07 September 2020: Clinical Research
Effect of Comorbidity on Outcomes of Patients with Advanced Non-Small Cell Lung Cancer Undergoing Anti-PD1 Immunotherapy
Xianghua Zeng123CDEG, Shicong Zhu12BCD, Cheng Xu4CF, Zhongyu Wang12CD, Xingxing Su12CD, Dong Zeng12CD, Haixia Long12AF*, Bo Zhu12AGDOI: 10.12659/MSM.922576
Med Sci Monit 2020; 26:e922576
Abstract
BACKGROUND: Comorbidities are reportedly related to the survival of patients with non-small cell lung cancer (NSCLC). The purpose of this study was to explore the impact of comorbidity, assessed by the Charlson comorbidity index (CCI) and the simplified comorbidity scores (SCS) on clinical outcomes of patients with NSCLC treated with immune checkpoint inhibitors.
MATERIAL AND METHODS: Sixty-six patients with NSCLC who received programmed cell death protein 1 (PD1) inhibitors in our institution in the past 2 years were enrolled in this retrospective study. Data on comorbidity (CCI and SCS) and clinical outcomes, including progression-free survival (PFS), immunotherapy responses, and immunotherapy-related adverse events, were analyzed.
RESULTS: The disease control rate was obviously higher among patients in the CCI <1 group than the CCI ≥1 group (P<0.001), but were similar between the SCS <8 group and SCS ≥8 group (P=0.585). The median PFS in the CCI <1 group was 271.0 days (95% CI: 214.3–327.7 days) compared with 232.0 days (95% CI: 66.2–397.8 days) for the CCI ≥1 group (P=0.0084). However, the median PFS showed no difference between the groups with SCS <8 at 271.0 days (95% CI: 138.7–403.3 days) versus SCS ≥8 at 222.0 days (95% CI: 196.2–247.8 days), P=0.2106). The incidence of adverse events was similar among patients with high versus low comorbidity indexes (CCI: 35.8% versus 23.6%, P=0.286, respectively; and SCS: 28.0% versus 29.3%, respectively, P=0.912).
CONCLUSIONS: The comorbidity burden might be a predictor for survival in patients with NSCLC undergoing PD1 inhibitor immunotherapy.
Keywords: Progression Free Survival, Comorbidity, Programmed Cell Death 1, non-small cell lung cancer, Aged, 80 and over, Immune Checkpoint Inhibitors, Progression-Free Survival
Background
Lung cancer is a top cause of cancer deaths worldwide, with about 781 000 new diagnoses each year in China [1]. Approximately 85% of all lung cancer patients have histological diagnosis of non-small cell lung cancer (NSCLC) [2]. In contrast with chemotherapy, immunotherapy targeting the pathway of programmed cell death receptor/ligand 1 (PD1/PD-L1) has been found to have clear and sustained effects on survival of patients with NSCLC and has accordingly been a recommended form of therapy in the past decade [3–5]. Inhibiting the interaction of PD-L1 constitutively expressed on tumor cells and PD1 expressed on activated T cells markedly enhances T cell function, resulting in anti-tumor activity [6]. The promising efficacy of PD1/PD-L1 inhibitors, including pembrolizumab, nivolumab and atezolizumab, in clinic trials has prompted their approval for the treatment of NSCLC by the US Food and Drug Administration [7–9].
The presence of comorbidities has been reported to exert great influence on anticancer effects in various malignancies, including NSCLC [10–12]. Nevertheless, the impact of comorbidity on the outcomes of NSCLC is still controversial [13,14]. Moreover, to the best of our knowledge, no studies have so far investigated the influence of comorbidities on outcomes during immunotherapy in patients with NSCLC. The simplified comorbidity score (SCS) and Charlson comorbidity index (CCI) are the 2 most extensively validated scoring systems for assessing comorbidities and predicting prognosis [15,16]. These 2 comorbidity indices have previously been used as prognostic factors in patients with various carcinomas [17–19]. Of interest, the SCS was designed specifically for lung cancer [20,21].
In the present study, we intended to explore the association of comorbidities evaluated by CCI and SCS with clinical outcomes, including survival and immune-related adverse events (irAEs), in a cohort of patients with advanced NSCLC undergoing immunotherapy with anti-PD1/PD-L1 agents in China.
