19 December 2022: Clinical Research
Retrospective Evaluation of Hematological and Clinical Factors Associated with 30-Day Mortality in 170 Patients Diagnosed with Intracerebral Hematoma in a Single Center in Turkey
Bulent Gulensoy1C*DOI: 10.12659/MSM.938674
Med Sci Monit 2022; 28:e938674
Abstract
BACKGROUND: This retrospective study from a single center in Turkey aimed to evaluate hematological and clinical factors related with 30-day mortality in patients diagnosed with intracerebral hematoma (ICH) between 2013 and 2021.
MATERIAL AND METHODS: All 170 consecutive patients (>18 years) admitted to the Intensive Care Unit (ICU) with spontaneous ICH were analyzed. Cranial computed tomography was performed in all patients. Venous blood samples were routinely obtained upon admission. Demographic characteristics, blood test results, imaging data, and survival data were retrieved from the institutional digital database. The primary goal of this study was to investigate the role of presenting demographic and clinical characteristics and blood tests in predicting 30-day mortality in patients with spontaneous ICH.
RESULTS: Receiver operating characteristic curve analysis showed that the Glasgow coma scale (GCS) score (≤9), hematoma volume (>13.4 cm³), hemoglobin (≤13.1 g/dL), international normalized ratio (>1.25), C-reactive protein (CRP) (>7.5 mg/L), and third-day neutrophil-to-lymphocyte ratio (>17.8) could be used to predict 30-day mortality. Patients with low GCS scores (≤9) had a 14.432-fold higher risk of death than other patients (OR: 14.432, 95% CI: 6.421-32.441, P<0.001). Patients with high CRP levels (>7.5) had a 3.323-fold higher risk of death than other patients (OR: 3.323, 95% CI: 1.491-7.405, P=0.003).
CONCLUSIONS: Tailoring scoring systems to include CRP may be beneficial for predicting spontaneous ICH prognosis. However, further studies assessing CRP and other inflammatory markers are necessary to assess whether inflammatory activity could be associated with worse outcomes in patients with ICH.
Keywords: C-Reactive Protein, Glasgow Coma Scale, Hospital Mortality, Intracranial Hemorrhage, Hypertensive, Humans, Turkey, Hematoma, Cerebral Hemorrhage, Prognosis
Background
Spontaneous intracerebral hematoma (ICH) accounts for 10% to 15% of the strokes in Western countries and Japan [1,2]. It is a condition with a high mortality risk (30–60%) [3,4] that occurs as a consequence of sudden rupture of degenerated intracranial vessels into the cerebral lobes, basal ganglia, thalamus, and brainstem [5]. Neuro-glial tissue at the bleeding site is irreversibly affected, and further growth of the hematoma worsens outcomes [6]. Advanced age, use of anticoagulant agents, presence of uncontrolled hypertension, and excessive alcohol use are the most common risk factors for the development of spontaneous ICH [6]. Despite increasing efforts to control blood pressure among patients with hypertension within the last decades, the increased use of oral anticoagulant and antiaggregant agents indicates that the incidence of spontaneous ICH will probably demonstrate a steady increase with time [7,8].
Magnetic resonance imaging (MRI) and computed tomography (CT) are the first-line choices for ICH diagnosis, but CT may be preferred owing to accessibility and the fact that findings can predict various characteristics [9]. Although well-defined management approaches exist [10], prognosis has been shown to be poor in patients demonstrating hematoma regrowth or re-bleeding, which occur at some degree in about three-quarters of patients within the first few hours of the index event [11]. Prevention of further complications in spontaneous ICH, through intensive medical treatment and/or surgical evacuation, is critical to reduce morbidity and mortality [12]. Factors associated with mortality and poor outcome include the aforementioned risk factors of ICH development, early termination of aggressive therapy, localization, hematoma shape and size, imaging findings, and several laboratory markers, including C-reactive protein (CRP), glucose, and cholesterol fractions [12–16].
Currently there are 3 externally-validated scoring systems used for risk stratification: the ICH score, ICH grading scale, and functional outcome risk stratification scale of intracerebral hemorrhage (FUNC). The ICH score and ICH grading scale can predict 30-day mortality rate, while the FUNC score can predict 90-day functional recovery [17–19]. However, these systems use only clinical characteristics and imaging findings for scoring. To increase predictive power, additional parameters such as the use of anticoagulant drugs [20] and the Full Outline of Unresponsiveness (FOUR) score [21] were added to these scoring systems over time. Using simple laboratory measurements to classify patients, instead of scoring methods that require tedious, time-consuming and costly examinations, would be highly convenient for the prediction of 30-day mortality in patients with spontaneous ICH. In this context, some clinical and radiological factors and, possibly, inflammatory or coagulation parameters can be expected to be associated with mortality rates in spontaneous ICH. Some studies have shown that blood glucose level, white blood cell (WBC) count, and international normalized ratio (INR) are associated with ICH mortality [22–24]. However, there is limited evidence regarding the role of laboratory markers in predicting mortality in spontaneous ICH.
