27 September 2023: Clinical Research
Audiometric Outcomes of Ventilation Drainage Treatment for Otitis Media with Effusion in Children: Implications for Speech Development and Hearing Loss
Kornela Partycka-PietrzykDOI: 10.12659/MSM.941350
Med Sci Monit 2023; 29:e941350
Abstract
BACKGROUND: Otitis media with effusion is the most commonly recognized condition in childhood. Chronic otitis media with accompanying hearing loss is particularly unfavorable in the first years of the child’s life because it can not only permanently damage the structure of the middle ear, but also adversely affect speech development and intellectual abilities in the child.
MATERIAL AND METHODS: This study, from a single center in Poland, included 201 children (372 ears) requiring surgical treatment due to otitis media with effusion. The condition was diagnosed by an ear, nose, and throat specialist, and each patient had a hearing test performed. The control group consisted of 21 patients (42 ears) with negative outcomes following an audiological interview.
RESULTS: Among all of the patients enrolled in the study, a normal tympanometry result was found in 60.6% of ears, and otoemission occurred in 63.3% of ears. The average hearing threshold in the study group was 22.01 Hz in the 500 Hz frequency range, while they were 16.76 Hz, 12.72 kHz, and 14.78 kHz for the corresponding 1 kHz, 2 kHz, and 4 kHz ranges, respectively.
CONCLUSIONS: Ventilation drainage is an effective treatment for otitis media with effusion. The presence of genetic disease has the greatest impact on the course of otitis media. These patients most often require reinsertion of a ventilation tube.
Keywords: Hearing Loss, Middle Ear Ventilation, Otitis Media with Effusion, Humans, Child, Speech, Otitis Media, Deafness, Drainage
Background
Otitis media with effusion (OME; Latin o
This is the most common condition in children that requires medical health care in the USA and in the majority of developed or developing countries [2]. Incidence rates of OME are variously reported in world statistics, depending on the country of origin, age group, diagnostic criteria adopted, and the instrumental techniques used for helping to make diagnoses [3–7]. One study has shown that at least 80% of children suffer from OME before the age of 4 years [8]. It is also recognized that OME occurs more frequently in the winter months than in summer. A study by Fiellau-Nikolajsen, however, reported that the actual course of OME is independent of the seasons [9]. OME is characterized by chronic fluid retention within the middle ear cavity without any symptoms of acute infection, such as earache, fever, or a flushed eardrum [10].
Nevertheless, this condition may arise as a direct consequence of such infection. Rates for diagnosing exudate following infection are 45% after 1 month and about 10% after 3 months in patients who have experienced an episode of acute otitis media [11].
After an acute otitis media episode in children, fluid builds up in the middle ear, inhibiting vibration of the tympanic membrane and subsequent transmission of sound into the inner ear. With this deficit, children may have a decreased ability to communicate in noisy environments. The child may show signs of inattention or decreased academic performance [12]. This disease resolves spontaneously within 3 months in 20–56% of cases [13], but recurs in 30–40% of children, and can last a year or more in 5–10% of such cases [5,7]. Because of the increasing OME rates, a systematic increase has been observed in the number of procedures performed using ventilation drainage of the tympanic cavity. It actually became the most frequently performed 1-day surgical procedure in 2006 for children under 15 years of age in the United States, at 667 000 cases per year [14].
The pathogenesis of OME is multifactorial and most likely arises from an interaction between both endogenous and exogenous factors. In patients with large adenoids, the adenoids can obstruct the Eustachian tube, resulting in a poorly ventilated middle ear. This type of blockage may result in OME. Because the adenoids are a lymphatic structure, it is possible that they can transmit bacteria into the Eustachian tube and allow the growth of biofilms. Such bacterial growth can cause inflammation that can lead to blockage and fluid build-up within the middle ear [12].
Its diagnosis is based upon a clinical interview and an ear, nose & throat (ENT) examination, with particular emphasis on otoscopy or video-otoscopy, along with additional investigations, such as impedance audiometry and tonal audiometry. Symptoms are discreet and easily overlooked at first, but only increase over time, meaning that in about 40–50% of cases the disease may go undiagnosed for quite some time [15]. A delayed speech development may arise as a consequence of persistent hearing loss during the first years of life. Also observed are worsening relationships with peers, attention deficit disorder, irritability, anxiety, and poorer academic performance in pre-school and school-age children. The hearing loss gives the misleading appearance that the child is disobedient and hyperactive, leading to it initially going unnoticed by the child’s surrounding social environment/milieu. For children in non-deaf families and communities, normal hearing is one of the basic conditions for balanced intellectual development and for normal contacts made by a child with his or her social milieu. Suffering from OME thereby also makes this condition a social problem [5,16–21]. The temporary alteration in the peripheral auditory system due to otitis media changes the quality of sound perception once the acoustic signal is observed in incomplete form [22].
Both individual and environmental factors influence the development of OME. The most commonly cited personal factors in the literature include anatomical and functional dysfunction of the auditory trumpet, malformations of the facial cranium, recurrent upper respiratory tract infections, hypertrophy of lymphatic tissue of Waldeyer’s ring, immunological disorders, allergy, gastric reflux disease, male gender, low birth weight <2500 g, prematurity, first episode of acute otitis media below 6 months of age, other conditions causing obstruction of the oropharyngeal inlet (nasal septum deviation, polyps, allergic edema of the nasal mucosa, nasopharyngeal tumors), horizontal breastfeeding position, artificial feeding, and the use of pacifiers. Environmental factors, on the other hand, include active and passive exposure to tobacco smoke by the child in organized centers of care (nurseries, kindergartens), poor socio-economic conditions of the family, and autumn and winter seasons [23].
For children with OME who suffer from hearing loss, the insertion of ventilation tubes helps the middle ear fluid to drain, aerates the middle ear, and balances the pressures on each side of the tympanic membrane, allowing for normal mobility and conduction of sound and thus improving the child’s ability to hear. The improvement in hearing is immediate in the majority of cases but occasionally complete resolution takes days to weeks. Ventilation tubes usually remain working within the tympanic membrane for 12 months on average, and usually spontaneously extrude with healing of the tympanic membrane. Following this, the child may remain free from OME; however, in a subset of children, OME can return and persist, requiring repeat insertion. Factors that can limit the effectiveness of ventilation tubes include blockage of the tube (with blood), difficulty or inability to place the tubes due to narrow ear canals (Down syndrome and cleft palate), and early extrusion [24].
Grommets are tiny plastic tubes that are inserted in the tympanic membrane (eardrum) by an ENT surgeon; in children this usually happens under general anesthesia as a day-case procedure. An operating microscope or other magnification is used to visualize the tympanic membrane where a small incision is made (myringotomy), middle ear fluid is aspirated (subject to need and surgical preference), and the grommet is placed in the incision. Grommets facilitate middle ear ventilation and provide a route for drainage of middle ear fluid; they reverse and prevent the formation of middle ear effusions by providing a surrogate to the under-functioning Eustachian tube and so create a less favorable environment for viruses and bacteria to cause recurrent middle ear infections [25,26].
The aim of the present study was to evaluate the effectiveness of surgical treatment of OME in children who receive ventilation drainage. The effectiveness of the treatment was determined by otoscopic examination and hearing tests.
