25 March 2024: Clinical Research
Effect of Standardized Bundle Care and Bundle Compliance on Reducing Surgical Site Infections: A Pragmatic Retrospective Cohort Study
Yu-san Chien12CDEF, Hsiang-ting Chen3BC, Hsiu-tzy Chiang4BC, Tz-shin Luo5EF, Hung-i Yeh6AD, Jin-Cherng Sheu7AF, Jiun-yi Li235ABCD*DOI: 10.12659/MSM.943493
Med Sci Monit 2024; 30:e943493
Abstract
BACKGROUND: Care bundles for infection control consist of a set of evidence-based measures to prevent infections. This retrospective study aimed to compare surgical site infections (SSIs) from a single hospital surveillance system between 2017 and 2020, before and after implementing a standardized care bundle across specialties in 2019. It also aimed to assess whether bundle compliance affects the rate of SSIs.
MATERIAL AND METHODS: A care bundle consisting of 4 components (peri-operative antibiotics use, peri-operative glycemic control, pre-operative skin preparation, and maintaining intra-operative body temperature) was launched in 2019. We compared the incidence rates of SSIs, standardized infection ratio (SIR), and clinical outcomes of surgical procedures enrolled in the surveillance system before and after introducing the bundle care. The level of bundle compliance, defined as the number of fully implemented bundle components, was evaluated.
RESULTS: We included 6059 procedures, with 2010 in the pre-bundle group and 4049 in the post-bundle group. Incidence rates of SSIs (1.7% vs 1.0%, P=0.013) and SIR (0.8 vs 1.48, P<0.01) were significantly lower in the post-bundle group. The incidence of SSIs was significantly lower when all bundle components were fully adhered to, compared with when only half of the components were adhered to (0.3% vs 4.0%, P<0.01).
CONCLUSIONS: SSIs decreased significantly after the application of a standardized care bundle for surgical procedures across specialties. Full adherence to all bundle components was the key to effectively reducing the risk of surgical site infections.
Keywords: Surgical Wound Infection, Patient Care Bundles, Guideline Adherence, Infection Control
Introduction
Surgical site infections (SSIs), defined as infections of superficial/deep incision or organ or space occurring after surgery [1,2], are common post-operative complications that account for 20% of all healthcare-associated infections, and are associated with a 2–11 times increased risk of mortality, with 75% of SSI-related death directly attributed to SSIs [3,4]. SSIs can lead to higher healthcare expenses, extended hospital stays, increased visits to the emergency department, and more readmissions [5].
Bundle care, a structured set of evidence-based clinical practices, has been introduced to prevent ventilator-associated pneumonia [6], catheter-related bloodstream infections [7], and catheter-associated urinary tract infections [8]. Results of much recent research that examined the efficacy of bundle care for SSIs were positive, but most of them focused on a single type of surgery, such as colorectal [9–11], cardiovascular [12], or cesarean delivery [13,14]. A recent meta-analysis of 13 uncontrolled before–after studies of various surgical procedures showed that use of a peri-operative care bundle could reduce SSI, but the effect was inconsistent with the pooled outcome of the 4 enrolled randomized control trials, and the interventions used in each study also varied [15]. Whether use of a standardized care bundle could help to prevent SSIs in a multi-specialty setting remained uncertain.
MacKay Memorial Hospital, a tertiary referral center in Taiwan, launched a prospective surveillance system in 2016 to track the incidence and outcomes of SSIs across the hospital, followed by a multi-faceted education campaign, and started an infection control care bundle program starting in 2019.
The goal of this study was to compare the incidence of surgical site infections in a single hospital surveillance system from 2017 to 2020, before and after implementing the standardized SSI care bundle, on surgical procedures across specialties in 2019. It also sought to determine whether compliance with the bundle components affects the rate of SSIs.
Material and Methods
ETHICS APPROVAL:
The study was approved by the Ethics Committee of Mackay Memorial Hospital (approval number 16MMHIS115). Because of the study’s retrospective nature, informed consent was waived.