In this retrospective study from a single center in Turkey, we aimed to evaluate hematological and some clinical factors related to 30-day mortality in patients with a diagnosis of ICH between 2013 and 2021.
Material and Methods
ETHICS STATEMENT:
The study was approved by the local ethics committee (Date: December 2, 2021, no: 2021/148).
STUDY DESIGN AND POPULATION:
All consecutive patients (>18 years) admitted to the Intensive Care Unit (ICU) of Lokman Hekim University Training Hospital between January 2013 and February 2021 with spontaneous ICH were analyzed in a retrospective manner. Patients with tumor-related or traumatic ICH, hemorrhagic stroke, subarachnoid hemorrhage, malignant disease, hematological disorders, and active infection and those receiving steroid agents were excluded.
DATA COLLECTION:
All patients underwent detailed systemic and neurological examination within the Emergency Department. Cranial CT was ordered for all patients prior to ICU admission. Venous blood samples for measurement of CRP, activated partial thromboplastin time (aPTT), INR, prothrombin time (PT) values, albumin levels, and complete blood count (CBC) were routinely obtained upon admission. Demographic characteristics, Glasgow coma scale (GCS), laboratory findings, imaging data (including ICH localization and hematoma volume), and survival data were retrieved from the institutional digital database and patient charts. The presence and absence of ischemic infarct, hypertension, arrhythmia, arteriovenous malformation, aneurysm, bleeding disorder, anticoagulant use, and accompanying infection were also recorded. The neutrophil-to-lymphocyte ratio (NLR) was calculated by dividing neutrophil and lymphocyte counts [25] obtained from CBC analyses at admission (first day) to the ICU and on the third day of ICU stay. The GCS is a tool used to assess and calculate a patient’s level of consciousness. It was measured as previously described [26].
ICH TREATMENT:
Craniotomy and surgical drainage of the hematoma were performed in critical situations, including large hematomas with mass effect or midline shift leading to progressive deterioration in consciousness and in patients with delayed neurological deterioration [27].
LABORATORY ANALYSIS:
All laboratory measurements were performed in the Biochemistry Department of Lokman Hekim University Training Hospital, using calibrated standard devices according to routine practice. The CBC was measured 1 h after venous blood was taken into potassium-ethylenediaminetetraacetic acid tubes using the ADVIA 2120i analyzer (Siemens AG, Munich, Germany). The hemoglobin level (reference range 13.5–18 g/dL), platelet count (reference range 150–400×103/μL), WBC count (reference range 4.5–11×103/μL), neutrophil count (reference range 2–7×103/μL), and lymphocyte count (reference range 0.6–3.4×103/μL) were obtained from routine CBC measurements.
The albumin level (reference range 3.5–5.2 g/dL) was measured using the bromocresol green method [28]. Plasma PT (reference range 11.5–15.5 s), aPTT (reference range 26.5–40 s), and INR levels were measured in an auto-analyzer (SYSMEX CA1500; Sysmex Corporation, Kobe, Japan) using commercial kits (Siemens Healthcare Diagnostics; Marburg, Germany). Blood CRP levels (reference range 0.0–5.0) were measured by immunonephelometry on an automated Dimension Vista analyzer (Siemens; Erlangen, Germany).
OUTCOMES:
The primary outcome measure of this study was to investigate the role of presenting demographic and clinical characteristics and blood tests in predicting 30-day mortality among patients with spontaneous ICH.
STATISTICAL ANALYSIS:
All analyses were performed on SPSS version 25 (IBM Corp, Armonk, NY, USA). Histogram and Q-Q plots were used to determine whether continuous variables were normally distributed. Continuous variables are summarized as mean±standard deviation if the variables were normally distributed or as median (first quartile – third quartile) if the variables were not normally distributed, while frequency (percentage) values were used for categorical variables. Continuous variables were analyzed with the independent samples
Results
PATIENTS’ GENERAL CHARACTERISTICS AND UNIVARIATE ANALYSIS RESULTS:
A total of 170 patients were analyzed retrospectively (mean age 66.2±13.9 years, 52.4% male). Mortality occurred within 30 days in 84 (49.4%) patients. The comparison of survivors and deceased patients is presented in Table 1.