Material and Methods
PARTICIPANTS:
The cohort of this retrospective study comprises 66 consecutive patients with NSCLC who were treated with PD1 inhibitors (pembrolizumab, nivolumab, and toripalimab) in the Institute of Cancer, Xinqiao Hospital of the Third Military Medical University, Chongqing, China and between February 2017 and November 2019. Pre-immunotherapy data on the following variables were recorded for analysis: age, sex, height, weight, tumor stage (TNM), pathological tumor type, smoking history, Eastern Cooperative Oncology Group performance status (ECOG PS), drinking status, and comorbid diseases.
This study was in compliance with the Declaration of Helsinki and also approved by the Ethics Committee of Xinqiao Hospital, Third Military Medical University (Chongqing, China). The recorded data were analyzed anonymously.
COMORBIDITY ASSESSMENT:
The CCI and SCS were used to assess the severity of comorbidities of all patients before commencement of PD1 inhibitors. As shown in Tables 1 and 2, the CCI and the SCS are individually weighted indexes of 19 and 7 different comorbid conditions, respectively, the maximum scores being 33 for the CCI and 20 for the SCS [19]. Three of the authors, all physicians in oncology, independently reviewed each patient’s comorbidities and calculated the CCI and SCI scores.
ASSESSMENT OF OUTCOMES:
Progression-free survival (PFS) is the primary endpoint to evaluate the efficacy of PD1 inhibitor immunotherapy according to iRECIST (immune responses Response Evaluation Criteria in Solid Tumors) [22]. In our study, PFS was referred to interval from the time of the most recent computed tomography (CT) or positron emission tomography (PET)-CT scan prior to the first cycle of immunotherapy to the time of tumor progression, death, or last follow-up. Secondary end points included but were not limited to a comparison of the overall response rate and disease control rate based on the comorbid status.
Organ specific immune-related adverse events (dermatological: rash and pruritus, gastrointestinal: diarrhea and colitis, hepatic: hepatitis, endocrine: hypophysitis and thyropathy, and respiratory: pneumonitis) throughout the study and until at least 1 month after the last cycle were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
STATISTICAL ANALYSES:
All data are expressed as the median (95% confidence interval [CI]), mean±standard deviation (SD), or number (percentage) as appropriate. Differences between groups were compared using by a χ2 test or Student’s
GraphPad Prism version 7 (GraphPad Software, San Diego, CA, USA) and SPSS 20.0 software (IBM Corp., Armonk, NY, USA) were used for all data analyses.
Results
EFFICACY:
The immunotherapy response rates are shown in Table 4. The disease control rate achieved by PD1 inhibitors was 81.8% in the entire cohort (36.4% partial response and 45.4% stable disease); however, no patients achieved complete responses. Progressive disease occurred in 12 patients (18.2%). Further subgroup analysis revealed that the disease control rate was much higher in the CCI <1 group than the CCI ≥1 group (P<0.001); but showed no significant difference between the SCS <8 group and the SCS ≥8 group (P=0.585). Overall response rates did not differ significantly between the CCI <1 group and the CCI ≥1 group (P=0.883) or between the SCS <8 group and the SCS ≥8 group (P=0.301). The median PFS of all patients was 264.0 days (95% CI: 211.8–316.2 days). As shown in Figure 2, the median PFS in the CCI <1 group was 271.0 days (95% CI: 214.3–327.7 days) compared with 232.0 days (95% CI: 66.2–397.8 days) in the CCI ≥1 group (P=0.0084). However, the median PFS did not differ between the SCS <8 group and the SCS ≥8 group (271.0 days; 95% CI: 138.7–403.3 days versus 222.0 days; 95% CI: 196.2–247.8 days, respectively; P=0.2106).
TOXICITY:
Data regarding irAEs in all patients are summarized in Table 5. In all, 20 patients (30.3%) developed irAEs, the most common being skin rash (n=6, 9.1%) and pneumonitis (n=6, 9.1%), followed by hypothyroidism (n=4, 6.1%), hepatitis (n=3, 4.5%), and colitis (n=1, 1.5%). Only 2 patients (3%) had severe pneumonitis (CTCAE: III), and none died from irAEs. irAEs occurred in 10 patients (26.3%) with CCI <1 and in 10 patients (35.7%) with CCI ≥1 (P=0.286). Similarly, the incidence of irAEs was comparable in the SCS <8 group (31.7%) and the SCS ≥8 group (28.0%) (P=0.912).
Discussion
Immunotherapy with anti-PD1/PD-L1 agents has progressed dramatically since it was found that these agents can achieve long-lasting responses in NSCLC. However, few studies have yet focused on assessing comorbidities and their influence on the prognosis of patients with NSCLC in developing countries. So far as we know, this study is the first study from China to use 2 different comorbidity scoring systems (i.e., CCI and SCS) to investigate whether comorbidities affected the outcomes of patients with NSCLC undergoing treatment with PD1 inhibitors. In our study, high CCI scores, not SCS scores, may have been associated with poor survival because of the shorter PFS, but there was no significant impact found on the incidence of irAEs in patients with NSCLC who received anti-PD1 treatment.
