume.
The authors compared this equation with that formulated by Gattinonis (Eq. 2) in 40 adult ARDS patients with volume-controlled ventilation and deep sedation. Both equations correlated well with R2 ≥0.98.
Chi et al proposed a simple formula including mean airway pressure (Pmean) [69] and tested it in the volume-controlled ventilation mode with a constant inspiratory flow and without inspiration-hold maneuver (Eq. 7).
where MV is the minute ventilation in L/min, Pmean is the mean airway pressure, PEEP is the positive end-expiratory pressure, and E: I the expiratory: inspiratory ratio
The formula was also validated against that by Gatitinonis (Eq. 2) in 50 ARDS and 50 non-ARDS patients using Tplat settings of 0 and 0.5 s. At both Tplat levels, the Pmean-derived MP correlated very well with the reference MP both in patients with or without ARDS. The equation also implies that providing that MV, PEEP and I: E (the inspiratory: expiratory ratio) settings are stable, the Pmean alone could reflect the dynamic changes of MP, which could be easily monitored by clinicians. The same authors suggest in their retrospective analysis that patients with elevated Pmean during the first 24 h of mechanical ventilation had worse outcomes, even if a low tidal volume strategy was applied [70]. Pmean was recognized as an independent risk factor for poor outcomes, with an odds ratio of 1.35 (95% CI 1.29–1.42).
ME IN PRESSURE-CONTROLLED VENTILATION:
In pressure-controlled ventilation, where the pressure in the respiratory tract remains constant and the flow decreases during ventilation (which is also associated with the decreased airway resistance and non-linear pressure–volume relationship), the Van Der Meijden equation can be used (Eq. 8) [71,72]. Besides Vt and RR, this equation also takes into account the respiratory system compliance and resistance, PEEP, DP, and Tinsp (inspiration time).
where RR is the respiratory rate, Vt is the tidal volume, PEEP is the end-expiratory pressure, DP is the driving pressure, Tinsp is the inspiration time, R is the resistance, and C is the compliance.
Compliance and resistance calculations are based on volume-controlled ventilation with a constant inspiratory flow of 30 L/min during the end-expiratory and end-inspiratory pauses. This equation assumes constant airway resistance during inspiration. However, the airway resistance is a function of the airflow and changes during inspiration, which reduces the accuracy of results obtained using this equation. However, the correlation of these results with the geometrical method was very good (mean difference of −0.001 J/min, with the upper and lower limits of agreement being 2.05 and −2.05 J/min, respectively) [64]. A simplified version of the same equation was proposed by Becher [73] (Eq. (9)):
where RR is the respiratory rate, Vt is the tidal volume, PEEP is the end-expiratory pressure, and DP is the driving pressure.
This simplification is based on the assumption that the pressure wave is ideally squared. Despite this significant simplification, this equation still correlated well with the geometrical method (r2=0.94, p<0.001) with a bias of −0.81 J/min, and with the upper and lower limits of agreement of 2.11 and −0.48 [64]. When compared with the original equation by Van Der Meijden, it performed well, with an even narrower confidence interval. Therefore, Chiumello et al concluded that the simplified version (Eq. 9) seems to better fit the need for bedside ME calculations than Eq. 8, even in terms of precision [64]. On the other hand, Van Der Meijden claimed that the Becher’s equation (Eq. 9) was less accurate than the original Equation 8 (limits of agreement 8.6% vs 13%) [71].
If the inspiratory pressure rise time is considered, the equation becomes more complex (Eq. (10)) [73]:
where RR is the respiratory rate, Vt is the tidal volume, PEEP is the end-expiratory pressure, DP is the driving pressure, R is the resistance, C is the compliance, and Tslope is the inspiration rise time.
Becher further compared the geometric method with the simplified equation (Eq. 9) they previously proposed and with the equation considering Tslope (Eq. 10) in a group of 42 patients [73]. The ME determined by the geometrical method was highly correlated with the results of both equations (Eq. 9 and 10). These results have shown that in bedside practice, Tslope is not as crucial and can be neglected.