Presenting mean systolic blood pressure (
Laboratory test results revealed that median PT (
PERFORMANCE OF THE VARIABLES TO PREDICT 30-DAY MORTALITY:
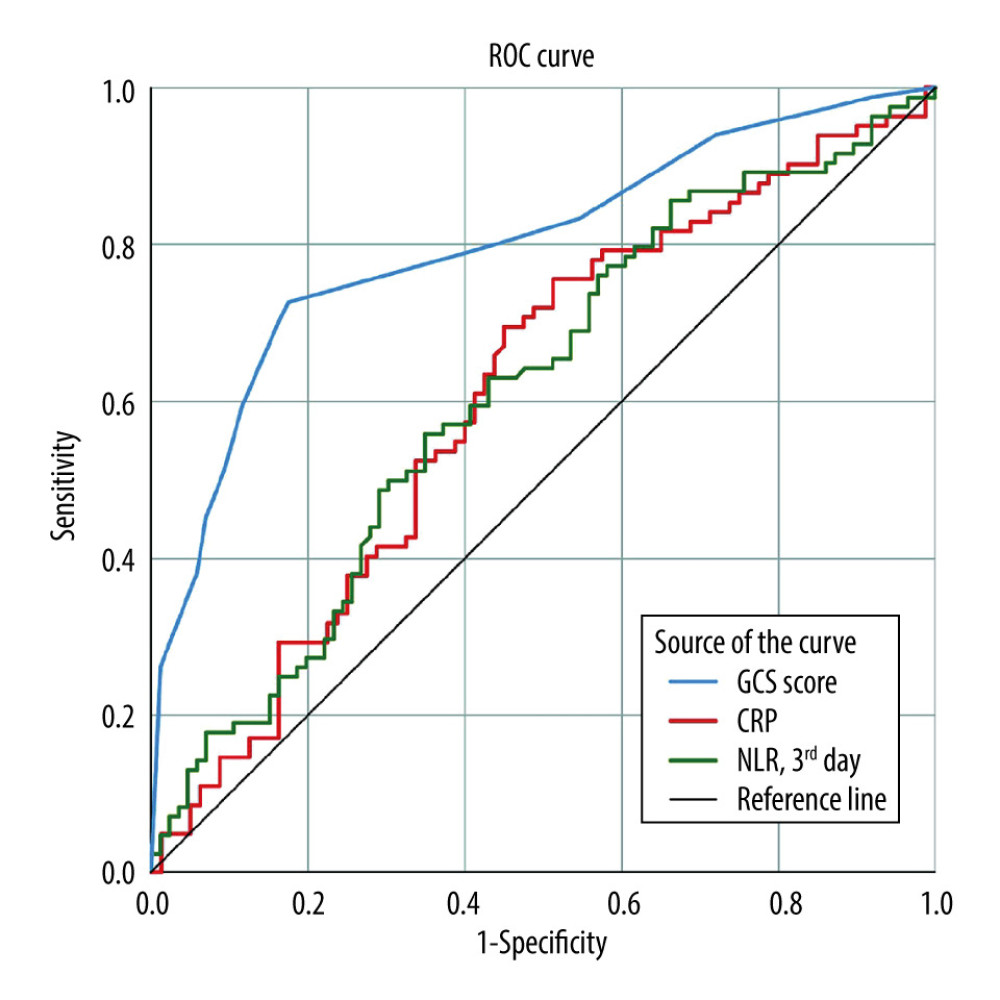
ROC curve analysis revealed the following predictive capabilities for 30-day mortality: GCS score ≤9 (sensitivity 72.6%, specificity 82.6%), hematoma volume >13.4 cm (sensitivity 56.9%, specificity 76.5%), hemoglobin ≤13.1 (sensitivity 66.7%, specificity 55.8%), INR >1.25 (sensitivity 46.8%, specificity 74.7%), CRP >7.5 mg/L (sensitivity 69.5%, specificity 55.0%), and third-day NLR >17.8 (sensitivity 56.0%, specificity 65.1%) (Table 2, Figure 1).
MULTIVARIABLE ANALYSIS RESULTS:
We performed multiple logistic regression analysis to determine the best predictive factors associated with 30-day mortality. Patients with low GCS scores (≤9) had a 14.432-fold higher risk of death than other patients (OR: 14.432, 95% CI: 6.421–32.441; P<0.001). Patients with high CRP levels (>7.5) had a 3.323-fold higher risk of death than other patients (OR: 3.323, 95% CI: 1.491–7.405; P=0.003) (Table 3). Other variables included in the model, age (P=0.348), sex (P=0.228), blood pressure (P=0.449), hematoma volume (P=0.995), presence of intraventricular hematoma (P=0.120), need for surgery during hospitalization (P=0.315), hemoglobin level (P=0.563), INR (P=0.401), and third-day NLR (P=0.129) were found to be nonsignificant (Table 3).