CCI, a comprehensive index of multi-morbidities, is a good indicator of a patient’s global status and has been demonstrated to affect the prognosis of patients with lung cancer [17,23]. The variables in the CCI model are readily available and scores can easily be calculated by physicians. Recent studies have reached controversial conclusions on the prognostic significance of the CCI [17,23,24]. Additionally, no studies have investigated the predictive significance of CCI in patients with NSCLC receiving treatment with PD1 inhibitors; accordingly, so we used a cutoff point of 1 for further investigation, as used by Pylvalainen et al. [18]. Our findings indicated that the PFS of patients with higher CCI scores (CCI ≥1) were inferior to those of patients with lower CCI scores (CCI <1) (
The SCS was first constructed by Pujol et al. in 2005 and was verified in a large population of patients with NSCLC. This scoring system is much more convenient in routine clinical practice because it incorporates only 6 factors. Smoking status has the largest weight in the index (7 points), followed by diabetes mellitus (5 points) and renal insufficiency (4 points). Using 8 points as the cutoff, we found that patients with higher SCS points had a shorter PFS than those with lower points, but the difference was not significant (
This study had several limitations. Firstly, it was a single institution retrospective study. Secondly, the sample size was relatively small because immunotherapy has only been administered in China in the past 3 years. Thus, the findings are speculative rather than definitive. Prospective studies with larger cohorts would be more convincing. Thirdly, the median follow-up time in the current study was too short in that most participants had not reached the end point, resulting in information bias.
Conclusions
To the best of our knowledge, we have demonstrated for the first time, that comorbidities might correlate with the prognosis in NSCLC patients treated with PD1 inhibitors. However, large-scale prospective research is still needed to confirm these findings considering the aforementioned study limitations. Additionally, an innovative comorbidity assessment model which incorporates immune diseases or other factors that affect the immunity of patients should be developed for predicting the efficacy of immunotherapy.
Figures
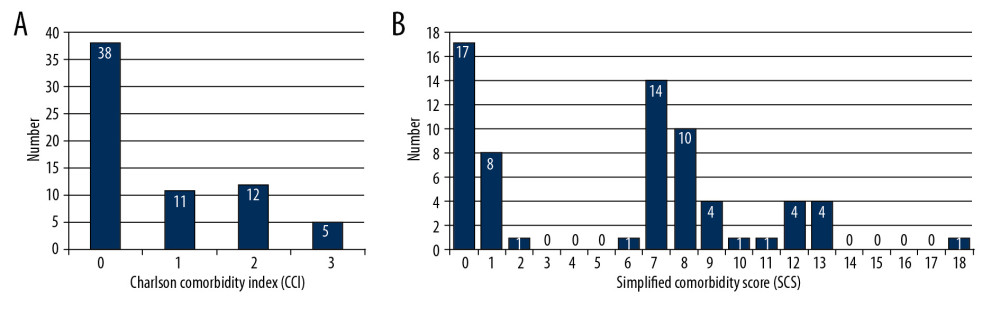 Figure 1. Distribution of comorbidity assessed by Charlson comorbidity index (A) and simplified comorbidity score (B) in patients with non-small cell lung cancer. Horizontal axis means the absolute points of each scoring system and vertical axis represents the number of patients.
Figure 1. Distribution of comorbidity assessed by Charlson comorbidity index (A) and simplified comorbidity score (B) in patients with non-small cell lung cancer. Horizontal axis means the absolute points of each scoring system and vertical axis represents the number of patients. 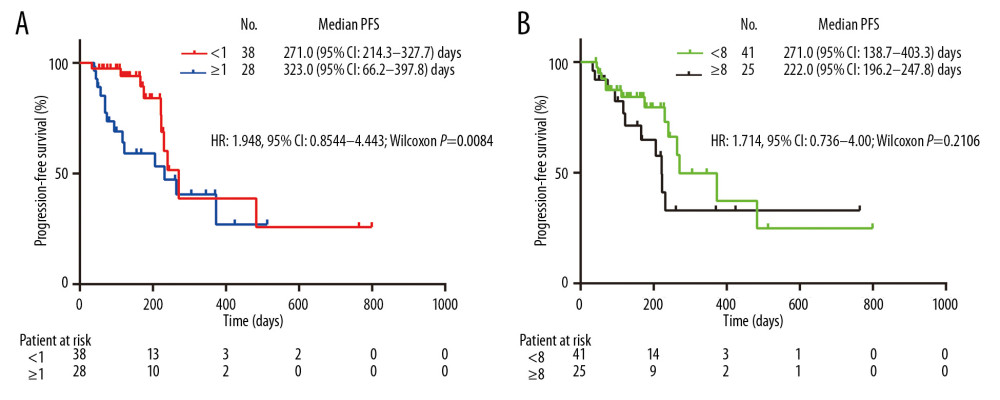 Figure 2. Kaplan-Meier plots of PFS in patients with non-small cell lung cancer undergoing PD1 inhibitors based on comorbidity conditions assessed by Charlson comorbidity index (A) and simplified comorbidity score (B). HR – hazard ratio; CI – confidence interval; PFS – progression-free survival.