Guérin et al described the influence of DP on patient mortality in a retrospective analysis of patients with ARDS in 2 RCT trials – the Acurasys [74] and Proseva [75] studies [76]. Patient mortality was lower if the ME during the first day of ventilation was ≤12 J/min, although the difference was not reported, likely not being too high (although it was statistically significant). However, they only evaluated the effect of ME on the first day of ventilation and not over the entire course of the treatment, which can be considered a limitation of that study. Moreover, they calculated ME using a greatly simplified equation (Eq. 11), which mainly considers the DP effect on ME.
where RR is the respiratory rate, Vt is the tidal volume, and DP is the driving pressure. This is essentially the same equation as Eq. (9), but does not consider PEEP. As PEEP in their study was not significantly different between the groups, this may be fine for the findings of their study; however, taking this into consideration is important when discussing the threshold of 12 J/min, as including the mean PEEP found in their study into the equation and calculating ME according to the Eq. (9), the applied ME would almost double.
ME IN FLOW-CONTROLLED VENTILATION (FCV):
Flow-controlled ventilation is a ventilatory mode where both inspiratory and expiratory flow rates are maintained at a constant level by regulating tracheal pressure, whereas tidal volume and respiratory rate vary depending on ventilator settings [77]. In a study with 10 ARDS patients, Grasseto et al reported reduced MP in FCV compared to volume-controlled ventilation as a consequence of lower inspiratory flow rates and breathing frequencies, which potentially reduces the dissipated energy [78]. However, they did not specify the method of MP measurement.
ME IN PRESSURE-SUPPORT VENTILATION (PSV):
All the abovementioned algorithms assume fully controlled ventilation without any spontaneous ventilation effort by the patient; therefore, these methods of mechanical energy determination can only be applied to patients who are sedated or even are on myorelaxation. Calculations of ME are more complicated in the case of PSV because the patient’s lung is stretched not only by the ventilator-generated pressure but also by the patient’s spontaneous effort. The pressure inflating the lung, therefore, is the sum of pressures generated by the ventilator and by the patient. It is impossible to easily measure the overall pressure and lung compliance, which necessitates the use of an esophageal catheter for Ptp measurement. If Ptp is not used and ME is calculated only on the basis of the ventilator-provided pressure values, ME could be significantly underestimated. To acquire the correct ME, the Ptp must be known and the calculation must be performed using the geometrical method. Spontaneously ventilating patients can be subjected to excessive ME and Ptp, putting them at risk of lung damage [79]. This phenomenon is called self-inflicted lung injury (SILI) and can influence treatment outcomes [80].
ME Thresholds Increasing VILI Probability
Cut-off values indicating an increased risk of VILI development have been proposed, but evidence for their clinical relevance is not strong and more human studies are needed to confirm these values. Moreover, much of the available data originate from animal models. The clinical relevance of such data to real-world ARDS patients is questionable, as these studies used healthy animals ventilated extremely aggressively. It is also important to distinguish Ptp-calculated ME (mechanical energy of the lung, MElung) from ME calculated for the whole respiratory system (MErs, ie, including lungs, airways, and thoracic wall).
Saffaran et al investigated the effect of multiple ventilation settings on reducing ME and DP, aiming to determine whether it is possible to minimize DP and ME while preserving gas exchange and respiratory rate [81]. They succeeded in reducing ME and DP in 66% and 23% of adults, respectively. Kassis et al applied predictive modeling to estimate the effects of modifying ventilator parameters on DP and MP in 2622 ARDS patients from the Medical Information Mart for Intensive Care IV database [82]. Reducing VT led to a decrease in DP and MP, with a more pronounced effect on MP in patients with lower lung compliance. On the other hand, strategies reducing RR consistently increased MP. This is not surprising when looking at Eq. (2), as RR is associated with MP with a growth factor of 1.4, but VT needed to be adjusted to maintain the minute ventilation, with a growth factor of 2. Rietveld et al investigated MP in volume-controlled and pressure-controlled ventilation with different pause times (Tplat) in 46 patients, finding that volume-controlled ventilation without pauses yielded the lowest MP [83]. Romitti et al ventilated healthy pigs for 48 h at MP of 3, 7, or 12 J/min [84]. Mechanical ventilation increased the lung weight, and worsened the lung histology, regardless of the ME. ME measurements using various ventilation settings were tested on healthy pigs by Cressoni et al using a geometrical method and Ptp [60]. For 54 h, healthy piglets were ventilated with identical constant high transpulmonary pressure and tidal volume, but the respiratory rate was set differently in individual groups. Ventilator setups with MElung greater than 12 J/min lead to VILI development (based on computed tomography scans). To further confirm this finding, the authors applied a low tidal volume with a high respiratory rate to reach a power greater than 12 J/min. This led to VILI development in all piglets in this group. Further, the influence of the inspiratory flow was investigated by adjusting the inspiratory-to-expiratory ratio. A higher flow was associated with VILI, which may be explained by the fact that under the same tidal volume, a shorter inspiratory time means a higher airway pressure, corresponding to a higher ME [8].
