05 June 2023: Database Analysis
Effect of Cefoperazone/Sulbactam on Blood Coagulation Function in Infected Emergency Department Patients and the Necessity |of Vitamin K1 (VK1) Preventive Intervention: A Single-Center, Retrospective Analysis
Xupeng Shao1ABCDEFG, Yuanyuan Ren1B, Na Xie1BC, Kailiang Fan1A*, Haiyan Sun1EF, Jiamin Lu2E, Jiuxiang Wei2F, Xincheng Tian2FDOI: 10.12659/MSM.939203
Med Sci Monit 2023; 29:e939203
Abstract
BACKGROUND: Owing to its broad-spectrum antibacterial activity, strong antibacterial effects, and β-lactamase stability, cefoperazone/sulbactam has been recognized as a first-line empirical drug for treating severe infections. However, its administration is also characterized by numerous adverse effects, including coagulation dysfunction. Here, we summarize past clinical treatment data to provide data support for clinical use of cefoperazone sulbactam.
MATERIAL AND METHODS: We retrospectively analyzed the clinical medical records of 820 patients treated with cefoperazone/sulbactam from January 2015 to December 2020. A retrospective cohort study design was used. We assessed the general data of patients, age and sex distribution, type of primary disease, and incidence and days of abnormal blood coagulation with cefoperazone sulbactam. The chi-square test and t test were used to analyze the effect of cefoperazone sulbactam on coagulation function and the effect of vitamin K intervention on prognosis.
RESULTS: The rate of coagulation dysfunction was 24.39% (200 patients). Among these 200 patients, 50 were treated with vitamin K1. With increasing patient age, the number of patients with cefoperazone/sulbactam-induced coagulation dysfunction increased (peak at 81-90 years). APACHE II of coagulation dysfunction (15.54±4.095) was significantly higher than that in the normal group. It occurred at days 2-19 after administration of 9.0 g/day of cefoperazone/sulbactam. Measured coagulation indices were significantly higher after treatment with cefoperazone/sulbactam than before treatment, including international normalized ratio, prothrombin time, and activated partial thrombin time (P<0.0001).
CONCLUSIONS: All coagulation indices decreased significantly after vitamin K1 intervention, indicating improved coagulation function, especially in patients with high APACHE II scores. Hence, regulated vitamin K1 administration can benefit patients with coagulation dysfunction in clinical treatment.
Keywords: Cefoperazone, Blood Coagulation Disorders, Vitamin K1 Oxide, Aged, 80 and over, Humans, Anti-Bacterial Agents, Blood Coagulation, Microbial Sensitivity Tests, Retrospective Studies, Sulbactam, Vitamin K 1, Male, Female, Adult, Middle Aged, Aged, Emergency Service, Hospital
Background
Cefoperazone/sulbactam is a widely used antibiotic combination for treating moderate to severe infections and is among the top 10 prescribed antibiotics for hospital-acquired infections [1]. Cefoperazone/sulbactam has broad-spectrum antibacterial activity, strong antibacterial effects, and β-lactamase stability. Cefoperazone prevents formation of the bacterial cell wall, and sulbactam is a β-lactamase inhibitor that reduces the resistance and activity of cefoperazone against bacteria. Thus, it has become a first-line empirical drug for treating severe infections.
The bacteria responsible for nosocomial infection are particularly multi-drug resistant [2]. Therefore, the combination of cefoperazone/sulbactam is widely used clinically to treat respiratory tract infections, urinary tract infections, peritonitis, cholecystitis, sepsis, meningitis, skin and soft tissue infections, bone and joint infections, pelvic inflammation, endometritis, gonorrhea, and other reproductive tract infections [3]. The adverse effects of cefoperazone/sulbactam include coagulation disorders, bleeding, abdominal pain, diarrhea, blood in urine, hemoptysis, skin and mucous membrane congestion, or ecchymosis [4]. Coagulation disorders, prolonged prothrombin time (PT), and activated partial thrombin time (APTT) have also been observed [5]. Kinasewitz et al found that inflammatory reaction and coagulation system changes were correlated with the severity and mortality rate of sepsis [6]. A study showed that 25.8% of patients administered cefoperazone/sulbactam exhibited coagulation dysfunction, which most frequently occurred 3–12 days after drug use [7]. Moreover, the incidence of coagulation dysfunction was higher in elderly and critically ill patients. Treatment with vitamin K1 improves coagulation function [8]. In this study, patients with coagulation dysfunction induced by cefoperazone/sulbactam in the Emergency Department of the Affiliated Hospital of Shandong University of Traditional Chinese Medicine from January 2015 to December 2020 were retrospectively analyzed to determine the effects of vitamin K1 on coagulation to provide a basis for clinical practice.
Material and Methods
STUDY DESIGN:
A retrospective analysis of 820 patients treated with cefoperazone/sulbactam in the Emergency Department of the Affiliated Hospital of Shandong University of Traditional Chinese Medicine from January 2015 to December 2020 was conducted and approved by the Institutional Review Board of the same facility (Ethics Review No. (017) KY in 2023).
DATA COLLECTION:
The general data of the patients were recorded, including sex, age, clinical diagnosis, time of coagulation dysfunction caused by cefoperazone/sulbactam, coagulation function indices (international normalized ratio [INR], prothrombin time [PT] and activated partial thromboplastin time [APTT]) before and after treatment with cefoperazone/sulbactam, and after treatment with vitamin K1.
DIAGNOSTIC CRITERIA OF COAGULATION DYSFUNCTION:
The normal ranges of coagulation function are a PT of 11–15 s and APTT of 28–43.5 s (STA series coagulation analyzer, coagulation method). According to the diagnostic criteria for coagulation dysfunction in experimental diagnostics, PT and APTT exceeding 3 s and 10 s of the normal upper limit, respectively, are defined as abnormal.