Figure 2. Kaplan-Meier plots of PFS in patients with non-small cell lung cancer undergoing PD1 inhibitors based on comorbidity conditions assessed by Charlson comorbidity index (A) and simplified comorbidity score (B). HR – hazard ratio; CI – confidence interval; PFS – progression-free survival. Tables
Table 1. The Charlson comorbidity index weights.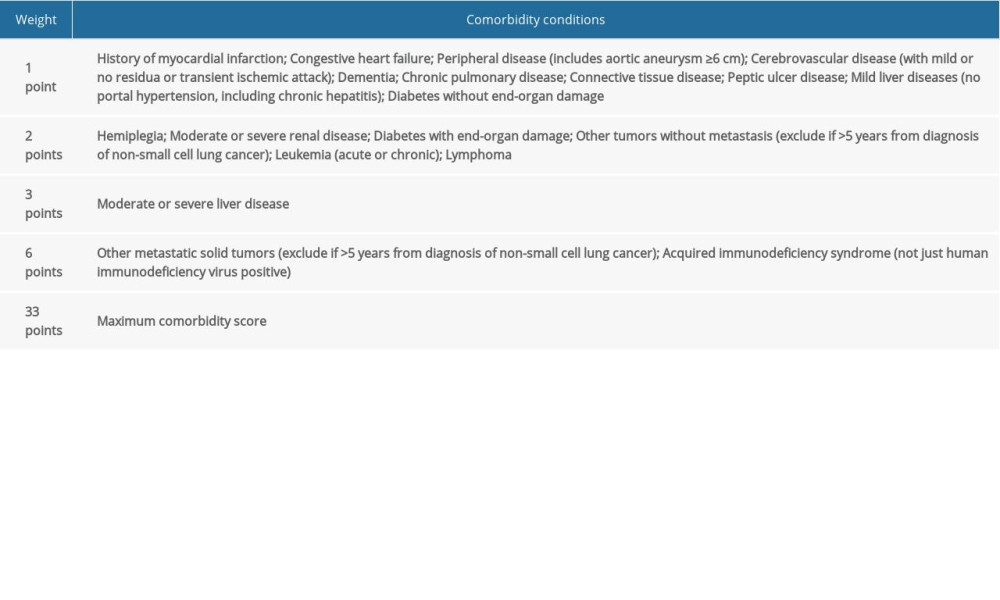 Table 2. The simplified comorbidity score weights.
Table 2. The simplified comorbidity score weights.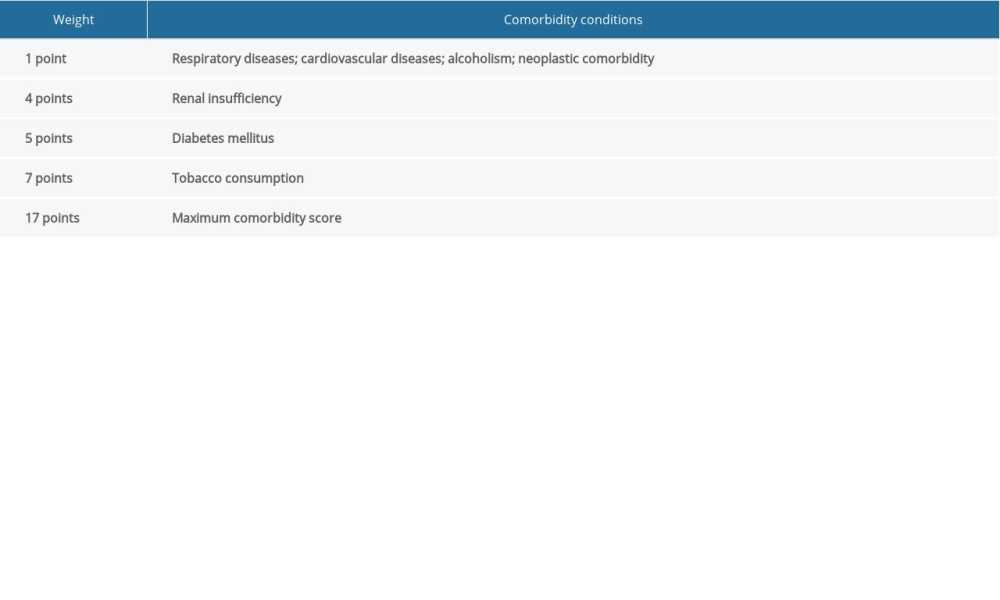 Table 3. Baseline and comparison of characteristics in patients with NSCLC based on the Charlson comorbidity index and simplified comorbidity score.