Material and Methods
CONSENT OF THE BIOETHICS COMMITTEE:
The study was approved by the Bioethics Committee of the Medical University of Lublin – KE-0254/7/2013. Informed and written consent was obtained from the parents/legal guardians of the participants to participate in this study. They were informed of the possibility of discontinuing participation in the study at any time during the study.
STUDY GROUP PROFILE:
This was a retrospective study from a single center in Poland of 201 children (372 ears) requiring surgical treatment due to OME using ventilation drainage (grommets). The study was conducted at the Department of Pediatric Otolaryngology, Phoniatrics, and Audiology, in the Medical University of Lublin, during 2006–2015. The study group consisted of 114 boys and 87 girls aged from 3 to 21 years and the evaluations were performed within 1 year after the end of treatment. The control group comprised 21 patients taken from the Department of Orthopaedics and Children’s Rehabilitation at the Medical University of Lublin, who had been negatively assessed following audiological interview.
CRITERIA FOR THE STUDY:
Inclusion criteria: history of ventilator tube insertion surgery, conductive hearing loss lasting longer than 3 months, type-B tympanometry before surgery, no otoacoustic response before surgery, conductive hearing loss of more than 40 dB.
STATISTICAL ANALYSES:
Study data were statistically processed using the ‘Statistica’ package (StatSoft). Quantitative variables were described in summary as the mean, standard deviation, median, upper and lower quartiles, and minimum and maximum values. The appropriate statistical tests were used to verify hypotheses. The normality of the data distributions was checked using the Shapiro-Wilk test. However, non-parametric tests were used, because the data were not normally distributed, and the compared groups were disproportionate in size.
The first part of the analysis was on 222 subjects divided into 2 groups which were compared by the Mann-Whitney U test (also termed the Wilcoxon Mann-Whitney test), to determine whether the medians were significantly different; assuming that the distributions of these 2 variables were closely similar. A Kruskal-Wallis rank analysis of variance (ANOVA) was used for comparison when there were more than 2 independent groups. This was a one-way ANOVA by ranks and is an extension of the Mann-Whitney U test to verify whether the differences between the medians of the studied variable are insignificant in several populations; also, assuming that the variables’ distributions were closely similar to each other. A test of independence was performed to test the relationship between 2 nominal variables: otherwise known as the Pearson’s Chi-square test. A
Results
STUDY GROUP DEMOGRAPHICS:
The study group consisted of n=222 child subjects (42.8% girls and 57.2% boys). There were 3 groups distinguished among the child subjects who underwent the procedure: OME patients with identified risk factors (n=130), OME patients without identified risk factors (n=71), and control (non-OME) patients (n=21). Boys appeared to dominate in all groups; however, there were no statistically significant differences between the groups in terms of sex distribution (Table 1). The youngest subject was 3 years old, whilst the eldest was 21 years old. The mean age of the entire group was 10 years. Allergy and passive smoking were the most common risk factors in children with risk factors present (n=130) (Table 2).
RESULTS OF OTOLOGICAL AND AUDIOLOGICAL EXAMINATION WITHIN THE ENTIRE STUDY GROUP:
A total of 414 ears were analyzed, of which the right ear accounted for 49.8% of cases, whilst the left accounted for the remaining 50.2%. Assessments were performed for the entire OME patient group (with or without risk factors), which consisted of 49.2% done on the right ear and 50.8% on the left for the group without risk factors, and 50% on the left and right ear in the group with risk factors. This was similar to the assessments performed in the control group (Table 3).
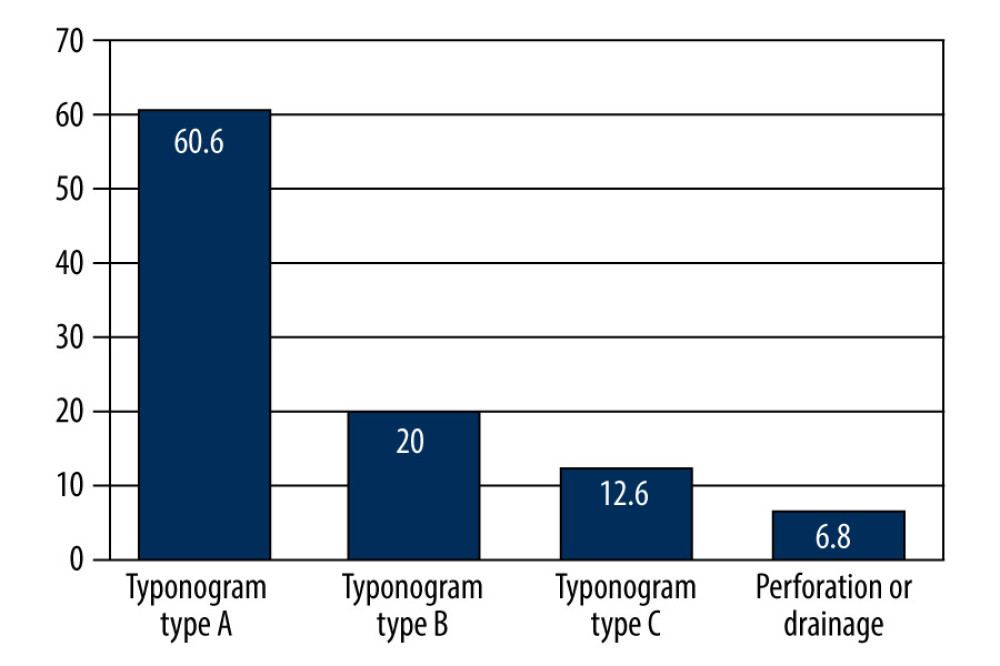
Within the entire study group (OME patients and controls), ears with a type-A tympanogram were found in 60.6% of cases, type-B tympanogram in 20%, and type C tympanogram in 12.6%, whilst 6.8% were not analyzed (Figure 1). Ipsilateral acoustic reflexes were present in 37.9% of ears, whilst they were absent in 57.2%. The analyses were not performed in 4.8% of cases due to technical reasons (eg, presence of a drain or perforation) (Table 4).
Otoemission occurred in 63.3% of ears, whilst it was absent in 28.7% of ears. The absence of otoemission was also found in 8% of the patients but for different reasons than active exudative otitis, ie, sensorineural hearing loss, perforations being present, or a ventilation drain in the tympanic membrane (Table 5).
