09 February 2024: Review Articles
Research Progress on Aortic Root Aneurysms
Tonghua Du1ABCD, Weitie Wang1ABCDEFG, Yong Wang1BCE, Hulin Piao1DEF, Kexiang Liu1ABCDEFG*DOI: 10.12659/MSM.943216
Med Sci Monit 2024; 30:e943216
Abstract
ABSTRACT: Aortic root aneurysms are one of the most common aortic root diseases, involving the aortic valve, aortic sinus, bilateral coronary arteries, and part of the ascending aorta. It is a life-threatening aortic disease with a high mortality rate of approximately 90%, due to aortic aneurysm rupture. Aortic valve insufficiency is one of the most common complications of aortic root aneurysms that can lead to acute left heart failure. The etiology of aortic root aneurysms is not yet completely clear and is mainly related to genetic diseases, such as Marfan syndrome and atherosclerosis. It can also occur secondary to aortic valve stenosis or a bivalve deformity. Surgery is the primary treatment for aortic root aneurysms, and aortic root replacement is a classic surgical method. However, the incidences of perioperative complications and mortality are relatively high, particularly in high-risk patients. In recent years, the anatomical structure of the aortic root has been gradually refined, and an in-depth understanding of root aneurysms has led to individualized treatment methods. Conservative drug therapy (β-receptor blockers, angiotensin-converting enzyme inhibitors, or angiotensin receptor blockers), Bentall and modified Bentall surgeries (Button technology, Cabrol surgery, and modified Cabrol surgery), valve-sparing aortic root replacement (David and Yacoub), personalized external aortic root support, and endovascular intervention therapy have significantly improved the perioperative and long-term survival rates of patients with aortic root aneurysms. However, different treatment methods have their own advantages and disadvantages. This review aimed to summarize the current research progress and treatment of aortic root aneurysms.
Keywords: aortic aneurysm, Thoracic Surgery, review
Background
Aortic root lesions are common in clinical practice, with a high mortality rate of approximately 90%, due to aortic aneurysm rupture [1]. The pathological change is in the aortic middle layer, caused by its own or environmental factors, resulting in aortic dilation. Aortic root lesions mainly include aneurysms and dissections. The etiology of aortic root aneurysms is not yet completely clear and is mainly related to genetic diseases, such as Marfan syndrome and atherosclerosis. It can also occur secondary to aortic valve stenosis or a bivalve deformity. Other arterial inflammatory diseases, such as Takayasu and Behçet diseases, are also associated with aneurysms. The arterial diameter will increase mainly due to aging (>50 years), sex (2–4 times more likely in males than in females), infection (arteritis), genetic predisposition, and smoking [2]. The main treatment methods include drug therapy, Bentall and modified Bentall surgeries, valve-sparing aortic root replacement (VSRR), personalized external aortic root support, and endovascular intervention therapy, all of which have achieved good results in clinical practice in recent years. Different treatment methods have their own advantages and disadvantages [3]; therefore, personalized treatment needs to be developed based on the patient’s condition. This topic has been reported in other papers; however, there remains a need to summarize the different approaches to treat aortic root lesions. This article reviews the research progress and summarizes the treatment prospects for aortic root aneurysms.
Overview of Aortic Root Aneurysms
ETIOLOGY:
Aortic root aneurysms are pathological aortic dilations >5 cm or >50% of the normal diameter, caused by various factors [4]. The etiology of aortic root aneurysms includes (1) congenital factors, such as Marfan syndrome; bicuspid aortic valve disease; and congenital connective tissue diseases, including Ehlers-Danlos syndrome and Loeys Dietz syndrome, and (2) secondary factors, such as aortic valve degeneration, rheumatic aortic valve disease, infection, trauma, and long-term hypertension. Additionally, smoking, hyperlipidemia, and cardiac dysfunction can cause aortic root expansion [5]. Infective endocarditis leads to aortic valve stenosis or incomplete closure, leading to hemodynamic changes, ultimately affecting the aortic root and ascending aortic wall, and causing aortic dilatation. Additionally, bacterial infections and Behcet disease of the aorta can damage the aortic wall, directly leading to aortic dilation and affecting the structures at the aortic root. When the aortic valve is narrowed, blood flow can be of high-pressure and speed, which significantly damages the aortic wall intima and increases the degree of valve stenosis. The cardiac output significantly increases in compensation to aortic valve insufficiency. The larger the degree of aortic valve insufficiency, the greater the impact force on the aortic wall. As time progresses, the intima will undergo more severe damage. Additionally, inflammatory cells inside or outside the plaque secrete cytokines, further amplifying inflammation and producing proteases, making the plaque unstable and destroying the extracellular matrix, leading to plaque rupture and hemorrhage or gradual deposition and enlargement of the atheromatous plaque itself, occupying the coronary artery lumen, and causing stenosis. The conversion of kinetic energy into potential energy after stenosis and the imbalance between intravascular pressure and vascular wall elasticity can also exacerbate aneurysm dilation [6].
ANATOMY OF THE AORTIC ROOT AND HEMODYNAMIC CHANGES:
The aortic root refers to the area between the junction of the sinus and aortic valve annulus, including the aortic valve annulus, aortic sinus, and junction of the sinus and canal. Three half-moon-shaped valves (left, right, and posterior semilunar valves) attached to the aortic valve ring form the aortic valve. The pocket-like space between the semilunar valve and aortic wall is also known as the aortic sinus, which is divided into the left, right, and posterior sinuses based on the valve position. The openings of the left and right coronary arteries are located above the free edge of the valve in the left and right sinuses, respectively. The opening and closing of the aortic valve leaflet are influenced by the anatomical structure and hemodynamic changes. When the aortic valve is opened during ventricular contraction, small eddies of blood are formed in the aortic sinus, which prevent valve adhesion and ensure blood supply to the coronary artery [7]. The 4-dimensional aortic root dynamics [8] show that during the cardiac cycle, the aortic root volume increases by an average of 33.7±2.7%, and by 36.7±3.3% before ejection. Expansion of the base starts from the isovolumic contraction period and continues to the junction of the sinus and duct. During the first third of the ejection period, the aortic root reaches its maximum dilation and then decreases to its minimum during mid-diastole. During the end-diastolic period, the aortic root volume expands again by 11.3±2.4%. This is sufficient to demonstrate the complexity of hemodynamic and elastic changes at the aortic root. Therefore, there is insufficient compliance for the artificial blood vessel transplantations to mimic the elastic mechanical characteristics of natural arterial tissues [9].
CLASSIFICATION:
Aortic root aneurysms are classified into 3 types based on the aneurysm location. Type 1 is ascending aortic aneurysm, in which the aortic root and sinus are normal; type 2 includes the proximal aortic root with extension to the arch; and type 3 includes the aortic root and sinus without ascending aortic and arch dilations [10]. Based on the involvement of the aneurysm, aortic root aneurysms are classified as follows. Aortic sinus aneurysm caused by vascular wall thinning and expansion in the aortic sinus and expansion into the surrounding cardiac cavity often occurs in the right coronary sinus (65–85%), followed by the non-coronary sinus (10–30%), and the left coronary sinus is the least common (<5%) [11,12]. Coronary artery aneurysm is a rare lesion that can be divided according to the aneurysm size: small (inner diameter <5 mm), medium (inner diameter of 5–8 mm), and giant (inner diameter >8 mm). Atherosclerosis is the most common cause of coronary aneurysms (50%) [12], followed by congenital (17%), and infectious (10%) etiologies [13].
