15 March 2024: Meta-Analysis
Accuracy of MRI-Based Radiomics in Diagnosis of Placenta Accreta Spectrum: A PRISMA Systematic Review and Meta-Analysis
Liqiong Huang1AE, Lin Ma1F, Qin Zhou1F, Yi Hu1B, Lirong Hu1B, Yang Luo1C, Yan Li1AD*DOI: 10.12659/MSM.943461
Med Sci Monit 2024; 30:e943461
Abstract
BACKGROUND: Placenta accreta syndrome (PAS) can lead to severe obstetric bleeding, and can be life-threatening. This study aimed to assess the precision of radiomics features derived from magnetic resonance imaging (MRI) for diagnosing PAS.
MATERIAL AND METHODS: A comprehensive search was conducted in the databases PubMed, Embase, Web of Science, and the Cochrane library from inception to October 2023. We included diagnostic accuracy studies utilizing radiomics-MRI in PAS patients, with histopathology serving as the reference standard. The overall diagnostic odds ratio (DOR), sensitivity, specificity, and area under the curve (AUC) were computed to gauge the diagnostic accuracy of MRI-based radiomic features in PAS patients. Quality assessment was performed using the Quality Assessment of Diagnostic Accuracy Studies 2. Statistical analyses were carried out using Stata 14.2, MetaDiSc 1.4, and Review Manager 5.3 software.
RESULTS: Seven studies involving 672 patients were incorporated. The aggregated DOR, sensitivity, specificity, and AUC for radiomics in detecting PAS were 78% (confidence interval32, 191), 87% (76%, 93%), 92% (89%, 94%), and 0.93 (0.91-0.95), respectively. The meta-analysis revealed notable heterogeneity among the included studies, with no evidence of a threshold effect. Subgroup analysis demonstrated that, in comparison to manual segmentation and validation groups with ≤100 cases and internal validation datasets, automated segmentation, validation groups with >100 cases, and external validation datasets exhibited superior diagnostic performance .
CONCLUSIONS: Our findings indicate that MRI-based radiomic features perform well in assessing the diagnostic risk of PAS during prenatal diagnosis. This noninvasive and convenient tool may prove valuable in facilitating the identification of PAS.
Keywords: Diagnosis, Placenta Accreta, Magnetic Resonance Imaging, Imaging Genomics, Meta-Analysis
Introduction
Placenta accreta spectrum (PAS) is abnormal adhesion of the placenta to the uterine wall, and its prevalence has been on the rise [1]. This phenomenon is closely associated with the escalating rates of cesarean section (CD) deliveries, a prominent risk factor for PAS [2]. Approximately 7% of maternal mortality is reported to be linked to PAS [3]. Recently, the International Federation of Gynecology and Obstetrics (FIGO) categorized PAS into placental adhesion (PA), placenta increta (PI), and penetrating placenta (PP) [4]. The detachment of aberrant placental tissue within the myometrium poses a significant risk of obstetric hemorrhage at delivery and severe maternal morbidity. Thus, precise preoperative identification of placental status in PAS patients becomes crucial to avoid unnecessary surgical interventions, facilitate treatment planning, and address the urgent need for prenatal PAS diagnosis. Presently, prenatal diagnosis has had improvements in critical factors affecting maternal and perinatal outcomes, particularly in the first trimester of pregnancy [5].
Ultrasound (US) is the first-line imaging modality for PAS screening and diagnosis [6]. Its noninvasive nature and the ability to perform it multiple times during pregnancy make ultrasound the primary choice for diagnosing and monitoring PAS throughout gestation. As a supplementary tool to ultrasound, MRI has higher spatial resolution, better display of uterine myometrium, and can accurately determine the depth of invasion of placental villous tissue into the myometrium. While ultrasound is a commonly used prenatal diagnostic tool for PAS, its accuracy is limited and influenced by the examiner’s expertise and technique. Notably, Aitken et al demonstrated that MRI has distinct advantages over ultrasound in predicting the depth of placental implantation and invasion into surrounding tissues [7]. Additionally, MRI allows for acquisition of permanent digital images, mitigating the impact of subjective factors.
In recent years, several published studies have leveraged radiomics models to predict the onset of PAS. Radiomics, an emerging field in medical imaging, involves converting lesion imaging information into calculated quantitative data to enhance the diagnostic capabilities of medicine [8]. MRI has seen increasing use in the prenatal care of PAS cases, especially for assessing disease extent and invasion depth [6]. Prenatal diagnostic evaluation using this technique, coupled with appropriate delivery and surgical planning, holds promise in reducing complications associated with PAS [9].
For this method to be considered in clinical practice, its effectiveness must be thoroughly investigated. Hence, the objective of this meta-analysis was to determine the diagnostic performance of MRI-based radiomics features in the preoperative prediction of implanted placental patients.
Material and Methods
LITERATURE SEARCH:
We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reporting our systematic review. PubMed, Embase, Web of Science, and the Cochrane library were systematically searched from inception to October 2023. Two authors independently reviewed titles and abstracts to identify potential trials, and the full text was further assessed to determine eligibility. Disagreements between the 2 reviewers were resolved through consensus or by involving a third independent arbiter. The retrieval strategy, based on Cochrane Handbook guidelines, was developed through collaborative discussions among researchers. The planned search terms were as follows: (“Placenta Accreta Spectrum” or “PAS” or “placenta accreta,” “placenta increta” or “placenta percret”) and (“radiomics” or “texture analysis”) and (“magnetic resonance imaging” or “MRI”) (Supplementary Table 1).
The protocol was registered on the International Prospective Register of Systematic Reviews under registration number CRD42023487957.
ETHICS REVIEW:
Ethics approval and patient consent were not required since this study was a meta-analysis based on published studies.
INCLUSION CRITERIA:
We included literature meeting the following criteria: (1) clinical studies on MRI-based radiomics diagnosis of placenta accreta spectrum, including randomized control trials (RCTs) and retrospective cohort studies (RCS); (2) studies in which all the patients underwent prenatal MRI and in which PAS status was confirmed by histopathology for all patients; (3) published in English; (4) full text available; (5) Studies reporting or providing sufficient information to calculate true-positive (TP), false-positive (FP), true-negative (TN), and false-negative (FN) values.
EXCLUSION CRITERIA:
Exclusion criteria were as follows: (1) abstracts, letters, editorials, conference articles, case reports, reviews, animal studies, and study protocols; (2) repeated studies and data; (3) research not using MRI-based radiomics; (4) studies with insufficient data integrity.
DATA EXTRACTION AND QUALITY ASSESSMENT:
Two independent reviewers extracted data, with mutual blindness to each other’s results. Differences were discussed until consensus was reached. Extracted information included study characteristics (first author, publication year, study period, number of patients), patient characteristics (age, country, gestational age), imaging characteristics (MRI vendor, imaging sequence, segmentation strategy, algorithms), and statistical analysis (TP, FP, FN, TN). In case of inadequate or missing data, attempts were made to contact corresponding authors via email for data integration. If data retrieval was unsuccessful, analysis was performed using available data, and potential impacts were discussed.
The Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool was used independently by 2 reviewers to evaluate the risk of bias in the selected studies. This tool assesses risk of bias in 4 key domains: patient selection, index test, reference standard, and flow and timing.