INCLUSION AND EXCLUSION CRITERIA:
The inclusion criteria were as follows: (1) meeting the diagnostic criteria of coagulation dysfunction, (2) treatment with cefoperazone/sulbactam, (3) availability of complete data on coagulation function before and after treatment, and (4) no history of abnormal coagulation function before the administration of cefoperazone/sulbactam.
The exclusion criteria were as follows: (1) not meeting the diagnostic criteria of coagulation dysfunction, (2) history of coagulation dysfunction before treatment (such as hematological disease and abnormal liver function), (3) incomplete collection of medical history data, and (4) use of anticoagulants and other drugs that affect coagulation function (eg, warfarin, aspirin, livasaban).
STATISTICAL ANALYSIS:
Data analysis was performed using SPSS software (version 26.0; SPSS, Chicago, IL, USA) and GraphPad Prism 9.2 software (GraphPad, Inc., San Diego, CA, USA). The results were expressed as the rate (%), and a chi-square (χ2) test was performed. The measurement data were expressed as the mean±standard deviation (±s). After the
The detailed patient selection process and study flow diagram are shown in Figure 1.
Results
INCLUSION OF CASES:
The detailed patient selection process and study flow diagram are shown in Figure 1. There were 200 cases with coagulation dysfunction, including 132 men and 68 women, with an average age of 79±11 years and time of onset of coagulopathy after drug administration of 7.380±4.62 days. All the patients were treated with cefoperazone/sulbactam (Sulperazon; Pfizer, Inc. New York, NY, USA; 1.5 g/piece) at a dosage of 9 g/day. Fifty patients with coagulation disorders were treated with intravenous injection of vitamin K1 (10 mg/day), and coagulation function indices were monitored.
AGE AND SEX DISTRIBUTIONS:
A total of 200 patients were enrolled, with an average age of 79±11 years. There were 132 men (66%) and 68 women (34%). Their ages were <60 years (6.00%), 60–70 years (12.00%), 71–80 years (26.5%), 81–90 years (48.00%), and >90 years (7.50%). The age and sex distributions are shown in Table 1 and Figure 2.
PROTOPATHY DISEASE TYPE:
At the time of enrollment, the primary disease types were as follows: 65 cases of infection (32.50%), including 48 cases of pneumonia (24.00%), 38 cases of cerebrovascular disease (19.00%), 10 cases of tumor (5.00%), 7 cases of fracture (3.50%), and 80 cases of other diseases (40.00%). Scores of Acute Physiology and Chronic Health Evaluation (APACHE II) were 15.54±4.095. The disease types are shown in Table 2 and Figure 3.
COAGULATION INDICES BEFORE AND AFTER TREATMENT WITH CEFOPERAZONE/SULBACTAM: The incidence of coagulation dysfunction in the study population was 24.39%. Coagulation indices before and after treatment with cefoperazone/sulbactam were compared; INR (1.183±0.180 vs 3.037±1.799), PT (14.97±1.688 vs 31.23±13.630), APTT (41.24±7.641 vs 67.98±21.720). The results are shown in Table 3. Statistical significance was set at P<0.001.
EFFECT OF CEFOPERAZONE/SULBACTAM ADMINISTRATION DURATION ON COAGULATION DYSFUNCTION: Coagulation dysfunction started from day 2. Overall, 195 cases were documented between days 2 and 19, accounting for approximately 99% of cases. INR (3.374±1.985 vs 1.438±0.424), PT (35.42±15.860 vs 17.54±4.095), APTT (69.17±21.940 vs 47.56±12.040). The results are shown in Figure 4.
COAGULATION AFTER INTERVENTION WITH VITAMIN K1:
Patients with coagulation dysfunction were therapeutically administered vitamin K1 to improve coagulation. The coagulation indices before and after intervention are shown in Table 4. Statistical significance was set at P<0.001.
After cefoperazone/sulbactam administration, the international normalized ratio (INR) increased significantly and prothrombin time (PT) and activated partial thrombin time (APTT) were prolonged, suggesting coagulation dysfunction (Figure 5). After intervention with vitamin K1, the INR decreased and PT and APTT were shortened, suggesting that coagulation function improved.
Discussion
Vitamin K1 Improved Coagulation Dysfunction Induced by Cefoperazone/Sulbactam
VITAMIN K1 IMPROVED COAGULATION DYSFUNCTION INDUCED BY CEFOPERAZONE/SULBACTAM:
The mechanism of cefoperazone/sulbactam-induced coagulation dysfunction may be related to the presence of the N-methylthiotetrazolium side chain in cefoperazone, which has a molecular structure similar to that of glutamate. It competitively binds glutamate carboxylase along with vitamin K1 in the liver microsomes. This structure affects the synthesis of vitamin K-dependent coagulation factors II, VII, IX, and X, thus causing coagulation dysfunction in the body [9]. The COOH group on the substituent of the 7 C atom of cephalosporin hinders platelet aggregation and causes bleeding.
Moreover, cefoperazone cannot be efficiently metabolized in the body, and more than 40% is discharged from the bile duct through the intestine. Cefoperazone inhibits normal intestinal flora and the intestinal synthesis of vitamin K and leads to vitamin K-dependent prothrombin hypoemia. These effects result in coagulation dysfunction, which is characterized by prolonged INR, PT, and APTT, and bleeding tendency [10].