RELATION WITH AORTIC VALVE DISEASE:
The aortic valve is a unidirectional valve located between the left ventricular outflow tract and ascending aorta, assisting in the unidirectional movement of blood flow during systole and preventing backflow to the heart during diastole [14]. Aortic valve diseases are classified into stenosis and regurgitation. A population study in 2018 showed an upward trend of aortic root aneurysms over time, with an incidence of 7.6 per 100 000 [15]. In a study by Della Corte et al [16], 76.6% of patients had ascending aortic dilation combined with aortic valve disease, and aortic regurgitation accounted for 55.7% of these patients. Therefore, surgeons need to evaluate aortic valve disease during the assessment of aortic root aneurysms, including surgical indications and contraindications, intraoperative support for cardiac function, and perioperative and long-term survival curves. With the exploration of the anatomy of the aortic root, ultrasound and imaging examinations, and hemodynamic interpretations, the interaction mechanism between aortic root aneurysms and aortic valve disease has gradually been discovered.
AORTIC ROOT ANEURYSMS CAUSED BY AORTIC VALVE DISEASE:
Bicuspid aortic valve (BAV) is the most common congenital aortic valve malformation, which leads to more complications and deaths than other congenital heart defects. Research has shown that the incidence of dilation of all segments from the aortic root to the aortic arch in patients with BAV ranges from 20% to 84% [11]. A BAV-concomitant aortic root aneurysm can be completely asymptomatic and found only on imaging examinations during regular health examinations. The diameters of the aortic root and ascending aorta of patients with BAV are significantly larger than those of patients with normal aortic tricuspid valve function [12]. This difference is related to the study population, evaluation techniques, differences in aortic root size thresholds, and disease heterogeneity. However, the growth rate of the ascending aorta diameter in patients with BAV (0.2–1.9 mm/year) is significantly higher than that of the normal aortic tricuspid valve population. In conclusion, the diameters of the aortic root and all segments of the ascending aorta in patients with BAV are larger than those in the normal tricuspid valve structure population [13]. The main reason for this phenomenon is the hemodynamic changes caused by BAV stenosis, especially in cases of concomitant hypertension, which leads to an increase in shear stress in the aortic sinus of blood flow to the contralateral surface, resulting in aneurysm-like dilation. This can lead to stenosis combined with aortic valve insufficiency, enhanced cardiac systolic function, and exacerbated aortic sinus dilation.
AORTIC VALVE DISEASE CAUSED BY AORTIC ROOT ANEURYSMS:
Marfan syndrome is an autosomal dominant connective tissue disease caused by FBN-1 mutations and is the main risk factor for aortic root aneurysms, with an incidence rate of 0.01% to 0.02% [14]. The growth rate of the aortic root diameter in patients with Marfan syndrome (2.6 mm/year) is significantly faster than that in those without it (1 mm/year). The growth rate of an aneurysm also increases with size, with a growth rate of approximately 4 mm/year for an aneurysm size of 60 mm and 4.6 mm/year for an aneurysm size of 70 mm [15]. Rupture is the most fatal complication of aortic root aneurysms. Gradual enlargement of the aortic root aneurysm can also lead to irreversible changes in the normal mitral valve, leading to reflux and further exacerbation of the left ventricular load. Simultaneously, blood can form eddies in the aneurysm, promoting its expansion. Acute enlargement or tearing of the valve leaflet can cause severe acute aortic valve insufficiency, leading to rapid progression and even death due to heart failure.
Marfan syndrome and BAV aortic disease share common histopathological characteristics, including mid-layer degeneration, increased matrix metalloproteinase (MMP) activity, and decreased fibrinogen 1 levels in the aortic wall. However, compared with that of BAV, the incidence of Marfan syndrome is very low [16]. Meanwhile, research has found that in patients with aortic stenosis and regurgitation, 43% with an aortic diameter of 45 to 49 mm do not require surgery, whereas 86% with an aortic diameter <40 mm do. This indicates that moderate aortic dilatation is an important risk factor for aortic complications after valve replacement surgery [17]. Therefore, the possibility of repairing a dilated ascending aorta while treating aortic valve disease during surgery remains controversial in clinical practice.
Surgical Indications
Regardless of the cause of the aortic aneurysm, once dilation begins, the expansion becomes progressive and irreversible. According to Laplace’s law, tension=blood pressure×aneurysm radius/2, which means that the pressure on the aortic aneurysm wall is proportional to its radius and the patient’s blood pressure. Therefore, hypertension and increased aortic radius can promote aortic dilation [17]. If the aneurysm expands further, the damaged aortic wall impacted by the blood flow forms an aortic root dissection. For such patients, if treatment is untimely, serious life-threatening complications, such as aneurysm rupture and pericardial tamponade, can occur.
In general, the aorta gradually expands with age (0.07–0.2 cm/year) [18]. The dilation speed of the ascending aorta is slower than that of the descending aorta [17]. Aortic dilation speed is influenced by hypertension, connective tissue disease, and the BAV. Davies et al [19] pointed out that the median diameter of the ruptured ascending aorta or aortic arch is approximately 6 cm. However, in patients with aortic dissection, rupture occurs even when the diameter is <5.5 cm. Svensson et al found that approximately 15% of patients with Marfan syndrome and 12.5% with BAVs develop dissection when the ascending aortic diameter is <5 cm [20]. Parish et al [21] reported that approximately 42% of patients without Marfan syndrome but with a tricuspid aortic valve developed type A aortic dissection when the ascending aortic diameter was <5 cm. In an international aortic dissection registration study [22], approximately 60% of patients with type A aortic dissection had an ascending aortic diameter <5.5 cm at disease onset, and 40% had an ascending aortic diameter <5 cm. However, aortic diameter alone cannot fully predict the occurrence of aortic dissection. Some studies have also indicated that aortic wall elasticity and rigidity are related to aortic dissection rupture. In 2014 [23], the American College of Cardiology and American Heart Association pointed out in their guidelines that the risk of aortic root rupture increases significantly when the aortic root dilates >5.5 cm. Therefore, surgical indication is defined as an ascending aortic diameter >5.5 cm. Some experts believe that early surgical treatment is needed when the active aortic root diameter is >5.0 cm, and the latest 2022 guidelines [24] indicate that surgery is indicated in patients with aortic root and ascending aortic aneurysms who have symptoms attributable to the aneurysm. Many of these patients develop an intimal tear of the aneurysm wall during surgery, which is prone to dissection and can lead to aortic ring enlargement and valve insufficiency. In asymptomatic patients with aortic root or ascending aortic aneurysm with a maximum diameter of ≥5.5 cm, surgery is indicated. In patients with an aortic root or ascending aortic aneurysm of <5.5 cm, whose growth rate confirmed by tomographic imaging is ≥0.3 cm/year in 2 consecutive years or ≥0.5 cm in 1 year, surgery is indicated.
In cases of type A aortic dissections, if the dissection involves the aortic valve leaflet, junction, and annulus, aortic root replacement can provide great convenience in both the operation and hemostasis process. However, in experienced large centers, techniques such as junction suspension and sinus reconstruction can be used to save aortic valve leaflets and avoid valve replacement.
Conservative Therapies
β-RECEPTOR BLOCKERS:
β-receptor blockers are the best prophylactic treatment for thoracic aortic aneurysms [25]. Shores et al [26] firstly proposed the use of b-receptor blockers in the treatment of Marfan syndrome and ascending aortic dilation in 1994. However, these drugs do not improve the underlying pathological process that leads to aortic wall dilation and, therefore, cannot fundamentally treat aortic root dilation.
ANGIOTENSIN RECEPTOR BLOCKERS:
Angiotensin receptor blockers (ARBs) are effective against ascending aortic dilation, due to their ability to inhibit overexpression of transforming growth factor β. Brooke et al [27] conducted a small sample clinical study on patients with Marfan syndrome who were on ARB treatment for 1 year before taking β-receptor blockers. After the use of ARBs, the rate of aortic sinus dilation significantly decreased, with an average rate of approximately 46 mm/year. However, the study was limited to patients with Marfan syndrome, with a small sample size.