STATISTICAL METHODS:
Stata 14.2 software was employed to calculate overall combined sensitivity (SEN), specificity (SPE), positive likelihood ratios (PLR), negative likelihood ratios (NLR), and diagnostic odds ratio (DOR) across the 7 articles. Funnel plots, forest plots, and summary receiver operating characteristic (sROC) curves were drawn to assess overall diagnostic accuracy, with the area under the sROC curve (AUC) serving as a measure. The I2 value was calculated to identify heterogeneity sources. MetaDiSc 1.4 software was used for threshold effect evaluation. Subgroup analysis used MetaDiSc 1.4 and Review Manager 5.3 software, calculated combined sensitivity, specificity, natural ratio, and odds ratio of different groups, with forest plots drawn. Deeks’ funnel plot asymmetry tests were used to evaluate potential publication bias, with statistical significance set at
Results
LITERATURE SEARCH:
A total of 105 potentially eligible citations were retrieved from the electronic search, with 59 duplicates identified. After title and abstract evaluation, 35 citations were excluded based on the pre-defined inclusion and exclusion criteria. Subsequently, 2 independent observers thoroughly assessed the full text of 11 articles, ultimately including 7 studies in the meta-analysis. The study selection process is visually depicted in Figure 1.
CHARACTERISTICS OF THE ELIGIBLE STUDIES:
Table 1 presents the baseline characteristics of the included studies, which encompassed sample sizes ranging from 34 to 163 participants, totaling 672 individuals. Geographically, most studies were conducted in Asia (n=6), with only 1 study conducted in Europe [16]. All articles reported radiomic characteristics based on T2-weighted imaging (WI) sequences. Among them, 5 studies utilized manual-type radiomics for diagnostic analysis, while 2 reported outcomes based on automated-type radiomics diagnosis. Philips MRI was employed in 4 studies, 2 studies utilized multiple MRI brands, and 1 study did not specify the MRI brand (detailed information on the MRI equipment is provided in Table 1).
QUALITY ASSESSMENT:
The methodological quality of the studies was evaluated using the QUADAS-2 assessment, as depicted in Figure 2. Notably, 2 studies were deemed at high risk of bias in the patient selection domain due to their non-randomized, case-control design. Three studies exhibited a high risk of bias in the index test domain, primarily related to the lack of blinding regarding pre-specificity threshold and radiomic approach to the reference standard. One study raised applicability concerns in patient selection due to significant baseline characteristic differences among patients (P<0.05) (Figure 2 and Supplementary Table 2).
DIAGNOSTIC ACCURACY OF RADIOMIC MRI:
Overall sensitivity and specificity were 87% (76%, 93%) and 92% (89%, 94%), respectively. The AUC, DOR, PLR, and NLR were 0.93 (0.91–0.95), 78 (32, 191), 11.0 (7.5, 16.0), and 0.14 (0.07, 0.27), respectively. Figures 3 and 4 illustrate the forest plots for sensitivity and specificity and the SROC curve. The utilization of an MRI-based radiomic features model increased the post-test probability to 73% from 20% with a PLR of 3 when the pretest was positive, and reduced the post-test probability to 3% with an NLR of 0.14 when the pretest was negative (Figure 5).
HETEROGENEITY ASSESSMENT:
The I2 statistic indicated high heterogeneity for sensitivity (I2=87.35%) and low heterogeneity for specificity (I2=11.11%). The Spearman correlation coefficient for the threshold effect was calculated as 0.288 with a
SUBGROUP ANALYSIS:
Results of the subgroup analysis are presented in Table 2. Studies employing manual segmentation had lower pooled sensitivity [76% (69%, 81%)] compared to automated segmentation [91% (83%, 96%)], with similar pooled specificity [93% (89%, 96%) vs 91% (84%, 95%)]. Studies with validation groups of <100 cases had higher pooled sensitivity and specificity [92% (85%, 96%), 94% (89%, 97%)] than those with >100 cases [74% (67%, 81%), 91% (87%, 94%)]. Subgroup analysis on different types of validation datasets indicated higher pooled sensitivity in internal datasets [92% (86%, 95%)] compared to internal and external joint datasets [66% (57%, 74%)], with similar pooled specificity [93% (89%, 96%) vs 91% (85%, 95%)]. Corresponding subgroup analysis plots are depicted in Figure 6.
RISK FACTORS:
The OR was 2.29 (95% CI: 0.88 to 5.94), indicating a more than 2-fold increase in the odds of the outcome occurring in the group of maternal age at delivery more than 35 weeks [12]. The meta-analysis yielded a pooled OR of 2.79 (95% CI: 1.44 to 5.42), suggesting a significant association with the history of cesarean section across the studies included [11,12,16]. The heterogeneity among the studies, as quantified by the I2 statistic, was 0, indicating no observed variability beyond chance. Moreover, Egger’s test for publication bias returned a non-significant P value of 0.138, suggesting no statistical evidence of publication bias within the collected data (Table 3).
PUBLICATION BIAS:
No significant risk of publication bias was detected through funnel plot analysis (P=0.17) (Figure 7).
Discussion
This meta-analysis is the first to investigate the utility of MRI-based radiomic features for ruling out PAS on a per-patient basis. Our comprehensive assessment incorporated data from over 672 women who had confirmed PAS through histopathology and who underwent prenatal MRI examination to assess the accuracy of MRI-based radiomics features for diagnosing PAS. Sensitivity and specificity both exceeded 85%. Subgroup analyses were conducted based on the analysis strategy, number of cases, and validation dataset.
In a recent investigation, Huien Lin et al [17] analyzed 10 primary studies, comprising 4 retrospective and 6 prospective studies, revealing a magnetic resonance imaging (MRI) diagnostic sensitivity and specificity for placenta implantation of 88% and 79%, respectively. Nevertheless, our experimental findings notably surpass these figures. D’Annino et al [18] studied 164 women who underwent both MRI and sonography, reporting ultrasound sensitivity and specificity values of 87.8% and 96.3%, respectively, and MRI sensitivity and specificity values of 92.9% and 93.5%, respectively. Notably, MRI demonstrated higher sensitivity and specificity than ultrasound, corroborating our outcomes. Meng et al [19] investigated 1085 patients who underwent ultrasound examination, revealing an overall sensitivity of 83% and specificity of 95%. In a subgroup of 183 patients who underwent MRI examination, the overall sensitivity and specificity were 82% and 88%, respectively. This discrepancy is attributed to the substantially higher prevalence of ultrasound examinations compared to MRI examinations. These data closely align with our results and show the potential enhancement in predicting placenta accreta spectrum (PAS) accuracy in pregnant women through utilization of MRI-based radiomic features.
Our research also found that the application of T2-weighted MRI imaging is particularly important for evaluating PAS. The sequence’s high sensitivity to fluid content is instrumental in identifying abnormal placental adherence to the uterine wall. This sensitivity is crucial for early detection, allowing for clear visualization of the placenta–myometrium interface, which is pivotal in diagnosing the extent of placental invasion [20], while the specificity of T2WI aids in distinguishing between normal placental tissue and areas of abnormal adherence or invasion, crucial for differentiating between the varying degrees of the syndrome.
