17 January 2024: Review Articles
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron Variant
Anna BednarekDOI: 10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
Abstract
ABSTRACT: Vaccinations are an essential element of public health programs around the world, which have a major impact on morbidity, mortality, and costs of the health care system. In recent years, with a better understanding of the effectiveness and safety of vaccinations, many recommendations have been developed for administering vaccines to adults. Countless physiological changes occur during pregnancy, including those affecting the immune system. Pregnant women are at increased risk of developing infections and resulting complications. According to research, vaccines are immunogenic and safe for pregnant women. Pregnancy is not an absolute contraindication to vaccination. After administration of vaccines to pregnant women, the concentration of antibodies increases, which can be transferred to the child in the second and third trimesters of pregnancy and provide protection in the first months of life. The Advisory Committee on Immunization Practices (ACIP), guidelines of the Centers for Disease Control and Prevention (CDC), and the American College of Obstetrics and Gynecology (ACOG) unanimously recommend vaccination of pregnant women if a safe vaccine is available and there is a risk of exposure of the woman to a disease that threatens herself or her developing baby. In everyday clinical practice, medical professionals should provide their patients with the necessary information on vaccinations, which may contribute to greater awareness and implementation of vaccinations. This article aims to review current global recommendations for the vaccination of pregnant and breastfeeding women, including against the Omicron variants of SARS-CoV-2.
Keywords: Health Planning Guidelines, Pregnant Women, Vaccination
Background
Vaccination against infectious diseases is a key component of public health programs around the world [1,2]. Their implementation requires appropriate awareness and acceptance of vaccines by parents of children, adolescents, and adults [3,4].
Women of childbearing age belong to the group of people with specific indications for vaccination [5]. Thanks to this, they are better prepared for pregnancy and the birth of a healthy baby [4]. Recommendations for vaccination of adolescents and people of childbearing age always require an individual approach depending on the history of vaccinations performed so far, documented in the immunization card, and the age of the woman who plans to become pregnant [6–8]. The immunization needs of women in different years of life are different [9,10]. The decision to vaccinate may also be preceded by serological tests of vaccine antibody titers [6–8]. This applies primarily to vaccination against rubella and chickenpox [11,12]. If an adult has not received all doses of vaccines, as recommended by national vaccination programs, they can be supplemented at any age [9,13,14].
This article aims to review current global recommendations for the vaccination of pregnant and breastfeeding women, including against the Omicron variants of SARS-CoV-2.
This literature review covers currently recommended vaccinations for women and men during the reproductive period (ie, against chickenpox, mumps, rubella, measles, hepatitis A and B, influenza, human papillomavirus, diphtheria, pertussis, tetanus, and meningococci), and for pregnant women and breastfeeding mothers (ie, against diphtheria, pertussis, tetanus, and influenza), including vaccines against COVID-19 in the context of the dominance of the Omicron variant. The implementation of these vaccinations is important due to the Polish Immunization Program [9], which includes most vaccinations in early childhood. Cultural changes affecting the planning of the first pregnancy after the age of 30 require the administration of booster doses for some inactivated vaccines administered in childhood or supplementation of mandatory and recommended vaccinations not administered during this period.
Functioning of the Immune System During Pregnancy
The gestation period is associated with increased susceptibility to infections and often their severe course, as well as adverse effects on the fetus resulting from infection with specific pathogens [15,16]. Pregnancy causes a decrease in the number of B1 cells, which influences on deficiency of IgM antibodies and a decrease in the functionality of B cells [3]. These changes weaken the innate immunity of the pregnant woman and increase her susceptibility to infections [17–19]. During pregnancy, the activity of the complement system also increases, causing an increase in the immune response to some antibodies (eg, against influenza), leading to various obstetric complications (eg, preeclampsia) [15]. At the same time, adaptation in the mother’s immune system is the site of many physiological changes that protect her and the fetus from pathogens [5,16,17]. The mother’s increased immune reactivity, while providing her protection, finds a balance to maintain a healthy pregnancy and avoid a harmful immune response against the allogeneic fetus. Although the mechanism of this phenomenon is not precisely understood, studies confirm a dynamic interaction between the immune systems of the mother and fetus, despite suppression of immunity in the mother [5,20–23].
At the same time, a state of increased innate immune response and reduced cellular response is observed during pregnancy, which is maintained through the transformation of the cytokine phenotype (ie, the transition from the dominance of Th1 lymphocytes to Th2). Reducing cellular immunity increases the likelihood of infection [15]. It is assumed that proinflammatory cytokines trigger an immune response and cause tissue damage. Typically, a viral infection, such as rubella or chickenpox, occurs as a result of an acute, primary infection during pregnancy, although pre-pregnancy infections may also reactivate [16]. Their incidence increases with gestational age, due to changes in the factors determining the mother’s immunity and the decreasing anatomical thickness of the placenta. In contrast, the clinical manifestations of infection and the risk of complications are more severe when the infection occurs in the early stages of pregnancy and is associated with maternal comorbidities [17,24].