Discussion
The main findings of this study are summarized as follows. The 30-day mortality rate of the study group was 49.4%. Patients demonstrating mortality within the first 30 days of spontaneous ICH presented with lower GCS score, systolic blood pressure, hemoglobin, and platelet count, while they had higher hematoma volume, aPTT, PT, INR, CRP, and third-day NLR levels compared with patients who survived. The frequency of intraventricular hematoma and need for surgical intervention was significantly higher among deceased patients than survivors. A GCS score of ≤9, hematoma volume >13.4 cm, hemoglobin ≤13.1 g/dL, INR >1.25, CRP >7.5 mg/L, and third-day NLR >17.8 were predictive of 30-day mortality with low but relevant sensitivity and specificity values. Multiple logistic regression analysis revealed that patients with a presenting GCS score of ≤9 and CRP >7.5 mg/L had higher risk of 30-day mortality.
Spontaneous ICH constitutes 10% to 15% of all strokes; however, the resultant morbidity and mortality is disproportionate with its incidence. Early diagnosis, risk stratification, and management are crucial to prevent excessive morbidity and mortality from spontaneous ICH. Although therapeutic options are well defined, they have limited influence on survival. Risk stratification based on presenting physical examination and imaging findings have been demonstrated to provide insight to the prognosis of spontaneous ICH, facilitate clinical decision making, and identify patients requiring more intensive treatment or surgery. Several prognostic models and sole risk factors have been introduced to date [13,17,18,29–32]. The risk stratification model by Chuang et al, which demonstrated a sensitivity of 82.5% and specificity of 80.2% for prediction of poor outcomes, included age, GCS score, serum glucose, history of hypertension, and dialysis dependency [33]. The FUNC score described by Rost et al included age, GCS score, ICH location, ICH volume, and pre-ICH cognitive impairment as factors associated with functional independence at 90 days, with higher scores showing better outcomes [18]. The emergency department ICH score developed by Zis et al used GCS score, ICH location, ICH volume, INR, and presence of intraventricular hemorrhage to predict mortality with a high sensitivity and specificity in patients with spontaneous ICH [22]. The relatively recent Intracerebral Hemorrhage Outcomes Project (ICHOP) score incorporates data from available ICH-related scores and other parameters (baseline functionality and APACHE II) and has been reported to predict 1-year unfavorable outcomes in patients [34]. More recently, the risk stratification model developed by Sembill et al, which includes age, presence of intraventricular hemorrhage, lobar ICH volume, National Institutes of Health Stroke Scale, non-lobar ICH volume, and oral anticoagulation use, has also been shown to be predictive of unfavorable outcomes at the first year [35]. Houben et al evaluated the power of a new ICH score they created (by adding the use of oral anticoagulants to the ICH score) in predicting 30-day mortality. They reported that the ICH score was a useful tool for estimating 30-day mortality in oral anticoagulant users and non-oral anticoagulant users, and that oral anticoagulant use was an independent predictor of 30-day mortality. However, they emphasized that adding the use of oral anticoagulants to the existing ICH score did not improve the prognostic performance of this score [20]. In another study, a new scoring system was defined by using the FOUR score, developed to help clinicians in the clinical evaluation and localization of lesions within the brain of patients with impaired consciousness, as the substitute of the GCS in the ICH score. It has been reported that both the traditional ICH and the newly-defined ICH score can predict 1-month mortality with comparable accuracy [21]. Safatli et al showed that GCS score on admission, baseline volume, and the localization of the hemorrhage were strong predictors for 30-day mortality in patients with spontaneous primary ICH. Their results showed that ICH score and ICH-GS accurately predicted 30-day mortality [19]. The study by Hemphill et al showed that GCS score, age greater than 80 years, volume of the hematoma, and infratentorial origin were independently associated with 30-day mortality in patients with spontaneous ICH [17]. Tuhrim et al reported that ventricular blood volume was an independent predictor in patients with supratentorial intracerebral hemorrhages [36]. The study by Romero et al showed that CT characteristics, number of spot signs, maximum axial dimension, and maximum attenuation could precisely predict unfavorable outcomes in patients with spontaneous ICH [37].
Clinical and neurological findings at presentation, imaging findings, and laboratory test results are included in these models; however, many of these risk stratification models are time consuming as a result of the need for detailed clinical and imaging assessment. Although all these risk stratifying models have advantages and disadvantages, they have been shown to predict outcomes in patients with spontaneous ICH with acceptable sensitivity and specificity. Moreover, risk stratifying should be done immediately prior to the initiation of the management. Nevertheless, all these risk stratifying tools are time consuming and not user-friendly, particularly in the emergency setting. This study therefore aimed to identify simple, readily-available, and user-friendly markers that could predict mortality in patients presenting with spontaneous ICH. We investigated whether demographic and clinical characteristics, ICH volume, anticoagulant use, and simple laboratory markers would be useful in this manner. Our findings showed that only GCS score and CRP were independently associated with 30-day mortality in our group of patients with spontaneous ICH. While a GCS score of ≤9 was found to be associated with a 14.432-fold increase in 30-day mortality, having a CRP value of >7.5 mg/L was associated with a 3.323-fold higher risk of death within 30 days in patients with spontaneous ICH. In this context, we consider that the presenting GCS score and CRP value might be used to discriminate patients at a higher risk for 30-day mortality and address more intensive care and management in this patient subset. Our findings also support previous data indicating that CRP was associated with mortality when evaluated together with the ICH score [15].