A subgroup analysis was undertaken to delve deeper into the factors influencing diagnostic accuracy. Our investigation revealed a significantly higher DOR in the automatic segmentation group compared to the manual segmentation group. Xuan et al employed Stochastic Gradient Descent (SGD) as the optimizer for training over 200 epochs, utilizing a trained U-net for automated placental tissue segmentation in MRI images, yielding elevated DOR [13]. This superiority is attributed to the inherent reliance on subjective judgments in manual segmentation, potentially diminishing reliability within and among observers, especially in scenarios of suboptimal image quality and limited observer knowledge and experience [21,22]. However, the automated method’s accuracy may be compromised when applied to MRI scans acquired at lower magnetic field intensities due to reduced gray matter tissue contrast, resulting in comparable specificity between the 2 groups [23].
Our findings indicated that the >100 validation group had significantly lower outcomes than the <100 validation group. An excessive number of participants can introduce numerous interfering factors, leading to larger errors. Among the included studies, those with >100 validation group encompassed nearly twice the number of patients in the <100 validation group (432 vs 240). Subgroup analysis based on different dataset types revealed higher accuracy in internal validation datasets compared to combined internal and external validation datasets. In studies by Peng and Wang, 2 centers were utilized, with one collecting data for training and internal validation, and the other for external validation [14,15]. Internal validation, being based on the model development cohort data, typically serves as a component of model development, while external validation involves assessing the model’s performance in new data, resulting in lower sensitivity and DOR for this group.
Radiomics promises to revolutionize diagnostics with its enhanced accuracy, providing in-depth tissue characterization that is pivotal for differentiating between varying degrees of the syndrome. This heightened diagnostic precision paves the way for more personalized treatment plans and predictive analytics, which could significantly improve patient outcomes. Despite all this, the integration of MRI-based radiomics into clinical practice presents a complex interplay of cost implications and substantial benefits. While MRI-based radiomics in the context of PAS incurs high initial costs, the long-term benefits, including potential cost savings through more effective treatment planning and reduced rates of complications, suggest a favorable cost-benefit ratio, making it a worthwhile investment for the future of healthcare [24,25].
Our study has several limitations. Firstly, the sample size was small, underscoring the necessity for expansion to enhance accuracy. Secondly, the lack of standardized judgment criteria for MRI imaging omics among technicians introduces a significant dependency on specialized academic centers and high-tech professionals. This lack of uniformity in criteria may have contributed to variability in interpretations. Thirdly, potential geographic bias is evident, as all included studies were from Asia. The absence of validation data from personnel in other regions introduced a potential bias that may have distorted the diagnostic value of MRI for placenta implantation, limiting the generalizability of findings beyond Asia. Lastly, there are insufficient data in the literature to independently analyze the diagnostic performance of MRI-based radiomics in patients with different subtypes of PAS, such as PA, PI, and PP. Future research should focus on this area to identify potential variations in accuracy.
Conclusions
This study substantiates that the utilization of MRI-based radiomic features yields superior accuracy in diagnosing PAS. However, to comprehensively evaluate the efficacy and diagnostic performance of this technique, well-designed prospective studies are imperative and should incorporate meticulous standardization of radiomics protocols, ensuring rigorous methodologies. Such endeavors are crucial to advancing the application of radiomics as a routine clinical tool, providing valuable supplementary information for clinical decision-making in the foreseeable future.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Figures
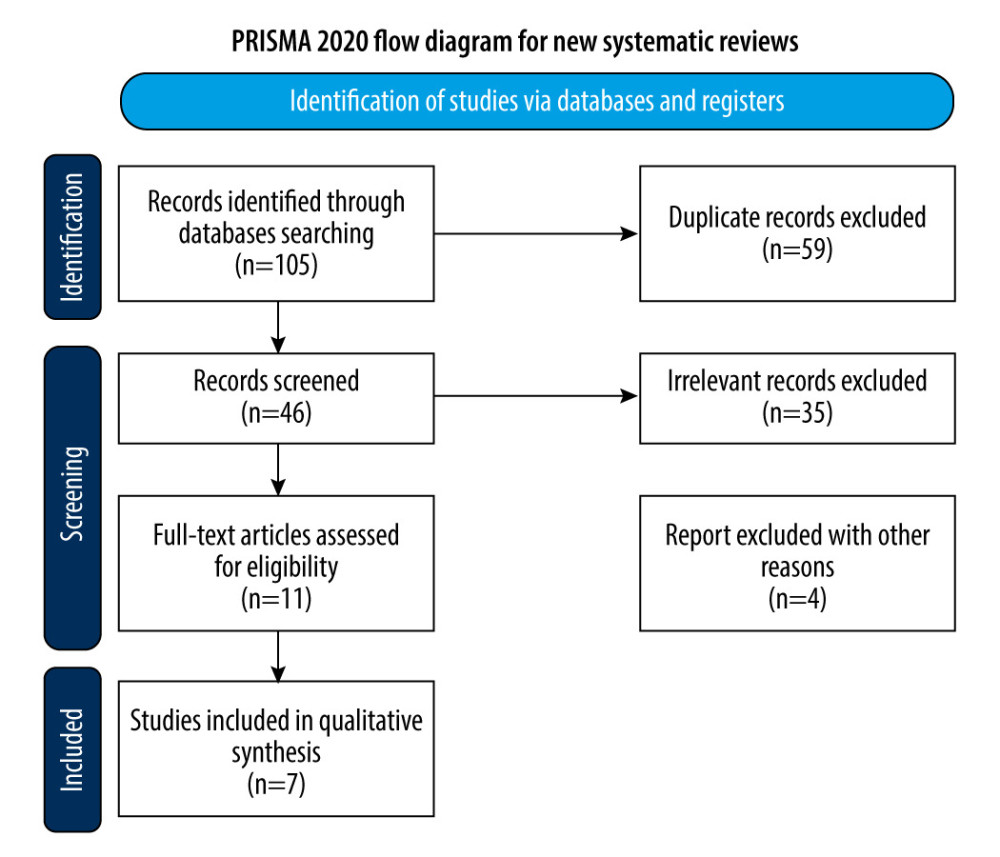 Figure 1. Flow diagram of study selection. The figure was created using Microsoft® Word (version 16.0.16626. 20170).