SSI SURVEILLANCE SYSTEM:
To monitor and understand the incidence and outcomes of surgical site infections at our hospital, a prospective surveillance registry was established in 2016. Major procedures from various surgical departments were gradually included, increasing from 4 in 2017 (coronary artery bypass, Cesarean section, colectomy, and appendectomy) to 19 in 2019 (all cardiovascular surgeries, hysterectomy, mastectomy, gastric or duodenal ulcer surgery, hepatectomy, spinal surgery, head and neck cancer excision, and craniectomy).
A total of 110 variables were obtained for each enrolled procedure, including patient characteristics, date, type, and duration of surgery, peri-operative blood glucose level, American Society of Anesthesiologists’ (ASA) physical status classification score [16], total anesthesia time, skin preparation method, hair removal methods, peri-operative antibiotic use, intra-operative body temperature, wound care information, microbiologic study results within 90 days of surgery, unexpected return to operation rooms during the same hospitalization, survival status at discharge, and length of hospital stay.
While data retrieval from existing electronic medical, nursing, anesthesia and operating room records was automated, SSI was identified and interpreted manually. The electronic profile of each procedure was reviewed and followed by an infection control nurse, and those with surgical site infections that meet the US CDC definition of SSI [1] within 30 days (or 90 days in patients with surgical implants) were manually registered.
SSI CARE BUNDLE:
In 2019, a multidisciplinary team at our hospital designed a standardized care bundle for SSIs based on the recommendations of the Taiwan CDC [17], selecting bundle components that could be applied to surgical procedures across specialties. The care bundle included 4 key components: adequate peri-operative antibiotic use, appropriate peri-operative glycemic control, pre-operative skin preparation, and maintaining intra-operative body temperature. The interventions of each bundle component are detailed below.
To ensure the implementation of the care bundle, surgeons, anesthesiologists, and nurses were required to attend education courses on a regular basis. Interventions from each bundle component were incorporated into the electronic clinical pathways for each enrolled surgery whenever possible. Pop-up notifications would appear on the computerized order entry system to remind doctors of items that had not yet been performed. Patients were routinely provided spoken and written instructions on how to perform pre-operative skin preparation and the nurses were required to supervise the performance.
STUDY DESIGN:
This was a retrospective cohort, before–after study using data from our hospital’s SSI surveillance system collected between January 1, 2017, and December 31, 2020. All surgical procedures reported to the registry during this period were used for analysis.
The main outcome measure was the standardized infection ratio (SIR) for SSIs, calculated by dividing the number of observed SSIs by the number of predicted SSIs. It is a validated risk-adjusted measure used to track the trend of healthcare-associated infections [18,19]. The predicted number of SSIs was calculated by adding up each patient’s probability of SSI, and the probability of each individual patient was estimated using statistically significant risk factors as predictors derived from a national database via a logistic regression model [20]. An SIR greater than 1.0 indicated more infections occurred than initially predicted, while an SIR less than 1.0 meant that fewer SSIs occurred than predicted.
Because bundle care for SSIs of our institute was initiated in 2019, operations recorded in the surveillance registry between 2017 to 2018 were placed in the pre-SSI bundle group, while procedures recorded from 2019 to 2020 were placed in the post-bundle group. The incidence of SSI, SIR, patient characteristics, and clinical outcomes were compared between the 2 groups.
As a process measure, the level of bundle compliance was defined as the total number of fully implemented bundle components, ranging from 0 to 4. Full implementation of a specific bundle component was defined as performance of all interventions listed above. The incidence of SSIs for different levels of bundle compliance was computed and compared.