MMP INHIBITORS:
MMP inhibitors reduced the serum MMP-2 and −9 levels and slowed the rate of aneurysm expansion in animal models [28,29]. Jackson et al [30] detected serum MMP expression in 109 patients with aortic diameters >4 cm and found that MMP-14 and −19 were highly expressed in the serum of these patients. Therefore, MMP can be considered as one of the reasons for aortic dilation. Doxycycline has shown effectiveness in treating small abdominal aortic aneurysms in clinical trials. Doxycycline mainly inhibits serum MMP-9; however, its efficacy in the thoracic aorta remains unclear. MMP inhibitors can be used in future basic research and clinical trials; however, their efficacy and related adverse effects remain unclear for clinical treatment.
STATINS:
Few prospective and randomized controlled studies have been conducted on statin use for aneurysm treatment. Two recent retrospective studies conducted at Yale University are related to the clinical efficacy of statins in ascending aortic dilation. Jovin et al [31] conducted a clinical follow-up of patients with ascending aortic aneurysms. During follow-up, the incidence of aneurysm rupture-related events was significantly reduced in patients who took statins on a regular basis, compared with those who did not take statins (20% vs 33%). Another retrospective study of patients with thoracic aortic aneurysms found a stronger protective effect of statins in descending aortic aneurysms. Long-term statin use can significantly prolong clinical follow-up and surgical procedures and reduce the occurrence of aortic-related complications (aortic dissection, aortic rupture, or death). Although drug therapy can slow the rate of ascending aortic dilation and delay the timing of surgical treatment, surgery remains the main treatment method. The focus in the past was mainly on the timing of surgical intervention; however, there has been no detailed discussion on the specific surgical treatment for patients.
Surgical Treatment
AORTIC ROOT PLASTY:
In 1958, Garamella performed aortic valve junction suspension surgery to treat aortic valve regurgitation secondary to a subcortical ventricular septal defect. Subsequently, this technology was applied in patients with mild aortic regurgitation caused by aortic dissection. Approximately 40% to 60% of patients with acute Stanford type A aortic dissection are associated with aortic valve regurgitation. The modified Bentall procedure for aortic root replacement is used to treat severe aortic valve disease [37,38]. For patients with aortic root dilation or Marfan syndrome, aortic valve regurgitation suspension is not recommended. The Bentall procedure is recommended for severe regurgitation, whereas other mild regurgitations can undergo aortic valve regurgitation suspension, thereby avoiding postoperative anticoagulation and related complications. Therefore, the surgical indication for aortic valve junction suspension must be limited to the absence of significant dilation of the aortic annulus and the normal morphology and structure of the valve leaflets. The main surgical point in aortic valve junction suspension surgery is the use of 4-0 Prolene, with a gasket to suspend the avulsed aortic valve junction. Aortic valve junction suspension surgery can require further surgical treatment, due to long-term aortic regurgitation. Therefore, this surgical procedure is not suitable in severe regurgitations or Stanford type A aortic dissections with aortic annulus dilation. Additionally, in some patients with Stanford type A aortic dissection involving the coronary opening, especially the right coronary opening, 4-0 Prolene with a gasket couple is used to fix the intima tear around the coronary opening.
BENTALL SURGERY:
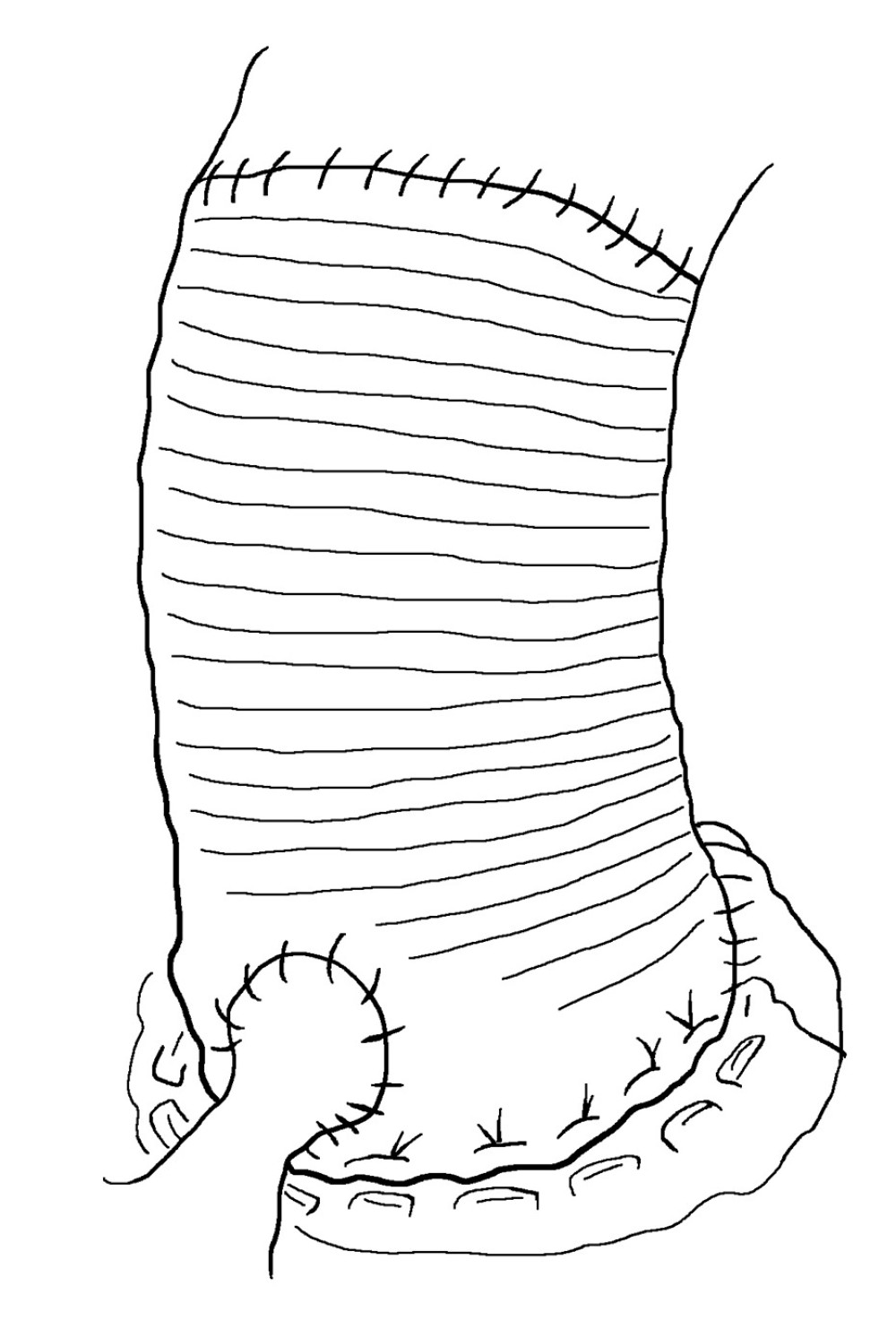
In 1968, Bentall and De Bono first described the surgical method of using artificial Dacron vessels with mechanical valves for valve replacement, ascending aortic replacement, and coronary artery replantation. This technology has been the standard surgical treatment for aortic root aneurysms for many years [39–41], and the development of this surgery has been ongoing for more than half a century. The mortality rate associated with Bentall surgery has decreased from 17% to 2% [17,18]. However, the promotion and application of this surgery are limited due to difficulties in coronary artery transplantation and anastomosis, excessive anastomotic tension, difficulty in managing anastomotic bleeding, and high postoperative complications [42–45].