Immune changes associated with pregnancy also affect the pathogenesis of autoimmune diseases, which can change the profile of symptoms in pregnant patients [17]. Changing the cytokine profile from a Th1-mediated phenotype to a Th2-engaging phenotype alleviates inflammatory autoimmune disease while exacerbating humoral autoimmune disease [18,24].
COVID-19 During Pregnancy and Breastfeeding
Due to altered maternal immunity, as well as adaptive changes in the respiratory, cardiovascular, and hematological systems, there is a risk of potential susceptibility of pregnant women to COVID-19 infection [14]. There is little evidence on various pregnancy disorders, including fetal growth limitations, preterm birth, and maternal mortality caused by COVID-19 infection per patient in the first trimester of pregnancy, but seasonal flu is known to be associated with higher rates of early miscarriages. Nevertheless, most studies did not observe a significant risk of severe COVID-19 infection among pregnant women than in the general population [8,25].
COVID-19 infection reduces the expression of angiotensin-converting enzyme-2 (ACE2), leading to increased angiotensin II activity followed by an increased immune response seen in COVID-19 patients [22]. The binding of the virus to ACE2 receptors reduces their dynamics on alveolar epithelial cells and activates the host’s adaptive immune response. Many inflammatory markers (mainly interleukin IL-1β, IL-4, IL-10, and interferon γ) are released, which disrupt the integrity of endothelial cells and thus hinder the vesicular-capillary barrier, often causing severe hypoxemia [7,26]. It seems that immune system adaptations during pregnancy may affect the response profile after vaccination against COVID-19. Although studies have shown the effectiveness of these vaccines in pregnant women, the small number of vaccinated expectant mothers makes it difficult to fully investigate this issue [27].
Undoubtedly, the gestation period is associated with a higher susceptibility to diseases that can be prevented by vaccination. Due to fluctuations in the hormonal balance of the body and modifications in the immune system, exposure to complications of various diseases, including infectious ones, is much higher in pregnant women than in the general population. Hence, vaccination of expectant mothers is a comprehensive element of antenatal care aimed at improving the health of both mother and child [4,5].
Recommendations for Vaccination of Adolescents and Adults in the Reproductive Period
To protect against bacterial and viral infections dangerous to both the pregnant, fetus, and newborn, vaccination against rubella, measles, mumps, chickenpox, pertussis, hepatitis A and B, meningococcus, HPV and influenza in adolescents and adults in the reproductive period should be considered. Table 1 presents recommendations for these vaccinations, taking into account local arrangements [6,7,9].
In many countries, it is recommended to vaccinate adults susceptible to VZV infection, and in Germany and the United States, serological tests are recommended for women of childbearing potential. If a negative result is obtained, it is recommended to carry out vaccination by the manufacturer’s recommendations [28].
The persistence of seroprotection in the case of rubella is estimated at >15 years. Therefore, it is recommended that the MMR vaccine be considered for the prevention of congenital rubella as well as measles and mumps in young women working in pediatric environments if >10 years have passed since the last MMR vaccination. Non-pregnant women who have received live vaccines (MMR, VZV) should be advised to avoid pregnancy for at least 4 weeks [29–33].
If vaccination against hepatitis B is required in adolescents and adults, a combined vaccine against hepatitis A and B is available in a 3-dose schedule for subjects >16 years of age [6,9].
The most effective method of preventing influenza is vaccination against this disease. Annual vaccination against influenza is recommended for all people over 6 months of age who have no contraindications to vaccination. Current strains for the production of influenza vaccines are recommended each year by the World Health Organization (WHO). Their validity period is one flu season. Inactivated and attenuated influenza vaccines are available. Both types of vaccines provide active immunization against 4 strains of influenza virus (2 subtypes A and 2 subtypes B) and are referred to as 4-valent vaccines [7]. The inactivated 4-valent influenza vaccine can be used in all people who do not have any special contraindications, while the attenuated vaccine can be used in healthy, non-pregnant women. In Poland, only 1 “live” intranasal influenza vaccine is used, recommended for children and adolescents from 6 months of age to 18 years of age [7,8,34].
Human
If a woman during the reproductive period has not had vaccinations against diphtheria, tetanus, and pertussis, then at any age they should be supplemented [40,41]. The vaccination schedule includes the administration of one dose of dTpa. After a period of 1–2 months, the Td vaccine should be administered, and after 6–12 months, another dose of Td (a total of 3 doses of primary vaccination). Booster doses of dTpa vaccine are recommended to be administered every 10 years [42,43].