STATISTICAL ANALYSIS:
Statistical analysis was performed using IBM SPSS Statistics software (version 22.0; IBM Corp., Armonk, NY USA). For categorical variables, results are expressed as n (%) and compared using Pearson’s chi-squared test or Fisher’s exact test. For continuous variables, results are reported as mean±standard deviation (SD) or median and interquartile (IQR) and compared using Student’s t-test and Mann-Whitney U test, as appropriate. Logistic regression models were used to explore independent risk factors for surgical site infections. Univariate analysis was done for each risk factor, and all biologically plausible variables with
Results
COMPARISON OF PRE-BUNDLE AND POST-BUNDLE GROUPS:
During the 4 years of the study period, 6059 procedures were enrolled in our study, including 2010 in the pre-bundle care group and 4049 in the post-bundle care group. Table 1 shows the comparison of these 2 groups. The patients in the post-bundle care group were older (54.9±16.3 vs 42.8±15.5, P<0.01), with more male (30.8% vs 25.3%, P<0.01), more smokers (16.7% vs 13.8%, P<0.01), more diabetics (15.0% vs 11.9%, P<0.01), and more with a ASA scores 3–5 (25.5% vs 20.7%, P<0.01), more operations that lasted ≥4 hours (13.3% vs 10.6%, P<0.01), more procedures with blood loss ≥1500 cc (0.8% vs 0.3%, P<0.01), and fewer emergency surgeries (10.2% vs 19.1%, P<0.01). All bundle components except adequate peri-operative temperature control were better implemented in the post-bundle care group.
Incidence rates of SSIs (1.7% vs 1.0%,
Figure 1 showed the quarterly number of enrolled procedures and the corresponding SIR during the study period. While the number of enrolled procedures gradually increased (from 675 to 2461), the SIR was trending downwards.
COMPARISON OF PATIENTS WITH AND WITHOUT SSIS:
Table 2 shows the comparison between patients with SSIs (SSI group) and those without (non-SSI group). In univariate analysis, the SSI group had more patients with ASA score III to IV (33.8% vs 28.9%, P=0.05), more emergency operations (23.0% vs 13.1%, P=0.02), longer duration of anesthesia (196.3 vs 172.7 minutes, P=0.02), longer operation time (159.0 vs 136.5 minutes, P=0.02), less pre-operative bathing (30.6% vs 43.8%, P=0.017), less use of clippers for hair removal (40.5% vs 56.7%, P<0.01), and more patients with post-operative glycemic level >180 mg/dL (40.7% vs 19.4%, P=0.01). Post-operative hospitals stay and total hospital stay were longer in the SSI group (8.2 days vs 5.7 days; 10.4 days vs 7.3 days, P<0.001). Only post-operative glycemic level >180 mg/dL on day 1 was associated with surgical site infections in multivariate analysis (odds ratio 8.1, 95 CI 1.003–64.845) (Table 3).
ASSOCIATION OF BUNDLE COMPLIANCE AND SSIS:
Figure 2 shows the association between the level of bundle compliance and the incidence of SSI. The incidence of SSI was significantly lower when all bundle components were fully adhered to, compared to when only half of the components were adhered to (0.3% vs 4.0%, P<0.01).
Discussion
In this large, single-center, retrospective cohort analysis of over 6000 surgical procedures, we discovered a significant drop in standardized infection ratio of surgical site infections after properly implementing a single uniformed SSI care bundle across specialties. We also discovered that the more thoroughly the bundle components were implemented, the lower the incidence of SSIs, highlighting the importance of bundle compliance.
Although the benefits of SSI care bundles have been observed in a wide range of specific surgical procedures, there is limited research on applying a standardized bundle care protocol to operations from different medical fields. Our positive finding suggested that this approach could be a practical way to systematically reduce the incidence of SSI in an institution.
According to previous research, a variety of risk factors that pertain to the patient or the surgery itself could affect the risk of SSIs, such as age, body weight, diabetes, smoking, pre-anesthesia physical status, and prolonged operation time [21,22]. In our study, patients in the post-bundle group were older, had more comorbidities, higher ASA scores, more operations exceeding 4 hours, and more surgical blood loss. It is plausible that without intervention, the post-bundle group would have been more susceptible to SSIs. However, our study findings revealed a contrary outcome, indicating that the advantages of bundle care persisted among the high-risk patients.