The effects of cefoperazone/sulbactam on PT, APTT, and TT are closely related to their concentrations; indeed, high concentrations of antibiotics are known to inhibit coagulation factors. In addition, secondary platelet function defects or quantity reduction cause endothelial cell damage and inflammatory reactions through the release of inflammatory transmitters and leukocyte adhesion deficiency, resulting in coagulation dysfunction [11]. Similarly, coagulation dysfunction caused by cefoperazone/sulbactam is mainly related to vitamin K deficiency in vivo. Therefore, the use of cefoperazone/sulbactam should be accompanied by monitoring of changes in INR, PT, and APTT, along with the administration of vitamin K1 when necessary.
APACHE II SCORES ON COAGULATION DYSFUNCTION INDUCED BY CEFOPERAZONE/SULBACTAM: The average APACHE II score of coagulation dysfunction induced by cefoperazone/sulbactam was 15.54±4.095. The APACHE II scoring system is a scientific and reliable scoring system recognized globally to evaluate the severity of critical patients and predict their prognoses. The higher the APACHE II scores, the worse the body condition and the higher the mortality. The correlation results showed that the higher the APACHE II scores, the higher the values of PT, APTT, and PLT. The levels of PT, APTT, and PLT in patients with sepsis were significantly positively correlated with their APACHE II scores. A report has found that blood coagulation dysfunction caused by cefoperazone/sulbactam in the group with high APACHE II scores was significantly higher than in the normal group [12]. The present study is consistent with previous research results.
Similarly, this study also suggests that the APACHE II scores of patients with coagulation dysfunction are significantly higher than that of the normal group. Analysis of the causes may be closely related to the primary disease of these patients. Primary diseases such as tumors, cerebrovascular disease, or infection also have a non-negligible effect on coagulation function. Therefore, it is important to carefully evaluate and clarify the causes and mechanisms of coagulation dysfunction as soon as possible for the rational clinical use of cefoperazone/sulbactam. Among patients with coagulation dysfunction, the primary disease type was an infection, most frequently pneumonia, followed by cerebrovascular diseases, tumors, and fractures. The infection causes a systemic inflammatory reaction. During disease progression, large amounts of thrombin and fibrinolytic proteins are formed, resulting in extensive microthrombosis in blood vessels, accompanied by the consumption of a large number of platelets and coagulation factors. Coagulation dysfunction can be corrected at this stage. This condition further develops into diffuse intravascular coagulation, which locally consumes a large number of coagulation factors, resulting in uncontrolled coagulation. Coagulation dysfunction occurs during the entire infection development process [13].
Disturbance of consciousness and swallowing dysfunction after cerebrovascular disease resulting in malnutrition and aspiration pneumonia are also high-risk factors for coagulation dysfunction. Moreover, after cerebrovascular disease, immune regulatory media is released to act on immune cells such as neutrophils, natural killer cells, T cells, and macrophagocytes, resulting in low immune function and uncomplicated infection [14]. Therefore, cefoperazone/sulbactam in the treatment of pneumonia, complicated with ischemic cerebrovascular disease, could prolong PT and APTT, which significantly impacts coagulation function. The risk of bleeding in the clinical use of these drugs must also be considered [14]. The tumor itself has an apparent coagulation state, but its immune function is low and thus is prone to infection. Coagulation dysfunction occurs following infection. Overall, in the treatment of infection using cefoperazone/sulbactam, complications resulting from primary diseases can affect the coagulation function.
EFFECT OF CEFOPERAZONE/SULBACTAM ADMINISTRATION DURATION ON COAGULATION DYSFUNCTION: We found that coagulation dysfunction occurred after 7.380±4.628 days of cefoperazone/sulbactam administration (Section 2). Additionally, the duration of administration is related to coagulation dysfunction. Thus, patients receiving cefoperazone/sulbactam were divided into 2 groups (>10 and <10 days) [15]. The duration of administration was an independent risk factor for coagulation dysfunction, and the risk associated with treatment for >10 days was 3.525-fold higher than that following treatment for <10 days. A longer inhibition time of normal intestinal flora, particularly that of Escherichia coli, may be associated with a longer duration of administration. Long-term and high-dose use of cefoperazone/sulbactam can lead to vitamin K deficiency and reduce vitamin K-dependent coagulation factors, resulting in coagulation dysfunction [16].
EFFECT OF AGE ON COAGULATION DYSFUNCTION INDUCED BY CEFOPERAZONE/SULBACTAM: The incidence of coagulation dysfunction increased with age, peaking at 81–90 years in this study. In terms of pharmacokinetics or pharmacodynamics, cefoperazone/sulbactam is mainly excreted through the kidney and biliary tract; approximately 25% of cefoperazone and 84% of sulbactam are excreted through the kidney, and the remaining are excreted through the biliary tract. Therefore, the use of cefoperazone/sulbactam is highly dependent on liver and kidney function. A decrease in liver microsomal enzyme activity, renal function, and drug clearance in elderly patients leads to drug accumulation in the body. Moreover, elderly patients often have poor nutritional status and intestinal flora disorders, making them prone to vitamin K deficiency and affecting coagulation function [17]. Studies have shown that cefoperazone/sulbactam does not lead to new abnormal liver and kidney function indices.
Similar to results reported in the literature, the present study showed that cefoperazone/sulbactam can cause coagulation abnormalities and that administration of vitamin K1 can reduce the occurrence of adverse drug reactions to some extent. In recent years, it has been found in clinical practice that coagulation abnormalities or bleeding due to the use of cefoperazone/sulbactam can be prevented and controlled. During, before, and after administration of the drug, monitoring patients’ coagulation indexes and using pre-given vitamin K1 or timely supplementation of vitamin K1 when abnormalities are detected have been shown to be useful for stopping the occurrence of coagulation dysfunction and reducing serious bleeding events. Nevertheless, there are shortcomings in this retrospective study; for instance, cefoperazone/sulbactam-induced coagulation dysfunction is associated with several factors such as age, immunocompromise, history of gastrointestinal disease, and dose of medication. Therefore, subsequent studies are needed for a more thorough exploration.