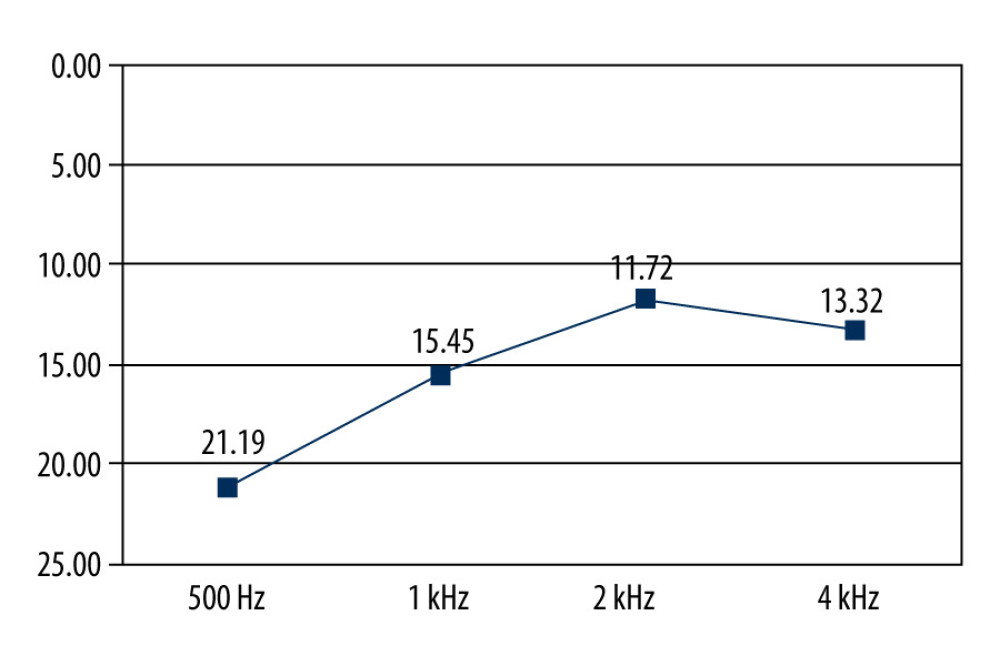
Hearing in 372 ears was examined using tonal audiometry. The average value for the hearing threshold was 22.01 Hz in the frequency range of 500 Hz, whilst they were respectively 16.76 Hz, 12.72 kHz, and 14.78 kHz for the respective ranges of 1 kHz, 2 kHz, and 4 kHz (Figure 2).
WHAT ARE THE EFFECTS OF TREATMENT IN CHILDREN WITH OR WITHOUT RISK FACTORS WHEN COMPARED WITH CONTROLS?:
The type-A tympanogram was found in 66.4% of ears in subjects without risk factors, in 52.5% of ears in those with risk factors, and in 90.5% of ears in the control group. The type-B tympanogram was found in 15.6% of ears in subjects without risk factors, and in 25.8% of ears where risk factors were absent. The tympanogram type C was, however, found in 12.5% of ears in subjects without risk factors but in 13.1% of ears in those subjects where risk factors were present, whereas it was observed in 9.5% of ears in the control group. These analyses were not, however, performed in 5.5% of the subjects without risk factors and in 8.6% of the subjects with risk factors. The differences found between the analyzed groups were statistically significant (Table 6).
Among the children without risk factors, 39.8% showed ipsilateral acoustic reflexes, whereas among the children with risk factors, 56.3% showed ipsilateral acoustic reflexes (3.9% were not analyzed). Rates of acoustic reflexes were 27.5% in the ears of the children with risk factors and 66.4% in the ears of the children without risk factors (6.1% were untested). In the controls, 92.9% of the subjects demonstrated the presence of acoustic reflexes, with only 7.1% of the subjects lacking these reflexes. Those children with risk factors recorded the lowest rates for the stapes reflex. These differences were found to be statistically significant (Table 7).
For children without risk factors, otoemission was present in 68% of ears, whilst it was absent in 25%. The absence of otoemission was also found in 7% of the ears of children without risk factors, but for different reasons than active exudative otitis, ie, sensorineural hearing loss, perforations, or a ventilation drain in the tympanic membrane. For those subjects with risk factors, otoemission occurred in 54.5% of ears, whilst it was absent in 35.7%. The absence of otoemission was also found in 9.8% of the ears of children with risk factors, but for different reasons than active exudative otitis. In the control group, otoemission was present in all ears. The lowest rates of otoemission were found in the OME patients with risk factors. Differences between the study groups were statistically significant (Table 8).
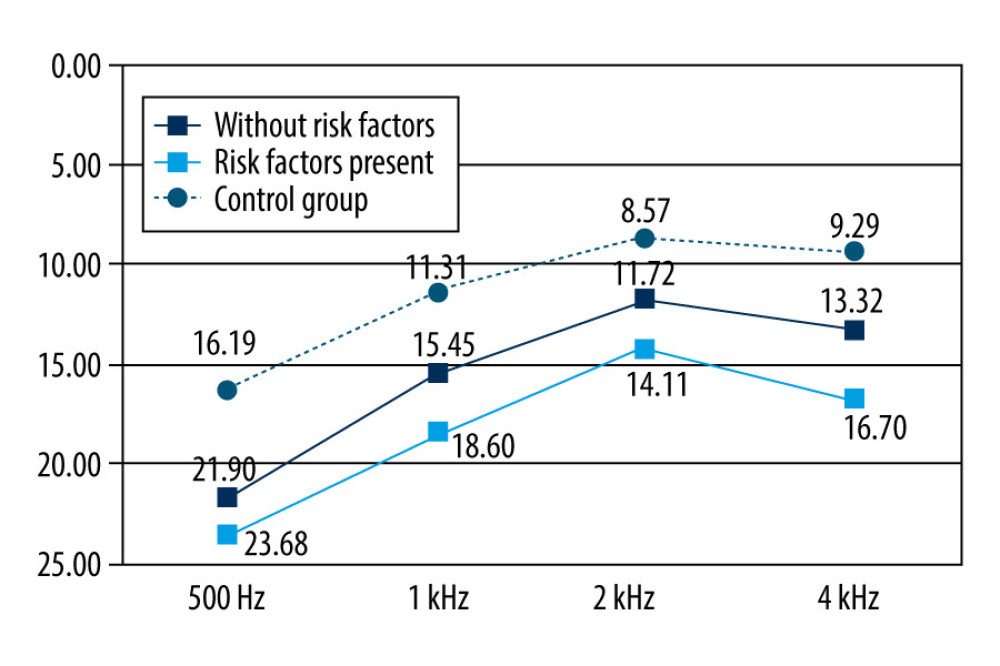
The means for the hearing thresholds for the frequency range of 500 Hz were 21.19 Hz, 23.68 Hz, and 16.19 Hz in the OME patients without risk factors, OME patients with risk factors, and control patients, respectively. At the 1 kHz frequency range, hearing threshold means were 15.45 kHz, 18.69 kHz, and 11.31 kHz in the OME patients without risk factors, OME patients with risk factors, and control patients, respectively. At the 2 kHz frequency range, the means for the hearing thresholds were 11.72 kHz, 14.11 kHz, and 8.57 Hz in the OME patients without risk factors, OME patients with risk factors, and control patients, respectively. At the 4 kHz frequency range, hearing threshold means were 13.32 kHz, 16.70 kHz, and 9.29 kHz in the OME patients without risk factors, OME patients with risk factors, and control patients, respectively (Figure 3).
The differences in mean hearing thresholds were found to be statistically significant between groups throughout the frequency ranges studied (500 Hz–4 kHz).
Discussion
A limitation of the study was the sizes of the included samples (leading to imprecise effect estimates) and subjective otoscopic assessment. The hearing tests, however, were objective because they were conducted on standardized equipment.