MP thresholds for VILI were tested in the aforementioned study by Guérin et al [76], whose threshold of 12 J/min, however, did not consider PEEP. Besides, only the initial MP on day 1 was considered and although the difference was significant, the degree of association (hazard ratio) between the groups with initial MP below and above that threshold was not reported.
Another retrospective observational study with a large cohort by Serpa et al showed an increase in the risk of death with MErs greater than 17 J/min [37]. Santer et al investigated ME during general anesthesia in a retrospective study of 230 767 elective, noncardiac adult surgical out- and inpatients whose MP was monitored [85]. The median intraoperative MP was 6.63 (interquartile range of 4.62 to 9.11) J/min and MP was higher in patients with postoperative respiratory failure compared to those without failure (adjusted odds ratio of 1.31 per 5 J/min increase; 95% CI, 1.21 to 1.42;
VILI itself is caused by the energy delivered to the lung parenchyma (MElung). However, this is only a portion of the total MErs and to be able to extract the pulmonary part of ME only, Ptp must be monitored. The relationship between MErs and MElung is usually linear, and separate thresholds for VILI incidence were established for MElung and MErs by Cressoni in an animal model [86]. These data cannot, however, be directly used in patients, and further investigation is needed.
Dianti et al described the effects of multiple parameters, including MP, on mortality in 9 trials comprising 4731 subjects, concluding that increased VT, DP, and MP were associated with increased mortality [87]. In a prospective study on 51 adults with moderate-to-severe ARDS, Haudebourg et al described the effect of a DP-guided (DP between 12 and 14 cm H2O) versus PBW (predicted body weight)-guided (6 ml/kg PBW) ventilation on MP [88]. DP-guided ventilation led to an increase of Vt from 6.1 mL/kg PBW [5.9–6.2] to 7.7 ml/kg PBW [6.2–8.7], and to a significant decrease in MP from 31.5 J/min [28–35.7] to 28.8 J/min [24.6–32.6] (
Although ME itself can provide valuable insight into the risk of VILI development, it cannot be perceived as an isolated parameter [89–91]. Rather, it should be understood as a complex sum of many factors, the mutual relationships of which need to be taken into account. For example, reducing PEEP in an effort to decrease ME could lead to lung collapse, which would impair lung compliance and, in effect, a higher DP would be needed to achieve the same Vt. This could, in effect, lead to the exact opposite result than the original intention, ie, an increase in ME. It is, therefore, reasonable to increase PEEP and titrate ventilatory settings to achieve the best combination of given parameters providing the best trade-off between satisfactory gas exchange and safe ME.
The ME concept has also other pitfalls and limitations [90]. The assumption of a linear relationship between ME and PEEP used in most equations could be mathematically incorrect. MP, as defined today, relates to the inspiratory phase only, although the expiratory phase may also play a role in the development of VILI [92].
ME Effects on Lung Parenchyma
It is important to note that the negative influence is only exerted by the portion of ME affecting lung parenchyma, while the portion affecting the endotracheal tube does not influence VILI development. Preexisting lung parenchyma condition is another important factor in the risk for VILI occurrence. Lungs with strong heterogeneous impairment or a small area that can be ventilated will, upon absorption of ME, suffer greater damage than a homogeneous lung with normal physiological volume [66]. Lung injury depends on the mass of the lung absorbing ME [40] and on the energy “intensity” affecting the ventilated lung area [93]. It must, therefore, be considered that high Vt in itself is not necessarily injurious and the ME concept could help distinguish hazardous ventilation from safe ventilation even at high VT [94]. This might explain why the outcomes of patients without ARDS (ie, those with a high functional residual capacity and high total lung volume) ventilated with high Vt (10 mL/kg) were not worse than those in patients ventilated with 6 mL/kg [95].