All people, regardless of gender and age, are exposed to meningococcal infection. Many cases of the disease occur in adolescents and young adults. The most dangerous is invasive meningococcal disease (IMD), which involves meningitis or sepsis [44]. Clinically, it develops very quickly and is fraught with high mortality and often permanent complications. Most cases of IMD are caused by meningococcal serogroups B and C. Recently, the participation of meningococcal serogroup W is also important. Vaccination against meningococcal group B includes the administration of 2 doses of vaccine and in the case of meningococcal vaccination of groups A, C, Y, and W one dose of vaccine [3,4,6].
Recommendations for Vaccination of Pregnant Women – Priorities and Prospects
Based on the available research results, and opinions of scientific societies and pro-health organizations, vaccination of pregnant women is safe for them and developing fetuses with selected inactivated vaccines, as well as administered in particularly justified situations of exposure to infection with other vaccines (eg, vaccination against COVID-19). Pregnant women are not given “live” vaccines [8,45]. Table 2 presents vaccinations recommended for pregnant and breastfeeding women, taking into account local recommendations [8,9,10,26].
If there is a possibility of exposure to an infectious disease that threatens the health of the mother or newborn, the benefits of vaccinating the pregnant woman are greater than the theoretical risks associated with them. Toxoids and inactivated antibacterial and antiviral vaccines are safe in pregnancy. They do not cause complications in either the mother or the fetus. In addition, according to available knowledge, the use of immunoglobulin preparations during pregnancy does not cause complications in the development of the fetus. In contrast, attenuated (“live”) antiviral or antibacterial vaccines are contraindicated during pregnancy, since there is theoretically a risk of causing infection in the fetus. So far, no data are available to suggest that giving a pregnant woman a vaccine containing ‘live’ viruses may be associated with fetal harm [46,47]. Pregnant women are recommended to be vaccinated against influenza tetanus and pertussis with a combined vaccine containing tetanus toxoid and reduced doses of diphtheria toxoid and acellular pertussis antigens (dTpa) [48]. Pregnant women planning to travel abroad or those who cannot avoid infectious diseases that are prevented by vaccination should be vaccinated. Breastfeeding is not a contraindication for the administration of inactivated and attenuated vaccines. If influenza vaccination and dTpa vaccination were not carried out during pregnancy, they can be given immediately after delivery [49–51].
All newborns can be protected by the active transplacental transport of antibodies specific to microbes with which the woman has previously been in contact or which she has developed after vaccination [10]. After administration of vaccines in pregnant women, the concentration of antibodies increases, which are then transferred to the child in the II and III trimesters. They protect the baby in the first months of life (ie, during the period of greater susceptibility to infectious diseases, resulting from the risk of exposure and the relative immaturity of the infant’s immune system) [5,43]. Although none of the registered vaccines in the United States has a specific indication for use in pregnant women, vaccination during pregnancy is recommended if the risk of complications of an infectious disease in the mother or child outweighs the risk of developing adverse reactions to vaccination [4,47].
All inactivated and live vaccines recommended for adults not given before or during pregnancy may be administered during breastfeeding. Family members of a pregnant woman can also be vaccinated with a combined vaccine against measles, mumps, and rubella because the live, weakened viruses contained in this vaccine do not spread from the vaccinated person to other people. Similarly, children whose mothers are pregnant against chickenpox can be vaccinated. There is no known evidence of a risk of transmission to the mother from a vaccinated child [7,11,12].
Worldwide, vaccination of pregnant women against tetanus was considered a major public health success, which reduced the incidence of tetanus in newborns by 96%. A combined vaccine against diphtheria, tetanus, and pertussis (dTap) was first recommended for pregnant women by the WHO in 2015 [40]. Pertussis vaccination is recommended in the form of a combined vaccine against diphtheria, tetanus, and pertussis [19,21]. The U.S. Immunization Advisory Committee (ACIP) recommends vaccinating pregnant women between 27 and 34 weeks of each pregnancy, regardless of previous vaccinations. This is to protect the newborn and infant by increasing the concentration of maternal antibodies (passive immunization). Whooping cough is a highly contagious disease, the most dangerous in the youngest children, mainly in the first 6 months of life. The risk of dying from whooping cough is highest in this age group. Vaccination of a pregnant woman protects the newborn and infant more effectively than the vaccine given to the child [23].
The effectiveness and safety of vaccination of pregnant women against influenza have been confirmed in numerous clinical studies. Thanks to the specific antibodies transmitted by the placenta, vaccination reduces the risk of premature birth, low birth weight of the baby, as well as the baby’s illness in the first 6 months of life, (ie, during the period when he cannot be vaccinated against influenza). Since 2005, the WHO has recommended vaccination of expectant mothers against seasonal influenza as a group at high risk of illness, severe disease, and increased risk of complications and hospitalization. The recommendation to vaccinate pregnant women with influenza has contributed to the acceptance of other vaccines for pregnant women in many countries. Vaccination against influenza should be recommended for pregnant women during the flu season [6,9,10,50].