Our study has several advantages. Firstly, compared with previous case reports and small uncontrolled studies, we used a control group to explore the risk of coagulation dysfunction caused by cefoperazone/sulbactam treatment. Secondly, we conducted a cohort study and analysis of relevant indicators to ensure the reliability of the results. However, although we plan to make some comparisons to explore the potential mechanism of abnormal coagulation function caused by cefoperazone/sulbactam, the data on cefoperazone and other sulbactam containing antibiotics are still insufficient. In addition, some confounding factors are difficult to measure and exclude.
However, this study has several uncontrolled and confounding factors due to an observational study based on retrospective data. This study was single-center retrospective analysis that did not include all types of patients and treatment methods. In addition, there are some problems with data inaccuracy and incompleteness. Further research involving more centers will provide a larger sample size to verify the conclusions of this study in the future.
Conclusions
We found that the rate of coagulation dysfunction was 24.39%. The index (INR, PT, and APTT) of patients was significantly prolonged, indicating that cefoperazone/sulbactam leads to an increased risk of coagulation dysfunction, especially during days 2–19. We also found that the use of cefoperazone/sulbactam was closely related to the patient’s age, peaking at 81–90 years. The administration of vitamin K1 significantly improved coagulation function. Notably, patients with infection, cerebrovascular diseases, tumors, and fractures are more prone to coagulation dysfunction. Therefore, during the clinical use of cefoperazone/sulbactam, particularly in elderly patients, liver and kidney function must be regularly monitored, long-term administration must be avoided, changes in the INR, PT, and APTT must be monitored, and vitamin K1 should be administered when necessary.
Figures
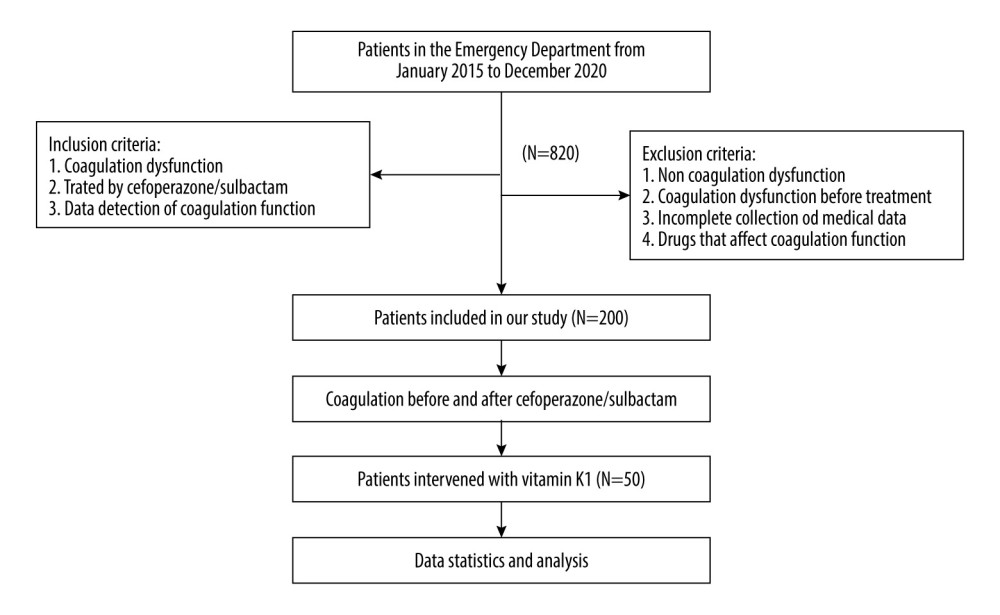 Figure 1. Detailed patient selection process and study flow diagram.
Figure 1. Detailed patient selection process and study flow diagram. 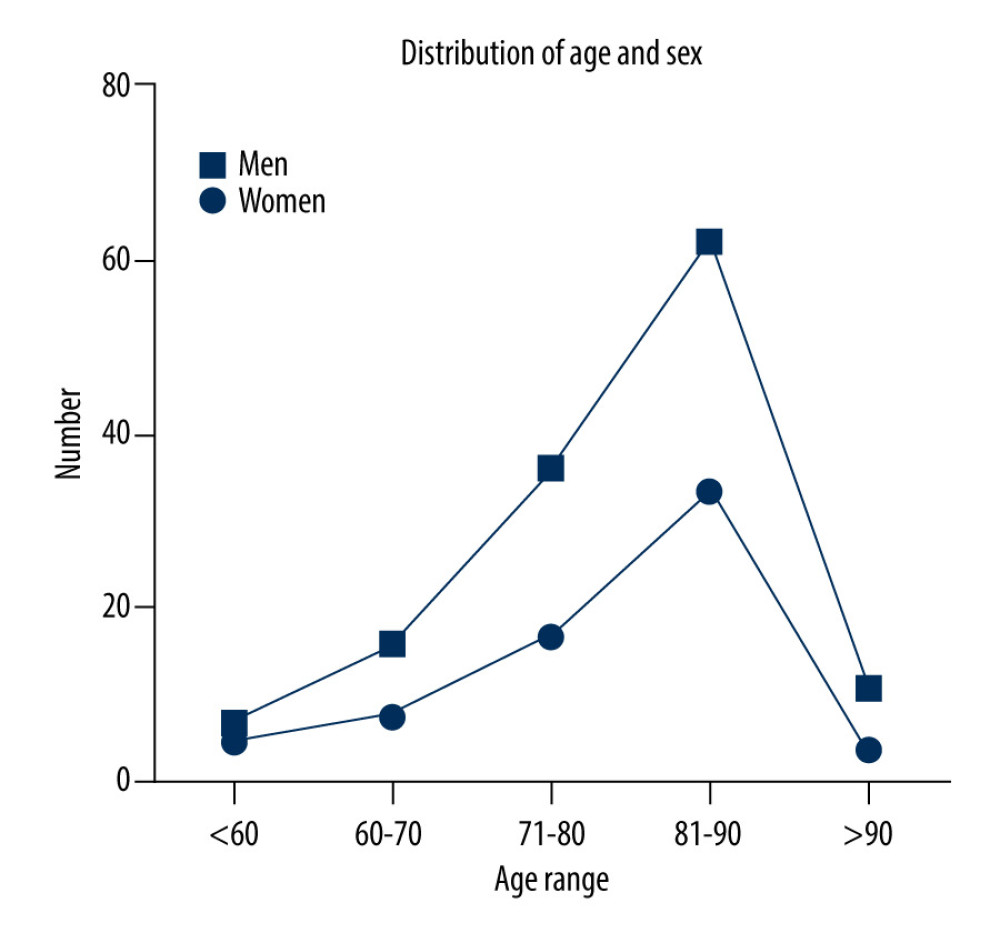 Figure 2. With increasing age, the number of people with coagulation dysfunction using cefoperazone/sulbactam increased, peaking at 81–90 years.
