30 October 2020: Clinical Research
Effect of Whole-Brain and Intensity-Modulated Radiotherapy on Serum Levels of miR-21 and Prognosis for Lung Cancer Metastatic to the Brain
Zhensheng Zhu1AEG*, Qiurong Li2ABD, Mingjuan Xu3CD, Zhongliang Qi1BFDOI: 10.12659/MSM.924640
Med Sci Monit 2020; 26:e924640
Abstract
BACKGROUND: The goal of the present study was to explore the influence of whole-brain radiotherapy (WBRT) and intensity-modulated radiotherapy (IMRT) on serum levels of miR-21 and prognosis for lung cancer that has metastasized to the brain.
MATERIAL AND METHODS: Two hundred patients with lung cancer metastatic to the brain were randomized, half to the control group and half to the observation group. The observation group received WBRT and reduced-field IMRT (WBRT+RF-IMRT) and the control group received conventional-field IMRT (CF-IMRT). The total effective rate after treatment was determined. Serum levels of miR-21 were measured before and after radiotherapy with reverse transcriptase-polymerase chain reaction. In addition, tumor marker levels were measured with enzyme-linked immunosorbent assay. The relationship between miR-21 levels and tumor marker levels was assessed with a Pearson correlation coefficient test. Five-year survival was estimated with Kaplan-Meier curves.
RESULTS: The total effective rate was higher in the observation group (86%) than in the control group (69%). Lower levels of miR-21 and tumor markers were seen in the observation group. Moreover, miR-21 levels were positively correlated with levels of tumor necrosis factor-a, neuron-specific enolase, SCC-Ag, and carcinoembryonic antigen. Low levels of miR-21 were associated with longer overall survival in patients with lung cancer metastatic to the brain.
CONCLUSIONS: WBRT+RF-IMRT is superior to CF-IMRT for lung cancer metastatic to the brain. MiR-21 may be a marker for prediction of the efficacy of radiotherapy in this disease setting.
Keywords: Aged, 80 and over, Carcinoma, Squamous Cell, Radiotherapy, Intensity-Modulated
Background
Lung cancer is one of the most dangerous malignancies, and morbidity and mortality associated with it have rapidly increased. Non-small cell lung cancer (NSCLC) accounts for 85% of all lung cancer cases. The prognosis is extremely poor, with a 5-year survival rate of only 15% [1]. Small cell lung cancer (SCLC) is a highly invasive neuroendocrine tumor. Brain metastasis (BM) frequently occurs in patients with lung cancer. About 15% of patients with SCLC have BM at diagnosis, and 40% develop it within 1 year after diagnosis. BM is a key feature of SCLC that significantly limits quality of life (QoL) and survival in patients [2]. Therefore, methods that effectively prevent and treat BM from lung cancer are of great significance.
Brain metastases from SCLC are usually multiple and easily lead to brain damage. Whole-brain radiotherapy (WBRT) is the conventional way to treat BM from SCLC without causing irreversible neurocognitive dysfunction [3]. Because of advances in radiotherapy instruments and technology, three-dimensional conformal radiotherapy (3D-CRT) is now being used to enhance the radiation dose to the lungs in treatment of stage IV NSCLC. With it, primary tumor cells can be killed while avoiding radiation-induced lung injuries and complications, thus inhibiting distant metastasis as much as possible while improving QoL [4] for patients. Clinical evidence is lacking, however, on the therapeutic efficacy of 3D-CRT [5].
Accumulating evidence has proven that microRNAs, (miRNAs) are vital to almost every aspect of tumor development, including growth, differentiation, angiogenesis, and metastasis [7,8]. miRNAs are non-coding RNAs that contain approximately 22 nucleotides. They regulate target gene expression through complementary base pairing, which results in mRNA degradation or inhibition of translation [8,9]. miR-21 is located on human chromosome 17q23.2 [10]. Plasma levels of miR-21 reportedly are upregulated in patients with chemotherapy-resistant NSCLC [11] and it also is highly expressed in tumor tissues [12]. Another study demonstrated that miR-21 levels are higher in patients with NSCLC who have BM than in those who do not have BM [13]. The aim of the present study was to explore the potential influence of miR-21 on assessment of the therapeutic efficacy and safety of intensity-modulated radiotherapy (IMRT) for BM from lung cancer.