Classic Bentall surgery uses a direct anastomosis method, in which the coronary artery opening is directly anastomosed to the aortic wall of artificial vessels (Figure 1). This method is simple, and aortic root-to-left atrial drainage can effectively prevent bleeding. However, the suture tension of the coronary artery anastomosis is high, which can lead to pseudoaneurysm [46]. The incidence of pseudoaneurysms during long-term follow-up is 8% to 15% in the literature [47]. Pseudoaneurysms are prone to concomitant cardiac dysfunction, and the reoperation risk is extremely high, especially in patients with poor conditions of the coronary artery opening anastomosis and those with a distance of <2 cm from the lower edge of the coronary artery opening to the aortic valve annulus. This is because once a homograft is inserted, coronary reconstruction is a major problem. Owing to the obstruction at the edge of the homograft, it is quite difficult to suture the coronary opening with the homograft.
MODIFED BENTALL SURGERY:
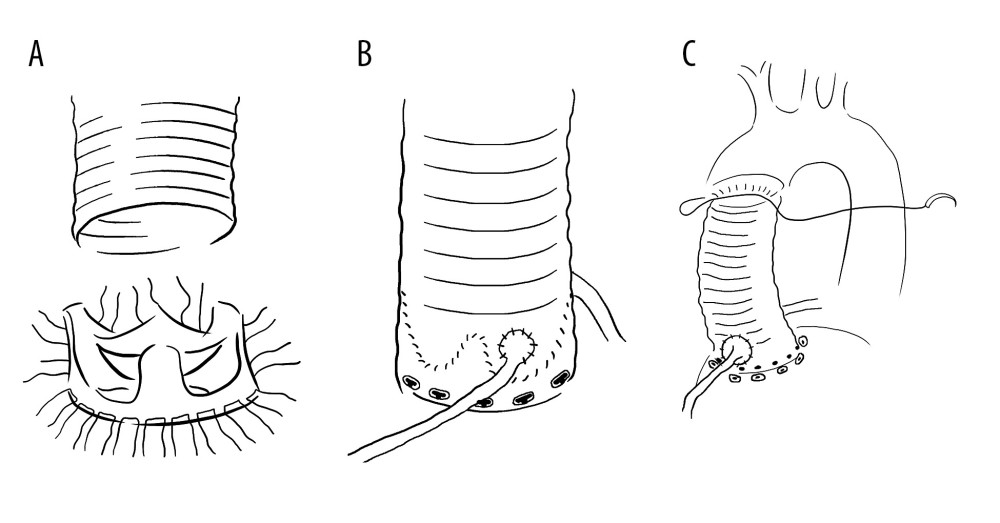
The original Bentall method involved suturing the coronary artery to a homograft, which is prone to bleeding risk [48–50]. Postoperative complications, such as coronary intimal tearing and pseudoaneurysm formation, occur frequently. The requirement of surgery after the Bentall operation is relatively high [51,52]. To address the problems of excessive tension at the anastomotic site and difficulty in managing anastomotic bleeding in traditional Bentall surgery, modified Bentall surgery mainly focuses on improving coronary artery anastomosis. Button technology is a well-known improved Bentall method [53–55], which mainly includes removing the aortic valve and liberating the left and right coronary arteries into a “button” shape. The diseased part of the aorta is replaced with a homograft, and holes in the artificial vessels are electrocauterized. The “button” of the coronary artery opening is continuously sutured with the artificial vessel using 5-0 polypropylene suture. Finally, the artificial vessel end is sutured to the aorta using 4-0 polypropylene. However, button technology has not yet resolved the issue of anastomotic tension, and complications such as coronary intimal tearing and pseudoaneurysm formation still occur [56–58].
Cabrol surgery is commonly used for internal coronary artery tear, especially in aortic dissection [59–61]. Due to the displacement of the coronary artery opening, severe calcification of the aortic vessel wall around the coronary artery opening, and severe adhesion during secondary surgery, it is difficult to isolate the coronary artery opening and anastomosis. For these patients, Cabrol et al modified the Bentall surgery, using a 10-cm long intermediate artificial vessel (usually 0.8 or 1.0 cm in diameter) to perform end-to-end anastomosis with the left and right coronary artery orifices, and then anastomosis with artificial blood vessel at the lateral position, which is called Cabrol surgery. Good surgical results have been achieved. This method increases the activity of the coronary artery orifices, significantly reduces the anastomotic tension between the coronary artery opening and artificial blood vessel, and reduces the risk of aneurysms, such as dilation of the coronary artery opening after anastomosis with the artificial blood vessel. It also exposes the anastomosis site, which makes hemostasis relatively easy but increases the risk of intermediary vessel angulation, folding, compression, and thrombosis, ultimately leading to myocardial ischemia, myocardial infarction, and even death. However, the Cabrol surgery is more complex and does not have significant advantages over the button method. Some studies have found that the button method has a significantly higher long-term survival rate than the Cabrol surgery. Therefore, Cabrol surgery is now less commonly performed, and some heart centers have stopped performing it.
Bentall surgery using the button method for coronary artery opening transplantation has a relatively lower incidence of reoperation than the classic Bentall surgery [62,63] and does not increase bleeding risk after surgery [64–66]. This improvement makes it relatively easy to expose the location of anastomotic bleeding; therefore, root wrapping and pressure hemostasis for root bleeding are better than classical Bentall surgery. However, the problem of anastomotic bleeding has not been effectively solved. In 1978, Cabrol et al solved the problem of aortic root anastomotic bleeding using aortic root drainage surgery. Subsequently, many surgeons have achieved good clinical outcomes [67,68]. Owing to the complications of taking anticoagulants after Bentall surgery, Bio-Bentall surgery was promoted in experienced heart centers. A comparative study of the Bio-Bentall and Bentall procedures found no significant difference in valve-related complications and health-related quality of life scores in 1- and 5-year survival rates [9]. Emergency surgery and coronary atherosclerotic heart disease have a significant impact on short-term outcomes, while coronary artery disease, old age, degenerative etiology, thrombosis, and bleeding are independent risk factors for long-term survival [10]. Animal experiments have shown that the aortic tissues of older adults are significantly stronger than that of pigs aged 6 to 9 months. The aortic root and sinus tissues of humans have similar but uneven hardness, compared with those of pigs. From a biomechanical perspective, differences exist between the arterial valves of pigs and those of older adults. Therefore, in terms of valve type, it is still necessary to make appropriate choices based on the patient conditions (age and underlying heart disease) and medical conditions (high patient compliance and regular follow-up anticoagulation indicators).
DAVID SURGERY:
Anticoagulation-related complications, such as bleeding or thrombosis, are the main limitations after Bentall surgery. However, valve degeneration is inevitable after Bio-Bentall surgery. For some young patients, especially women with reproductive needs, the quality of life is severely affected [69] by Bio-Bentall and Bentall surgeries. The emergence of VSRR surgery effectively solved this problem. In the 1990s, aortic root replantation (David surgery) [70] (Figure 2) and aortic root remodeling (Yacoub surgery) (Figure 3) surgeries [71] were invented. Although VSRR is more complex and technically demanding than the Bentall surgery, personalized VSRR surgery provides more opportunities and possibilities for the development of modern cardiac surgery. VSRR have a lower incidence and mortality rate of postoperative complications, especially in terms of quality of life. During a follow-up period >10 years, the majority of patients maintained good aortic valve stability [72,73]. Regardless of the underlying pathology, valve preservation has not only a good survival rate and valve durability, but can also reduce the incidence of ventricular hypertrophy and prevent ventricular remodelling, compared with Bentall or Bio-Bentall surgery [74].