Figure 1. Flow diagram of study selection. The figure was created using Microsoft® Word (version 16.0.16626. 20170). 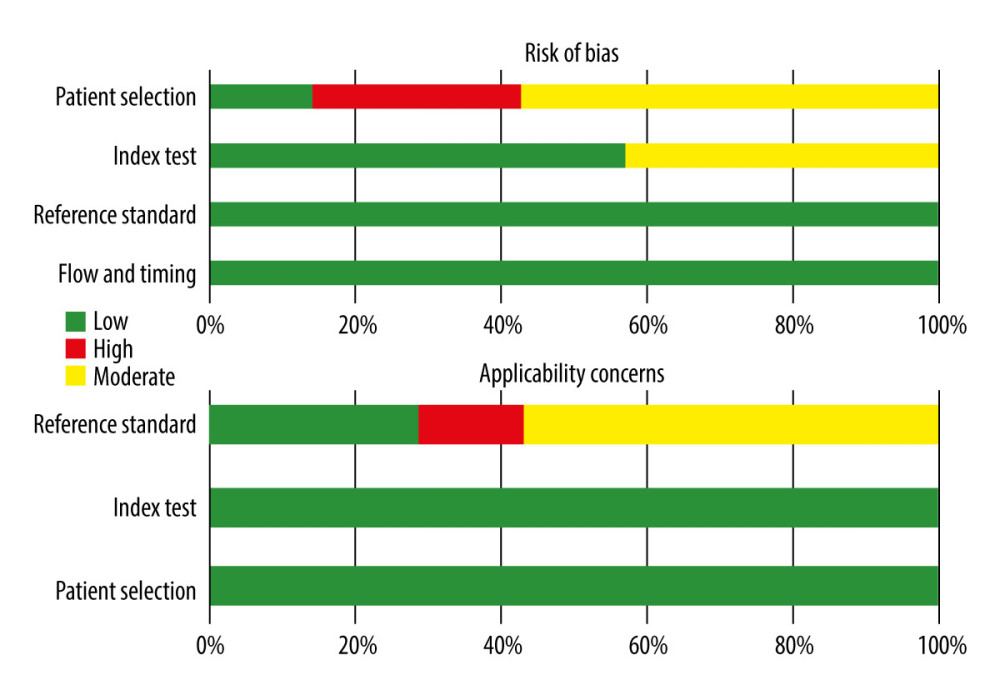 Figure 2. Risk of bias summary. The figure was created using Microsoft® Excel® 2021MSO (version 16.0.17126.20132).
Figure 2. Risk of bias summary. The figure was created using Microsoft® Excel® 2021MSO (version 16.0.17126.20132). 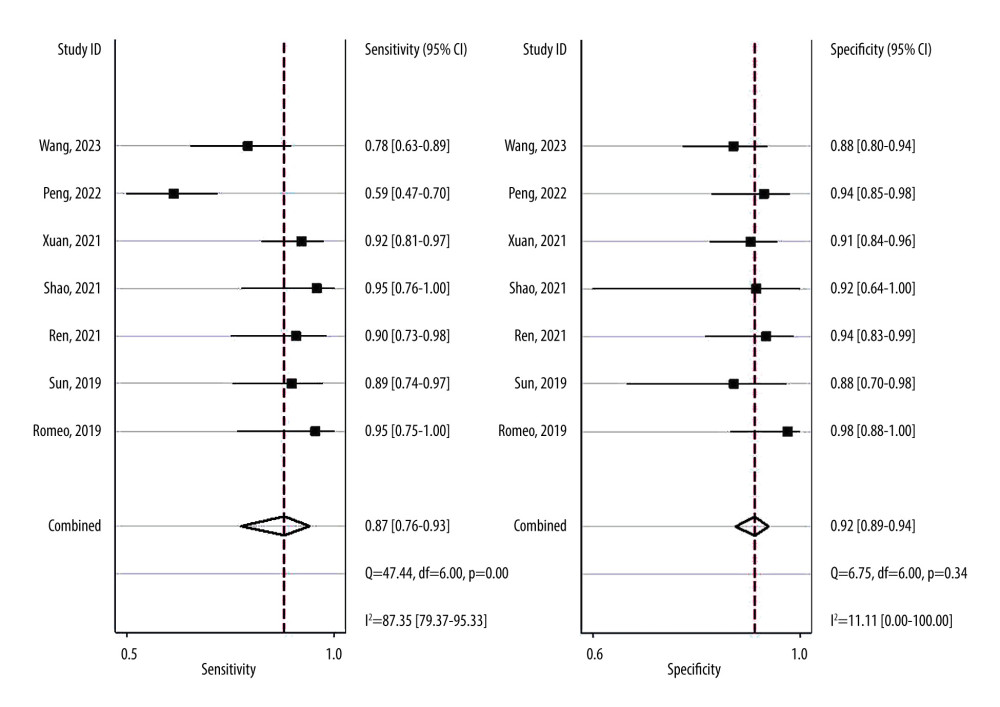 Figure 3. Forest plots of the sensitivity and specificity with corresponding heterogeneity statistics. Statistical analyses were carried out using Stata software (version 14.2).
Figure 3. Forest plots of the sensitivity and specificity with corresponding heterogeneity statistics. Statistical analyses were carried out using Stata software (version 14.2). 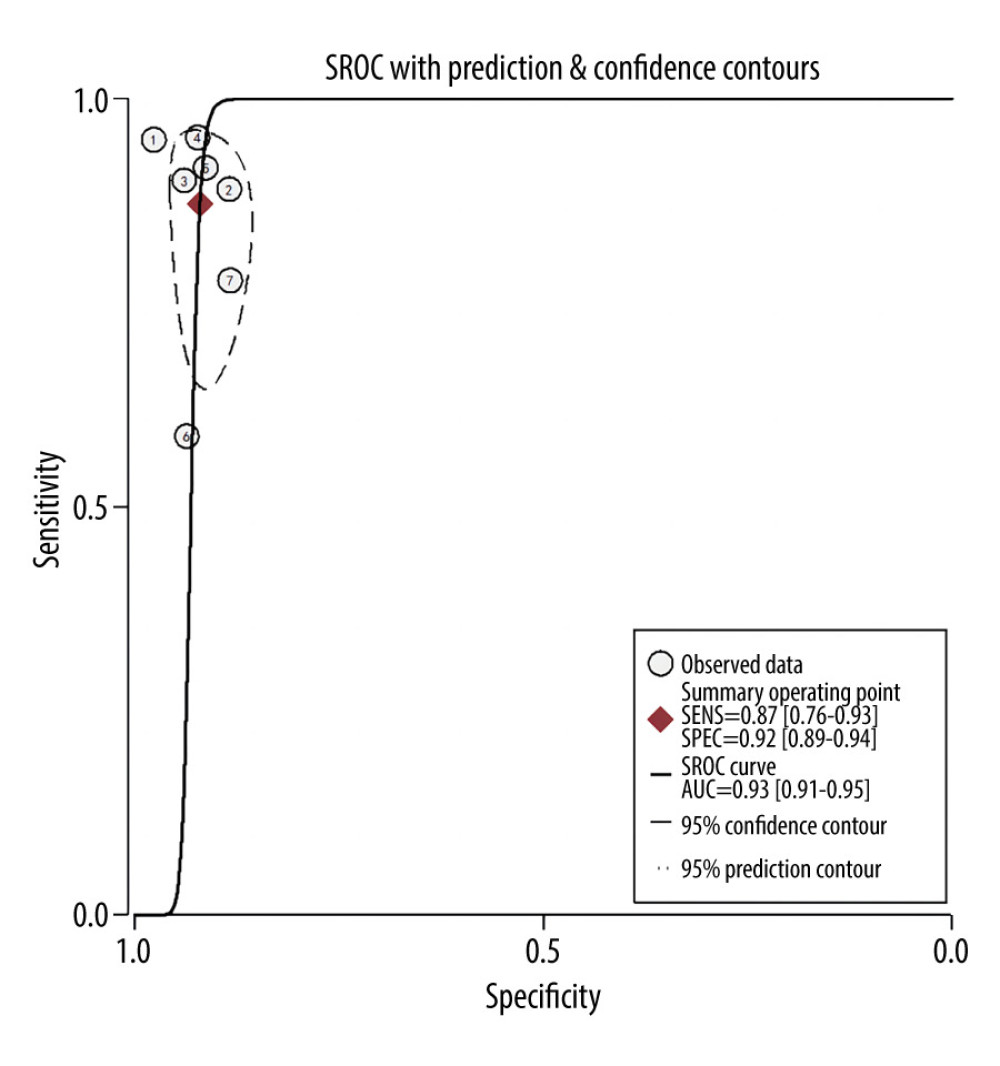 Figure 4. Summary receiver operating characteristic (SROC) curve of the predictive accuracy of PAS. Statistical analyses were carried out using Stata software (version 14.2).