Material and Methods
BASELINE CHARACTERISTICS:
Two hundred patients with BM from lung cancer who were admitted to Jinan Hospital of Integrated Traditional Chinese and Western Medicine between June 2013 and May 2017 were recruited and randomly assigned to control and observation groups using PEMS3.1 software. The former group included 53 men and 47 women with an average age of 61.54±5.31 years (range, 52–77 years). The histology of the cases in the control group was as follows: 23 large-cell carcinomas, 28 squamous cell carcinomas (SCCs), and 49 adenocarcinomas. Of the patients in that group, 41 had a single metastasis and 59 had multiple metastases. The observation group included 51 men and 49 women with an average age of 62.53±6.91 years (range, 53–80 years). There were 25 cases of large-cell carcinoma, 27 SCCs, and 48 adenocarcinomas. Fifty-five patients in the observation group had a single metastasis and 55 had multiple metastases. No significant differences in age, sex, histological subtype, or number of metastases were found between the groups (P>0.05) (Schedule 1). Approval was obtained from the Ethics Committee of Jinan Hospital of Integrated Traditional Chinese and Western Medicine and informed consent was obtained from the subjects.
INCLUSION AND EXCLUSION CRITERIA:
Patients were included in the study if they: (1) had BM from lung cancer that was pathologically confirmed with computed tomography (CT) or magnetic resonance imaging; (2) had an estimated life expectancy of ≥3 months; (3) had completed a course of radiotherapy; and (4) had metastases in other organs that were relatively stable. Patients were excluded from the study if they: (1) were younger than age 18 years; (2) had dysfunction in organs other than the brain, other malignancies, immune disease, severe infection or mental disorders; (3) had a history of brain radiotherapy or chemotherapy; or (4) refused treatment.
THERAPEUTIC STRATEGY:
Varian 23EX and 600CD linear accelerators were used to deliver radiotherapy. The treatment planning system (TPS) used to plan 3-dimensional radiotherapy was the 10.0Eclipse 3. With the patient in the supine position, the patient’s head was held steady in a thermoplastic mask and scanned with spiral CT. The simulator was positioned with the Philips Mx8000 CT scanner. Scanning was done in 3-mm intervals, from the top of the skull to 5 cm below the skull baseline. Results of scanning were processed with the TPS.
First, WBRT was performed on the observation group. The planned target volume (PTV) was a 2-mm expansion of the clinical target volume. The PTV was treated with 3 Gy of radiation per day for 5 days per week, to a total dose of 30 Gy of WBRT. Reduced-field IMRT (RF-IMRT) subsequently was administered, based on BM identified in the CT images, at a dose of 2 Gy per day for 5 days per week. The total dose of RF-IMRT administered was 50 Gy. Patients in the control group underwent conventional-field IMRT (CF-IMRT) targeting BM, at a dose of 2 Gy per day for 5 days per week. The total dose of CF-IMRT administered was 56 to 60 Gy. Radiation dosage to organs such as the spinal cord, eyes, and brain stem was within the range considered safe. During radiotherapy, patients received treatment for symptoms, including mannitol to prevent dehydration.
ENZYME-LINKED IMMUNOSORBENT ASSAY:
A 3-mL venous blood sample was taken from each patient in the morning while still in a fasting state. All the samples were placed in anticoagulated-coated Eppendorf™ tubes and centrifuged at 2000 r/min for 5 min. The upper layer of serum was purified and stored at −80°C. Relative levels of tumor necrosis factor (TNF)-α, neuron-specific enolase (NSE), SCC-Ag, and carcinoembryonic antigen (CEA) were measured using an enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, Minnesota, U.S.A.). Briefly, the standard sample was diluted twice. Samples from the patients were incubated for 30 min with the diluted standard sample, antibodies, and enzymes. The samples were then washed and stained in the dark for 10 min and induction was performed with terminate solution. For 5 to 30 min, all samples were subjected to 450-nm optical density measurement with an Infinite M200 Pro plate reader (TECAN, Switzerland). Levels of TNF-α, NSE, SCC-Ag, and CEA were assessed relative to standard curves.