Table 3. Baseline and comparison of characteristics in patients with NSCLC based on the Charlson comorbidity index and simplified comorbidity score.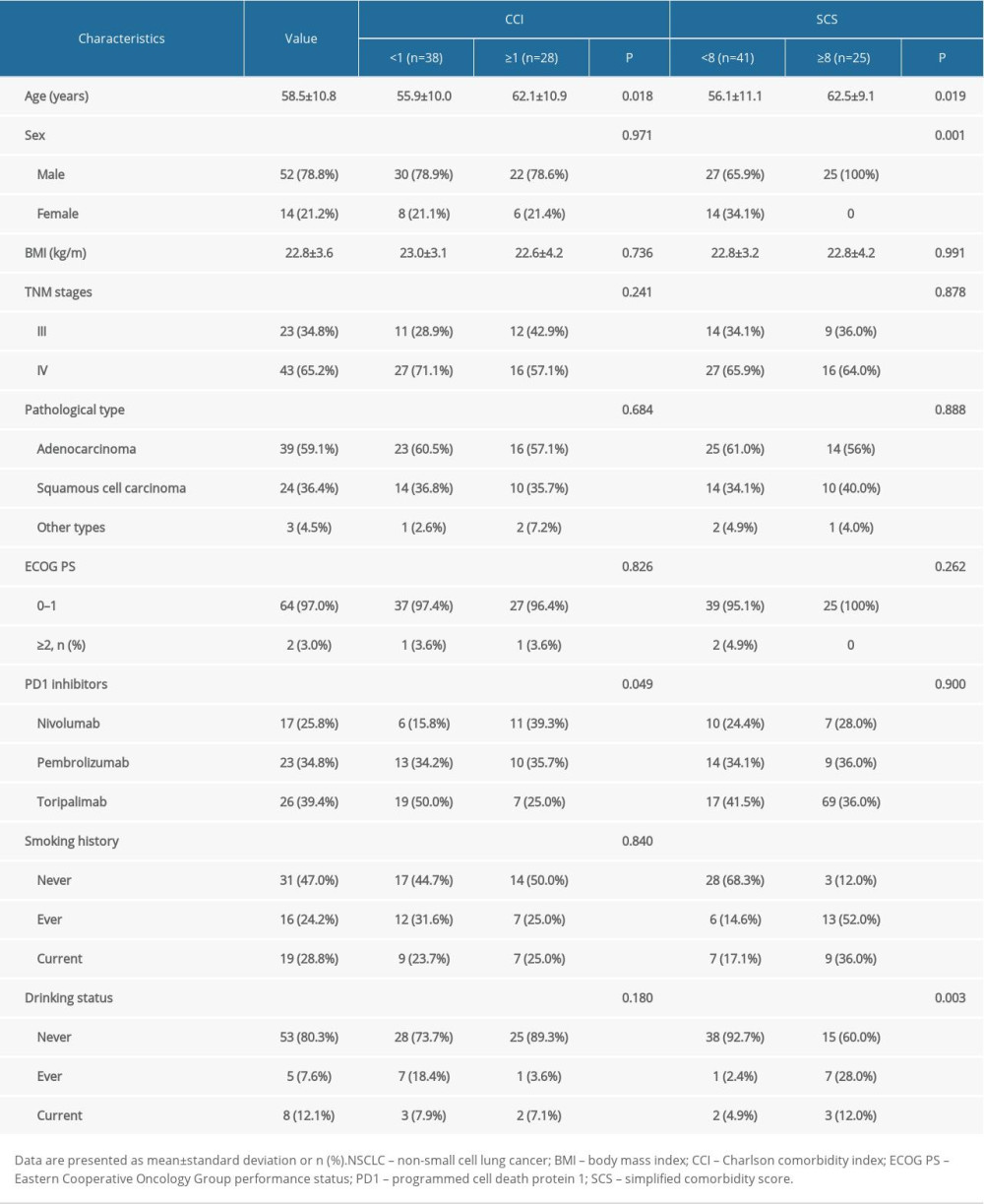 Table 4. Tumor response in patients with NSCLC treated with PD1 inhibitors.