Vaccinations Against COVID-19, Including Against Omicron Variants of SARS-CoV-2, Pregnant and Breastfeeding Women
The first recommendations for COVID-19 vaccination for pregnant women by the
In pregnant women, typical (as in the general population) local and systemic adverse reactions can be expected (ie, pain, swelling and redness at the injection site, chills, fever, fatigue, headache, and muscle and joint pain). The risk of myocarditis associated with mRNA vaccination is similar to that observed in the general population (approx. 1/million), as is the possibility of anaphylaxis (2–5/million). This allowed experts to conclude that the benefits of vaccination against COVID-19 in pregnant women at particular risk of the disease outweigh the theoretical risks associated with it. By April 2022, recommendations for vaccination against COVID-19 in pregnant women were issued in 162 countries around the world, including Poland. Preliminary data also suggest that vaccination of the mother during pregnancy also reduces the risk of contracting COVID-19 in the infant during the first 4–6 months of life [9,10].
In more than 3 years of the pandemic, the COVID-19 epidemiological situation has changed significantly. Thanks to widespread vaccination or infection, or both of these factors (so-called hybrid immunity), significant population immunity has been created. It is estimated that about 90% of the world’s population is seropositive. In March 2023, the WHO published new recommendations for the implementation of national COVID-19 vaccination programs in the context of the dominance of the SARS-CoV-2 Omicron variant and its sub-variants and increasing population immunity. They also take into account recommendations for vaccination of pregnant women [8,26].
Currently, most SARS-CoV-2 infections are caused by the Omicron variant and its subvariant (
Also, Novillo and Martínez-Varea [26] based on a systematic review on COVID-19 vaccines during pregnancy and breastfeeding, which analyzed 1,893 articles, 33 of which met the inclusion criteria for the study, demonstrated the safety of COVID-19 vaccinations during pregnancy and breastfeeding. pregnancy and breastfeeding. Minor side effects were pain at the injection site and fatigue. This indicates the effectiveness of vaccinations, given that they reduce the risk of severe COVID-19 in pregnant women. Moreover, passive immunity, both in terms of cellular and humoral immune response, to the COVID-19 vaccine has been proven. Vaccination is associated with a greater and more stable immunoglobulin G (IgG) response and infection with a rapid and long-lasting immunoglobulin A (IgA) response. Therefore, vaccination against COVID-19 is strongly recommended for pregnant and breastfeeding women to protect mothers and newborns.
The WHO recommends that national vaccination programs consider the concomitant administration of an updated COVID-19 vaccine adapted to circulating virus variants together with the seasonal influenza vaccine. Vaccination against COVID-19 can be done at any time, before or after other vaccines recommended for pregnant women. Coadministration of vaccines is widely recommended and safe, including in pregnant women. According to the WHO, expectant mothers belong to a special group in the classification of people covered by vaccination priority. Effective implementation of vaccination against COVID-19 in privileged populations, including during pregnancy, is crucial to achieving optimal effects of the universal vaccination program [3,6,9].
New vaccines against group B streptococcus and respiratory syncytium virus (RSV) are currently being studied in pregnant women to protect newborns. There is more and more data on the vaccination of pregnant women against pneumococcus, meningococcus, and
The Role of the Doctor, Midwife, and Nurse in the Care of a Pregnant Woman Regarding Immunization
Persons caring for pregnant women (doctors, nurses, midwives) should conduct antenatal education on the prevention of infectious diseases in the perinatal period, including the possibility of preventive vaccination (Table 3) [6,9,10]. According to the applicable rules, the nurse performing the vaccination is obliged to check whether each pregnant woman applying for vaccination has undergone a qualified medical examination, which aims to detect possible contraindications to vaccination. Such examination shall be valid for 24 hours. Vaccination always takes place in treatment rooms and is performed by qualified nurses or midwives. Vaccines are most often administered intramuscularly (most vaccines) or subcutaneously in the deltoid muscle. If several vaccines are planned during one visit, each injection should be given in a different anatomical region [59].