A variable investigated in the study was the type of tympanogram. Type A was found in 66.4% of ears in OME patients without risk factors and in 52.5% of ears of OME patients with risk factors. These results correspond to outcomes reported in the literature in studies on Polish children; namely, 43% to 76.1% [27–30]. The type-B tympanogram also correlated with the occurrence of risk factors. A study by Thornthon et al used chinchillas as an animal model to investigate the relationship between the decreased susceptibility of the tympanic membrane in the presence of fluid within the tympanic cavity. They used a standard probe signal at a frequency of 226 Hz, and took measurements before and after filling the middle ear cavity with defined amounts of silicone oil: 0.5–24ml. A type-A tympanogram was observed in 75% of ears studied at the smallest oil volume applied (0.5 ml). These tympanograms demonstrated a sharp and narrow peak that oscillated around 0 dPa showing that this minimum amount of fluid does not affect sound transmission in the middle ear. When the applied oil volumes were increased to 1.0 ml, there was a noticeable shortening and broadening of these curves. The tympanogram type changed from A to B only after 1.5 ml of oil had been administered, which in fact almost completely filled the tympanic cavity. Interestingly, an increased sensitivity of the test was observed when the probe signal was used at a higher frequency of 1400 Hz. Type-B tympanograms were found in 67% of cases when 1 ml of fluid had been applied [31]. The properties of the stimulus used were seen to affect the susceptibility of the tympanic membrane regardless of the amount of fluid in the tympanic cavity. The available literature shows that the incidence of tympanogram C ranges from 12.6%–18% [31,32], compared with rates of 12.5% and 13.1% found in our study in those without and with otological risk factors, respectively.
The susceptibility of the tympanic membrane to injury was also assessed by De Beer et al using tympanometry. They noticed that OME causes a significant increase in the susceptibility of the tympanic membrane to injury in childhood, and this increase is highest in children treated using ventilation drainage. It was thus considered that using tympanostomy tubes causes changes in the structure of the middle ear, leading to injury resulting from the strain on the tympanic membrane imposed by the weight of the tubes [32]. This notion was confirmed by a 2010 study on a group of children treated by this method at the Department of Pediatric Otolaryngology at the Medical University of Bialystok, Poland, which found increased susceptibility of the tympanic membrane to injury in every third child so treated. This was observed both in children with visible lesions in their otoscopic images and in those having a normal image of the tympanic membrane [33]. In contrast, a different position was taken in a study by Sade, which explained that the increased susceptibility to the qualitative changes in the tympanic membrane was influenced by enzymes and inflammatory mediators present in the exudate, ie, the formation of atrophic fields and the absence of a double fibrous layer [34].
Our study recorded the ipsilateral stapes reflex in only 37.9% of patients with OME.. Moreover, in patients with risk factors, only 27.5% of the ears managed to register a contraction of the stapes muscle following ipsilateral activation. The absence of an ipsilateral reflex has also been demonstrated in studies by Hassmann-Poznańska et al, in which this phenomenon was noted in 16.9% of atrophic or myringosclerosis eardrums, 33.3% of membranes with retraction pockets, and 10.8% of ears without otoscopic changes. Researchers have explained the absence of the ipsilateral stapes reflex by the high susceptibility of the tympanic membrane to injury [34]. A study by Gravel et al has, however, presented different results in a group of 132 patient subjects. In these subjects, a normal threshold was achieved in the stapes reflex with ipsilateral stimulation and an elevated threshold during contralateral stimulation. They suggested that this phenomenon is not the result of damage to the structures of the middle ear, but instead is an adverse effect of conductive hearing loss during the first years of life on the development of the auditory pathway at the level of the cochlea and the brainstem [35]. The children-subjects from the test and control groups also underwent otoacoustic emission testing, in which responses from the external auditory cells were statistically more common amongst children without risk factors. This correlates with the tympanometric test results obtained in the present study. The type-B tympanometric curve was found to be statistically more common amongst children suffering from the cleft palate defect. Indeed, studies in the literature have demonstrated similar findings, showing significantly reduced otoemission amplitudes in type-B tympanograms, which are therefore impossible to register. This is associated with both an impaired transmission of the sound stimulus itself and in the retransmission of the otoemission [36,37]. A study by Amedee, however, presents a different hypothesis, which states that it is the density of exudate (serous or mucous) that has the greatest effect on the presence or absence of transient EOAE. Nevertheless, there was no correlation between the type of tympanogram and the possibility of recording an otoemission [38].
The best results obtained from the tonal audiometry tests were in the control group subjects, in whom average conduction hearing thresholds were, respectively, 16.19 dB, 11.31 dB, 8.57 dB, and 9.29 dB for the frequencies of 500 Hz, 1 kHz, 2 kHz, and 4 kHz.
In a study by Lous, treatment with grommets improved hearing levels, especially during the first 6 months. In randomized controlled trials that studied the effect of grommet insertion alone, the mean hearing levels improved by around 9 dB (95% CI, 4 dB to 14 dB) after the first 6 months, and 6 dB (95% CI, 3 dB to 9 dB) after 12 months [39]. By comparison, a study by Sipila on 5-year-old children, showed a hearing loss greater than 20 dB in 2.7% of ears (pure tone average for air conduction) [13]. Other centers demonstrated significant variation, ranging from 1% to 20.9% [40,41]. There were, however, no significant differences in hearing in a long-term hearing assessment of people after treatment for chronic OME (25 years after surgery) compared with the norms established for a healthy population adjusted for age and sex. The method of treatment, whether myringotomy or ventilation tube, had no effect on long-term hearing levels [42,43].
The influence of a positive interview was clearly seen on the outcomes of tonal audiometry in the presence of risk factors. The most favorable results were obtained at all the tested frequencies in those children after drainage, when risk factors were absent vs when they were present.
An interesting study was conducted by Skarżyńska et al. The purpose of this analysis was to clinically evaluate the efficacy of surgical access (tube placement with adenotomy) compared with a nonsurgical approach (watchful waiting) during a 12-month followup period. In the nonsurgical group, the treatment effect was more variable than in the surgical group. In the non-surgical group, 10 children achieved 1 year (365 days) without recurrence, but about the same number achieved less than 30 days without recurrence, and 9 children achieved no more than 60 days without recurrence. The certainty of treatment in group 1 (surgical) was higher than in group 2 (watchful waiting), and the number of days without recurrence was also significantly higher in group 1 than in group 2 [44].
Kaytez et al suggest probiotics as a complementary therapy, in the form of dietary supplements. Considering their study’s otomicroscopic and histological findings, probiotics have a curative effect on the prevention and treatment of OME. This effect may be related to the anti-inflammatory effects of probiotics. Therefore, probiotics can be widely used in the age group at risk for OME as a complementary therapy, in the form of dietary supplements [45].
Sogebi et al found that at least 1 in every 4 children with enlarged adenoids had an additional burden of asymptomatic OME. An implication of this finding is that enlarged adenoids and OME are commonly associated. Thus, children with adenoid enlargement should be screened for OME and managed accordingly for achievement of the best outcome in the children. Active screening is more pertinent in children who have increased age and weight, since these characteristics have been found to be associated with asymptomatic OME [46].