QUANTITATIVE REAL-TIME POLYMERASE CHAIN REACTION:
The TRIzol™ protocol (Invitrogen, Carlsbad, California, U.S.A.) was used to isolate RNA from the serum samples. Through reverse transcription of RNA using an avian myeloblastosis virus reverse transcriptase (AMV) kit (Takara, Kusatsu, Japan), the extracted complementary deoxyribose nucleic acid (cDNA) was used for polymerase chain reaction (PCR) detection with the 2×SYBR® Green PCR Master Mix (Takara, Kusatsu, Japan). A quantitative real-time PCR (qRT-PCR) system was prepared, using the SYBR® Premix DimerEvaser and including 10.0 μL of deoxynucleoside triphosphate, 0.4 μL of forward primer (10.0 μmol/L), 0.4 μL of reverse primer (10.0 μmol/L), 2.0 μL of cDNA, 0.4 μL of ROX, and 6.8 μL of double-distilled water. The system was subjected to qRT-PCR at 94°C for 5 min before denaturation, followed by 40 cycles at 94°C for 10 s for denaturation, 58°C for 30 s for annealing, and 72°C for an additional 30 s. Relative levels in each sample were determined using the ABI7900 Fast Real-Time System (Applied Biosystems, Massachusetts, U.S.A.) and calculated using the 2−ΔΔCT method and normalized to the internal reference U6. Sequences of miR-21 and U6 were as follows:
EVALUATION OF TUMOR EFFICACY:
RECIST1.1 was used to evaluate solid tumor efficacy, based on tumor size calculated as the product of the maximum diameter and maximum vertical diameters [14]. Tumor response was divided into 4 categories: (1) complete response (CR), defined as complete disappearance of tumor that was sustained for >1 month; (2) partial response (PR), defined as tumor shrinkage of ≥50%; (3) stable disease (SD), defined as tumor shrinkage from 25% to <50%; and (4) progressive disease (PD), defined as tumor shrinkage of ≥25% and >1 new tumor lesion. The total effective rate=(case numbers of CR, PR, and SD)/total case number×100%.
FOLLOW-UP:
Monthly telephone calls, outpatient visits, inpatient testing, and other methods were used to follow up patients. Overall survival (OS), defined as the time from the first treatment to death from any cause, was recorded. Follow-up was terminated at death or at the end of 5 years.
STATISTICAL ANALYSIS:
Statistical Product and Service Solutions (SPSS) 19.0 software (IBM, Armonk, New York, U.S.A.) was used for all statistical analyses. Data were expressed as means±SD (standard deviation). A
Results
COMPARISON OF TUMOR EFFICACY:
In the control group, 11 patients had CRs, 22 had PRs, 17 had SD, and 19 had PD. In the observation group, 20 patients had CRs, 35 had PRs, 21 had SD, and 10 had PD. The total effective rate was significantly higher in the observation group (86%) than in the control group (69%) (χ2=8.287, P=0.006, Table 1). That suggests that WBRT+RF-IMRT was superior to RF-IMRT in treatment of BM from lung cancer.
SERUM LEVELS OF MIR-21, TNF-α, NSE, SCC-AG, AND CEA:
miR-21 levels were comparable between groups before radiotherapy (P>0.05) and markedly lower after treatment (P<0.05). The decline was more pronounced in the observation group (P<0.05). Before radiotherapy, no significant differences in serum levels of TNF-α, NSE, SCC-Ag, or CEA were found between the groups (P>0.05). All of the levels were downregulated after treatment (P<0.05), especially in the observation group (Table 2). WBRT+RF-IMRT was more effective than RF-IMRT in reducing expression of miR-21 and tumor markers.