Figure 2. With increasing age, the number of people with coagulation dysfunction using cefoperazone/sulbactam increased, peaking at 81–90 years. 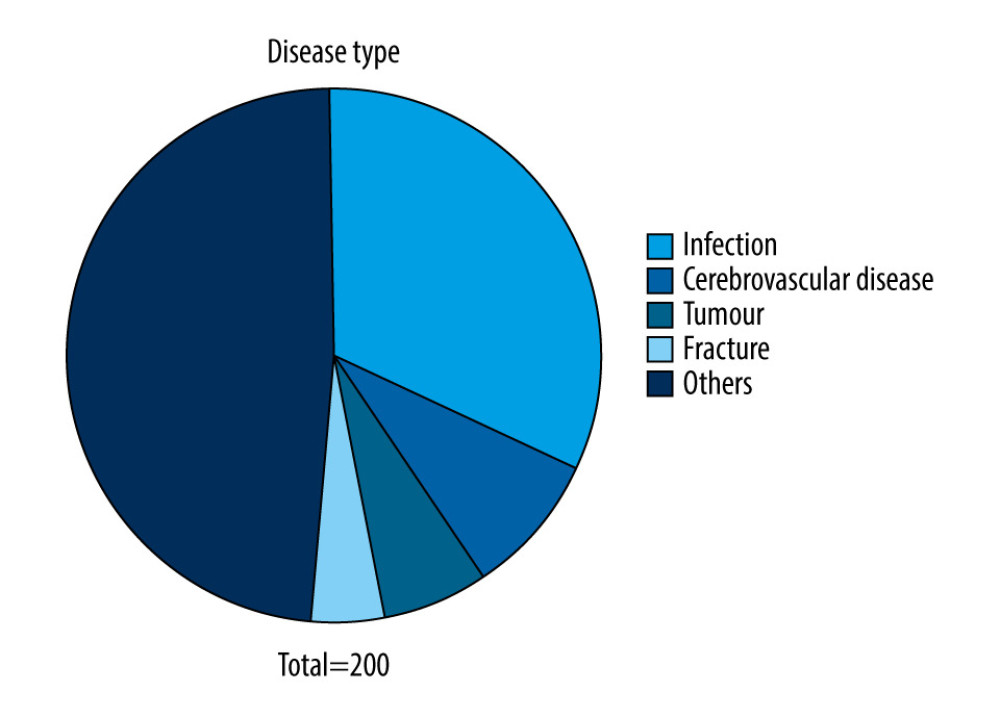 Figure 3. Among the patients with coagulation dysfunction, the most common type of primary disease was an infection, particularly pneumonia, followed by cerebrovascular disease, tumor, and fracture.
Figure 3. Among the patients with coagulation dysfunction, the most common type of primary disease was an infection, particularly pneumonia, followed by cerebrovascular disease, tumor, and fracture. 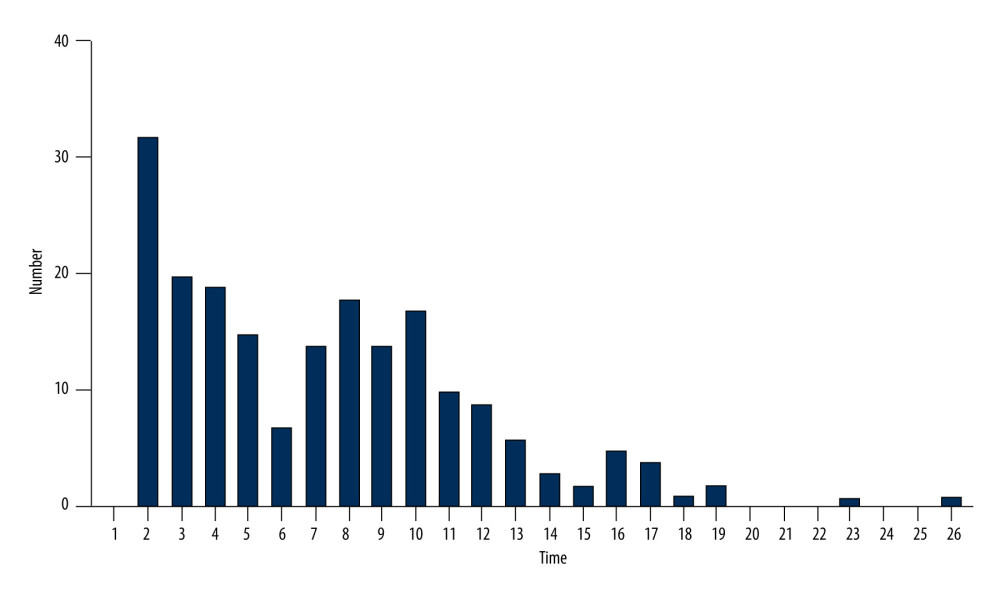 Figure 4. Coagulation dysfunction started from day 2. Overall, 195 cases were documented between days 2 and 19, accounting for approximately 99% of cases.