Future Directions
The ME concept summarizes the effects of multiple parameters on the total load on the lung parenchyma by the ventilator. So far, no high-quality RCTs have tested ME as a key parameter for VILI development or mortality rate. In the future, such a study is needed so that ME can be taken as a strong parameter for clinical practice. When using ME in clinical practice, one should be aware of limitations of the particular equation used and consider it in the implications for treatment. ME could be used to evaluate the overall effect of mechanical ventilation and to help set the ventilator parameters to reduce the risk of VILI. For this purpose, physicians should aim to ensure sufficient gas exchange with the lowest possible ME. The absolute numerical value of ME alone, however, may not produce sufficiently precise information to always guide safe ventilatory practice, and patient-specific parameters should be also taken into account [96]. If the usefulness of the ME concept is supported by sufficiently strong data, ME could be calculated using, eg, mobile apps; even better, it could be easily incorporated into the software of mechanical ventilators.
Conclusions
Simplified equations show promise and could be suitable for use in pressure-controlled and volume-controlled ventilation settings. The decrease in calculation accuracy caused by such simplification was shown to be low enough not to limit bedside use in everyday practice, as it does not outweigh the practicability of being easily able to calculate ME at the bedside using a mobile phone or handheld calculator [61,97]. For volume-controlled ventilation, the equation by Giosa et al (Eq. 5) could be used with good accuracy while avoiding the necessity of the inspiration-hold maneuver. For pressure-controlled ventilation, the very simple equation by Becher et al (Eq. 9) could be used with satisfactory accuracy. The equations assume fully controlled ventilation without spontaneity. Continual ME measurement by a dedicated calculator or device that could use a more complex equation (or calculate ME according to the geometric method in real time) would possibly yield even better results and has been even implemented in some ventilators [98]. However, the degree of clinical improvement when using continuous monitoring and the clinical relevance of using such a more complex method have yet to be established.
References
1. Grasselli G, Calfee CS, Camporota L, ESICM guidelines on acute respiratory distress syndrome: definition, phenotyping and respiratory support strategies: Intensive Care Med, 2023; 49(7); 727-59
2. Slutsky AS, Ranieri VM, Ventilator-induced lung injury: N Engl J Med, 2013; 369(22); 2126-36
3. Brower RG, Matthay MA, Morris A, Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome: N Engl J Med, 2000; 342(18); 1301-8
4. Amato MBP, Meade MO, Slutsky AS, Driving pressure and survival in the acute respiratory distress syndrome: N Engl J Med, 2015; 372(8); 747-55
5. Lumb PD, Wright LD: Ventilator management Wylie and Churchill-Davidsons: A practice of anesthesia; 1079-90 Published online January 1, 2023
6. Hotchkiss JR, Blanch L, Murias G, Effects of decreased respiratory frequency on ventilator-induced lung injury: Am J Respir Crit Care Med, 2000; 161(2 Pt 1); 463-68
7. Nascimento Baez Garcia CS, Abreu SC, Lassance Soares RM, Pulmonary morphofunctional effects of mechanical ventilation with high inspiratory air flow: Crit Care Med, 2008; 36(1); 232-39
8. Protti A, Maraffi T, Milesi M, Role of strain rate in the pathogenesis of ventilator-induced lung edema: Crit Care Med, 2016; 44(9); e838-e45
9. Spieth PM, Silva PL, Garcia CSNB, Modulation of stress versus time product during mechanical ventilation influences inflammation as well as alveolar epithelial and endothelial response in rats: Anesthesiology, 2015; 122(1); 106-16