SURGICAL INDICATIONS OF DAVID SURGERY:
The quality of the aortic valve leaflet is the key to the success of David surgery. The valves can be evaluated by transthoracic ultrasound observation, intraoperative esophageal ultrasound reconfirmation, and intraoperative incision of the ascending aorta, for direct exploration. Intraoperative esophageal ultrasound can clearly observe the quantity, quality, morphology, and nature of the aortic valve leaflets, which helps confirm the surgical method. When the number of leaflets is repeatedly confirmed to be normal; the leaflets are soft, without thickening, curling, calcification, or prolapse; and the reflux is mainly central, David surgery is performed. When the diameter of the aortic root is >60 mm and is accompanied by moderate aortic valve regurgitation, the aortic valve leaflets can be accompanied by severe pathological changes. The final choice of surgical method is direct exploration of the aortic valve during surgery.
:
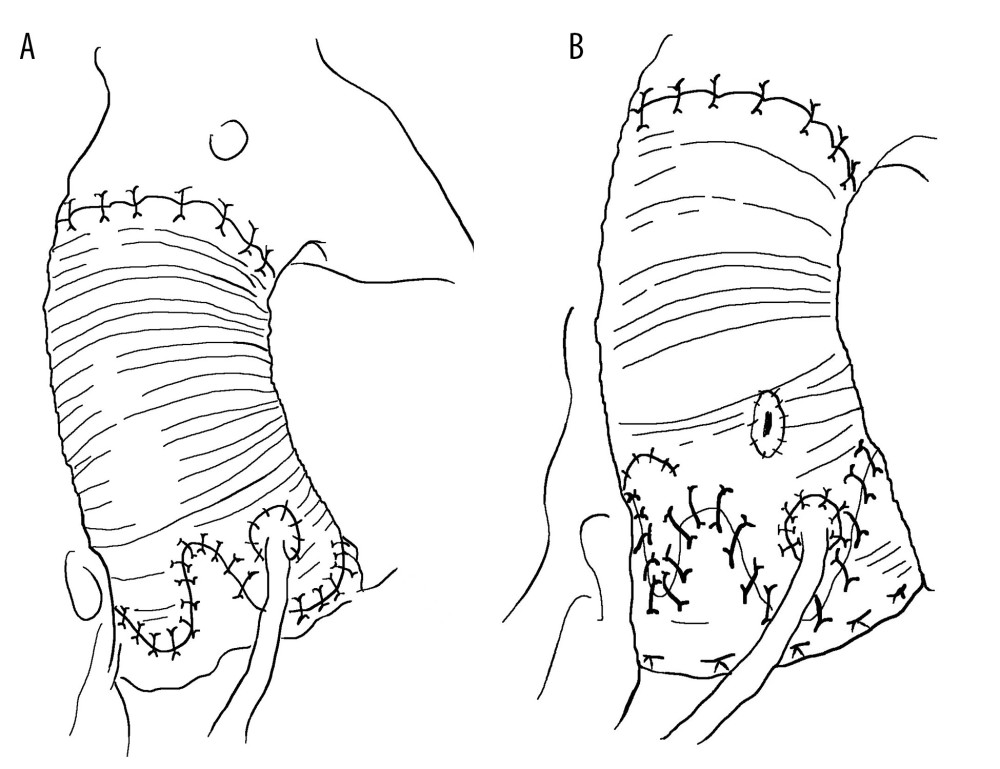
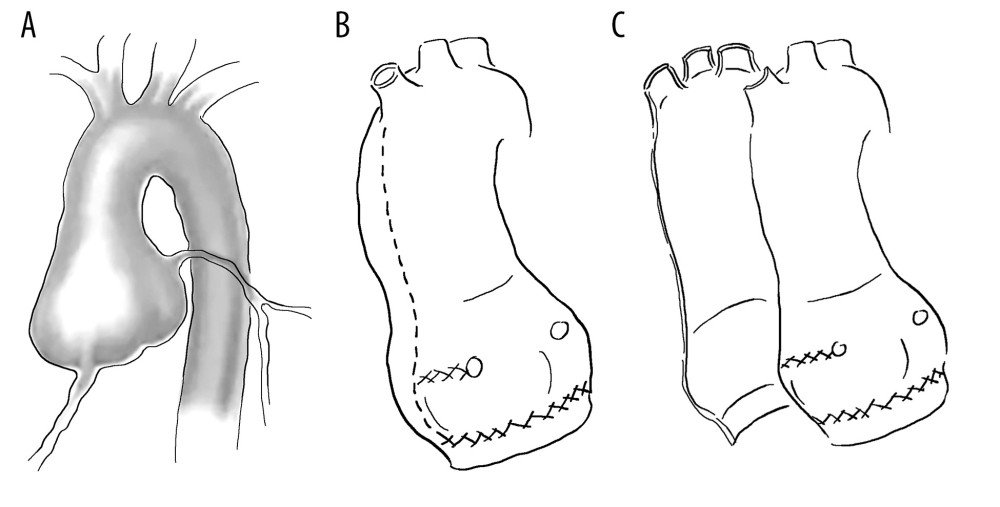
There are 4 types of David surgery. (1) David type I (replantation method): David et al proposed a method for preserving the aortic valve during aortic root replacement surgery in 1982. The detailed method involved directly anastomosing the artificial blood vessel with the aortic valve ring, suspending the valve junction, and fixing it inside the artificial blood vessel. This method effectively fixes the aortic valve ring with artificial blood vessels without causing aortic valve insufficiency, owing to aortic valve ring dilation. However, collisions between the valve leaflet and artificial blood vessels can accelerate aortic valve damage. In 1995, Cochran et al modified the David I procedure by trimming the proximal artificial blood vessel into a shallow wavy shape and fixing it to the aortic valve ring to form an artificial aortic sinus. The aortic valve junction was sutured to blood vessels. This can prevent the expansion of the aortic valve ring while avoiding collisions between the valve leaflet and artificial blood vessel. (2) David type II (remodeling method): In 1983, Yacoub et al proposed a shaping method for preserving the aortic valve root replacement. The detailed method involves trimming the end of the artificial blood vessel into 3 wavy shapes and suturing it to the aortic aneurysm wall. This method preserves the shape of the aortic sinus and can reduce collisions between the aortic valve and artificial blood vessels. However, there is a possibility of dilation of the aortic annulus. (3) David III (valve ring fixation plastic surgery): In 1996, David introduced the improved David III surgical method. Compared with David II surgery, improvements have been made using Teflon strips to strengthen and fix the aortic valve annulus, mainly focusing on the fibrous structure of the annulus. David III surgery can effectively prevent expansion of the aortic valve annulus after David II surgery. In 2006, Lansac et al improved the David II procedure by using an artificial vascular ring to reinforce the aortic root during plastic surgery, thereby preventing dilation of the aortic valve ring; this is also known as the Lansac method. (4) David IV type (replantation method for reconstructing the aortic sinus): Modified David I-type surgery aims to reconstruct the aortic sinus and avoid collisions between valve leaflets and artificial blood vessels. The surgical method involves the use of an artificial blood vessel with a diameter increase of 4 to 8 mm compared with the aortic valve ring or sinus junction. The proximal end of the artificial blood vessel is first narrowed to fit the valve ring, and proximal anastomosis is then performed. With continuous attention to the role of the aortic sinus and development of new materials, artificial blood vessels with aortic sinuses have emerged. There is no need for a large proximal constriction of artificial blood vessels, simplifying David IV surgery without increasing surgical difficulty and achieving good clinical results.