The study’s multivariate analysis revealed that glycemic control on post-operative day 1 was the sole significant risk factor for SSIs. The odds ratio was 8.1, but the confidence interval was wide (1.003–64.845), which could be attributed to the limited number of patients in the SSI group. Although a meta-analysis of randomized control trials showed favorable outcomes for surgical site infections with tight peri-operative glycemic control, especially when insulin was administered during the post-operative phase [23], additional studies may be necessary to confirm the relevance of blood glucose levels on post-operative day 1.
No other individual component of the SSI care bundle was linked to reducing SSIs. We found that the more bundle components were implemented thoroughly, the lower the risk of SSIs, highlighting the importance of bundle compliance. Similar results were seen in a national survey in the Netherlands, which demonstrated a 13% risk reduction for implementation of each of the 4 bundle elements recommended by the Dutch Hospital Patient Safety Program [23]. However, because uploading data to their surveillance system was not mandatory, only 29% of all included surgeries had complete reporting. The actual incidence of SSIs and the effect of bundle compliance could be under- or overestimated. In the present study, missing data was less an issue since data retrieval of our SSI registry was automated and the source of information was the existing electronic medical system. Bundle compliance might have a greater significance than previously registered.
There are several limitations to our study. First, as an uncontrolled before–after study that evaluated the incidence of SSIs before and after use of a care bundle, it could be challenging to determine whether the outcome was affected by other confounders or whether it occurred independently of other changes over time, such as advancements in surgical techniques and equipment or improvement in post-operative care. Second, we applied the same interventions to 19 surgical operations across specialties. There might be procedure-specific precautions that could further reduce infection rates that were not included in this study. Finally, this was a single-center experience in a well-staffed facility with efficient computerized medical record systems. It could be difficult for other institutions to conduct the same study, obtain the same amount of data for analysis, and to replicate the results.
Conclusions
Incidence of SSI significantly decreased after a standardized care bundle was applied to multiple surgical procedures from various medical specialties. Full adherence to the bundle protocol was the key to effectively reducing the risk of SSIs.
Figures
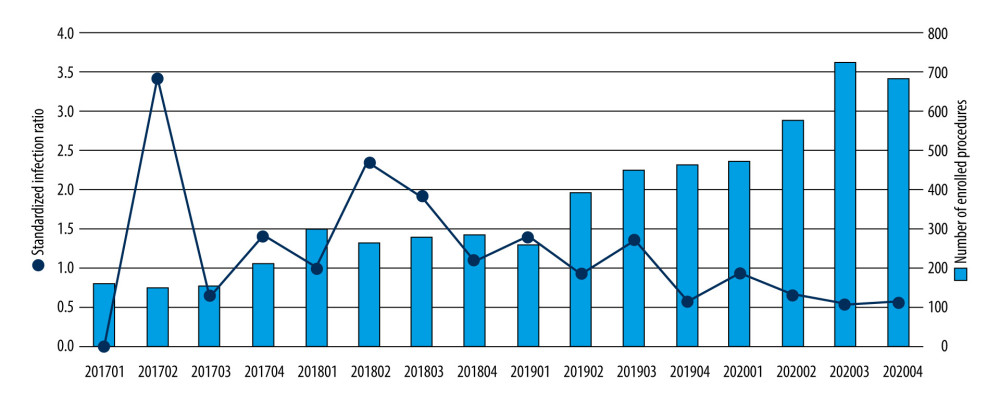 Figure 1. Quarterly number of enrolled procedures, and the trend of standardized infection ratio during the study period (2017–2020). This figure was generated using SPSS Statistics software, version 22.0; IBM Corp., Armonk, NY USA.