In the new Polish standard of perinatal care [10], developed by the Minister of Health and by the Polish Society of Gynecology, an important element is prenatal education for undertaking pro-health behaviors during pregnancy, childbirth, and postpartum, as well as caring for a newborn and infant. Prevention of infectious diseases, including vaccination, is an integral part of this care. In preparation for pregnancy, it is recommended to supplement vaccination against rubella and hepatitis B. Recommendations for vaccination during pregnancy are in line with the recommendations of the
Future Directions
The field of maternal vaccinations is developing rapidly. In pregnant and breastfeeding women, vaccination achieves double immunization (ie, active artificial immunization for the mother and passive natural immunization for the fetus). There is increasing evidence confirming the safety, immunogenicity, and effectiveness of vaccinations during pregnancy, including against SARS-CoV-2. However, many challenges and key knowledge gaps remain, including the acceptance of an increasing number of vaccines for use in pregnancy, the optimal timing of vaccination, the impact of antigen type on antibody transfer across the placenta, correlations with protection against key pathogens, and the impact on subsequent infant immune responses for vaccination. Despite these challenges, vaccinations during pregnancy play an important role in protecting women’s health or fetuses and infants from infection.
Conclusions
Pregnancy is a stressful situation for the mother’s body. The maternal immune system must achieve immunological tolerance towards the fetus, which results in a reduced response to infections, including COVID-19. The best strategy to fight infections during pregnancy and breastfeeding is immunization through vaccination.
Understanding vaccine safety is critical when making vaccine decisions. Our goal was to present data on the current recommendations for vaccination of people in the reproductive period and pregnant women, including COVID-19 vaccines. Many observational studies confirm the safety of vaccination of adults and during pregnancy against COVID-19, and the benefits of vaccination far outweigh the potential risks associated with it. There is a need to strengthen the role of healthcare providers over people in the reproductive period and during pregnancy vaccinations, which is associated with the need to improve the preparation of medical staff to meet these expectations.
It is important to develop effective strategies for information policy on vaccination based on sound scientific foundations and denying negative media reports. The priority should be to increase knowledge and awareness about the safety and effectiveness of vaccines among pregnant women and their families, which influence individual decisions regarding active immunoprophylaxis of expectant mothers. The ultimate goal should be to ensure sustainable access to existing immunization programs for adults and expectant mothers.
Tables
Table 1. Vaccinations are recommended for adolescents, adults, and women in the reproductive period [6,7,9].![Vaccinations are recommended for adolescents, adults, and women in the reproductive period [6,7,9].](https://jours.isi-science.com/imageXml.php?i=t1-medscimonit-30-e942799.jpg&idArt=942799&w=1000) Table 2. Vaccinations recommended for pregnant women and during breastfeeding [8,9,10,26].
Table 2. Vaccinations recommended for pregnant women and during breastfeeding [8,9,10,26].![Vaccinations recommended for pregnant women and during breastfeeding [8,9,10,26].](https://jours.isi-science.com/imageXml.php?i=t2-medscimonit-30-e942799.jpg&idArt=942799&w=1000) Table 3. The scope of tasks related to preventive vaccination of adolescents, people in the reproductive period, and pregnant women is carried out by a doctor and a nurse or midwife [6,9,10].