OUTCOMES OF DAVID SURGERY:
Early follow-up results showed a total mortality rate of approximately 1.3%, with 97% to 99% of patients exempted from secondary surgery [78]. In 2000, Leyh [79,80] reported early postoperative outcomes in patients with aortic dissection who underwent David surgery, which showed low perioperative mortality and good clinical prognosis. In 2003, a clinical physician [81] pointed out that David surgery is more suitable for patients with aortic dissection. Surgery can completely remove the affected tissue and facilitate intraoperative hemostasis, and the hemostasis range is limited to the proximal anastomosis of the coronary artery and aorta. The postoperative reoperation rate is low and anticoagulant drugs are not required after surgery. In 2000, De Paulis [82] first reported that an antral artificial blood vessel can reduce the tension on the autologous valve. In 2015, Lizhong et al [83] conducted a 9-year clinical outcome analysis of the intraoperative use of artificial blood vessels with sinuses for the treatment of aortic dissection or ascending aortic aneurysms. The perioperative mortality rate was 2.6% and the 10-year postoperative exemption rate from reoperation was 90%. Gaudino et al [84] found no in-hospital or early mortality in patients undergoing VSRR, with an overall operative mortality of 0.2% and an incidence of major postoperative complications of 0.5%. Svensson et al [85] showed an overall operative mortality of 0.16% and rare occurrence major adverse events. In a recent update by David et al [86], the operative mortality rate of VSRR was 1.1%. Other groups reported a VSRR operative mortality of 1.8% to 4% [87–89].
Endovascular Therapy
In recent years, with the development of thoracic endovascular repair (TEVAR) and improved techniques for treating thoracic aortic diseases, endovascular repair of the ascending aorta has become a research hotspot [90]. In 2002, Cribier et al [91] successfully performed the first transcatheter aortic valve replacement (TAVR), which is widely used because of its advantages of minimal trauma, fast postoperative recovery, and a stable anchoring area. In 2014, Rylski et al [92] proposed the concept of integrated intracavitary therapy with TEVAR and TAVR, and the surgical concepts of Endo-Wheat and Endo-Bentall emerged. Endo-Wheat uses a straight ascending aortic graft and an artificial heart valve that combines TEVAR and TAVR techniques [90]. The Endo-Bentall technique uses a composite graft to simultaneously reconstruct the ascending aorta, aortic valve, and coronary artery [93]. However, due to the complex anatomical structure of the aortic root, the long-term patency of left and right coronary artery reconstruction using endovascular grafts for the treatment of aortic root aneurysms combined with aortic valve disease remains unknown. Additionally, whether the aortic sinus needs to be reconstructed is still unclear and is mainly determined by the specific location of the aortic root aneurysm. If the aneurysm is located in the aortic ring and body of the aortic sinus, the choice is not to reconstruct the aortic sinus, but to use a graft directly crossing the aortic sinus, bilateral coronary artery fenestration, or an integrated graft with bilateral coronary artery branches. If the aneurysm is located at the junction of the aortic sinus, endovascular reconstruction is often performed to preserve the aortic sinus function. In summary, the implementation of endovascular treatment expands the scope of indications for aortic root aneurysms combined with aortic valve disease and has the advantages of minimal surgical trauma and fast postoperative recovery. However, further exploration is needed to address the issues of personalized graft customization, long-term observation of aortic sinus reconstruction and physiological function, and treatment strategies for patients with concomitant coronary stenosis or aneurysm-like dilation.
Personalized External Aortic Root Support
Personalized external aortic stents were first introduced in 2004 (Figure 4), primarily for the conservative treatment of patients with Marfan syndrome. The stent was designed and manufactured using a computer, based on the shape of the patient’s aortic root. The manufacturing and clinical application of this stent began between 2000 and 2004. Since the advent of personalized external aortic stents, computer-aided design, manufacturing methods, and surgical techniques have remained unchanged, reflecting the superiority of this surgical technique. After a prospective evaluation of 20 patients [94], the technology passed the health technology evaluation conducted by the National Institute of Health and Health Excellence in the United Kingdom.
Based on the results of cross-sectional digital images, computer-aided design parameters, and modeling of the patient’s aorta, the production of thermoplastic materials can be completed quickly, resulting in the production of an extracorporeal stent (a macroporous polymer mesh stent), suitable for individual patients. The stent is located around the aorta and tightly connected from the proximal aortic interventricular septal junction to the distal brachiocephalic artery. Surgery is performed with the heart beating, usually without extracorporeal circulation. Between 2004 and 2011, 30 patients underwent this surgery and during the follow-up process, no deaths, cerebrovascular diseases, or adverse events related to the aorta or valves were reported [95].
Conclusions
The anatomical structure of the aortic root is complex, and there is currently no consensus on the physiological function of the aortic sinus in patients with aortic root aneurysms. Systematic surgical planning is necessary when treating aortic root aneurysms using concomitant aortic valves. Conservative treatment delays disease progression and surgical intervention remains the “criterion standard” of treatment. Significant progress has been made in traditional and modified Bentall surgeries. More complex VSRR are also being performed in an increasing number of heart centers. Personalized and comprehensive treatment plans tailored to different populations will become a new direction for the treatment of aortic root aneurysms.
References
1. Anderson RH, Clinical anatomy of the aortic root: Heart, 2000; 84; 670-73
2. Chung JC, Pathology and pathophysiology of the aortic root: Ann Cardiothorac Surg, 2023; 12(3); 159-67
3. Jin XY, Yuan L, Petrou M, The evolution of surgical and medical treatment of aortic root aneurysm: Front Med, 2014; 8(4); 427-32
4. Salameh MJ, Black JH, Ratchford EV, Thoracic aortic aneurysm: Vasc Med, 2018; 23(6); 573-78
5. Putotto C, Pulvirenti F, Pugnaloni F, Clinical risk factors for aortic root dilation in patients with 22q11.2 deletion syndrome: A longitudinal single-center study: Genes (Basel), 2022; 13(12); 2334
6. Góis J, Higuchi M, Reis M, Infectious agents, inflammation, and growth factors: How do they interact in the progression or stabilization of mild human atherosclerotic lesions?: Ann Vasc Surg, 2006; 20(5); 638-45
7. Koch TM, Reddy BD, Zilla P, Aortic valve leaflet mechanical properties facilitate diastolic valve function: Comput Methods Biomech Biomed Engin, 2010; 13(2); 225-34
8. Lansac E, Lim HS, Shomura Y, A four-dimensional study of the aortic root dynamics: Eur J Cardiothorac Surg, 2002; 22(4); 497-503