We consider that our paper in its current form provides valuable data contributing to the body of literature dealing with ICH and risk factors associated with poor outcome. However, this study has some limitations to be mentioned. The retrospective design and relatively small sample size are the main drawbacks of this study. Lack of CT angiography and absence of more sophisticated parameters of ICH growth such as ‘spot sign’ or ‘leakage sign’ are among the other limitations of this study. The study includes data for an 8-year period. In today’s world, in which the diagnosis and treatment methods of diseases are developing and changing rapidly, this period can be considered as a relatively long time. Since the retrospective design of the study allowed us to use only the information recorded in the database, we could not include possible informal changes in the diagnosis and treatment methods of the patients.
Conclusions
In conclusion, a low GCS score and high CRP level were found to be independently associated with 30-day mortality in patients with spontaneous ICH. Thus, tailoring scoring systems to include CRP may be beneficial for predicting spontaneous ICH prognosis. However, further studies assessing CRP and other inflammatory markers are necessary to assess whether inflammatory activity could be associated with worse outcomes in patients with ICH.
Tables
Table 1. Summary of patients’ clinicodemographic characteristics and laboratory measurements with regard to 30-day mortality in patients with spontaneous intracerebral hematoma.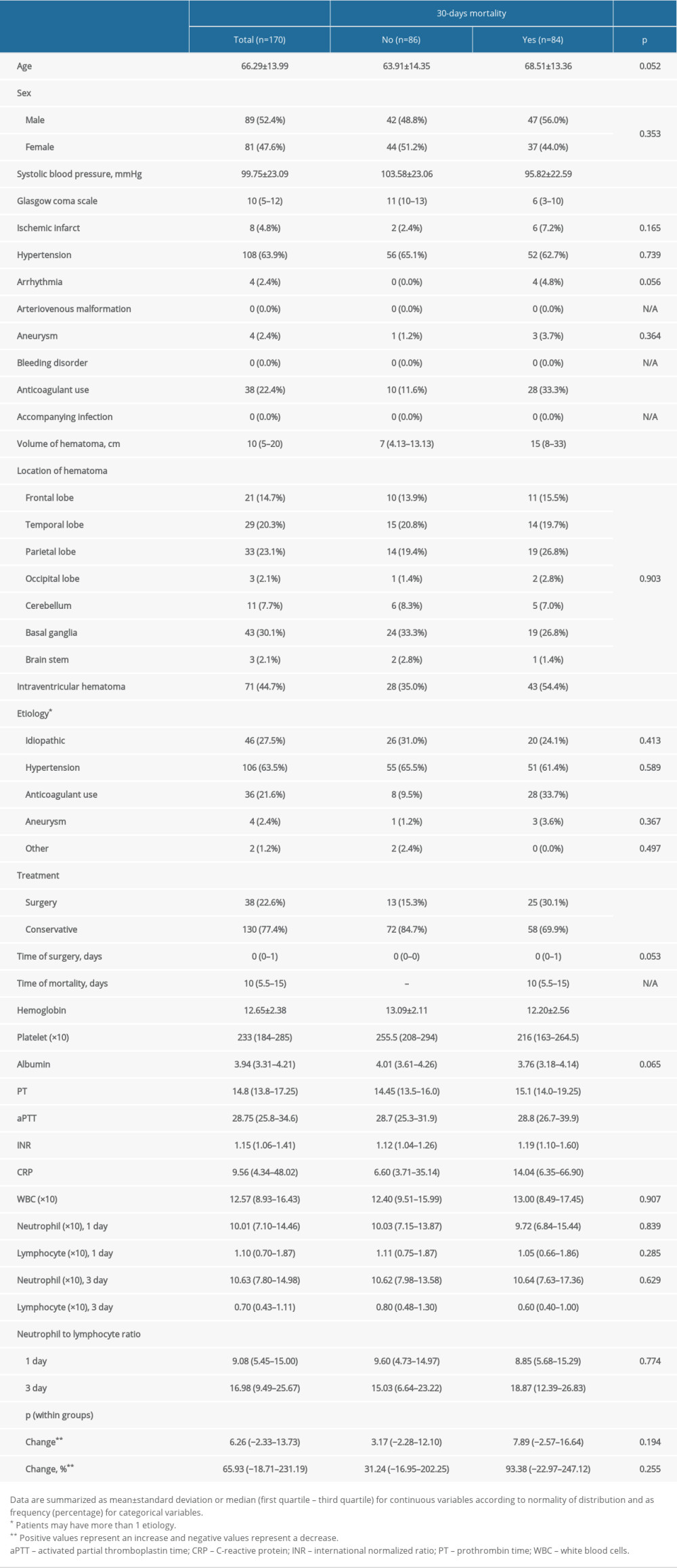 Table 2. Predictive performance of significant variables to predict 30-day mortality in patients with spontaneous intracerebral hematoma.