CORRELATION BETWEEN MIR-21 LEVELS AND TUMOR MARKERS:
The relationship between miR-21 levels and tumor marker levels was assessed with a Pearson coefficient correlation test. MiR-21 levels were positively correlated with levels of TNF-α (r=0.531), NSE (r=0.773), SCC-Ag (r=0.406), and CEA (r=0.783) (P<0.05, Table 3).
COMPARISON OF OS:
Kaplan-Meier survival curves were created based on the 5-year follow-up data. OS was much longer in the observation group than in the control group (hazard ratio [HR]=5.550, P=0.0185, Figure 1A). IMRT improved OS in BM from lung cancer. To further explore the influence of miR-21 levels on OS, 200 patients with BM from lung cancer were broken into 2 groups based on median level of miR-21 after radiotherapy. Prognosis was poor in patients with tumors that had high levels of miR-21 expression (HR=8.471, P=0.0372, Figure 1B). As a result, miR-21 was unfavorable as a marker of survival in BM from lung cancer.
Discussion
Lung cancer is the leading cause of death from cancer and BM is the main reason for the disease’s high mortality rate [15]. BMs reportedly are seen in 50% to 60% of patients with lung cancer [16]. They cause severe damage to the central nervous system and significantly decrease QoL for patients [17,18]. Use of WBRT to treat BM from lung cancer is now common [19,20]. In recent years, use of 3D-CRT has become more popular [5], but its efficacy has not been validated in clinical trials.
TNF-α is a proinflammatory cytokine that is involved in inflammatory and immune responses of living organisms. It facilitates the growth and metastasis of cancer cells [21]. Serum NSE is ubiquitous in nerve cells and can be used to diagnose neuroendocrine tumors and predict their likelihood of recurrence [22]. SCC-Ag is a cytoplasmic glycoprotein seen specifically in SCC that can be used as a specific marker for the disease [22,23]. CEA is a tumor marker used in lung cancer to evaluate treatment efficacy, monitor disease, and predict prognosis [24]. Our findings showed declines in the previously described tumor markers after radiotherapy, especially in the observation group, which suggests that WBRT+RF-IMRT has significant efficacy in treatment of BM from lung cancer.
miR-21 is upregulated in many malignant tumors [25]. In one study, upregulation of the marker was reported in patients with but not without BM from NSCLC. MiR-21 has diagnostic and prognostic values in BM from NSCLC. Receiver operating characteristic curves showed that the sensitivity and specificity of miR-21 in diagnosing BM from NSCLC were 92.3% and 60.7%, respectively.
Because of the limited follow-up time and other factors associated with the present study, our findings may not be comprehensive. The occurrence of multiple metastases in the brain may be indicative of systemic metastases. Controlling the primary disease, therefore, has a significant effect on patient prognosis. A study with a larger sample size is needed to validate our conclusions.
Conclusions
WBRT+RF-IMRT is superior to RF-IMRT for treatment of BM from lung cancer. MiR-21 may be a marker for prediction of radiotherapy efficacy in treatment of BM from lung cancer.