9. Spadaccio C, Nappi F, Al-Attar N, Old myths, new concerns: The long-term effects of ascending aorta replacement with Dacron grafts. Not all that glitters is gold: J Cardiovasc Transl Res, 2016; 9(4); 334-42
10. McKellar SH, Sundt TM, Valve replacement options in the setting of an ascending aortic aneurysm: Future Cardiol, 2009; 5(4); 375-83
11. Rasool F, Khan MA, Amanullah M, Sinus of valsalva aneurysm rupturing into main pulmonary artery: A rare paediatric cardiac emergency: J Pak Med Assoc, 2018; 68(7); 1113-14
12. Kawasaki T, Kosaki F, Okawa S, A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan: Pediatrics, 1974; 54(3); 271-76
13. Pahlavan PS, Niroomand F, Coronary artery aneurysm: A review: Clin Cardiol, 2006; 29(10); 439-43
14. Piazza N, de Jaegere P, Schultz C, Anatomy of the aortic valvar complex and its implications for transcatheter implantation of the aortic valve: Circ Cardiovasc Interv, 2008; 1(1); 74-81
15. McClure RS, Brogly SB, Lajkosz K, Epidemiology and management of thoracic aortic dissections and thoracic aortic aneurysms in Ontario, Canada: A population-based study: J Thorac Cardiovasc Surg, 2018; 155(6); 2254-64e4
16. Della Corte A, Romano G, Tizzano F, Echocardiographic anatomy of ascending aorta dilatation: Correlations with aortic valve morphology and function: Int J Cardiol, 2006; 113(3); 320-26
17. Rizzo JA, Coady MA, Elefteriades JA, Procedures for estimating growth rates in thoracic aortic aneurysms: J Clin Epidemiol, 1998; 51(9); 747-54
18. Patel HJ, Deeb GM, Ascending and arch aorta: Pathology, natural history, and treatment: Circulation, 2008; 118(2); 188-95
19. Davies RR, Goldstein LJ, Coady MA, Yearly rupture or dissection rates for thoracic aortic aneurysms: Simple prediction based on size: Ann Thorac Surg, 2002; 73(1); 17-27 discussion 27–28
20. Svensson LG, Kim KH, Lytle BW, Relationship of aortic cross-sectional area to height ratio and the risk of aortic dissection in patients with bicuspid aortic valves: J Thorac Cardiovasc Surg, 2003; 126(3); 892-93
21. Parish LM, Gorman JH, Kahn S, Aortic size in acute type A dissection: Implications for preventive ascending aortic replacement: Eur J Cardiothorac Surg, 2009; 35(6); 941-45 discussion 945-46
22. Pape LA, Tsai TT, Isselbacher EM, Aortic diameter > or =5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD): Circulation, 2007; 116(10); 1120-27
23. Nishimura RA, Otto CM, Bonow RO, 2014 AHA/ACC Guideline for the management of patients with valvular heart disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: Circulation, 2014; 129(23); 2440-92
24. Isselbacher EM, Preventza O, Hamilton Black J, 2022 ACC/AHA Guideline for the diagnosis and management of aortic disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines: Circulation, 2022; 146(24); e334-e482
25. Attenhofer Jost CH, Greutmann M, Connolly HM, Medical treatment of aortic aneurysms in Marfan syndrome and other heritable conditions: Curr Cardiol Rev, 2014; 10(2); 161-71
26. Shores J, Berger KR, Murphy EA, Progression of aortic dilatation and the benefit of long-term beta-adrenergic blockade in Marfan’s syndrome: N Engl J Med, 1994; 330(19); 1335-41
27. Brooke BS, Habashi JP, Judge DP, Angiotensin II blockade and aortic-root dilation in Marfan’s syndrome: N Engl J Med, 2008; 358(26); 2787-95
28. Xiong W, Knispel RA, Dietz HC, Doxycycline delays aneurysm rupture in a mouse model of Marfan syndrome: J Vasc Surg, 2008; 47(1); 166-72 discussion 172
29. Chung AW, Yang HH, Radomski MW, Long-term doxycycline is more effective than atenolol to prevent thoracic aortic aneurysm in marfan syndrome through the inhibition of matrix metalloproteinase-2 and −9: Circ Res, 2008; 102(8); e73-85
30. Jackson V, Olsson T, Kurtovic S, Matrix metalloproteinase 14 and 19 expression is associated with thoracic aortic aneurysms: J Thorac Cardiovasc Surg, 2012; 144(2); 459-66
31. Jovin IS, Duggal M, Ebisu K, Comparison of the effect on long-term outcomes in patients with thoracic aortic aneurysms of taking versus not taking a statin drug: Am J Cardiol, 2012; 109(7); 1050-54
32. Mazine A, Chu MWA, El-Hamamsy I, Valve-sparing aortic root replacement: A primer for cardiologists: Curr Opin Cardiol, 2022; 37(2); 156-64
33. Iannacone EM, Lau C, Soletti G, Aortic valve-sparing root replacement or Bentall?: Ann Cardiothorac Surg, 2023; 12(3); 168-78
34. Maskell P, Brimfield M, Ahmed A, In patients undergoing valve-sparing aortic root replacement, is reimplantation superior to remodelling?: Interact Cardiovasc Thorac Surg, 2021; 32(3); 441-46
35. Cherry C, DeBord S, Hickey C, The modified Bentall procedure for aortic root replacement: AORN J, 2006; 84(1); 52-55 quiz 71–74
36. Khachatryan Z, Leontyev S, Magomedov K, Management of aortic root in type A dissection: Bentall approach: J Card Surg, 2021; 36(5); 1779-85
37. Tabayashi K, Fukujyu T, Turu Y, Replacement of the ascending aorta and aortic valve with a composite graft: Operative and long-term results: Tohoku J Exp Med, 1998; 184; 257-66
38. Tamura K, Arai H, Kawaguchi S, Long-term results of modified bentall procedure using flanged composite aortic prosthesis: Ann Thorac Cardiovasc Surg, 2013; 19; 126-30
39. Abdulameer H, Al Taii H, Al-Kindi SG, Milner R, Epidemiology of fatal ruptured aortic aneurysms in the United States (1999–2016): J Vasc Surg, 2019; 69(2); 378-384e2
40. Kim TS, Na CY, Oh SS, Kim JH, Long-term mortality and morbidity after button Bentall operation: J Card Surg, 2013; 28(3); 280-84
41. Joo HC, Chang BC, Youn YN, Clinical experience with the Bentall procedure: 28 years: Yonsei Med J, 2012; 53(5); 915-23 [Erratum in: Yonsei Med J. 2017;58(1):261]
42. Mookhoek A, Korteland NM, Arabkhani B, Bentall procedure: A systematic review and meta-analysis: Ann Thorac Surg, 2016; 101(5); 1684-89
43. Mazine A, David TE, Lafreniere-Roula M, Early outcomes of the Bentall procedure after previous cardiac surgery: J Thorac Cardiovasc Surg, 2021; 162(4); 1063-71
44. Yang B, Patel HJ, Sorek C, Sixteen-year experience of David and Bentall procedures in acute type A aortic dissection: Ann Thorac Surg, 2018; 105(3); 779-84
45. Nagai T, Arakawa J, Tabata H, Systolic collapse of aortic composite graft after Bentall operation: A sign of pseudoaneurysm formation: Echocardiography, 2017; 34(6); 942-44
46. Saul D, Kandula V, Donuru A, Large aortic pseudoaneurysm after Bentall procedure in a patient with Marfan’s syndrome: Ann Pediatr Cardiol, 2022; 15(3); 314-16
47. Manenti A, Zizzo M, Fedeli C, Coronary artery pseudoaneurysm after Bentall procedure: Ann Thorac Surg, 2016; 102(4); 1409
48. Song MH, Tokuda Y, Nakayama T, A simple method of inspection of proximal bleeding in Bentall procedure: Asian Cardiovasc Thorac Ann, 2008; 16(4); 329-30
49. Chen LW, Dai XF, Wu XJ, A modified composite valve Dacron graft for prevention of postoperative bleeding from the proximal anastomosis after Bentall procedure: Ann Thorac Surg, 2009; 88(5); 1705-7
50. Sponga S, Di Mauro M, Malvindi PG, Surgery for Bentall endocarditis: Short- and midterm outcomes from a multicentre registry: Eur J Cardiothorac Surg, 2020; 58(4); 839-46
51. Koza Y, Kaya U, Tas MH, An aortic pseudoaneurysm following Bentall procedure: Aorta (Stamford), 2014; 2(5); 200-1
52. Serraino GF, Zanobini M, Beghi C, Perspective. Reoperative Bentall: Choice of conduits: Indian J Thorac Cardiovasc Surg, 2019; 35(Suppl 2); 127-29