Table 2. Predictive performance of significant variables to predict 30-day mortality in patients with spontaneous intracerebral hematoma.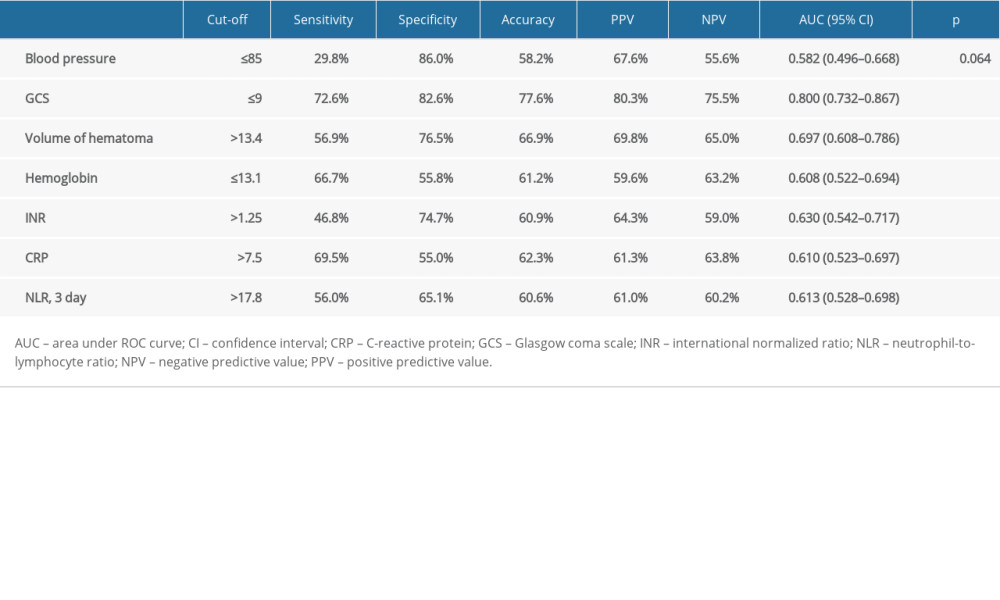 Table 3. Factors independently associated with 30-days mortality in patients with spontaneous intracerebral hematoma, determined by multiple logistic regression analysis.
Table 3. Factors independently associated with 30-days mortality in patients with spontaneous intracerebral hematoma, determined by multiple logistic regression analysis.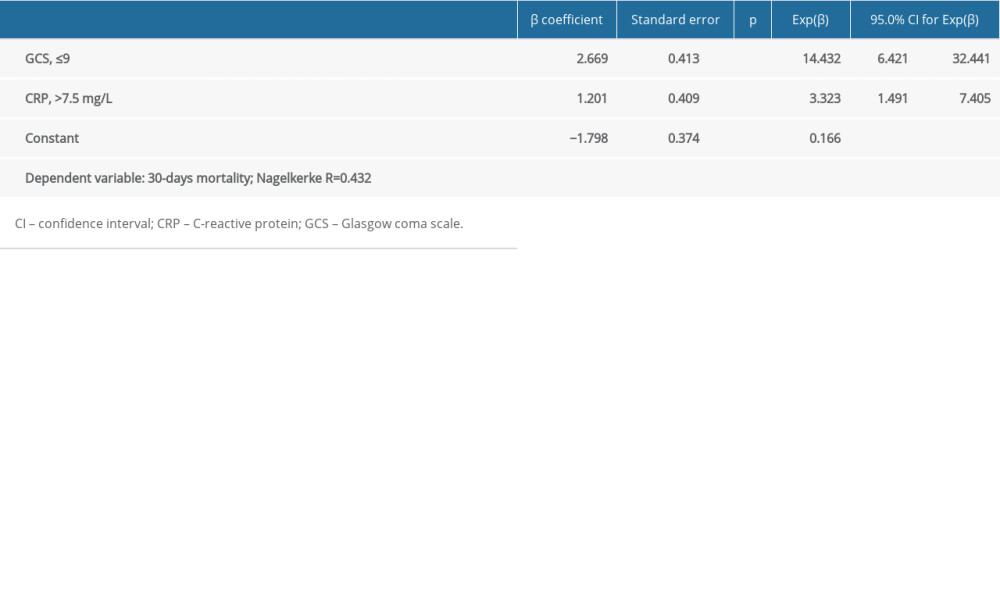
References
1. Qureshi AI, Mendelow AD, Hanley DF, Intracerebral haemorrhage: Lancet, 2009; 373(9675); 1632-44
2. Hemphill JC, Greenberg S, Anderson CAmerican Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursingsemicolon1; Council on Clinical Cardiology, Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American heart Association/American stroke association: Stroke, 2015; 46(7); 2032-60
3. Moulin S, Cordonnier C, Prognosis and outcome of intracerebral haemorrhage: Front Neurol Neurosci, 2015; 37; 182-92
4. Fernando SM, Qureshi D, Talarico R, Intracerebral hemorrhage incidence, mortality, and association with oral anticoagulation use: A population study: Stroke, 2021; 52(5); 1673-81
5. Morioka M, Orito K, Management of spontaneous intracerebral hematoma: Neurol Med Chir (Tokyo), 2017; 57(11); 563
6. O’donnell MJ, Chin SL, Rangarajan S, Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): A case-control study: Lancet, 2016; 388(10046); 761-75
7. Toyoda K, Bleeding with Antithrombotic Therapy (BAT) Study Group: Dual antithrombotic therapy increases severe bleeding events in patients with stroke and cardiovascular disease: A prospective, multicenter, observational study: Stroke, 2008; 39; 1740-45
8. Ruff CT, Giugliano RP, Antman EM, Management of bleeding with non-vitamin K antagonist oral anticoagulants in the era of specific reversal agents: Circulation, 2016; 134(3); 248-61
9. Phan TG, Krishnadas N, Lai VWY, Meta-analysis of accuracy of the spot sign for predicting hematoma growth and clinical outcomes: Stroke, 2019; 50(8); 2030-36
10. Qureshi AI, Tuhrim S, Broderick JP, Spontaneous intracerebral hemorrhage: N Engl J Med, 2001; 344(19); 1450-60
11. Hankey GJ, Sudlow CL, Dunbabin DW, Thienopyridines or aspirin to prevent stroke and other serious vascular events in patients at high risk of vascular disease? A systematic review of the evidence from randomized trials: Stroke, 2000; 31(7); 1779-84
12. Woo D, Comeau ME, Venema SU, Risk factors associated with mortality and neurologic disability after intracerebral hemorrhage in a racially and ethnically diverse cohort: JAMA Netw Open, 2022; 5(3); e221103
13. Broderick JP, Brott TG, Duldner JE, Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality: Stroke, 1993; 24(7); 987-93
14. Parry-Jones AR, Sammut-Powell C, Paroutoglou K, An intracerebral hemorrhage care bundle is associated with lower case fatality: Ann Neurol, 2019; 86(4); 495-503
15. Di Napoli M, Godoy DA, Campi V, C-reactive protein level measurement improves mortality prediction when added to the spontaneous intracerebral hemorrhage score: Stroke, 2011; 42(5); 1230-36