Figure 4. Coagulation dysfunction started from day 2. Overall, 195 cases were documented between days 2 and 19, accounting for approximately 99% of cases. 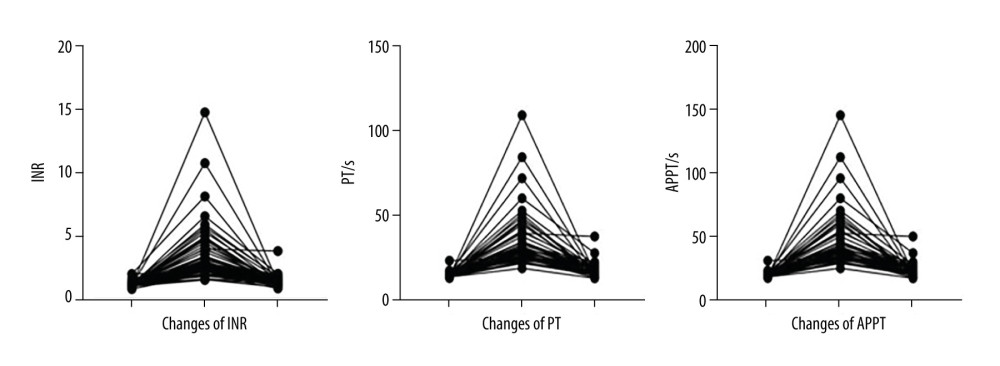 Figure 5. After cefoperazone/sulbactam administration, the international normalized ratio (INR) increased significantly and prothrombin time (PT) and activated partial thrombin time (APTT) were prolonged, suggesting coagulation dysfunction. After treatment with vitamin K1, the INR decreased and PT and APTT were shortened, suggesting that coagulation function improved.
Figure 5. After cefoperazone/sulbactam administration, the international normalized ratio (INR) increased significantly and prothrombin time (PT) and activated partial thrombin time (APTT) were prolonged, suggesting coagulation dysfunction. After treatment with vitamin K1, the INR decreased and PT and APTT were shortened, suggesting that coagulation function improved. References
1. Ouyang W, Xue H, Chen Y, Clinical characteristics and antimicrobial patterns in complicated intra-abdominal infections: A 6-year epidemiological study in southern China: Int J Antimicrob Agents, 2016; 47; 210-16
2. Yang Z-H, Zhu X-Y, Xue L, Comparison of clinical efficacy of imipenem and cefoperazone/sulbactam in treatment of hospital-acquired pneumonia in patients with hematologic malignancies: Chin J Nosocomiol, 2016; 26; 1539-40
3. , Expert Consensus of β-Lactam/β-lactamase inhibitor combinations on clinical application, 2020 Edition: Nal Med J China, 2020(10); 738-47
4. Chen LJ, Hsiao FY, Shen LJ, Use of hypoprothrombinemia-inducing cephalosporins and the risk of hemorrhagic events: A nationwide nested case-control study: PLoS One, 2016; 11; e0158407
5. Wang W, Liu Y, Yu C, Cefoperazone-sulbactam and risk of coagulation disorders or bleeding: A retrospective cohort study: Expert Opin Drug Saf, 2020; 19; 339-47
6. Kinasewitz GT, Yan SB, Basson B, Universal changes in biomarkers of coagulation and inflammation occur in patients with severe sepsis, regardless of causative microorganism: Crit Care, 2004; 8(2); 82
7. Bin W, Xiaoqin D, Chunhong Z, Effect of sulbactam-cefoperazone on coagulation function and intervention: Chin J Crit Care Med, 2013; 33; 228-30
8. Cai Z-Y, Yang W, He Y-Y, Cefoperazone/sulbactam-induced abdominal wall hematoma and upper gastrointestinal bleeding: A case report and review of the literature: Drug Saf Case Rep, 2016; 3; 2
9. Wong RSM, Cheng G, Chan NPH, Use of cefoperazone still needs a caution for bleeding from induced vitamin K deficiency: Am J Hematol, 2006; 81; 76
10. Wang YY, Yu JH, Duan JL, Analysis of influencing factors of coagulation caused by cefoperazone sulbactam: Chin Gen Pract, 2020; 23(S1); 138-40
11. Tang JH, Chen KY, Xu MZ, To explore the effect of cefoperazone/sulbactam on coagulation: Chin Contin Educ, 2015; 7; 162-63
12. Li YY, Shi L, Wang YH, Analysis on factors affecting dysfunction of blood coagulation caused by cefoperazone sodium-sulbactam sodium: Chin J Ration Drug Use, 2018; 15; 78-80
13. Fullerton JN, Thompson K, Shetty AThe Australian and New Zealand Intensive Care Society Clinical Trials Group; The George Institute for Global Health, New sepsis definition changes incidence of sepsis in the Intensive Care Unit: Crit Care Resusc, 2017; 19; 9-13
14. Emergency Medicine Branch of Chinese Stroke Society, Stroke Middle School Group of Emergency Medicine Branch of Chinese Medical Association, Emergency Medicine Branch of Chinese Geriatric Society, et al, Chinese expert consensus on the diagnosis and treatment of stroke associated pneumonia (2019 update): Chin J Emerg Med, 2019; 28; 1476-84
15. Liang H, Meng GY, Zhong LQ, Analysis of influence factors coagulation abnormalities induced by cefoperazone sodium and sulbactam sodium in patients with ischemic cerebrovascular disease caused: China Pharm, 2020; 29; 154-56
16. Hu J, Xiao YH, Zheng Y, Clinical characteristics and risk factors of tigecycline-associated hypofibrinogenaemia in critically ill patients: Eur J Clin Pharmacol, 2020; 76; 913-22
17. Li DR, Gao WB, Zhu JH, Case of disfunction of blood clotting induced by cefoperazone sulbactam sodium: Chin J Emerg Med, 2018; 27; 555-57
Figures
 Figure 1. Detailed patient selection process and study flow diagram.