References
1. Mok TS, Wu Y, Ahn M, Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer: N Engl J Med, 2017; 376; 629-40
2. Kalemkerian GP, Akerley W, Bogner P, Small cell lung cancer: J Natl Compr Canc Netw, 2013; 11; 78-98
3. Brown PD, Ahluwalia MS, Khan OH, Whole-Brain radiotherapy for brain metastases: Evolution or revolution?: J Clin Oncol, 2018; 36; 483-91
4. Gevaert T, Steenbeke F, Pellegri L, Evaluation of a dedicated brain metastases treatment planning optimization for radiosurgery: A new treatment paradigm?: Radiat Oncol, 2016; 11; 13
5. Tukiendorf A, Mansournia MA, Wydmanski J, Wolny-Rokicka E, Association between stereotactic radiotherapy and death from brain metastases of epithelial ovarian cancer: A gliwice data re-analysis with penalization: Asian Pac J Cancer Prev, 2017; 18; 1113-16
6. Croce CM, Causes and consequences of microRNA dysregulation in cancer: Nat Rev Genet, 2009; 10; 704-14
7. Wang F, Ren X, Zhang X, Role of microRNA-150 in solid tumors: Oncol Lett, 2015; 10; 11-16
8. Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T, Identification of novel genes coding for small expressed RNAs: Science, 2001; 294; 853-58
9. Lytle JR, Yario TA, Steitz JA, Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5′ UTR as in the 3′ UTR: Proc Natl Acad Sci USA, 2007; 104; 9667-72
10. Lagos-Quintana M, Rauhut R, Identification of novel genes coding for small expressed RNAs: Science, 2001; 294; 853-58
11. Wei J, Gao W, Zhu CJ, Identification of plasma microRNA-21 as a biomarker for early detection and chemosensitivity of non-small cell lung cancer: Chin J Cancer, 2011; 30; 407-14
12. Gao W, Lu X, Liu L, MiRNA-21: A biomarker predictive for platinum-based adjuvant chemotherapy response in patients with non-small cell lung cancer: Cancer Biol Ther, 2012; 13; 330-40
13. Dong J, Zhang Z, Gu T, The role of microRNA-21 in predicting brain metastases from non-small cell lung cancer: Onco Targets Ther, 2017; 10; 185-94
14. Schwartz LH, Bogaerts J, Ford R, Evaluation of lymph nodes with RECIST 1.1: Eur J Cancer, 2009; 45; 261-67