Table 4. Tumor response in patients with NSCLC treated with PD1 inhibitors.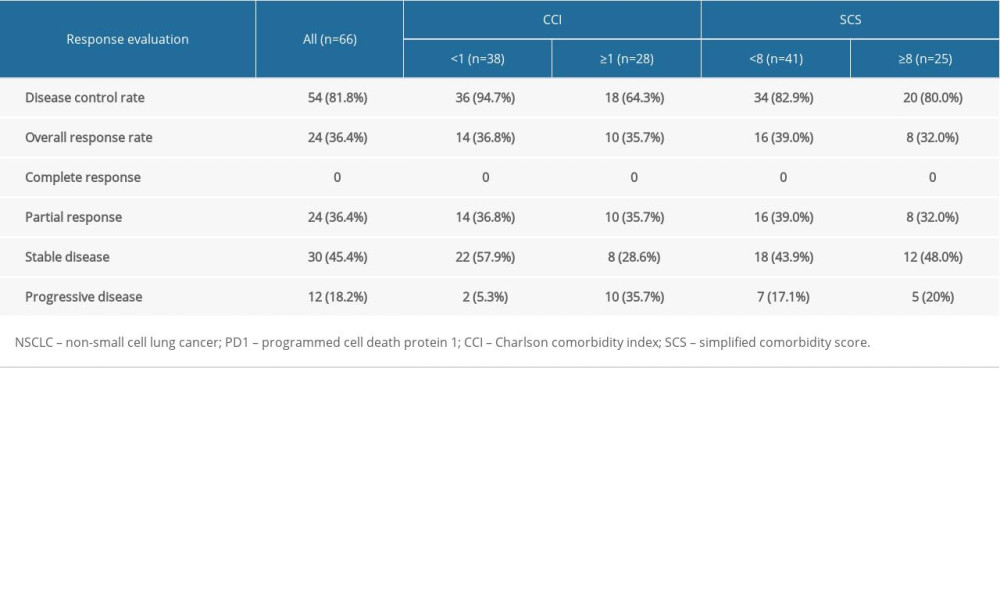 Table 5. Incidence of immune-related adverse events in all recipients of PD1 inhibitors.
Table 5. Incidence of immune-related adverse events in all recipients of PD1 inhibitors.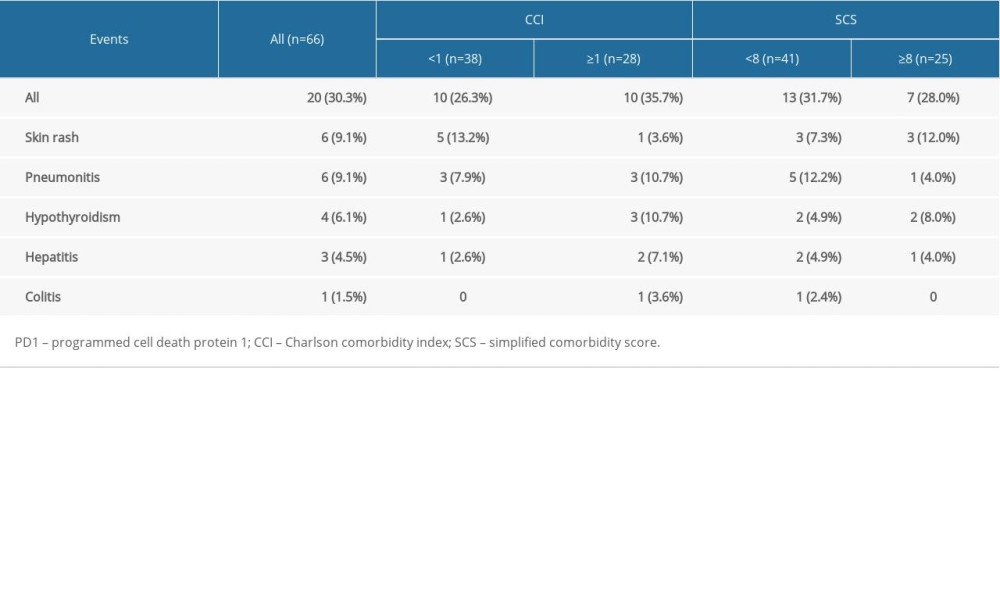
References
1. Chinese Medical Association guidelines for clinical diagnosis and treatment of lung cancer (Edition 2018): Zhonghua Zhong Liu Za Zhi, 2018; 40; 935-64 [In Chinese]
2. Bray F, Ferlay J, Soerjomataram I, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries: Cancer J Clin, 2018; 68; 394-424
3. Brahmer J, Reckamp KL, Baas P, Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer: N Engl J Med, 2015; 373; 123-35
4. Lisberg A, Cummings A, Goldman JW, A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor naive patients with advanced NSCLC: J Thorac Oncol, 2018; 13; 1138-45
5. Rittmeyer A, Barlesi F, Waterkamp D, Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial: Lancet, 2017; 389; 255-65
6. Bandini M, Marchioni M, Preisser F, Comprehensive analysis of in-hospital delirium after major surgical oncology procedures: Can Urol Assoc J, 2019; 14(3); E84-93
7. Herbst RS, Baas P, Kim DW, Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial: Lancet, 2016; 387; 1540-50
8. Fehrenbacher L, Spira A, Ballinger M, Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial: Lancet, 2016; 387; 1837-46
9. Kazandjian D, Khozin S, Blumenthal G, Benefit-risk summary of nivolumab for patients with metastatic squamous cell lung cancer after platinum-based chemotherapy: A report from the US Food and Drug Administration: JAMA Oncol, 2016; 2; 118-22
10. Asmis TR, Ding K, Seymour L, Age and comorbidity as independent prognostic factors in the treatment of non-small-cell lung cancer: A review of National Cancer Institute of Canada Clinical Trials Group trials: J Clin Oncol, 2008; 26; 54-59
11. Calvo-Espinos C, De Gaona-Lana ER, Gonzalez-Anguren C, Lama-Gay M, Assessment of the impact of comorbidity on the survival of cancer patients treated by palliative care teams: Palliat Support Care, 2015; 13; 1049-55
12. Kang HW, Kim SM, Kim WT, The age-adjusted Charlson comorbidity index as a predictor of overall survival of surgically treated non-metastatic clear cell renal cell carcinoma: J Cancer Res Clin Oncol, 2020; 146; 187-96
13. Bauml J, Mick R, Zhang Y, Determinants of survival in advanced non-small-cell lung cancer in the era of targeted therapies: Clin Lung Cancer, 2013; 14; 581-91
14. Read WL, Tierney RM, Page NC, Differential prognostic impact of comorbidity: J Clin Oncol, 2004; 22; 3099-103
15. Brusselaers N, Lagergren J, The Charlson Comorbidity Index in registry-based research: Methods Inf Med, 2017; 56; 401-6
16. Colinet B, Jacot W, Bertrand D, A new simplified comorbidity score as a prognostic factor in non-small-cell lung cancer patients: Description and comparison with the Charlson’s index: Br J Cancer, 2005; 93; 1098-105