16. Rodriguez-Luna D, Rubiera M, Ribo M, Serum low-density lipoprotein cholesterol level predicts hematoma growth and clinical outcome after acute intracerebral hemorrhage: Stroke, 2011; 42(9); 2447-52
17. Hemphill JC, Bonovich DC, Besmertis L, The ICH score: A simple, reliable grading scale for intracerebral hemorrhage: Stroke, 2001; 32(4); 891-97
18. Rost NS, Smith EE, Chang Y, Prediction of functional outcome in patients with primary intracerebral hemorrhage: the FUNC score: Stroke, 2008; 39(8); 2304-9
19. Safatli DA, Günther A, Schlattmann P, Predictors of 30-day mortality in patients with spontaneous primary intracerebral hemorrhage: Surg Neurol Int, 2016; 7(Suppl 18); S510
20. Houben R, Schreuder FH, Bekelaar KJ, Predicting prognosis of intracerebral hemorrhage (ICH): Performance of ICH score is not improved by adding oral anticoagulant use: Front Neurol, 2018; 9; 100
21. Braksick SA, Hemphill JC, Mandrekar J, Application of the FOUR score in intracerebral hemorrhage risk analysis: J Stroke Cerebrovasc Dis, 2018; 27(6); 1565-69
22. Zis P, Leivadeas P, Michas D, Predicting 30-day case fatality of primary inoperable intracerebral hemorrhage based on findings at the emergency department: J Stroke Cerebrovasc Dis, 2014; 23(7); 1928-33
23. Zou J, Chen H, Liu C, Development and validation of a nomogram to predict the 30-day mortality risk of patients with intracerebral hemorrhage: Front Neurosci, 2022; 16; 942100
24. Huang X, Wang D, Zhang Q, Development and validation of a clinical-based signature to predict the 90-day functional outcome for spontaneous intracerebral hemorrhage: Front Aging Neurosci, 2022; 14; 904085
25. Goyal N, Tsivgoulis G, Chang JJ, Admission neutrophil-to-lymphocyte ratio as a prognostic biomarker of outcomes in large vessel occlusion strokes: Stroke, 2018; 49(8); 1985-87
26. Mehta R, Chinthapalli K, Glasgow coma scale explained: BMJ, 2019; 365; I1296
27. Kim JY, Bae H-J, Spontaneous intracerebral hemorrhage: Management: J Stroke, 2017; 19(1); 28
28. Hao N, Cheng B-C, Yang H-T, Time-varying serum albumin levels and all-cause mortality in prevalent peritoneal dialysis patients: A 5-year observational study: BMC Nephrol, 2019; 20(1); 254
29. Juvela S, Risk factors for impaired outcome after spontaneous intracerebral hemorrhage: Arch Neurol, 1995; 52(12); 1193-200
30. Qureshi AI, Safdar K, Weil EJ, Predictors of early deterioration and mortality in black Americans with spontaneous intracerebral hemorrhage: Stroke, 1995; 26(10); 1764-67
31. Tuhrim S, Dambrosia JM, Price TR, Intracerebral hemorrhage: External validation and extension of a model for prediction of 30-day survival: Ann Neurol, 1991; 29(6); 658-63
32. Ruiz-Sandoval JL, Chiquete E, Romero-Vargas S, Grading scale for prediction of outcome in primary intracerebral hemorrhages: Stroke, 2007; 38(5); 1641-44
33. Chuang Y-C, Chen Y-M, Peng S-K, Risk stratification for predicting 30-day mortality of intracerebral hemorrhage: Int J Qual Health Care, 2009; 21(6); 441-47
34. Gupta VP, Garton AL, Sisti JA, Prognosticating functional outcome after intracerebral hemorrhage: the ICHOP score: World Neurosurg, 2017; 101; 577-83