Mohhiudin showed that the strategy of ventilation tubes only (VT-only) is more effective and less costly than a strategy of hearing aids plus ventilation tubes (HA+VT), while the incremental cost-effectiveness ratio for the VT-only strategy compared with a hearing aid-only (HA-only) strategy was £5,086 per quality-adjusted life-year (QALY) gained. At the willingness-to-pay threshold of £20,000 per QALY, the probability that the VT-only strategy is likely to be more cost-effective was 0.58. The expected value of perfect information (EVPI) at the population level of around £9.5 million at the willingness-to-pay threshold of £20,000 indicated that future research in this area is potentially worthwhile, while the expected value of partially perfect information (EVPPI) analysis indicated considerable uncertainty surrounding the parameters used for computing the QALY score, for which more precise estimates would be most valuable. The VT-only strategy is a cost-effective option when compared with the HA-only and HA+VT strategies, but the need for additional information from future studies is evident, to inform this surgical treatment choice. Future studies of surgical and non-surgical treatment of OME in childhood should evaluate the economic impact of pertinent interventions to provide greater context [47].
Conclusions
Our findings led us to draw the following conclusions. It is recognized that ventilation drainage is a good means for treating OME, despite the occurrence of adverse events. The presence of risk factors affects the results of treatment. Children in the no-risk-factors group obtained better hearing test results than children with risk factors.
Tables
Table 1. Gender distribution in the study groups.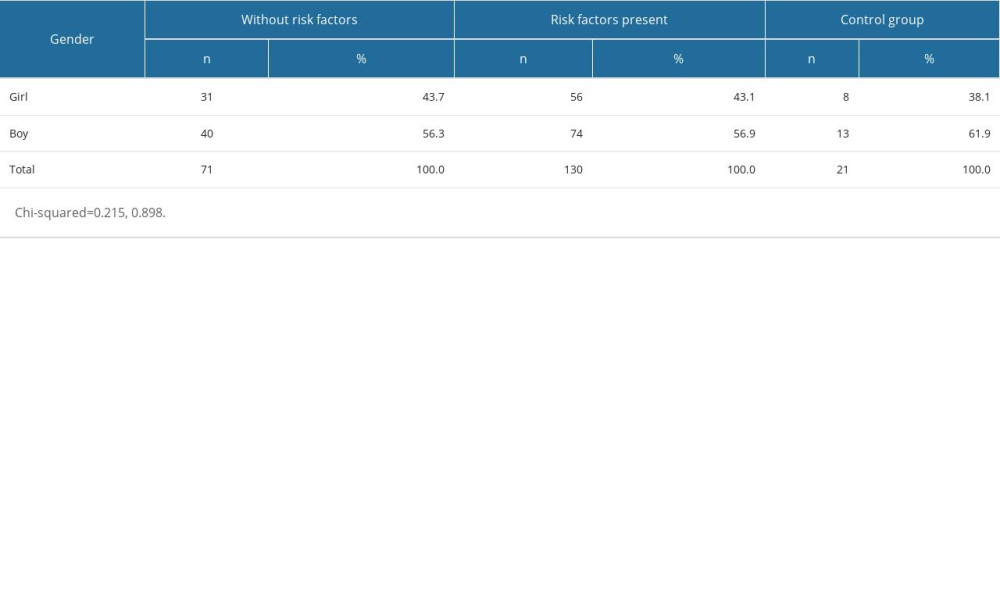 Table 2. Risk factor rates in the study groups.
Table 2. Risk factor rates in the study groups.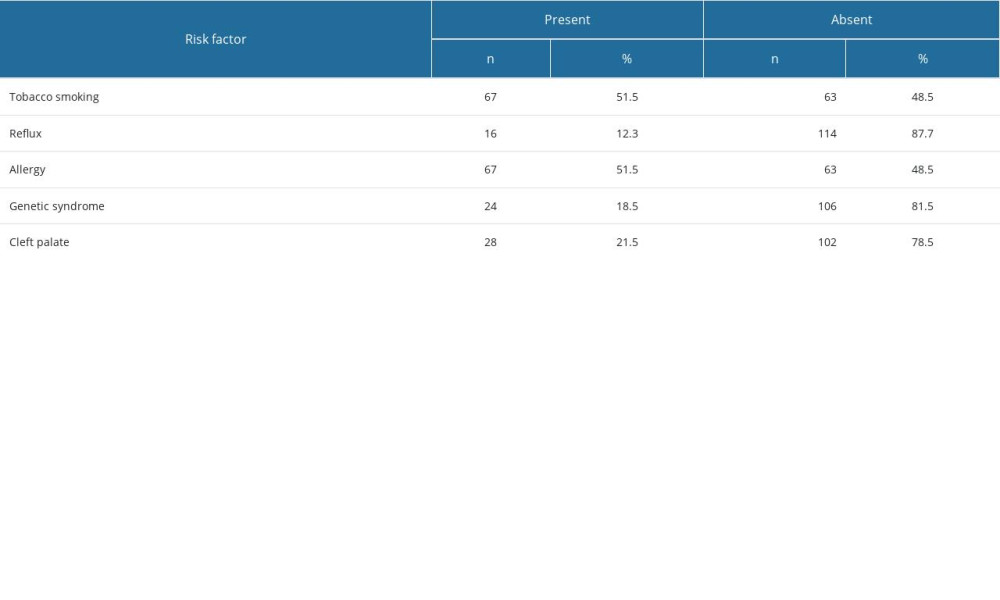 Table 3. Ear-side orientation in patients with and without risk factors.
Table 3. Ear-side orientation in patients with and without risk factors.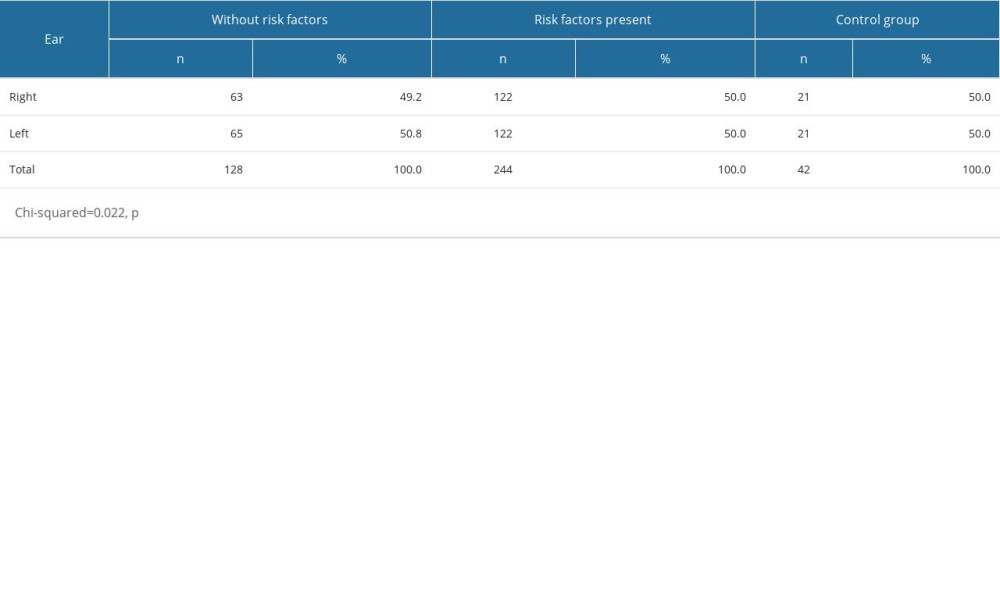 Table 4. Acoustic reflex results in the study population.