Figure 4. Summary receiver operating characteristic (SROC) curve of the predictive accuracy of PAS. Statistical analyses were carried out using Stata software (version 14.2). 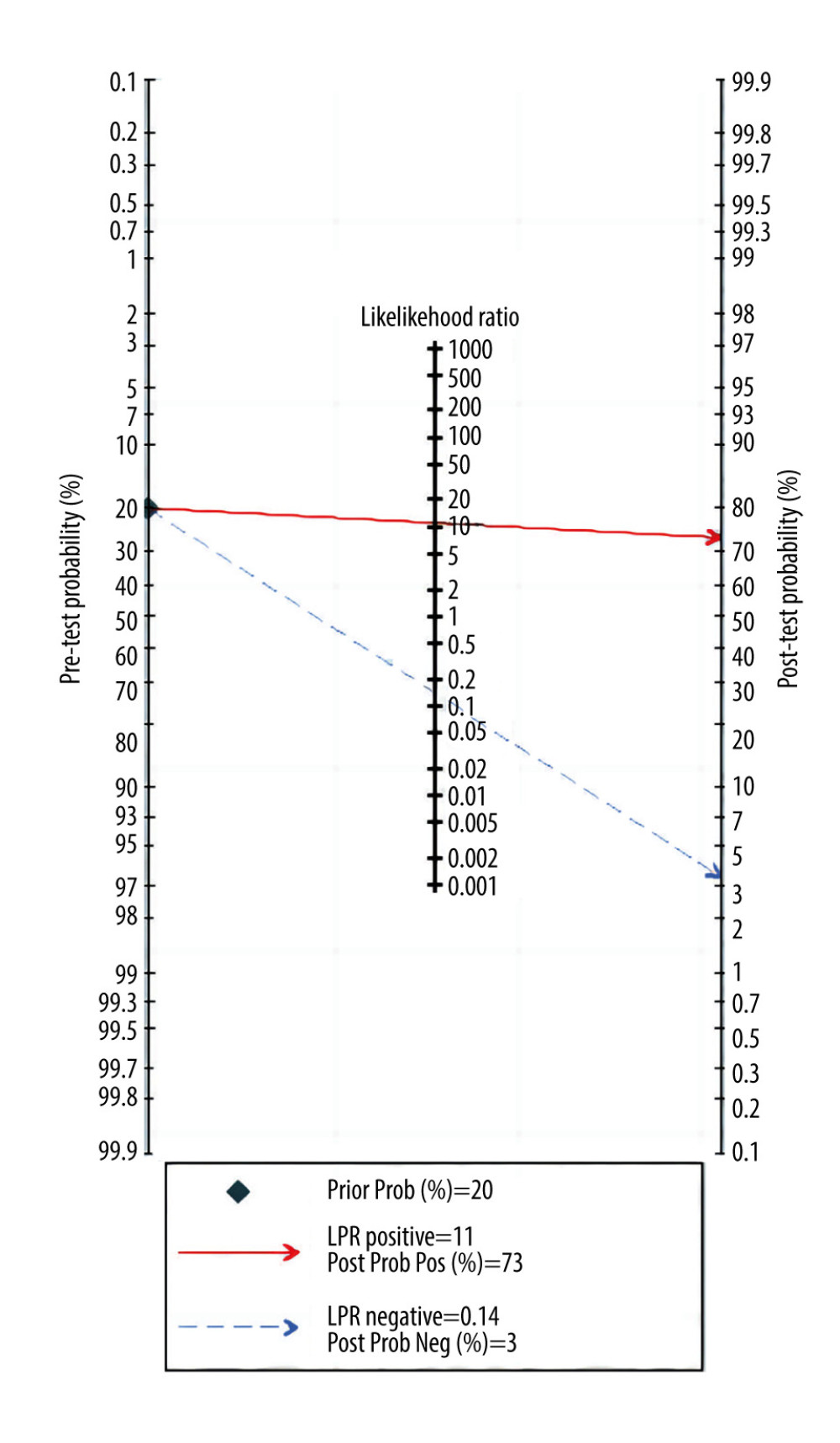 Figure 5. Fagan plots for assessing the clinical utility. Statistical analyses were carried out using Stata software (version 14.2).
Figure 5. Fagan plots for assessing the clinical utility. Statistical analyses were carried out using Stata software (version 14.2). 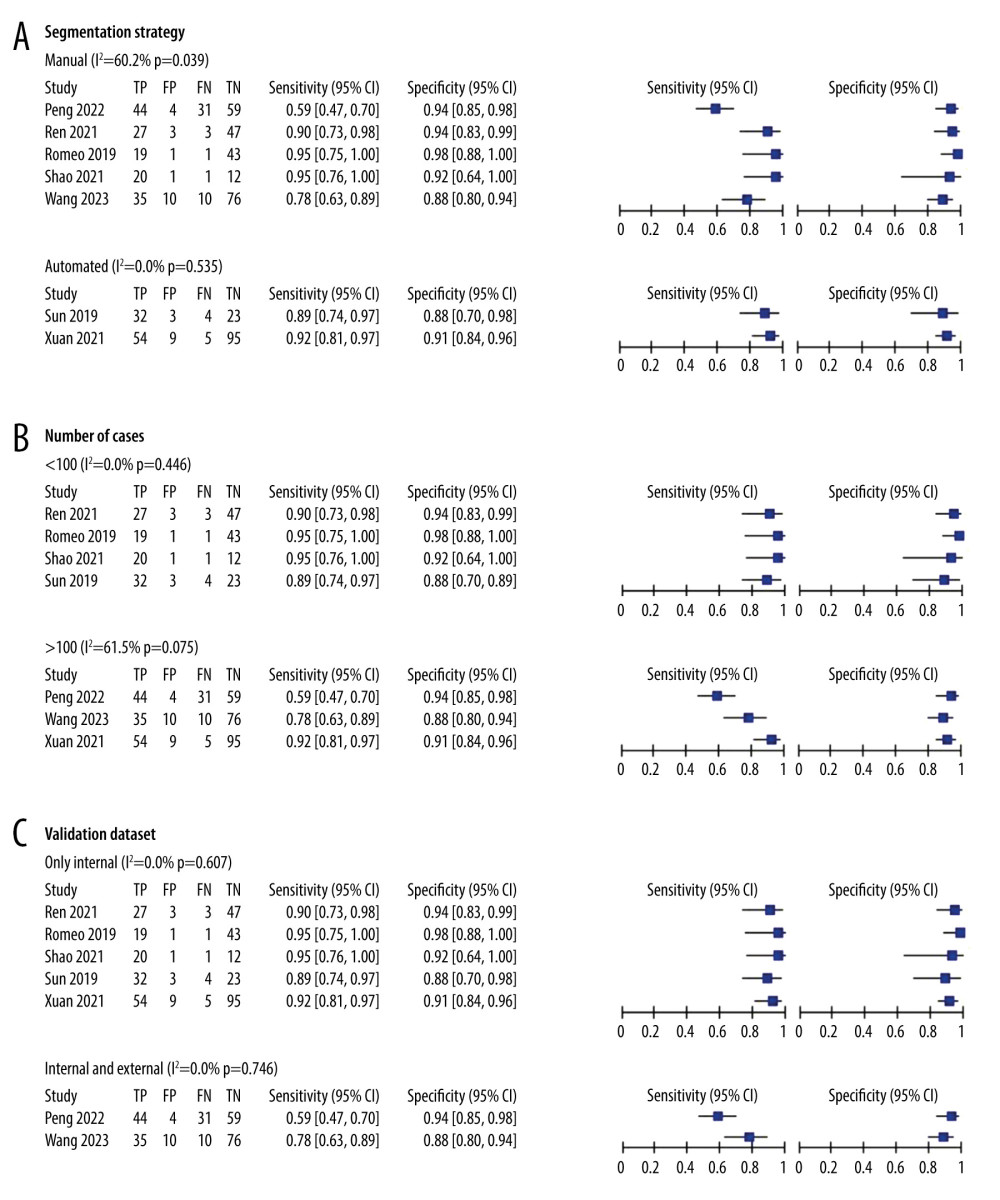 Figure 6. (A–C) Forest plot of multiple univariable meta-regression and subgroup analyses for SEN and SPE. Statistical analyses were carried out using Review Manager software (version 5.3).