10. Gattinoni L, Marini JJ, Collino F, The future of mechanical ventilation: Lessons from the present and the past: Crit Care, 2017; 21(1); 183
11. Peters RM, The energy cost (work) of breathing: Ann Thorac Surg, 1969; 7(1); 51-67
12. Paudel R, Trinkle CA, Waters CM, Mechanical power: A new concept in mechanical ventilation HHS public access: Am J Med Sci, 2021; 362(6); 537-45
13. Cabello B, Mancebo J, Cabello B, Work of breathing: Intensive Care Med, 2006; 32; 1311-14
14. Belliato M, Epis F, Cremascoli L, Mechanical power during veno-venous extracorporeal membrane oxygenation initiation: A pilot-study: Membranes (Basel), 2021; 11(1); 1-11
15. Hoppe K, Khan E, Meybohm P, Riese T, Mechanical power of ventilation and driving pressure: two undervalued parameters for pre extracorporeal membrane oxygenation ventilation and during daily management?: Crit Care, 2023; 27(1); 111
16. Gattinoni L, Tonetti T, Cressoni M, Ventilator-related causes of lung injury: The mechanical power: Intensive Care Med, 2016; 42(10); 1567-75
17. Araos J, Alegria L, Garcia P, Near-apneic ventilation decreases lung injury and fibroproliferation in an acute respiratory distress syndrome model with extracorporeal membrane oxygenation: Am J Respir Crit Care Med, 2019; 199(5); 603-12
18. Xie Y, Wang Y, Liu K, Li X, Correlation analysis between mechanical power, transforming growth factor-β1, and connective tissue growth factor levels in acute respiratory distress syndrome patients and their clinical significance in pulmonary structural remodeling: Medicine, 2019; 98(29); e16531
19. Li HH, Wang CW, Chang CH, Relationship between mechanical ventilation and histological fibrosis in patients with acute respiratory distress syndrome undergoing open lung biopsy: J Pers Med, 2022; 12(3); 474
20. Xie Y, Zheng H, Mou Z, High expression of CXCL10/CXCR3 in ventilator-induced lung injury caused by high mechanical power: Biomed Res Int, 2022; 2022; 6803154
21. Fuller BM, Page D, Stephens RJ, Pulmonary mechanics and mortality in mechanically ventilated patients without acute respiratory distress syndrome: A cohort study: Shock, 2018; 49(3); 311-16
22. Parhar KKS, Zjadewicz K, Soo A, Epidemiology, mechanical power, and 3-year outcomes in acute respiratory distress syndrome patients using standardized screening. An observational cohort study: Ann Am Thorac Soc, 2019; 16(10); 1263-72
23. Bhalla AK, Klein MJ, Modesto I, Alapont V, Mechanical power in pediatric acute respiratory distress syndrome: A PARDIE study: Crit Care, 2022; 26(1); 2
24. Proulx F, Emeriaud G, François T, Oxygenation defects, ventilatory ratio, and mechanical power during severe pediatric acute respiratory distress syndrome: Longitudinal time sequence analyses in a single-center retrospective cohort: Pediatr Crit Care Med, 2022; 23(1); 22-33
25. Wu HP, Chu CM, Chuang LP, The association between mechanical power and mortality in patients with pneumonia using pressure-targeted ventilation: Diagnostics (Basel), 2021; 11(10); 1862
26. Tonna JE, Peltan ID, Brown SM, Positive end-expiratory pressure and respiratory rate modify the association of mechanical power and driving pressure with mortality among patients with acute respiratory distress syndrome: Crit Care Explor, 2021; 3(12); e0583
27. Fariña-González TF, Núñez-Reiz A, Yordanov-Zlatkov V, Hourly analysis of mechanical ventilation parameters in critically ill adult COVID-19 patients: Association with mortality: J Intensive Care Med, 2022; 37(12); 1606-13
28. Chiumello D, Froio S, Mistraletti G, Gas exchange, specific lung elastance and mechanical power in the early and persistent ARDS: J Crit Care, 2020; 55; 42-47
29. Das A, Camporota L, Hardman JG, Bates DG, What links ventilator driving pressure with survival in the acute respiratory distress syndrome? A computational study: Respir Res, 2019; 20(1); 29
30. Tonna JE, Peltan I, Brown SM, Mechanical power and driving pressure as predictors of mortality among patients with ARDS: Intensive Care Med, 2020; 46(10); 1941