Figure 1. Detailed patient selection process and study flow diagram. Figure 2. With increasing age, the number of people with coagulation dysfunction using cefoperazone/sulbactam increased, peaking at 81–90 years.
Figure 2. With increasing age, the number of people with coagulation dysfunction using cefoperazone/sulbactam increased, peaking at 81–90 years. Figure 3. Among the patients with coagulation dysfunction, the most common type of primary disease was an infection, particularly pneumonia, followed by cerebrovascular disease, tumor, and fracture.
Figure 3. Among the patients with coagulation dysfunction, the most common type of primary disease was an infection, particularly pneumonia, followed by cerebrovascular disease, tumor, and fracture. Figure 4. Coagulation dysfunction started from day 2. Overall, 195 cases were documented between days 2 and 19, accounting for approximately 99% of cases.
Figure 4. Coagulation dysfunction started from day 2. Overall, 195 cases were documented between days 2 and 19, accounting for approximately 99% of cases. Figure 5. After cefoperazone/sulbactam administration, the international normalized ratio (INR) increased significantly and prothrombin time (PT) and activated partial thrombin time (APTT) were prolonged, suggesting coagulation dysfunction. After treatment with vitamin K1, the INR decreased and PT and APTT were shortened, suggesting that coagulation function improved.
Figure 5. After cefoperazone/sulbactam administration, the international normalized ratio (INR) increased significantly and prothrombin time (PT) and activated partial thrombin time (APTT) were prolonged, suggesting coagulation dysfunction. After treatment with vitamin K1, the INR decreased and PT and APTT were shortened, suggesting that coagulation function improved. Tables
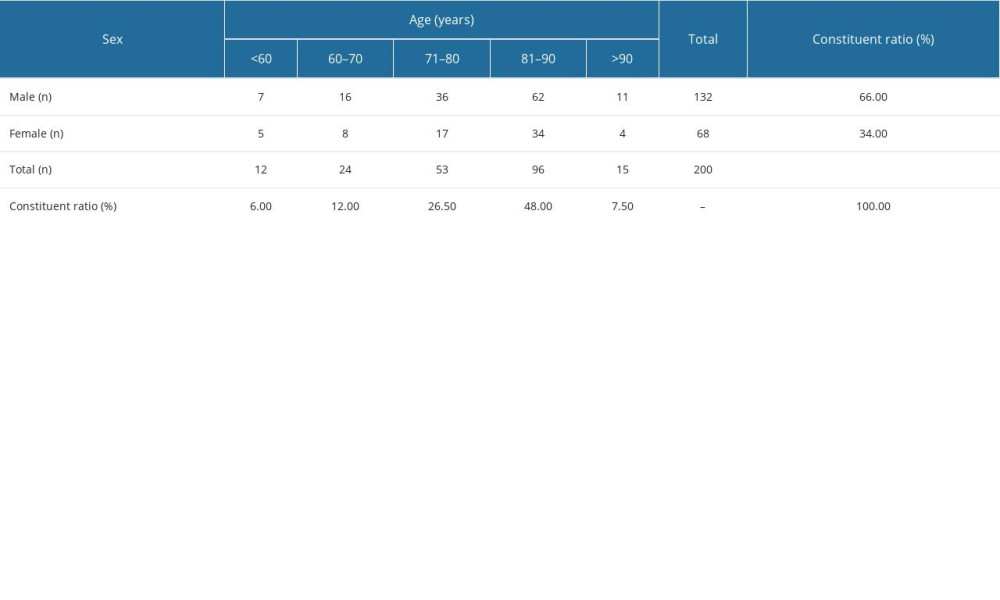 Table 1. Age and sex distribution of patients.
Table 1. Age and sex distribution of patients.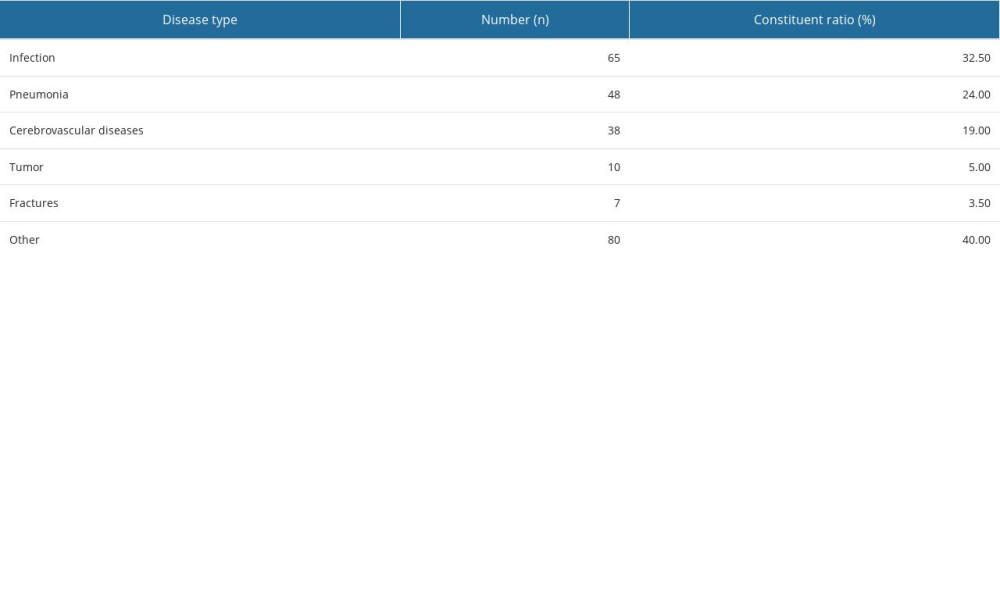 Table 2. Disease type.