Figure 6. (A–C) Forest plot of multiple univariable meta-regression and subgroup analyses for SEN and SPE. Statistical analyses were carried out using Review Manager software (version 5.3). 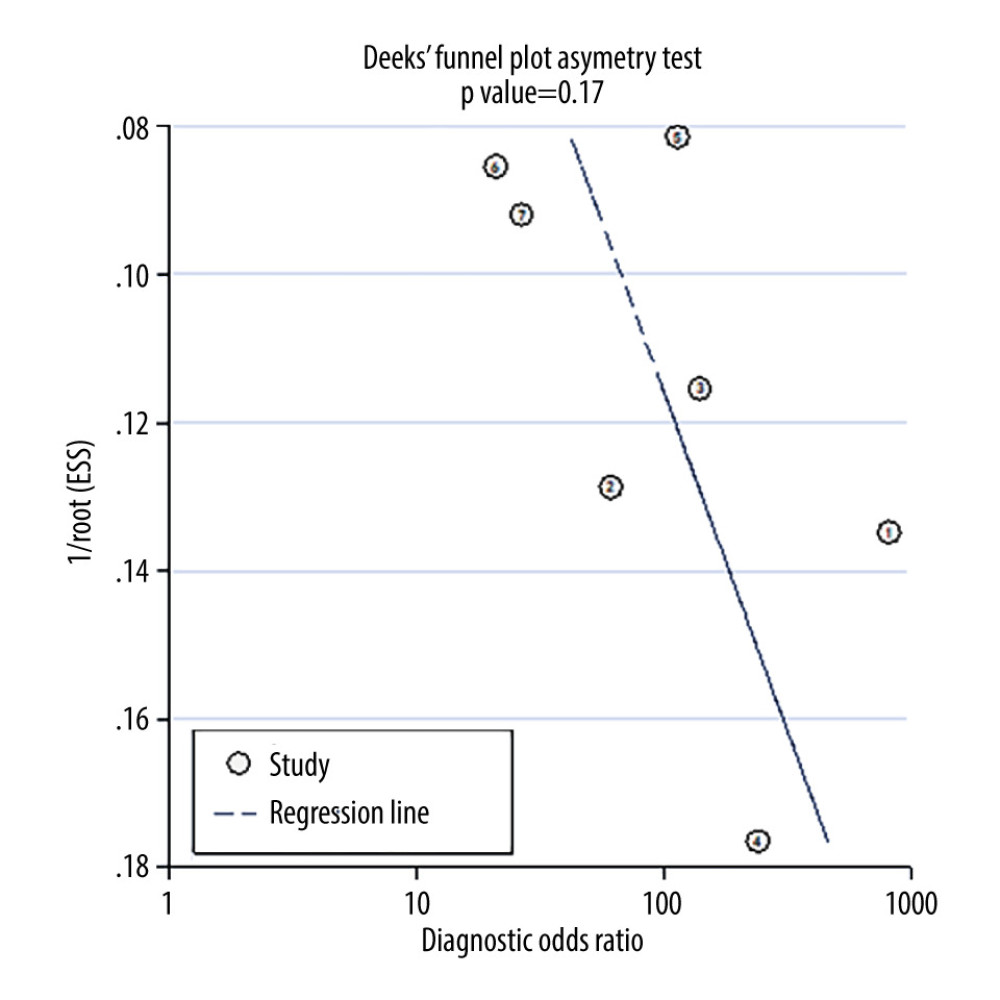 Figure 7. Deek’s funnel plot for assessment of publication bias. Statistical analyses were carried out using Stata software (version 14.2).