Figure 1. Quarterly number of enrolled procedures, and the trend of standardized infection ratio during the study period (2017–2020). This figure was generated using SPSS Statistics software, version 22.0; IBM Corp., Armonk, NY USA. 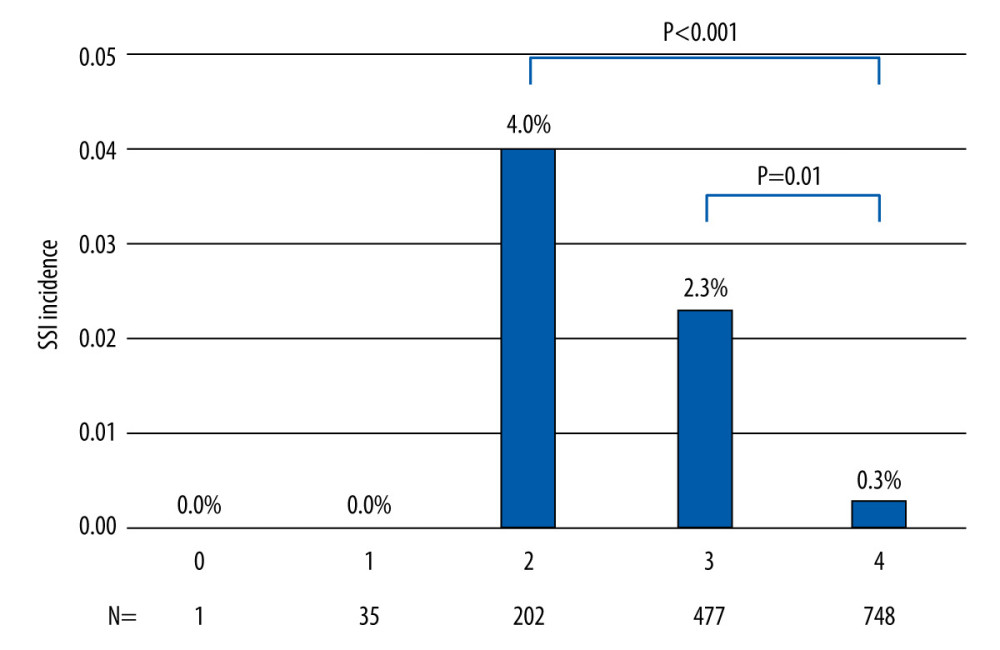 Figure 2. The level of bundle compliance, presented by the number bundle components that was fully implemented, was associated with the incidence of surgical site infection. This figure was generated using SPSS Statistics software, version 22.0; IBM Corp., Armonk, NY USA.
Figure 2. The level of bundle compliance, presented by the number bundle components that was fully implemented, was associated with the incidence of surgical site infection. This figure was generated using SPSS Statistics software, version 22.0; IBM Corp., Armonk, NY USA. References
1. Berríos-Torres SI, Umscheid CA, Bratzler DW, Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017: JAMA Surgery, 2017; 152(8); 784-91
2. Ban KA, Minei JP, Laronga C, American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 Update: J Am Coll Surg, 2017; 224(1); 59-74
3. Awad SS, Adherence to surgical care improvement project measures and post-operative surgical site infections: Surg Infect (Larchmt), 2012; 13(4); 234-37
4. Magill SS, Edwards JR, Bamberg W, Multistate point-prevalence survey of health care-associated infections: N Engl J Med, 2014; 370(13); 1198-208
5. Mastrogianni M, Katsoulas T, Galanis P, The impact of care bundles on ventilator-associated pneumonia (VAP) prevention in adult ICUs: A systematic review: Antibiotics (Basel), 2023; 12(2); 227
6. Selby LM, Rupp ME, Cawcutt KA, Prevention of central-line associated bloodstream infections: 2021 update: Infect Dis Clin North Am, 2021; 35(4); 841-56
7. Musco S, Giammò A, Savoca F, How to prevent catheter-associated urinary tract infections: A reappraisal of Vico’s theory-is history repeating itself?: J Clin Med, 2022; 11(12); 3415
8. Crolla RMPH, van der Laan L, Veen EJ, Reduction of surgical site infections after implementation of a bundle of care: PLoS One, 2012; 7(9); e44599
9. Leaper DJ, Holy CE, Spencer M, Assessment of the risk and economic burden of surgical site infection following colorectal surgery using a US longitudinal database: Is there a role for innovative antimicrobial wound closure technology to reduce the risk of infection?: Dis Colon Rectum, 2020; 63(12); 1628-38
10. Lohsiriwat V, High compliance with surgical site infection (SSI) prevention bundle reduces incisional SSI after colorectal surgery: Ann Coloproctol, 2021; 37(3); 146-52
11. Jayakumar S, Khoynezhad A, Jahangiri M, Surgical site infections in cardiac surgery: Crit Care Clin, 2020; 36(4); 581-92
12. Davidson C, Enns J, Dempster C, Impact of a surgical site infection bundle on cesarean delivery infection rates: Am J Infect Control, 2020; 48(5); 555-59