Table 2. Disease type.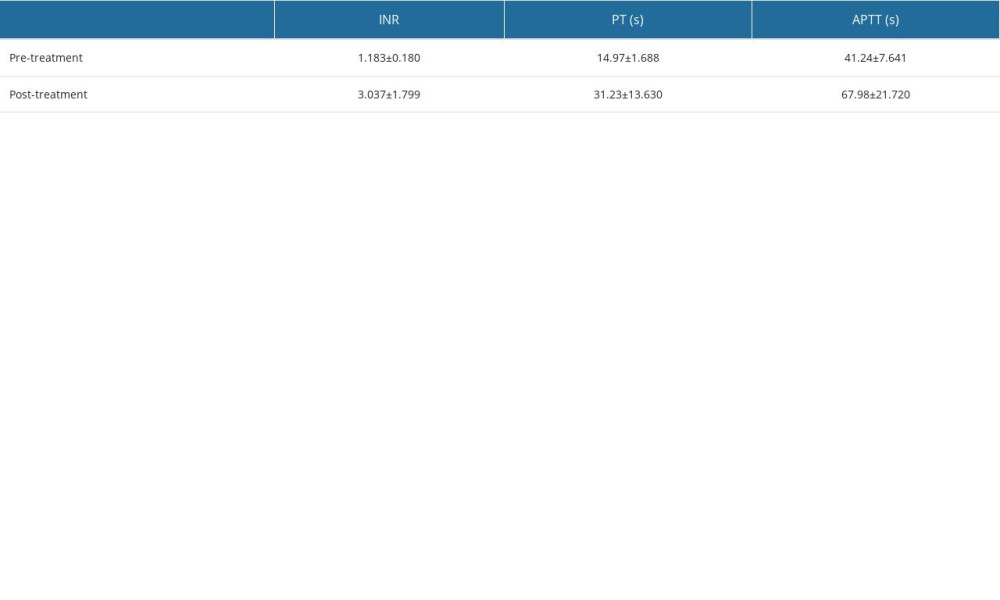 Table 3. Coagulation indices before and after treatment with cefoperazone/sulbactam.
Table 3. Coagulation indices before and after treatment with cefoperazone/sulbactam.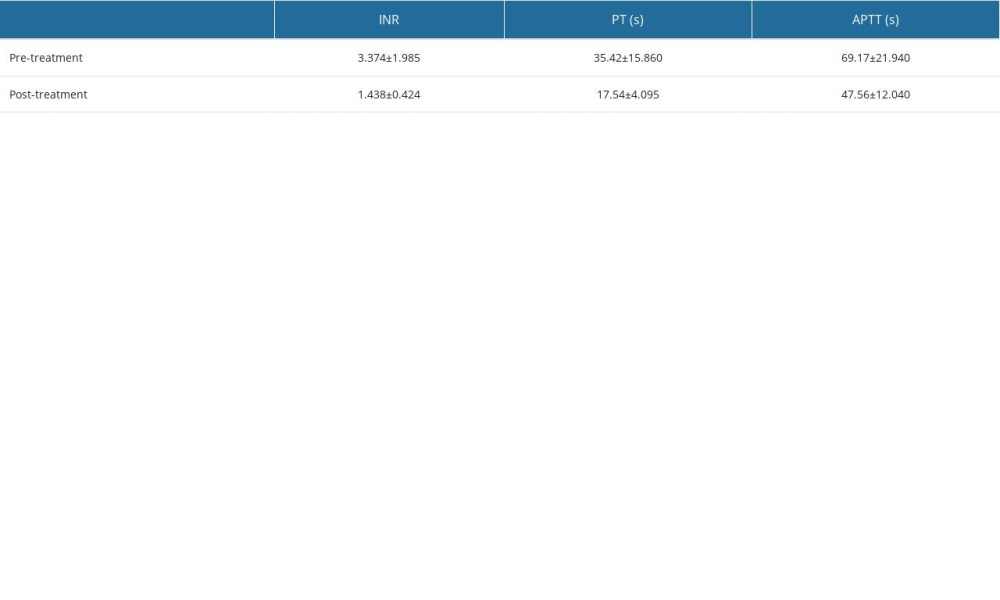 Table 4. Effect of vitamin K1 intervention on coagulation indices.
Table 4. Effect of vitamin K1 intervention on coagulation indices. Table 1. Age and sex distribution of patients.
Table 1. Age and sex distribution of patients. Table 2. Disease type.
Table 2. Disease type. Table 3. Coagulation indices before and after treatment with cefoperazone/sulbactam.
Table 3. Coagulation indices before and after treatment with cefoperazone/sulbactam. Table 4. Effect of vitamin K1 intervention on coagulation indices.
Table 4. Effect of vitamin K1 intervention on coagulation indices. In Press
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