Figure 7. Deek’s funnel plot for assessment of publication bias. Statistical analyses were carried out using Stata software (version 14.2). References
1. Wortman AC, Alexander JM, Placenta accreta, increta, and percreta: Obstet Gynecol Clin North Am, 2013; 40(1); 137-54
2. Bloomfield V, Rogers S, Leyland N, Placenta accreta spectrum: CMAJ, 2020; 192(34); E980
3. O’Brien JM, Barton JR, Donaldson ES, The management of placenta percreta: Conservative and operative strategies: Am J Obstet Gynecol, 1996; 175(6); 1632-38
4. Jauniaux E, Ayres-de-Campos D, Langhoff-Roos JFIGO Placenta Accreta Diagnosis and Management Expert Consensus Panel, FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders: Int J Gynaecol Obstet, 2019; 146(1); 20-24
5. Bartels HC, Postle JD, Downey P, Brennan DJ, Placenta accreta spectrum: A review of pathology, molecular biology, and biomarkers: Dis Markers, 2018; 2018; 1507674
6. Yu FNY, Leung KY, Antenatal diagnosis of placenta accreta spectrum (PAS) disorders: Best Pract Res Clin Obstet Gynaecol, 2021; 72; 13-24
7. De Oliveira Carniello M, Oliveira Brito LG, Sarian LO, Bennini JR, Diagnosis of placenta accreta spectrum in high-risk women using ultrasonography or magnetic resonance imaging: Systematic review and meta-analysis: Ultrasound Obstet Gynecol, 2022; 59(4); 428-36
8. Davey MG, Davey MS, Boland MR, Radiomic differentiation of breast cancer molecular subtypes using pre-operative breast imaging – a systematic review and meta-analysis: Eur J Radiol, 2021; 144; 109996
9. Maged AM, El-Mazny A, Kamal N, Diagnostic accuracy of ultrasound in the diagnosis of Placenta accreta spectrum: Systematic review and meta-analysis: BMC Pregnancy Childbirth, 2023; 23(1); 354
10. Sun H, Qu H, Chen L, Identification of suspicious invasive placentation based on clinical MRI data using textural features and automated machine learning: Eur Radiol, 2019; 29(11); 6152-62
11. Ren H, Mori N, Mugikura S, Prediction of placenta accreta spectrum using texture analysis on coronal and sagittal T2-weighted imaging: Abdom Radiol (NY), 2021; 46(11); 5344-52
12. Shao Q, Xuan R, Wang Y, Deep learning and radiomics analysis for prediction of placenta invasion based on T2WI: Math Biosci Eng, 2021; 18(5); 6198-215
13. Xuan R, Li T, Wang Y, Prenatal prediction and typing of placental invasion using MRI deep and radiomic features: Biomed Eng Online, 2021; 20(1); 56
14. Peng L, Zhang X, Liu J, MRI-radiomics-clinical-based nomogram for prenatal prediction of the placenta accreta spectrum disorders: Eur Radiol, 2022; 32(11); 7532-43
15. Wang H, Wang Y, Zhang H, A deep learning pipeline using prior knowledge for automatic evaluation of placenta accreta spectrum disorders with MRI: J Magn Reson Imaging, 2024; 59(2); 483-93
16. Romeo V, Ricciardi C, Cuocolo R, Machine learning analysis of MRI-derived texture features to predict placenta accreta spectrum in patients with placenta previa: Magn Reson Imaging, 2019; 64; 71-76
17. Lin H, Li L, Lin Y, Wang W, Accuracy of magnetic resonance imaging in diagnosing placenta accreta: A systematic review and meta-analysis: Comput Math Methods Med, 2022; 2022; 2751559
18. D’Antonio F, Iacovella C, Palacios-Jaraquemada J, Prenatal identification of invasive placentation using magnetic resonance imaging: Systematic review and meta-analysis: Ultrasound Obstet Gynecol, 2014; 44(1); 8-16
19. Meng X, Xie L, Song W, Comparing the diagnostic value of ultrasound and magnetic resonance imaging for placenta accreta: A systematic review and meta-analysis: Ultrasound Med Biol, 2013; 39(11); 1958-65
20. Lu Y, Zhou L, Wang X, Magnetic resonance imaging-based radiomics nomogram to predict intraoperative hemorrhage of placenta previa: Am J Perinatol, 2023 [Online ahead of print]
21. Warfield SK, Zou KH, Wells WM, Simultaneous truth and performance level estimation (STAPLE): An algorithm for the validation of image segmentation: IEEE Trans Med Imaging, 2004; 23(7); 903-21
22. Jack CR, Bentley MD, Twomey CK, Zinsmeister AR, MR imaging-based volume measurements of the hippocampal formation and anterior temporal lobe: Validation studies: Radiology, 1990; 176(1); 205-9
23. Kruggel F, Turner J, Muftuler LTAlzheimer’s Disease Neuroimaging Initiative, Impact of scanner hardware and imaging protocol on image quality and compartment volume precision in the ADNI cohort: Neuroimage, 2010; 49(3); 2123-33
24. van Timmeren JE, Cester D, Tanadini-Lang S, Radiomics in medical imaging-“how-to” guide and critical reflection: Insights Imaging, 2020; 11(1); 91
25. Lambin P, Leijenaar RTH, Deist TM, Radiomics: The bridge between medical imaging and personalized medicine: Nat Rev Clin Oncol, 2017; 14(12); 749-62
Figures
 Figure 1. Flow diagram of study selection. The figure was created using Microsoft® Word (version 16.0.16626. 20170).
Figure 1. Flow diagram of study selection. The figure was created using Microsoft® Word (version 16.0.16626. 20170). Figure 2. Risk of bias summary. The figure was created using Microsoft® Excel® 2021MSO (version 16.0.17126.20132).
Figure 2. Risk of bias summary. The figure was created using Microsoft® Excel® 2021MSO (version 16.0.17126.20132). Figure 3. Forest plots of the sensitivity and specificity with corresponding heterogeneity statistics. Statistical analyses were carried out using Stata software (version 14.2).
Figure 3. Forest plots of the sensitivity and specificity with corresponding heterogeneity statistics. Statistical analyses were carried out using Stata software (version 14.2). Figure 4. Summary receiver operating characteristic (SROC) curve of the predictive accuracy of PAS. Statistical analyses were carried out using Stata software (version 14.2).
Figure 4. Summary receiver operating characteristic (SROC) curve of the predictive accuracy of PAS. Statistical analyses were carried out using Stata software (version 14.2). Figure 5. Fagan plots for assessing the clinical utility. Statistical analyses were carried out using Stata software (version 14.2).
Figure 5. Fagan plots for assessing the clinical utility. Statistical analyses were carried out using Stata software (version 14.2). Figure 6. (A–C) Forest plot of multiple univariable meta-regression and subgroup analyses for SEN and SPE. Statistical analyses were carried out using Review Manager software (version 5.3).
Figure 6. (A–C) Forest plot of multiple univariable meta-regression and subgroup analyses for SEN and SPE. Statistical analyses were carried out using Review Manager software (version 5.3). Figure 7. Deek’s funnel plot for assessment of publication bias. Statistical analyses were carried out using Stata software (version 14.2).
Figure 7. Deek’s funnel plot for assessment of publication bias. Statistical analyses were carried out using Stata software (version 14.2). Tables
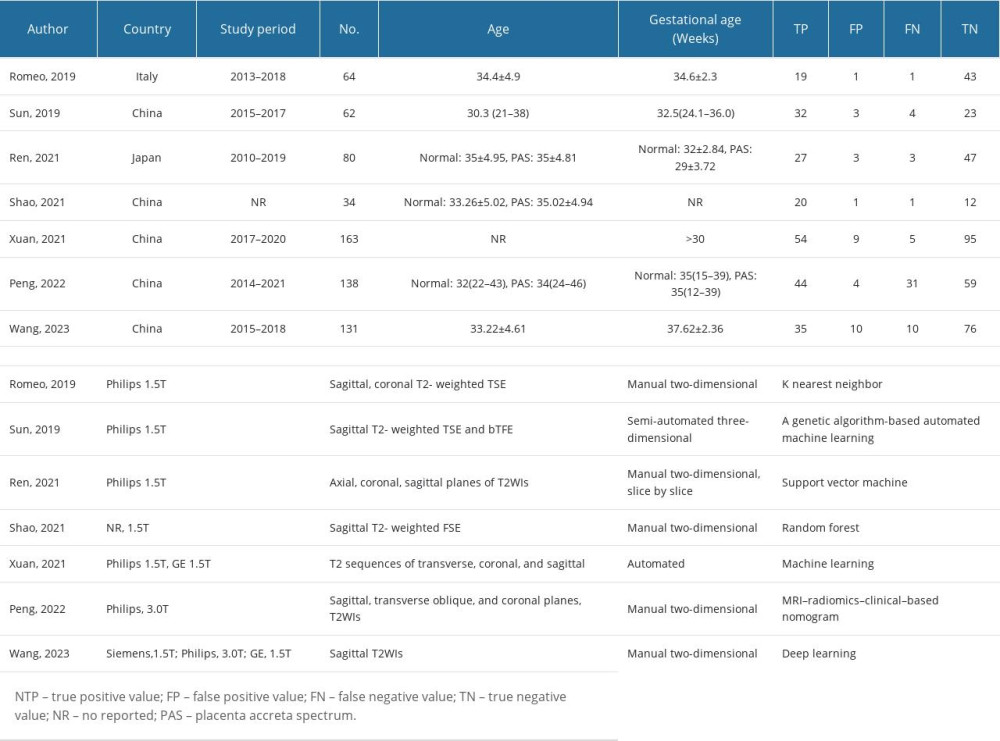 Table 1. The baseline characteristic of included studies.