13. Wang CN, Foo J, Huang IT, Identifying more risk factors for surgical site infection following cesarean section: Eur J Obstet Gynecol Reprod Biol, 2020; 251; 282-84
14. Wolfhagen N, Boldingh QJJ, Boermeester MA, de Jonge SW, Perioperative care bundles for the prevention of surgical-site infections: Meta-analysis: Br J Surg, 2022; 109(10); 933-42
15. Doyle DJ, Hendrix JM, EHG : American Society of Anesthesiologists Classification, 2022, Treasure Island (FL), StatPearls Publishing [updated 2022 Dec 4: cited 2023]: Available from: https://www.ncbi.nlm.nih.gov/books/NBK441940/
16. Huang SJ, Wu LH, Chen YH, Prevention of surgical site infection: Local challenges and strategies of implementation: Infection Control Journal, 2018; 28(1); 17-26
17. Jodra VM, Rodela AR, Martinez FM, Fresneña NL, Group QCIW, Standardized infection ratios for three general surgery procedures: A comparison between Spanish hospitals and US centers participating in the National Nosocomial Infections Surveillance System: Infect Control Hosp Epidemiol, 2003; 24(10); 744-48
18. Chinn R, Lempp JM, Huang SS, Standardized infection ratio for surgical site infection after colon surgery: Discord in models measuring healthcare quality: Infect Control Hosp Epidemiol, 2016; 37(11); 1378-82
19. Centers for Disease Control and Prevention (CDC): The National Healthcare Safety Network (NHSN) Standardized Infection Ratio, A Guide to the SIR, 2022 Available from: https://www.cdc.gov/nhsn/pdfs/ps-analysis-resources/nhsn-sir-guide.pdf
20. Cheadle WG, Risk factors for surgical site infection: Surg Infect (Larchmt), 2006; 7(Suppl 1); S7-11
21. Ansari S, Hassan M, Barry HD, Risk factors associated with surgical site infections: A retrospective report from a developing country: Cureus, 2019; 11(6); e4801
22. Lai J, Li Q, He Y, Glycemic control regimens in the prevention of surgical site infections: A meta-analysis of randomized clinical trials: Front Surg, 2022; 9; 855409
23. Koek MBG, Hopmans TEM, Soetens LC, Adhering to a national surgical care bundle reduces the risk of surgical site infections: PLoS One, 2017; 12(9); e0184200
Figures
 Figure 1. Quarterly number of enrolled procedures, and the trend of standardized infection ratio during the study period (2017–2020). This figure was generated using SPSS Statistics software, version 22.0; IBM Corp., Armonk, NY USA.
Figure 1. Quarterly number of enrolled procedures, and the trend of standardized infection ratio during the study period (2017–2020). This figure was generated using SPSS Statistics software, version 22.0; IBM Corp., Armonk, NY USA. Figure 2. The level of bundle compliance, presented by the number bundle components that was fully implemented, was associated with the incidence of surgical site infection. This figure was generated using SPSS Statistics software, version 22.0; IBM Corp., Armonk, NY USA.