17. Kang J, Ning MS, Feng H, Predicting 5-year progression and survival outcomes for early stage non-small cell lung cancer treated with stereotactic ablative radiation therapy: Development and validation of robust prognostic nomograms: Int J Radiat Oncol Biol Phys, 2020; 106; 90-99
18. Pylvalainen J, Talala K, Murtola T, Charlson Comorbidity Index based on hospital episode statistics performs adequately in predicting mortality, but its discriminative ability diminishes over time: Clin Epidemiol, 2019; 11; 923-32
19. Singh N, Singh PS, Aggarwal AN, Behera D, Comorbidity assessment using Charlson Comorbidity Index and Simplified Comorbidity Score and its association with clinical outcomes during first-line chemotherapy for lung cancer: Clin Lung Cancer, 2016; 17; 205-213.e1
20. Ball D, Thursfield V, Irving L, Evaluation of the Simplified Comorbidity Score (Colinet) as a prognostic indicator for patients with lung cancer: A cancer registry study: Lung Cancer, 2013; 82; 358-61
21. Alexander M, Evans SM, Stirling RG, The influence of comorbidity and the Simplified Comorbidity Score on overall survival in non-small cell lung cancer – a prospective cohort study: J Thorac Oncol, 2016; 11; 748-57
22. Eisenhauer EA, Therasse P, Bogaerts J, New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1): Eur J Cancer, 2009; 45; 228-47
23. Dreyer J, Bremer M, Henkenberens C, Comorbidity indexing for prediction of the clinical outcome after stereotactic body radiation therapy in non-small cell lung cancer: Radiat Oncol, 2018; 13; 213
24. Bernard S, Inderjeeth C, Raymond W, Higher Charlson Comorbidity Index scores do not influence Functional Independence Measure score gains in older rehabilitation patients: Australas J Ageing, 2016; 35; 236-41
25. Polo Friz H, Corno V, Orenti A, Comorbidity assessment as predictor of short and long-term mortality in elderly patients with hemodynamically stable acute pulmonary embolism: J Thromb Thrombolysis, 2017; 44; 316-23
26. Manig L, Kasmann L, Janssen S, Simplified Comorbidity Score and Eastern Cooperative Oncology Group Performance Score predicts survival in patients receiving organ-preserving treatment for bladder cancer: Anticancer Res, 2017; 37; 2693-96
27. El-Osta H, Jafri S, Predictors for clinical benefit of immune checkpoint inhibitors in advanced non-small-cell lung cancer: A meta-analysis: Immunotherapy, 2019; 11; 189-99
28. Solomon B, Young RJ, Rischin D, Head and neck squamous cell carcinoma: Genomics and emerging biomarkers for immunomodulatory cancer treatments: Semin Cancer Biol, 2018; 52; 228-40
29. Poynter JN, Haile RW, Siegmund KD, Associations between smoking, alcohol consumption, and colorectal cancer, overall and by tumor microsatellite instability status: Cancer Epidemiol Biomarkers Prev, 2009; 18; 2745-50
30. Valiathan R, Miguez MJ, Patel B, Tobacco smoking increases immune activation and impairs T-cell function in HIV infected patients on antiretrovirals: A cross-sectional pilot study: PLoS One, 2014; 9; e97698