35. Sembill JA, Gerner ST, Volbers B, Severity assessment in maximally treated ICH patients: the max-ICH score: Neurology, 2017; 89(5); 423-31
36. Tuhrim S, Horowitz DR, Sacher M, Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage: Crit Care Med, 1999; 27(3); 617-21
37. Romero JM, Brouwers HB, Lu J, Prospective validation of the computed tomographic angiography spot sign score for intracerebral hemorrhage: Stroke, 2013; 44(11); 3097-102
Tables
 Table 1. Summary of patients’ clinicodemographic characteristics and laboratory measurements with regard to 30-day mortality in patients with spontaneous intracerebral hematoma.
Table 1. Summary of patients’ clinicodemographic characteristics and laboratory measurements with regard to 30-day mortality in patients with spontaneous intracerebral hematoma. Table 2. Predictive performance of significant variables to predict 30-day mortality in patients with spontaneous intracerebral hematoma.
Table 2. Predictive performance of significant variables to predict 30-day mortality in patients with spontaneous intracerebral hematoma. Table 3. Factors independently associated with 30-days mortality in patients with spontaneous intracerebral hematoma, determined by multiple logistic regression analysis.
Table 3. Factors independently associated with 30-days mortality in patients with spontaneous intracerebral hematoma, determined by multiple logistic regression analysis. Table 1. Summary of patients’ clinicodemographic characteristics and laboratory measurements with regard to 30-day mortality in patients with spontaneous intracerebral hematoma.
Table 1. Summary of patients’ clinicodemographic characteristics and laboratory measurements with regard to 30-day mortality in patients with spontaneous intracerebral hematoma. Table 2. Predictive performance of significant variables to predict 30-day mortality in patients with spontaneous intracerebral hematoma.
Table 2. Predictive performance of significant variables to predict 30-day mortality in patients with spontaneous intracerebral hematoma. Table 3. Factors independently associated with 30-days mortality in patients with spontaneous intracerebral hematoma, determined by multiple logistic regression analysis.
Table 3. Factors independently associated with 30-days mortality in patients with spontaneous intracerebral hematoma, determined by multiple logistic regression analysis. In Press
12 Mar 2024 : Database Analysis
Risk Factors of Age-Related Macular Degeneration in a Population-Based Study: Results from SHIP-TREND-1 (St...Med Sci Monit In Press; DOI: 10.12659/MSM.943140
12 Mar 2024 : Clinical Research
Preoperative Blood Transfusion Requirements for Hemorrhoidal Severe Anemia: A Retrospective Study of 128 Pa...Med Sci Monit In Press; DOI: 10.12659/MSM.943126
12 Mar 2024 : Clinical Research
Tissue Inhibitors of Metalloproteinase 1 (TIMP-1) and 3 (TIMP-3) as New Markers of Acute Kidney Injury Afte...Med Sci Monit In Press; DOI: 10.12659/MSM.943500
12 Mar 2024 : Review article
Optimizing Behçet Uveitis Management: A Review of Personalized Immunosuppressive StrategiesMed Sci Monit In Press; DOI: 10.12659/MSM.943240
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952