Figure 2. The level of bundle compliance, presented by the number bundle components that was fully implemented, was associated with the incidence of surgical site infection. This figure was generated using SPSS Statistics software, version 22.0; IBM Corp., Armonk, NY USA. Tables
 Table 1. Comparison of characters and clinical outcomes of pre-bundle group and post-bundle group.
Table 1. Comparison of characters and clinical outcomes of pre-bundle group and post-bundle group.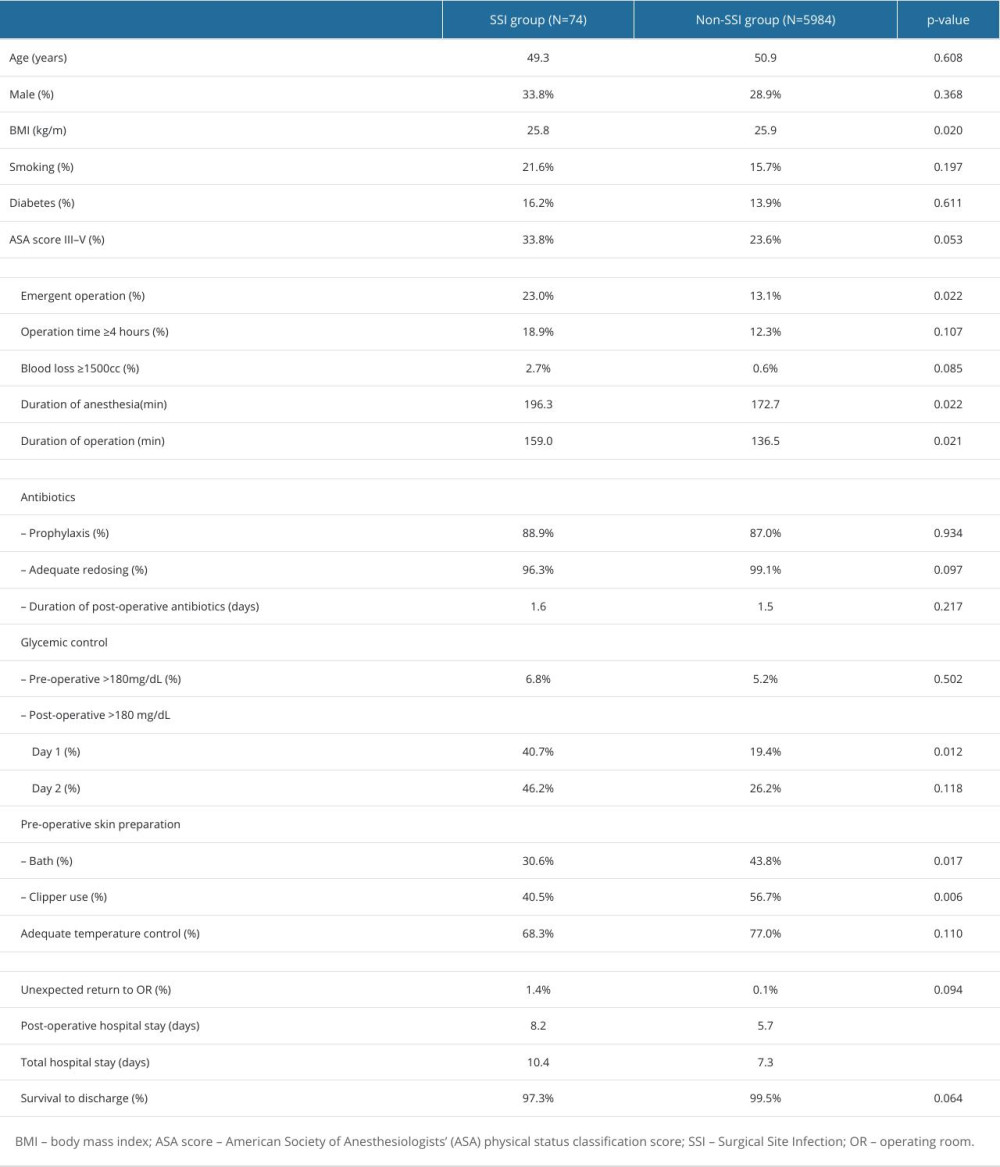 Table 2. Comparison of patients with and without surgical site infections.