Table 4. Acoustic reflex results in the study population.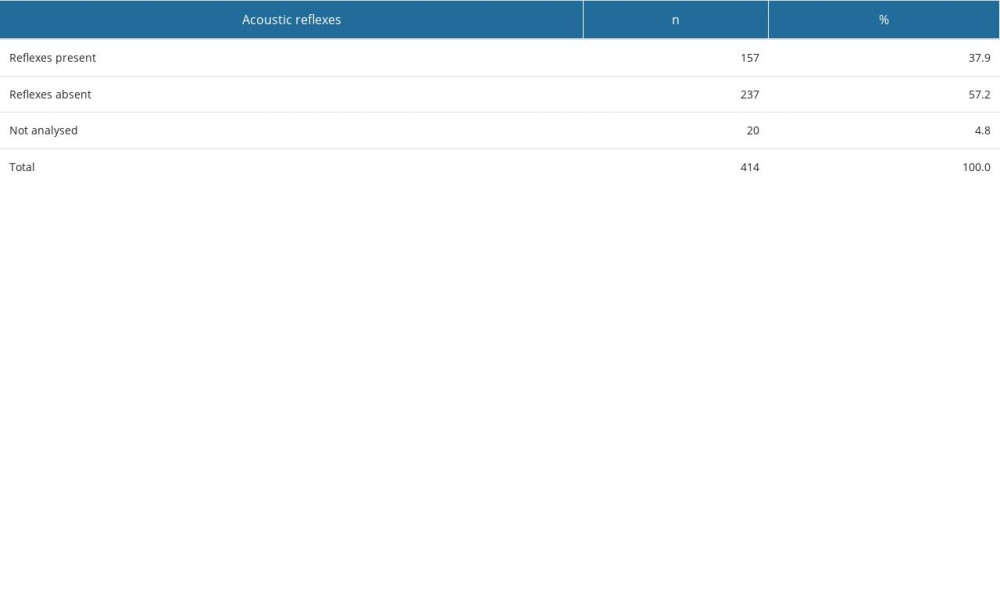 Table 5. Otoemission results in the study population.
Table 5. Otoemission results in the study population.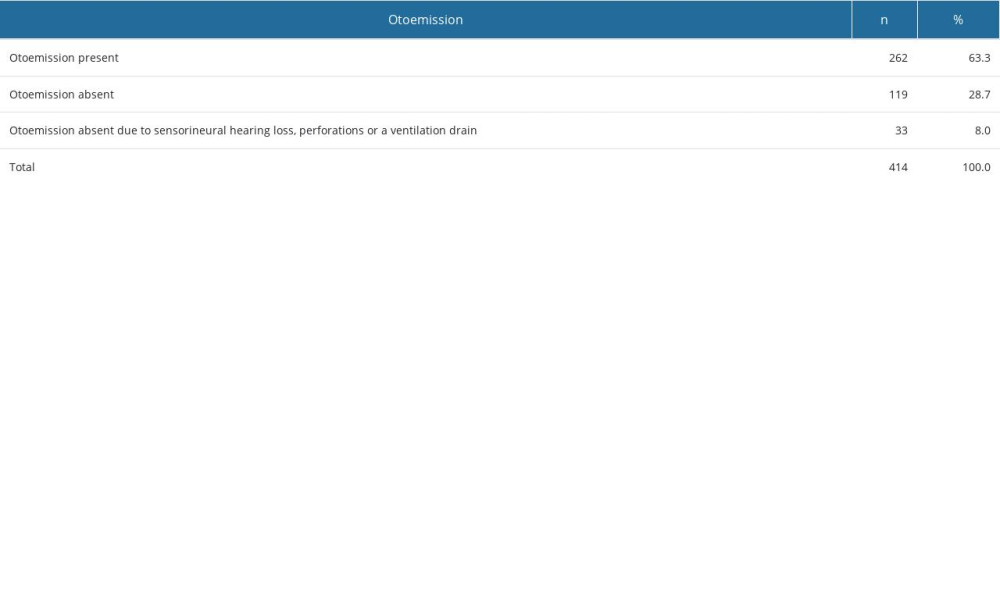 Table 6. Distribution of tympanogram types in patients with and without risk factors.
Table 6. Distribution of tympanogram types in patients with and without risk factors.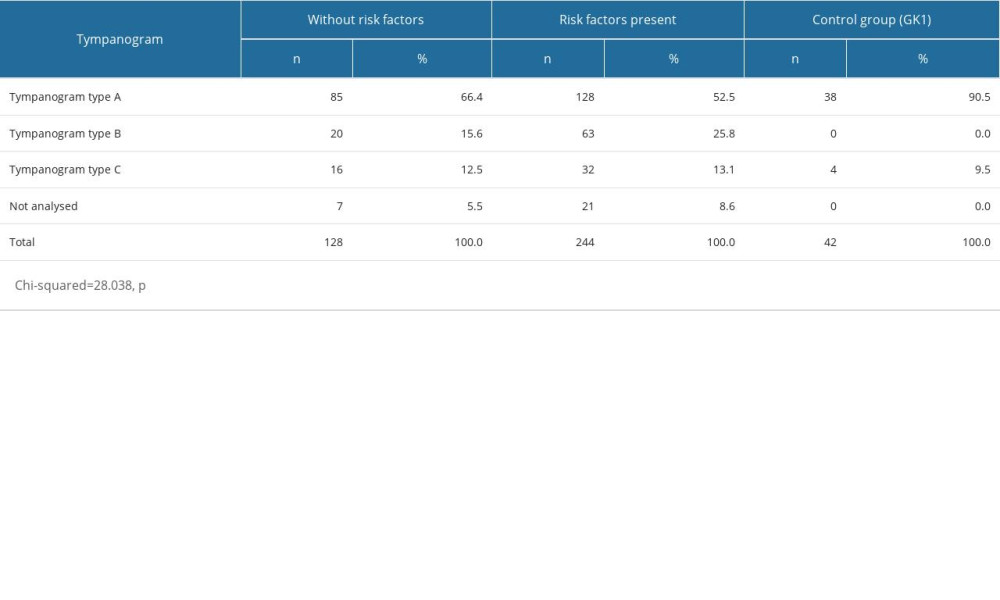 Table 7. Acoustic reflex results in the study group and controls.