Table 3. The scope of tasks related to preventive vaccination of adolescents, people in the reproductive period, and pregnant women is carried out by a doctor and a nurse or midwife [6,9,10].![The scope of tasks related to preventive vaccination of adolescents, people in the reproductive period, and pregnant women is carried out by a doctor and a nurse or midwife [6,9,10].](https://jours.isi-science.com/imageXml.php?i=t3-medscimonit-30-e942799.jpg&idArt=942799&w=1000)
References
1. Ratzan SC, Vaccine Literacy: A new shot for advancing health: J Health Commun, 2011; 16; 227-29
2. Wilson RJ, Paterson P, Jarrett C, Larson HJ, Understanding factors influencing vaccination acceptance during pregnancy globally: A literature review: Vaccine, 2015; 33(47); 6420-29
3. Badua AR, Caraquel KJ, Cruz M, Narvaez RA, Vaccine literacy: A concept analysis: Int J Ment Health Nurs, 2022; 31(4); 857-67
4. Gidengil C, Goetz MB, Newberry S, Safety of vaccines used for routine immunization in the United States: An updated systematic review and meta-analysis: Vaccine, 2021; 23; 3696-716
5. Marshall H, McMillan M, Andrews RM, Vaccines in pregnancy: The dual benefit for pregnant women and infants: Hum Vaccines Immunother, 2016; 12; 848-56
6. : Advisory Committee on Immunization Practices (ACIP) Recommendations Last Reviewed: March 13, 2023https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/index.htm
7. Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices (ACIP): Vaccine-Specific Recommendations Jan 28, 2022 Available from: https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/index.html
8. Centers for Disease Control and Prevention (CDC): COVID-19. Pregnancy or breastfeeding September 22, 2023 Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html
9. : Polish Protective Vaccination Program-Announcement of the Chief Sanitary Inspector of October 28, 2022 regarding the Protective Vaccination Program for 2023 [in Polish]https://dziennikmz.mz.gov.pl/DUM_MZ/2022/113/oryginal/akt.pdf
10. , Regulation of the Minister of Health of September 11, 2018 on the Organizational Standard of Perinatal Care: OJ, 2018; 1756 Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20180001756/O/D20181756.pdf
11. Azami M, Jaafari Z, Soleymani A, Rubella immunity in pregnant Iranian women: A systematic review and meta-analysis: Int J Fertil Steril, 2019; 13(3); 169-77
12. Ahn KH, Park YJ, Hong SC, Congenital varicella syndrome: A systematic review: J Obstet Gynaecol, 2016; 36(5); 563-66
13. Zhang E, Dai Z, Wang S, Vaccine literacy and vaccination: A systematic review: Int J Public Health, 2023; 14(68); 1605606
14. Omidvar S, Firouzbakht M, Acceptance of COVID-19 vaccine and determinant factors in the Iranian population: A web-based study: BMC Health Serv Res, 2022; 22(1); 652
15. Davies B, Olivier J, Amponsah-Dacosta E, Health systems determinants of delivery and uptake of maternal vaccines in low- and middle-income countries: A qualitative systematic review: Vaccines, 2023; 11; 869
16. Abu-Raya B, Michalski C, Sadarangani M, Lavoie PM, Maternal immunological adaptation during normal pregnancy: Front Immunol, 2020; 11; 575197
17. Morelli SS, Mandal M, Goldsmith LT, The maternal immune system during pregnancy and its influence on fetal development: Res Rep Biol, 2015; 6; 171-89
18. Wang H, Wang LL, Zhao SJ, IL-10: A bridge between immune cells and metabolism during pregnancy: J Reprod Immunol, 2022; 154; 103750
19. Bisset KA, Paterson P, Strategies for increasing uptake of vaccination in pregnancy in high-income countries: A systematic review: Vaccine, 2018; 36; 2751-59