Table 2. Comparison of patients with and without surgical site infections.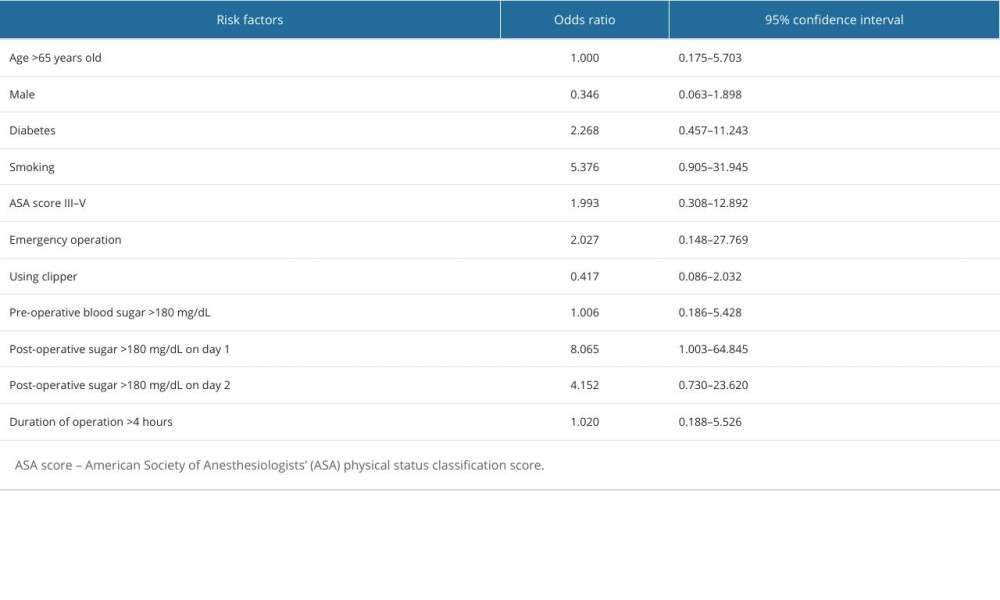 Table 3. Multivariate analysis of risk factors for surgical site infection.
Table 3. Multivariate analysis of risk factors for surgical site infection. Table 1. Comparison of characters and clinical outcomes of pre-bundle group and post-bundle group.
Table 1. Comparison of characters and clinical outcomes of pre-bundle group and post-bundle group. Table 2. Comparison of patients with and without surgical site infections.
Table 2. Comparison of patients with and without surgical site infections. Table 3. Multivariate analysis of risk factors for surgical site infection.
Table 3. Multivariate analysis of risk factors for surgical site infection. In Press
14 Mar 2024 : Clinical Research
Renal Dysfunction Increases Risk of Adverse Cardiovascular Events in 5-Year Follow-Up Study of Intermediate...Med Sci Monit In Press; DOI: 10.12659/MSM.943956
15 Mar 2024 : Clinical Research
Impact of One-Lung Ventilation on Oxygenation and Ventilation Time in Thoracoscopic Heart Surgery: A Compar...Med Sci Monit In Press; DOI: 10.12659/MSM.943089
14 Mar 2024 : Clinical Research
Differential DHA and EPA Levels in Women with Preterm and Term Births: A Tertiary Hospital Study in IndonesiaMed Sci Monit In Press; DOI: 10.12659/MSM.943895
15 Mar 2024 : Clinical Research
Evaluation of an Optimized Workflow for the Radiofrequency Catheter Ablation of Paroxysmal Atrial FibrillationMed Sci Monit In Press; DOI: 10.12659/MSM.943526
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