Table 7. Acoustic reflex results in the study group and controls.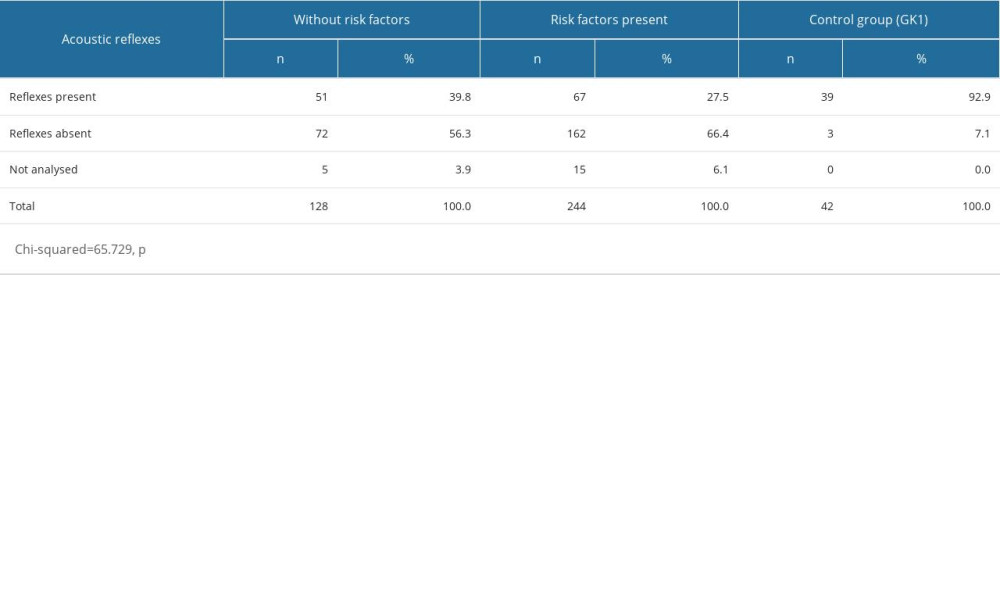 Table 8. Otoemission results in the study group and controls.
Table 8. Otoemission results in the study group and controls.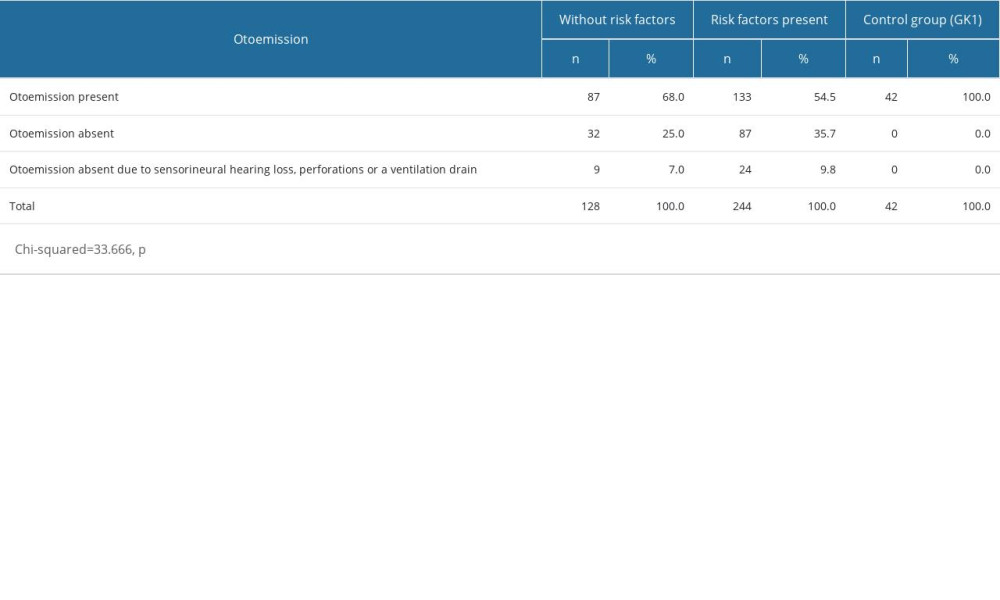
References
1. Gordon MA, Grunstein E, Burton WB, The effect of the season on otitis media with effusion resolution rates in the New York Metropolitan area: Int J Pediatr Otorhinolaryngol, 2004; 68(2); 191-95
2. Casselbrant ML, Mandel EM, Kurs-Lasky M, Otitis media in a population of black American and white American infants, 0–2 years of age: Int J Pediatr Otorhinolaryngol, 1995; 33(1); 1-16
3. Casselbrant ML, Mandel EM, Epidemiology: Evidence-Based Otitis Media, 2003, BC Decker
4. Caylan R, Bektas D, Atalay C, Prevalence and risk factors of otitis media with effusion in Trabzon, a city in northeastern Turkey, with an emphasis on the recommendation of OME screening: Eur Arch Otorhinolaryngol, 2006; 263(5); 404-8
5. Martines F, Bentivegna D, Di Piazza F, The point prevalence of otitis media with effusion among primary school children in Western Sicily: Eur Arch Otorhinolaryngol, 2010; 267(5); 709-14
6. Pelikan Z, Role of nasal allergy in chronic secretory otitis media: Curr Allergy Asthma Rep, 2009; 9(2); 107-13
7. Rosenfeld RM, Culpepper L, Doyle KJ, Clinical practice guideline: Otitis media with effusion: Otolaryngol Head Neck Surg, 2004; 130(5 Suppl); S95-118
8. Zielhuis GA, Rach GH, Broek P, The occurrence of otitis media with effusion in Dutch pre-school children: Clin Otolaryngol, 1990; 15(2); 147-53
9. Fiellau-Nikolajsen M, Tympanometry and secretory otitis media. Observations on diagnosis, epidemiology, treatment, and prevention in prospective cohort studies of three-year-old children: Acta Otolaryngol Suppl, 1983; 394; 1-73
10. Casselbrant ML, Brostoff LM, Cantekin EI, Otitis media with effusion in preschool children: Laryngoscope, 1985; 95(4); 428-36
11. Teele DW, Klein JO, Rosner B, Epidemiology of otitis media during the first seven years of life in children in greater Boston: A prospective, cohort study: J Infect Dis, 1989; 160(1); 83-94
12. Searight FT, Singh R, Peterson DC, Otitis media with effusion: StatPearls [Internet] May 20, 2023, Treasure Island (FL), StatPearls Publishing
13. Sipilä M: Occurrence, risk factors and consequences of otitis media in children, 1991, University of Tampere
14. Cullen KA, Hall MU, Golosinskyi A, Ambulatory surgery in the United States, 2006: Natl Health Stat, 2006(11); 1-25
15. Midgley EJ, Dewey C, Pryce K, The frequency of otitis media with effusion in British pre-school children: A guide for treatment. ALSPAC Study Team: Clin Otolaryngol Allied Sci, 2000; 25(6); 485-91
16. Ahn JH, Yoon TH, Pae KH, Clinical manifestations and risk factors of children receiving triple ventilating tube insertions for treatment of recurrent otitis media with effusion: Pediatrics, 2006; 117(6); e1119-23
17. Coatesworth AP, Addis RJ, Beverley DW, Ear nose and throat diseases in the Bedouin of the South Sinai Desert: A cross-sectional survey and discussion of healthcare needs: J Laryngol Otol, 2002; 116(2); 83-86
18. Fireman P, Otitis media and eustachian tube dysfunction: Connection to allergic rhinitis: J Allergy Clin Immunol, 1997; 99(2); s787-s97
19. Gryczyńska D, Przewlekłe zapalenie ucha środkowego z wysiękiem: Audiologia Kliniczna Mediton, 2005; 75-78 [in Polish]
20. Lubianca Neto JF, Hemb L, Silva DB, Systematic literature review of modifiable risk factors for recurrent acute otitis media in childhood: J Pediatr (Rio J), 2006; 82(2); 87-96
21. Sophia A, Isaac R, Rebekah G, Risk factors for otitis media among preschool, rural Indian children: Int J Pediatr Otorhinolaryngol, 2010; 74(6); 677-83
22. Gravel JS, Wallace IF, Listening and language at 4 years of age: effects of early otitis media: J Speech Hear Res, 1992; 35(3); 588-95
23. Borges LR, Paschoal JR, Colella-Santos MF, (Central) auditory processing: The impact of otitis media: Clinics (Sao Paulo), 2013; 68(7); 954-59
24. MacKeith S, Mulvaney CA, Galbraith K, Ventilation tubes (grommets) for otitis media with effusion (OME) in children: Cochrane Database Syst Rev, 2022; 2022(3); CD015215
25. Venekamp RP, Mick P, Schilder AG, Nunez DA, Grommets (ventilation tubes) for recurrent acute otitis media in children: Cochrane Database Syst Rev, 2018; 5(5); CD012017
26. Behrbohm H, Kaschke O, Nawka T: Choroby ucha, nosa i gardła z chirurgią głowy i szyi, 2011, Elsevier UrbanPartner in Polish
27. Jerger J, Clinical experience with impedance audiometry: Arch Otolaryngol Head Neck Surg, 1970; 92(4); 311-24
28. Namysłowski G, Fira R, Audiometria impedancyjna: Audiologia Kliniczna Mediton, 2005; 137-48 [in Polish]
29. Maw AR, Bawden R, Tympanic membrane atrophy, scarring, atelectasis and attic retraction in persistent, untreated otitis media with effusion and following ventilation tube insertion: Int J Pediatr Otorhinolaryngol, 1994; 30(3); 189-204
30. Zielnik-Jurkiewicz B, Olszewska-Sosińska O, Rakowska M, Odległe wyniki leczenia drenażem wentylacyjnym przewlekłego wysiękowego zapalenia ucha środkowego: Otolaryngol Pol, 2006; 60(2); 181-85 [in Polish]