31. Urner M, Jüni P, Hansen B, Time-varying intensity of mechanical ventilation and mortality in patients with acute respiratory failure: a registry-based, prospective cohort study: Lancet Respir Med, 2020; 8(9); 905
32. van Meenen DMP, Algera AG, Schuijt MTU, Effect of mechanical power on mortality in invasively ventilated ICU patients without the acute respiratory distress syndrome: An analysis of three randomised clinical trials: Eur J Anaesthesiol, 2023; 40(1); 21-28
33. Chi Y, Zhang Q, Yuan S, Twenty-four-hour mechanical power variation rate is associated with mortality among critically ill patients with acute respiratory failure: A retrospective cohort study: BMC Pulm Med, 2021; 21(1); 331
34. Costa ELV, Slutsky AS, Brochard LJ, Ventilatory variables and mechanical power in patients with acute respiratory distress syndrome: Am J Respir Crit Care Med, 2021; 204; 303-11
35. Sentruk E, Ugur S, Celik Y, The power of mechanical ventilation may predict mortality in critically ill patients: Minerva Anestesiol, 2023; 89(7–8); 663-70
36. Cavalcanti AB, Suzumura ÉA, Laranjeira LN, Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: A randomized clinical trial: JAMA, 2017; 318(14); 1335-45
37. Serpa Neto A, Deliberato RO, Johnson AEW, Mechanical power of ventilation is associated with mortality in critically ill patients: An analysis of patients in two observational cohorts: Intensive Care Med, 2018; 44(11); 1914-22
38. Chiumello D, Carlesso E, Cadringher P, Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome: Am J Respir Crit Care Med, 2008; 178(4); 346-55
39. Azizi BA, Munoz-Acuna R, Suleiman A, Mechanical power and 30-day mortality in mechanically ventilated, critically ill patients with and without Coronavirus Disease-2019: A hospital registry study: J Intensive Care, 2023; 11(1); 14
40. Zhang Z, Zheng B, Liu N, Mechanical power normalized to predicted body weight as a predictor of mortality in patients with acute respiratory distress syndrome: Intensive Care Med, 2019; 45(6); 856-64
41. Silva PL, Ball L, Rocco PRM, Pelosi P, Power to mechanical power to minimize ventilator-induced lung injury?: Intensive Care Med Exp, 2019; 7(Suppl 1); 38
42. Zhu Y, Peng W, Zhen S, Jiang X, Mechanical power normalized to predicted body weight is associated with mortality in critically ill patients: A cohort study: BMC Anesthesiol, 2021; 21(1); 278
43. Marini JJ, Gattinoni L, Rocco PRM, Estimating the damaging power of high-stress ventilation: Respir Care, 2020; 65(7); 1046-52
44. Tawfik PN, Evans MD, Dries DJ, Marini JJ, Reliable estimates of power delivery during mechanical ventilation utilizing easily obtained bedside parameters: Respir Care, 2022; 67(2); 177-83
45. Tonetti T, Cressoni M, Collino F, Volutrauma, atelectrauma, and mechanical power: Crit Care Med, 2017; 45(3); e327-e28
46. Ito Y, Takeuchi M, Inata Y, Normalization to predicted body weight may underestimate mechanical energy in pediatric acute respiratory distress syndrome: Am J Respir Crit Care Med, 2022; 205(11); 1360-63
47. Shelley B, Marczin N, Do we have the “power” to “drive” down the incidence of pulmonary complications after thoracic surgery: Br J Anaesth, 2023; 130(1); e37-e40
48. Kneyber MCJ, Ilia S, Koopman AA, Energy transmission in mechanically ventilated children: A translational study: Crit Care, 2020; 24(1); 1-8
49. Jimenez JV, Munroe E, Weirauch AJ, Electric impedance tomography-guided PEEP titration reduces mechanical power in ARDS: A randomized crossover pilot trial: Crit Care, 2023; 27(1); 21
50. Pelosi P, Ball L, Barbas CSV, Personalized mechanical ventilation in acute respiratory distress syndrome: Crit Care, 2021; 25(1); 1-10
51. Marini JJ, Rocco PRM, Gattinoni L, Static and dynamic contributors to ventilator-induced lung injury in clinical practice. pressure, energy, and power: Am J Respir Crit Care Med, 2020; 201(7); 767-74