20. Berger BE, Omer SB, Could the United States experience rubella outbreaks as a result of vaccine refusal and disease importation?: Hum Vaccine, 2010; 12; 1016-20
21. DiTosto JD, Weiss RE, Yee LM, Badreldin N, Association of Tdap vaccine guidelines with vaccine uptake during pregnancy: PLoS One, 2021; 16; e0254863
22. Shimabukuro TT, Kim SY, Myers TR, Preliminary findings of mRNA COVID-19 vaccine safety in pregnant persons: N Engl J Med, 2021; 384; 2273-82
23. Bishop K, McMorrow M, Meiring S, An evaluation of an influenza vaccination campaign targeting pregnant women in 27 clinics in two provinces of South Africa, 2015–2018: BMC Health Serv Res, 2021; 21; 941
24. Obuchowska A, Standyło A, Obuchowska K, Cytokine storms in the course of COVID-19 and haemophagocytic lymphohistiocytosis in pregnant and postpartum women: Biomolecules, 2021; 11; 1202
25. Wastnedge EAN, Reynolds RM, van Boeckel SR, Pregnancy and COVID-19: Physiol Rev, 2021; 101; 303-18
26. Novillo B, Martínez-Varea A, COVID-19 vaccines during pregnancy and breastfeeding: A systematic review: J Pers Med, 2023; 13(1); 40
27. Wei SQ, Bilodeau-Bertrand M, Liu S, Auger N, The impact of COVID-19 on pregnancy outcomes: A systematic review and meta-analysis: CMAJ, 2021; 193; E540-E48
28. Erazo CE, Erazo CV, Grijalva MJ, Moncayo AL, Knowledge, attitudes and practices on influenza vaccination during pregnancy in Quito, Ecuador: BMC Public Health, 2021; 21; 72
29. Marin M, Marti M, Kambhampati A, Global varicella vaccine effectiveness: A meta-analysis: Pediatrics, 2016; 137; e20153741
30. Demicheli V, Rivetti A, Debalini MG, Pietrantonj CD, Vaccines for measles, mumps and rubella in children: Cochrane Database Syst Rev, 2012; 2; CD004407
31. , Mumps virus vaccines: WHO position paper (cyt. 24.08.2023)http://www.who.int/wer/2007/wer8207.pdf
32. Zimmerman L, Rogalska J, Wannemuehler KA, Toward rubella elimination in Poland: Need for supplemental immunization activities, enhanced surveillance, and further integration with measles elimination efforts: J Infect Dis, 2011; 204; 389-95
33. Gagliardi AM, Andriolo BN, Torloni MR, Soares BGO, Vaccines for preventing herpes zoster in older adults: Cochrane Database Syst Rev, 2019; 3(3); CD008858
34. Demicheli V, Jefferson T, Ferroni E, Vaccines for preventing influenza in healthy adults: Cochrane Database Syst Rev, 2018; 2(2); CD001269
35. Ortiz RR, Smith A, Coyne-Beasley T, A systematic literature review to examine the potential for social media to impact HPV vaccine uptake and awareness, knowledge, and attitudes about HPV and HPV vaccination: Hum Vaccin Immunother, 2019; 15; 1465-75
36. Bergman H, Buckley BS, Villanueva G, Comparison of different human papillomavirus (HPV) vaccine types and dose schedules for prevention of HPV-related disease in females and males: Cochrane Database Syst Rev, 2019(11); CD013479
37. Akumbom AM, Lee JJ, Reynolds NR, Cost and effectiveness of HPV vaccine delivery strategies: A systematic review: Prev Med Rep, 2022; 18; 101734
38. : Recommendations of the Ministry of Health regarding vaccination against HPV as part of the universal program www.mp.pl/szkolenia/artykuly/wytyczne/323956,zalecenia-ministerstwa-zdrowia-dotyczace-zdrowie-powiedzko-hpv-w-ramach-programu-powszechnego
39. , Human papillomavirus vaccines: WHO position paper (2022 update): The Weekly Epidemiological Record, 2022; 50; 645-72
40. Pool V, Mege L, Abou-Ali A, Arthus reaction as an adverse event following Tdap vaccination: Vaccines (Basel), 2020; 8; 385
41. Etti M, Calvert A, Galiza E, Maternal vaccination: A review of current evidence and recommendations: Am J Obstet Gynecol, 2022; 226; 459-74