31. Thornton JL, Chevallier KM, Koka K, Conductive hearing loss induced by experimental middle-ear effusion in a chinchilla model reveals impaired tympanic membrane-coupled ossicular chain movement: J Assoc Res Otolaryngol, 2013; 14(4); 451-64
32. de Beer B, Snik A, Schilder AGM, The effect of otitis media in childhood on the development of middle ear admittance on reaching adulthood: Arch Otolaryngol Head Neck Surg, 2005; 131(9); 777-81
33. Hassmann-Poznańska E, Goździewski A, Piszcz M, Wpływ zmian strukturalnych błony bebenkowej na wyniki audiometriii impedancyjnej: Otolaryngol Pol, 2010; 64(5); 307-12 [in Polish]
34. Sadé J, Atelectatic tympanic membrane: histologic study: Ann Otol Rhinol Laryngol, 1993; 102(9); 712-16
35. Gravel JS, Roberts JE, Roush J, Early otitis media with effusion, hearing loss, and auditory processes at school age: Ear Hear, 2006; 27(4); 353-68
36. Smurzyński J, Podstawy badań otoemisji akustycznej: Audiofonologia, 1995; 7; 5-19 [in Polish]
37. Topolska MM, Hassmann-Poznańska E, Musiatowicz MP, Wpływ stanu ucha środkowego na wywołane emisje otoakustyczne: Otol Pol, 1998; 52(4); 451-55 [in Polish]
38. Amedee RG, The effects of chronic otitis media with effusion on the measurement of transiently evoked otoacoustic emissions: Laryngoscope, 1995; 105(6); 589-95
39. Venekamp RP, Mick P, Schilder AG, Nunez DA, Grommets (ventilation tubes) for recurrent acute otitis media in children: Cochrane Database Syst Rev, 2018; 5(5); CD012017
40. Gundersen T, Tonning FM, Ventilating tubes in the middle ear: Arch Otolaryngol, 1976; 102(4); 198-99
41. Karma P, Sipila M, Kokko E, Long-term results of tympanostomy treatment in chronic secretory otitis media: Acta Otolaryngol, 1982; 93(Sup386); 163-65
42. Sederberg-Olsen JF, Sederberg-Olsen AE, Jensen AM, Late results of treatment with ventilation tubes for secretory otitis media in ENT practice: Acta Otolaryngol, 1989; 108(5–6); 448-55
43. Khodaverdi M, Jørgensen G, Lange T, Hearing 25 years after surgical treatment of otitis media with effusion in early childhood: Int J Pediatr Otorhinolaryngol, 2013; 77(2); 241-47
44. Skarżyńska MB, Gos E, Czajka N, Effectiveness of surgical approach of insertion ventilation tubes (tympanostomy) and adenoidectomy in comparison with non-surgical approach (watchful waiting approach) in children at the age between 1 and 6 and who suffer from otitis media with effusion (OME) in 12-month period of observation-the retrospective analysis: Int J Environ Res Public Health, 2021; 18(23); 12502
45. Kaytez SK, Ocal R, Yumusak N, Effect of probiotics in experimental otitis media with effusion: Int J Pediatr Otorhinolaryngol, 2020; 132(109922); 109922
46. Sogebi OA, Oyewole EA, Ogunbanwo O, Asymptomatic otitis media with effusion in children with adenoid enlargement: J Natl Med Assoc, 2021; 113(2); 158-64
47. Mohiuddin S, Schilder A, Bruce I, Economic evaluation of surgical insertion of ventilation tubes for the management of persistent bilateral otitis media with effusion in children: BMC Health Serv Res, 2014; 14; 253
Figures
Tables
 Table 1. Gender distribution in the study groups.
Table 1. Gender distribution in the study groups. Table 2. Risk factor rates in the study groups.
Table 2. Risk factor rates in the study groups. Table 3. Ear-side orientation in patients with and without risk factors.
Table 3. Ear-side orientation in patients with and without risk factors. Table 4. Acoustic reflex results in the study population.
Table 4. Acoustic reflex results in the study population. Table 5. Otoemission results in the study population.
Table 5. Otoemission results in the study population. Table 6. Distribution of tympanogram types in patients with and without risk factors.
Table 6. Distribution of tympanogram types in patients with and without risk factors. Table 7. Acoustic reflex results in the study group and controls.
Table 7. Acoustic reflex results in the study group and controls. Table 8. Otoemission results in the study group and controls.
Table 8. Otoemission results in the study group and controls. Table 1. Gender distribution in the study groups.
Table 1. Gender distribution in the study groups. Table 2. Risk factor rates in the study groups.
Table 2. Risk factor rates in the study groups. Table 3. Ear-side orientation in patients with and without risk factors.
Table 3. Ear-side orientation in patients with and without risk factors. Table 4. Acoustic reflex results in the study population.
Table 4. Acoustic reflex results in the study population. Table 5. Otoemission results in the study population.
Table 5. Otoemission results in the study population. Table 6. Distribution of tympanogram types in patients with and without risk factors.
Table 6. Distribution of tympanogram types in patients with and without risk factors. Table 7. Acoustic reflex results in the study group and controls.
Table 7. Acoustic reflex results in the study group and controls. Table 8. Otoemission results in the study group and controls.
Table 8. Otoemission results in the study group and controls. In Press
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
12 Mar 2024 : Clinical Research
Comparing Neuromuscular Blockade Measurement Between Upper Arm (TOF Cuff®) and Eyelid (TOF Scan®) Using Miv...Med Sci Monit In Press; DOI: 10.12659/MSM.943630
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952