52. Neto AS, Nagtzaam L, Schultz MJ, Ventilation with lower tidal volumes for critically ill patients without the acute respiratory distress syndrome: A systematic translational review and meta-analysis: Curr Opin Crit Care, 2014; 20(1); 25-32
53. Umbrello M, Marino A, Chiumello D, Tidal volume in acute respiratory distress syndrome: How best to select it: Ann Transl Med, 2017; 5(14); 287
54. Marini JJ, How i optimize power to avoid VILI: Crit Care, 2019; 23(1); 1-3
55. Xie Y, Cao L, Qian Y, Effect of deep sedation on mechanical power in moderate to severe acute respiratory distress syndrome: A prospective self-control study: Biomed Res Int, 2020; 2020; 2729354
56. Becher T, Adelmeier A, Frerichs I, Adaptive mechanical ventilation with automated minimization of mechanical power-a pilot randomized cross-over study: Crit Care, 2019; 23(1); 338
57. Gattinoni L, Quintel M, How ARDS should be treated: Crit Care, 2016; 20(1); 86
58. Chi Y, He HW, Long Y, Progress of mechanical power in the Intensive Care Unit: Chin Med J (Engl), 2020; 133(18); 2197-204
59. Marini JJ, Rodriguez RM, Lamb V, Bedside estimation of the inspiratory work of breathing during mechanical ventilation: Chest, 1986; 89(1); 56-63
60. Cressoni M, Gotti M, Chiurazzi C, Mechanical power and development of ventilator-induced lung injury: Anesthesiology, 2016; 124(5); 1100-8
61. Louis B, Guérin C, Comparison of geometric and algebraic methods to determine mechanical power in patients with acute respiratory distress syndrome: Intensive Care Med, 2019; 45(5); 738-40
62. Wu SH, Kor CT, Mao IC, Accuracy of calculating mechanical power of ventilation by one commonly used equation: J Clin Monit Comput, 2022; 36(6); 1753-59
63. Collino F, Rapetti F, Vasques F, Positive end-expiratory pressure and mechanical power: Anesthesiology, 2019; 130(1); 119-30
64. Chiumello D, Gotti M, Guanziroli M, Bedside calculation of mechanical power during volume-and pressure-controlled mechanical ventilation: Crit Care, 2020; 24(1); 417
65. Giosa L, Busana M, Pasticci I, Mechanical power at a glance: A simple surrogate for volume-controlled ventilation: Intensive Care Med Exp, 2019; 7(1); 61
66. Gattinoni L, Tonetti T, Quintel M, Regional physiology of ARDS: Crit Care, 2017; 21(3); 9-14
67. Aşar S, Acicbe Ö, Çukurova Z, Bedside dynamic calculation of mechanical power: A validation study: J Crit Care, 2020; 56; 167-70
68. Cabello B, Mancebo J, Work of breathing: Intensive Care Med, 2006; 32(9); 1311-14
69. Chi Y, He H, Long Y, A simple method of mechanical power calculation: Using mean airway pressure to replace plateau pressure: J Clin Monit Comput, 2021; 35(5); 1139-47
70. Long Y, Su L, Zhang Q, Elevated mean airway pressure and central venous pressure in the first day of mechanical ventilation indicated poor outcome: Crit Care Med, 2017; 45(5); e485-e92
71. van der Meijden S, Molenaar M, Somhorst P, Schoe A, Calculating mechanical power for pressure-controlled ventilation: Intensive Care Med, 2019; 45; 1495-97
72. Aşar S, Acicbe O, Sabaz MS, Simplified calculation of mechanical power for pressure controlled ventilation in COVID-19 ARDS patients: Minerva Anestesiol, 2022; 88(1–2); 42-50
73. Becher T, van der Staay M, Schädler D, Calculation of mechanical power for pressure-controlled ventilation: Intensive Care Med, 2019; 45; 1321-23
74. Papazian L, Forel JM, Gacouin A, Neuromuscular blockers in early acute respiratory distress syndrome: N Engl J Med, 2010; 363(12); 1107-16
75. Guérin C, Reignier J, Richard JC, Prone positioning in severe acute respiratory distress syndrome: N Engl J Med, 2013; 23(6); 2159-68
76. Guérin C, Papazian L, Reignier J, Effect of driving pressure on mortality in ARDS patients during lung protective mechanical ventilation in two randomized controlled trials: Crit Care, 2016; 20(1); 384
77. Barnes T, van Asseldonk D, Enk D, Minimisation of dissipated energy in the airways during mechanical ventilation by using constant inspiratory and expiratory flows – flow-controlled ventilation (FCV): Med Hypotheses, 2018; 121; 167-76