42. Fernández-Cano MI, Arreciado Marañón A, Reyes-Lacalle A, Influenza and pertussis maternal vaccination coverage and influencing factors in Spain: A study based on primary care records registry: Int J Environ Res Public Health, 2022; 19; 4391
43. Regan AK, The safety of maternal immunization: Hum Vaccines Immunother, 2016; 12; 3132-36
44. Granoff DM, Pelton S, Harrison LH, Meningococcal vaccines: Vaccines Elsevier, 2013; 388-418
45. Blakeway H, Prasad S, Kalafat E, COVID-19 Vaccination during pregnancy: Coverage and safety: Am J Obstet Gynecol, 2022; 226; 236e1-e14
46. Albrecht M, Arck PC, Vertically transferred immunity in neonates: Mothers, mechanisms and mediators: Front Immunol, 2020; 11; 555
47. Maertens K, Schutter SD, Braeckman T, A review: Vaccine, 2014; 32; 1786-92
48. Tessier E, Campbell H, Ribeiro S, Impact of extending the timing of maternal pertussis vaccination on hospitalized infant pertussis in England, 2014–2018: Clin Infect Dis, 2021; 73; e2502-e8
49. Sebghati M, Khalil A, Uptake of vaccination in pregnancy: Best Pract Res Clin Obstet Gynaecol, 2021; 76; 53-65
50. Eick AA, Uyeki TM, Klimov A, Maternal influenza vaccination and effect of influenza virus infection in young infants: Arch Pediatr Adolesc Med, 2011; 65; 104-11
51. Shakib JH, Korgenski K, Presson AP, Influenza in infants born to women vaccinated during pregnancy: Pediatrics, 2016; 137; 6
52. Zavala E, Krubiner CB, Jaffe EF, Global disparities in public health guidance for the use of COVID-19 vaccines in pregnancy: BMJ Glob Health, 2022; 7; e007730
53. : EMA and ECDC statement on updating COVID-19 vaccines to target new SARS-CoV-2 virus variants www.ema.europa.eu/en/news/ema-ecdc-statement-updating-covid-19-vaccines-target-new-sars-cov-2-virus-variants
54. : Threat Assessment Brief: Implications for the EU/EEA of the spread of the SARS-CoV-2 Omicron XBB.1.5 sub-lineage www.ecdc.europa.eu/en/publications-data/covid-19-threat-assessment-brief-implications-spread-omicron-xbb
55. Giles M, Mantel C, Muñoz F, Vaccine implementation factors affecting maternal tetanus immunization in low-and middle-income countries: Results of the Maternal Immunization and Antenatal Care Situational Analysis (MIACSA) project: Vaccine, 2020; 38; 5268-77
56. Munoz FM, Jamieson DJ, Maternal immunization: Obstet Gynecol, 2019; 133; 739-53
57. Swamy GK, Metz TD, Edwards KM, Safety and immunogenicity of an investigational maternal trivalent group B streptococcus vaccine in pregnant women and their infants: Results from a randomized placebo-controlled phase II trial: Vaccine, 2020; 38; 6930-40
58. Carreras-Abad C, Ramkhelawon L, Heath PT, Doare KL, A vaccine against group B streptococcus: Recent advances: Infect Drug Resist, 2020; 29(13); 1263-72
59. Kroger A, Bahta L, Hunter P: General best practice guidelines for immunization (updated May 4, 2021) www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html
Tables
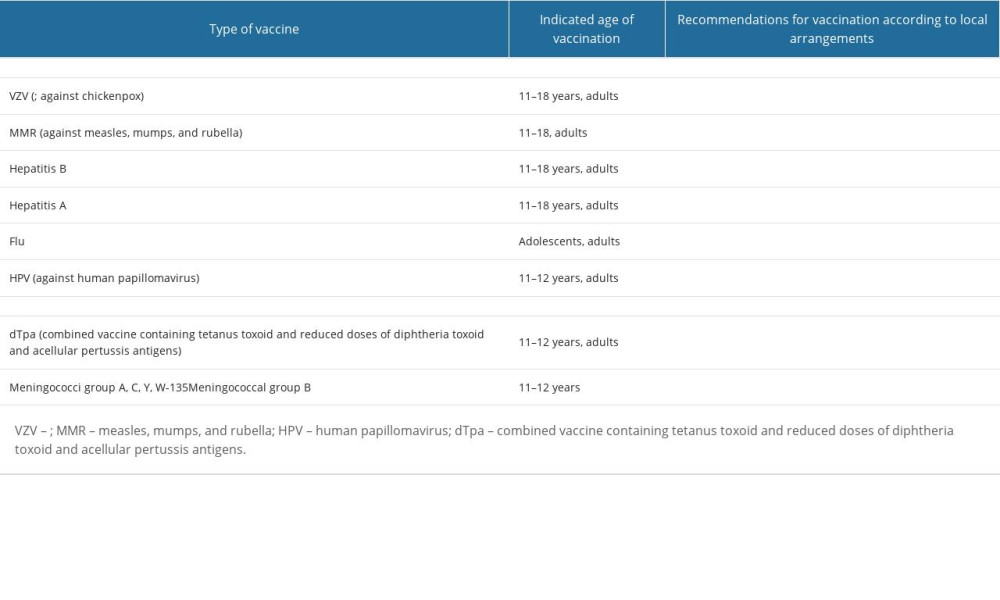 Table 1. Vaccinations are recommended for adolescents, adults, and women in the reproductive period [6,7,9].
Table 1. Vaccinations are recommended for adolescents, adults, and women in the reproductive period [6,7,9].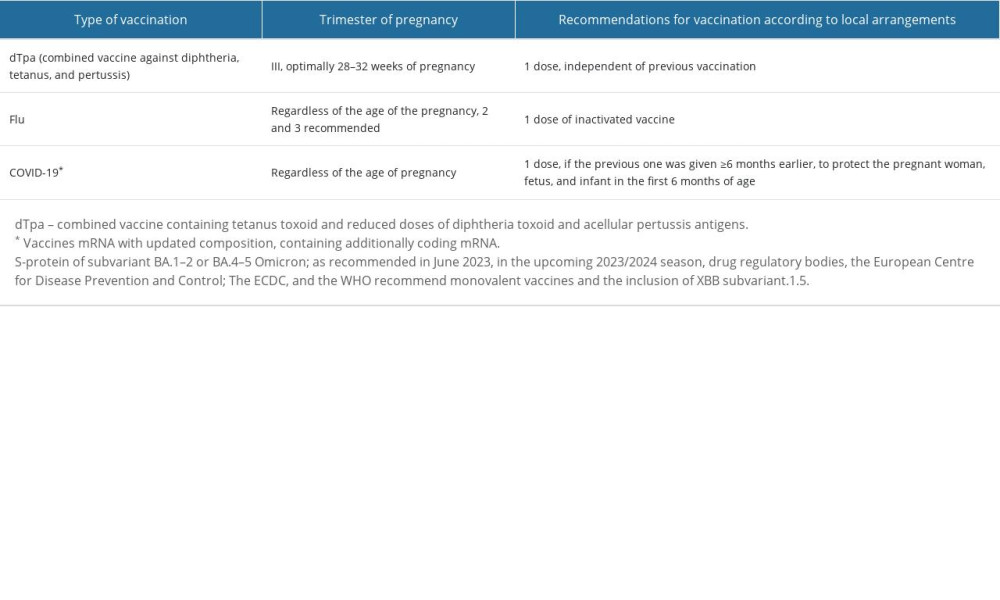 Table 2. Vaccinations recommended for pregnant women and during breastfeeding [8,9,10,26].
Table 2. Vaccinations recommended for pregnant women and during breastfeeding [8,9,10,26]. Table 3. The scope of tasks related to preventive vaccination of adolescents, people in the reproductive period, and pregnant women is carried out by a doctor and a nurse or midwife [6,9,10].
Table 3. The scope of tasks related to preventive vaccination of adolescents, people in the reproductive period, and pregnant women is carried out by a doctor and a nurse or midwife [6,9,10]. In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