Figures
 Figure 1. Distribution of comorbidity assessed by Charlson comorbidity index (A) and simplified comorbidity score (B) in patients with non-small cell lung cancer. Horizontal axis means the absolute points of each scoring system and vertical axis represents the number of patients.
Figure 1. Distribution of comorbidity assessed by Charlson comorbidity index (A) and simplified comorbidity score (B) in patients with non-small cell lung cancer. Horizontal axis means the absolute points of each scoring system and vertical axis represents the number of patients. Figure 2. Kaplan-Meier plots of PFS in patients with non-small cell lung cancer undergoing PD1 inhibitors based on comorbidity conditions assessed by Charlson comorbidity index (A) and simplified comorbidity score (B). HR – hazard ratio; CI – confidence interval; PFS – progression-free survival.
Figure 2. Kaplan-Meier plots of PFS in patients with non-small cell lung cancer undergoing PD1 inhibitors based on comorbidity conditions assessed by Charlson comorbidity index (A) and simplified comorbidity score (B). HR – hazard ratio; CI – confidence interval; PFS – progression-free survival. Tables
 Table 1. The Charlson comorbidity index weights.
Table 1. The Charlson comorbidity index weights. Table 2. The simplified comorbidity score weights.
Table 2. The simplified comorbidity score weights. Table 3. Baseline and comparison of characteristics in patients with NSCLC based on the Charlson comorbidity index and simplified comorbidity score.
Table 3. Baseline and comparison of characteristics in patients with NSCLC based on the Charlson comorbidity index and simplified comorbidity score. Table 4. Tumor response in patients with NSCLC treated with PD1 inhibitors.
Table 4. Tumor response in patients with NSCLC treated with PD1 inhibitors. Table 5. Incidence of immune-related adverse events in all recipients of PD1 inhibitors.
Table 5. Incidence of immune-related adverse events in all recipients of PD1 inhibitors. Table 1. The Charlson comorbidity index weights.
Table 1. The Charlson comorbidity index weights. Table 2. The simplified comorbidity score weights.
Table 2. The simplified comorbidity score weights. Table 3. Baseline and comparison of characteristics in patients with NSCLC based on the Charlson comorbidity index and simplified comorbidity score.
Table 3. Baseline and comparison of characteristics in patients with NSCLC based on the Charlson comorbidity index and simplified comorbidity score. Table 4. Tumor response in patients with NSCLC treated with PD1 inhibitors.
Table 4. Tumor response in patients with NSCLC treated with PD1 inhibitors. Table 5. Incidence of immune-related adverse events in all recipients of PD1 inhibitors.
Table 5. Incidence of immune-related adverse events in all recipients of PD1 inhibitors. In Press
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