53. Kim TS, Na CY, Oh SS, Long-term mortality and morbidity after button Bentall operation: J Card Surg, 2013; 28(3); 280-84
54. Milano AD, Bortolotti U, Coronary button dehiscence after the modified Bentall procedure: J Card Surg, 2022; 37(9); 2928
55. Sivakumar K, Sagar P, Post-Bentall ascending aortic pseudoaneurysm due to coronary button dehiscence: Ann Thorac Surg, 2021; 111(3); e161-e64
56. Nguyen KA, Keshavamurthy S, Yoon J, Right coronary artery button pseudoaneurysm after the modified Bentall procedure: Cureus, 2023; 15(6); e40144
57. Van HD, Pham TB, Chau CL, Modified Bentall procedure: A 15-year single-center clinical experience: Asian Cardiovasc Thorac Ann, 2022; 30(7); 779-87
58. Jo JJ, Kim YS, Kim GJ, True aneurysm of the common coronary button in a Marfan patient with an anomalous right coronary artery after a Bentall procedure: A case report: J Chest Surg, 2022; 55(3); 243-45
59. Lu P, Zhang J, Li S, Flanged Bentall and Cabrol procedures for complex aortic root diseases: Int Heart J, 2023; 64(3); 487-90
60. Li C, Liu Y, Qi R, Repair of aortic regurgitation due to Takayasu arteritis: Heart Surg Forum, 2013; 16(1); E24-26
61. Calcaterra D, Jazayeri MA, Turek JW, Aortic root reconstruction with a new Dacron graft featuring prefabricated coronary side branches: Lessons learned from the Cabrol procedure: Aorta (Stamford), 2017; 5(1); 1-10
62. Michielon G, Salvador L, Da Col U, Modified button-Bentall operation for aortic root replacement: The miniskirt technique: Ann Thorac Surg, 2001; 72(3); S1059-64
63. Li H, Song Y, Liu X, Short-term outcomes of a novel modified Bentall procedure in acute type A aortic dissection: J Cardiovasc Surg (Torino), 2021; 62(4); 385-90
64. Hussain G, Ahmad N, Ahmad S, New modification of modified bentall procedure (a single centre experience): Pak J Med Sci, 2015; 31(6); 1318-21
65. Della Corte A, Baldascino F, La Marca F, Hemostatic modifications of the Bentall procedure: imbricated proximal suture and fibrin sealant reduce postoperative morbidity and mortality rates: Tex Heart Inst J, 2012; 39(2); 206-10
66. Etz CD, Bischoff MS, Bodian C, The Bentall procedure: is it the gold standard? A series of 597 consecutive cases: J Thorac Cardiovasc Surg, 2010; 140(6 Suppl); S64-S70
67. Kim TS, Na CY, Oh SS, Long-term mortality and morbidity after button Bentall operation: J Card Surg, 2013; 28(3); 280-84
68. Li K, Wang Q, Pham T, Quantification of structural compliance of aged human and porcine aortic root tissues: J Biomed Mater Res A, 2014; 102(7); 2365-74
69. Zacek P, Holubec T, Vobornik M, Quality of life after aortic valve repair is similar to Ross patients and superior to mechanical valve replacement: A cross-sectional study: BMC Cardiovasc Disord, 2016; 16; 63
70. David TE, Feindel CM, An aortic valve-sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta: J Thorac Cardiovasc Surg, 1992; 103(4); 617-21
71. Pepper J, Yacoub M, Valve conserving operation for aortic regurgitation: J Card Surg, 1997; 12(2 Suppl); 151-56
72. Monsefi N, Zierer A, Risteski P, Long-term results of aortic valve resuspension in patients with aortic valve insufficiency and aortic root aneurysm: Interact Cardiovasc Thorac Surg, 2014; 18(4); 432-37
73. Bori Bata AK, D’Ostrevy N, Pereira B, Valve-sparing aortic root replacement-midterm outcomes and quality of life: Cardiovasc Diagn Ther, 2017; 7(6); 572-80
74. Svensson LG, Pillai ST, Rajeswaran J, Long-term survival, valve durability, and reoperation for 4 aortic root procedures combined with ascending aorta replacement: J Thorac Cardiovasc Surg, 2016; 151(3); 764-74
75. Beckmann E, Martens A, Pertz J, Valve-sparing David I procedure in acute aortic type A dissection: A 20-year experience with more than 100 patients: Eur J Cardiothorac Surg, 2017; 52(2); 319-24
76. Magro P, Marques M, Abecacis M, David operation in the bicuspid aortic valve population: Rev Port Cir Cardiotorac Vasc, 2017; 24(3–4); 131
77. Deas DS, Lou X, Leshnower BG, Preoperative eccentric aortic regurgitation and outcomes following valve-sparing root replacement: Semin Thorac Cardiovasc Surg, 2021; 33(3); 627-34
78. David TE, Ivanov J, Armstrong S, Aortic valve-sparing operations in patients with aneurysms of the aortic root or ascending aorta: Ann Thorac Surg, 2002; 74(5); S1758-61 discussion S1792–99
79. Leyh RG, Schmidtke C, Bartels C, Valve-sparing aortic root replacement (remodeling/reimplantation) in acute type A dissection: Ann Thorac Surg, 2000; 70(1); 21-24
80. Graeter TP, Langer F, Nikoloudakis N, Valve-preserving operation in acute aortic dissection type A: Ann Thorac Surg, 2000; 70(5); 1460-65
81. Miller DC, Valve-sparing aortic root replacement in patients with the Marfan syndrome: J Thorac Cardiovasc Surg, 2003; 125(4); 773-78
82. De Paulis R, De Matteis GM, Nardi P, A new aortic Dacron conduit for surgical treatment of aortic root pathology: Ital Heart J, 2000; 1(7); 457-63
83. Xu L, Gao F, Li P, Early and midterm outcomes of the VSSR procedure with De Paulis Valsalva graft: A Chinese single-center experience in 38 patients: J Cardiothorac Surg, 2015; 10; 167
84. Gaudino M, Lau C, Munjal M, Contemporary outcomes of surgery for aortic root aneurysms: A propensity-matched comparison of valve-sparing and composite valve graft replacement: J Thorac Cardiovasc Surg, 2015; 150; 1120-29
85. Svensson LG, Rosinski BF, Tucker NJ, Comparison of outcomes of patients undergoing reimplantation versus Bentall root procedure: Aorta (Stamford), 2022; 10; 57-68
86. David TE, David CM, Ouzounian M, A progress report on reimplantation of the aortic valve: J Thorac Cardiovasc Surg, 2021; 161; 890-99
87. Shrestha M, Boethig D, Krüger H, Valve-sparing aortic root replacement using a straight tube graft (David I procedure): J Thorac Cardiovasc Surg, 2023; 166(5); 1387-97
88. Manganiello S, Soquet J, Mugnier A, David procedure: A 21-year experience with 300 patients: Ann Thorac Surg, 2023; 115; 1403-10
89. Leshnower BG, Guyton RA, Myung RJ, Expanding the indications for the David V aortic root replacement: Early results: J Thorac Cardiovasc Surg, 2012; 143; 879-84
90. Rohlffs F, Grandi A, Panuccio G, Endovascular options for the ascending aorta and aortic arch: A scoping review: Ann Vasc Surg, 2023; 94; 102-18
91. Cribier A, Eltchaninoff H, Bash A, Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: First human case description: Circulation, 2002; 106(24); 3006-8
92. Rylski B, Szeto WY, Bavaria JE, Development of a single endovascular device for aortic valve replacement and ascending aortic repair: J Card Surg, 2014; 29(3); 371-76
93. Felipe Gaia D, Bernal O, Castilho E, First-in-human Endo-Bentall procedure for simultaneous treatment of the ascending aorta and aortic valve: JACC Case Rep, 2020; 2(3); 480-85
94. Treasure T, Crowe S, Chan KM, A method for early evaluation of a recently introduced technology by deriving a comparative group from existing clinical data: A case study in external support of the Marfan aortic root: BMJ Open, 2012; 2(2); e000725
95. Treasure T, Takkenberg JJ, Golesworthy T, Personalised external aortic root support (PEARS) in Marfan syndrome: Analysis of 1–9 year outcomes by intention-totreat in a cohort of the first 30 consecutive patients to receive a novel tissue and valve-conserving procedure, compared with the published results of aortic root replacement: Heart, 2014; 100(12); 969-75
In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952