Table 1. The baseline characteristic of included studies.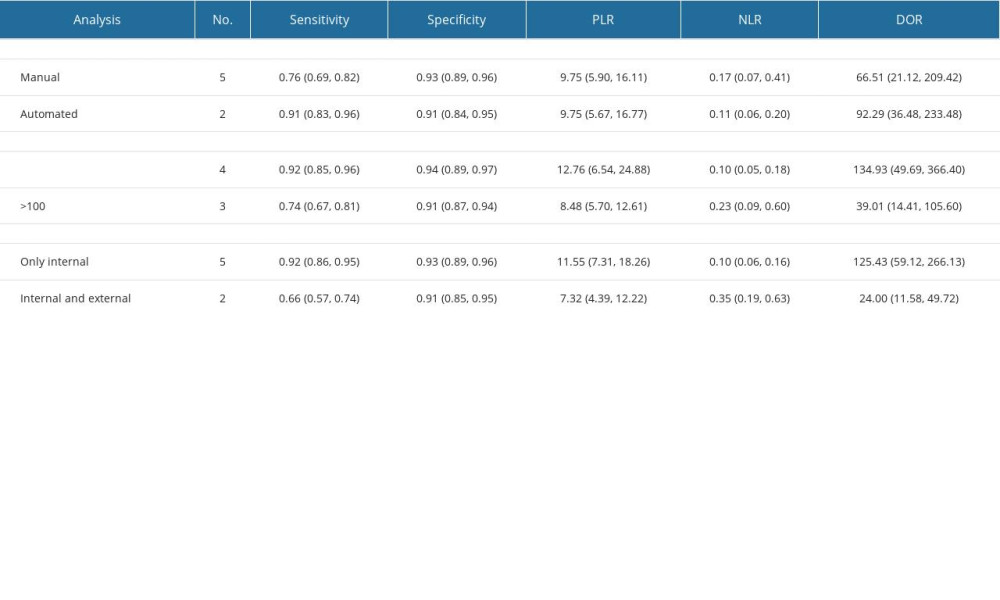 Table 2. The results of subgroup analysis.
Table 2. The results of subgroup analysis.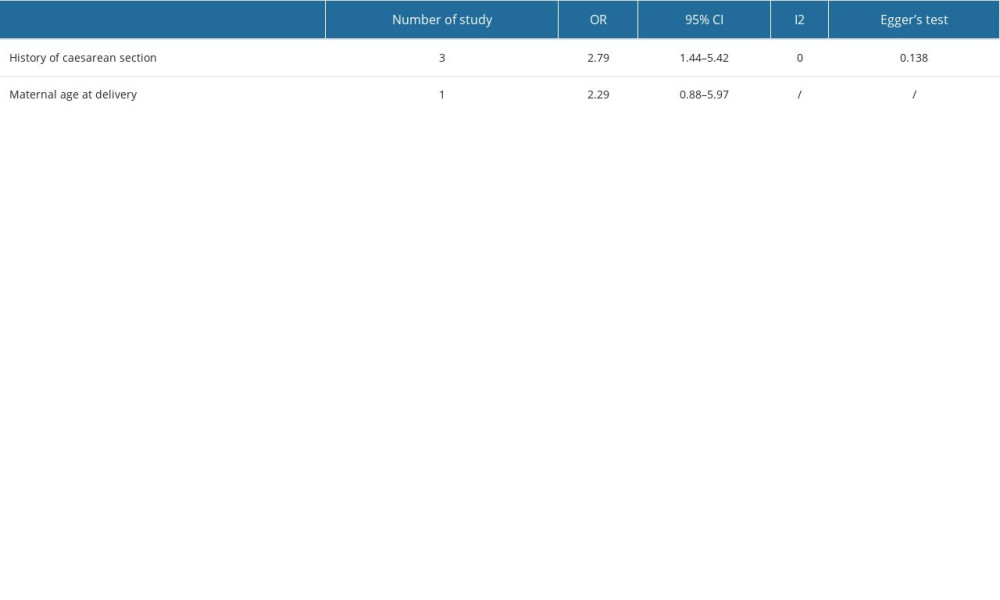 Table 3. Risk factors of placenta accreta syndrome.
Table 3. Risk factors of placenta accreta syndrome. Table 1. The baseline characteristic of included studies.
Table 1. The baseline characteristic of included studies. Table 2. The results of subgroup analysis.
Table 2. The results of subgroup analysis. Table 3. Risk factors of placenta accreta syndrome.
Table 3. Risk factors of placenta accreta syndrome. Supplementary Table 1. Search strategy
Supplementary Table 1. Search strategy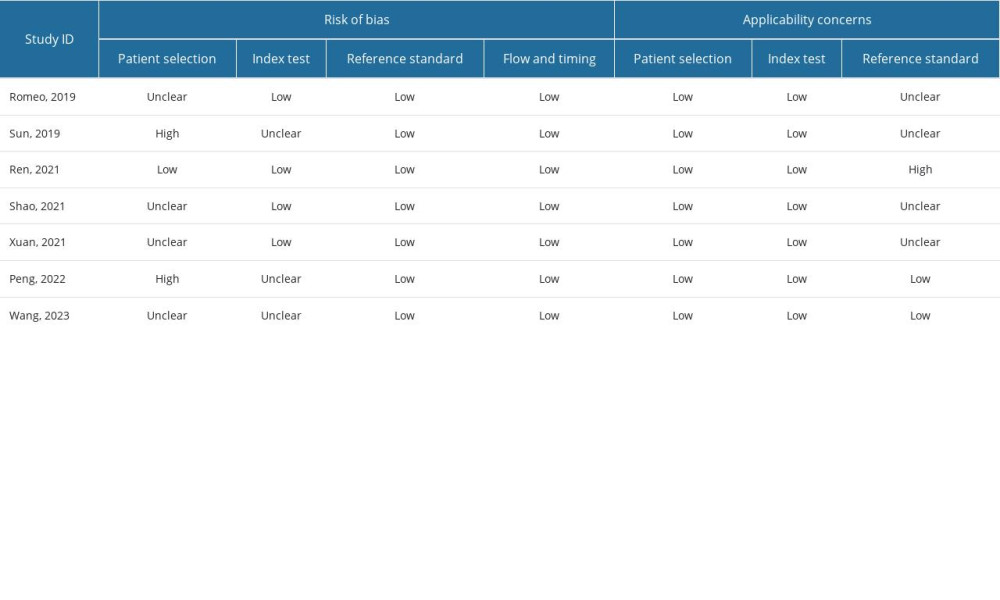 Supplementary Table 2. Quality assessment of each study.
Supplementary Table 2. Quality assessment of each study. In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