78. Grassetto A, Pettenuzzo T, Badii F, Flow-controlled ventilation may reduce mechanical power and increase ventilatory efficiency in severe coronavirus disease-19 acute respiratory distress syndrome: Pulmonology, 2023; 29(2); 154-56
79. Gattinoni L, Giosa L, Bonifazi M, Targeting transpulmonary pressure to prevent ventilator-induced lung injury: Expert Rev Respir Med, 2019; 13(8); 73-46
80. Carteaux G, Parfait M, Combet M, Patient-self inflicted lung injury: A practical review: J Clin Med, 2021; 10(12); 2738
81. Saffaran S, Das A, Laffey JG, Utility of driving pressure and mechanical power to guide protective ventilator settings in two cohorts of adult and pediatric patients with acute respiratory distress syndrome: A computational investigation: Crit Care Med, 2020; 48(7); 1001-8
82. Baedorf Kassis EN, Hu S, Lu M, Titration of ventilator settings to target driving pressure and mechanical power: Respir Care, 2022; 68(2); 199-207
83. Rietveld PJ, Snoep JWM, Lamping M, Mechanical power differs between pressure-controlled ventilation and different volume-controlled ventilation modes: Crit Care Explor, 2022; 4(8); e0741
84. Romitti F, Busana M, Palumbo MM, Mechanical power thresholds during mechanical ventilation: An experimental study: Physiol Rep, 2022; 10(6); e15225
85. Santer P, Wachtendorf LJ, Suleiman A, Mechanical power during general anesthesia and postoperative respiratory failure: A multicenter retrospective cohort study: Anesthesiology, 2022; 137(1); 41-54
86. Cressoni M, Gotti M, Chiurazzi C, Supplemental digital content 1 mechanical power and the development of ventilator-induced lung injury: Anesthesiology, 2016; 124(5); 1100-8
87. Dianti J, Matelski J, Tisminetzky M, Comparing the effects of tidal volume, driving pressure, and mechanical power on mortality in trials of lung-protective mechanical ventilation: Respir Care, 2021; 66(2); 221-27
88. Haudebourg AF, Tuffet S, Perier F, Driving pressure-guided ventilation decreases the mechanical power compared to predicted body weight-guided ventilation in the Acute Respiratory Distress Syndrome: Crit Care, 2022; 26(1); 1-9
89. Gama De Abreu M, Sessler DI, Mechanical power: Correlate or cause of ventilator-induced lung injury?: Anesthesiology, 2022; 137(1); 6-8
90. Huhle R, Neto AS, Schultz MJ, deAbreu MG, Is mechanical power the final word on ventilator-induced lung injury?-no: Ann Transl Med, 2018; 6(19); 394
91. Tonetti T, Vasques F, Rapetti F, Driving pressure and mechanical power: New targets for VILI prevention: Ann Transl Med, 2017; 5(14); 286
92. Busana M, Zinnato C, Romitti F, Energy dissipation during expiration and ventilator-induced lung injury: An experimental animal study: J Appl Physiol, 2022; 133(5); 1212-19
93. Vasques F, Duscio E, Pasticci I, Is the mechanical power the final word on ventilator-induced lung injury? – we are not sure: Ann Transl Med, 2018; 6(19); 395
94. Nieman GF, Satalin J, Andrews P, Lung stress, strain, and energy load: Engineering concepts to understand the mechanism of ventilator-induced lung injury (VILI): Intensive Care Med Exp, 2016; 4(1); 16
95. Simonis FD, Serpa Neto A, Binnekade JM, Effect of a low vs intermediate tidal volume strategy on ventilator-free days in Intensive Care Unit Patients without ARDS: A randomized clinical trial: JAMA, 2018; 320(18); 1872-80
96. Marini JJ, Thornton LT, Rocco PRM, Practical assessment of risk of VILI from ventilating power: A conceptual model: Crit Care, 2023; 27(1); 157
97. Senzi A, Bindi M, Cappellini I, COVID-19 and VILI: Developing a mobile app for measurement of mechanical power at a glance: Intensive Care Med Exp, 2021; 9(1); 6
98. Chiumello D, Coppola S, Formenti P, A validation study of a continuous automatic measurement of the mechanical power in ARDS patients: J Crit Care, 2022; 67; 21-25
In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952