15. Siegel RL, Miller KD, Jemal A, Cancer statistics, 2018: Cancer J Clin, 2018; 68; 7-30
16. Nayak L, Lee EQ, Wen PY, Epidemiology of brain metastases: Curr Oncol Rep, 2012; 14; 48-54
17. Oh Y, Taylor S, Bekele BN, Number of metastatic sites is a strong predictor of survival in patients with nonsmall cell lung cancer with or without brain metastases: Cancer-Am Cancer Soc, 2009; 115; 2930-38
18. Novello S, Barlesi F, Califano R, Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up: Ann Oncol, 2016; 27; v1-27
19. Ferrarese F, Baggio V, Zorat PL, Fiore D, Treatment and prophylaxis for brain metastases from non-small cell lung cancer: Whole brain radiation treatment versus stereotactic radiosurgery: Ann Oncol, 2006; 17(Suppl 2); i71-72
20. Baykara M, Kurt G, Buyukberber S, Management of brain metastases from non-small cell lung cancer: J Cancer Res Ther, 2014; 10; 915-21
21. Girouard J, Belgorosky D, Hamelin-Morrissette J, Molecular therapy with derivatives of amino benzoic acid inhibits tumor growth and metastasis in murine models of bladder cancer through inhibition of TNFalpha/NFKappaB and iNOS/NO pathways: Biochem Pharmacol, 2019; 176; 113778
22. Yang Q, Zhang P, Wu R, Identifying the Best marker combination in CEA, CA125, CY211, NSE, and SCC for lung cancer screening by combining ROC curve and logistic regression analyses: Is it feasible?: Dis Markers, 2018; 2018 2082840
23. Molina R, Marrades RM, Auge JM, Assessment of a combined panel of six serum tumor markers for lung cancer: Am J Respir Crit Care Med, 2016; 193; 427-37
24. You C, Qian X, He YDevelopment of a lung cancer vaccine by transfecting dendritic cells with rAAV/CEA: Nan Fang Yi Ke Da Xue Xue Bao, 2014; 34; 487-91 [in Chinese]
25. Pfeffer SR, Yang CH, Pfeffer LM, The role of miR-21 in cancer: Drug Dev Res, 2015; 76; 270-77
Tables
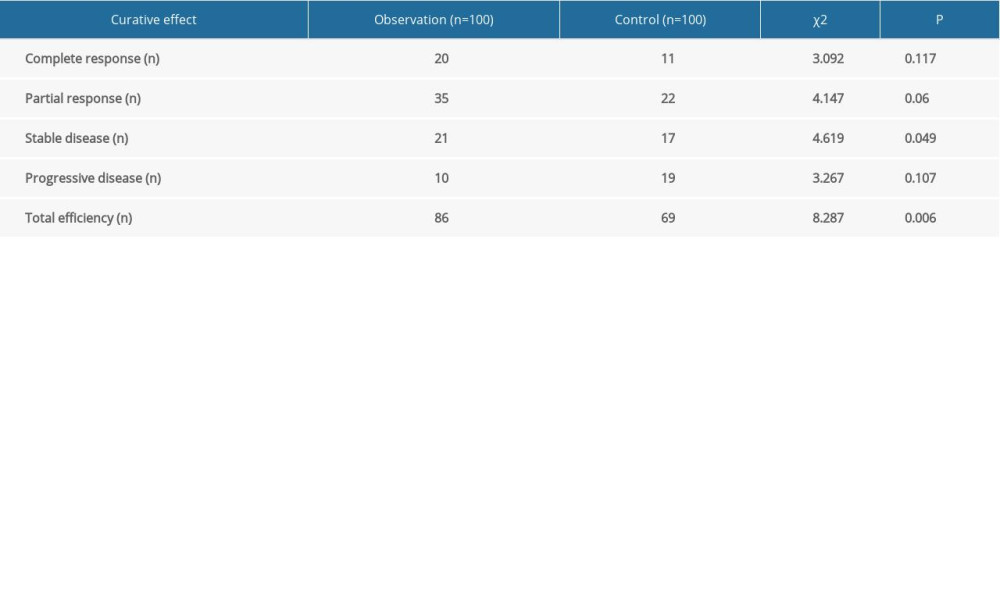 Table 1. Comparison of tumor efficacy.
Table 1. Comparison of tumor efficacy.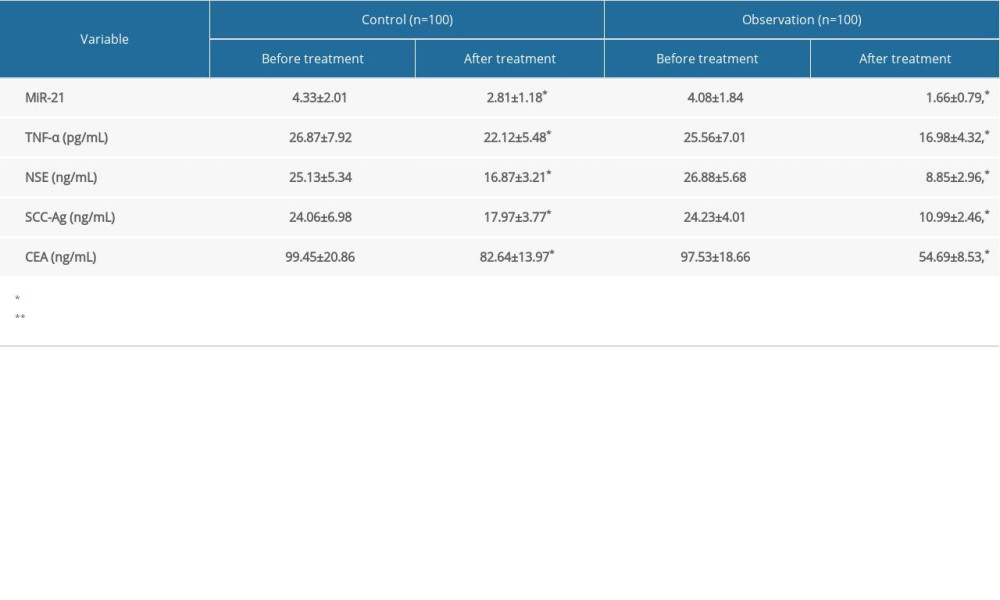 Table 2. Serum levels of miR-21, TNF-α, NSE, SCC-Ag and CEA.
Table 2. Serum levels of miR-21, TNF-α, NSE, SCC-Ag and CEA.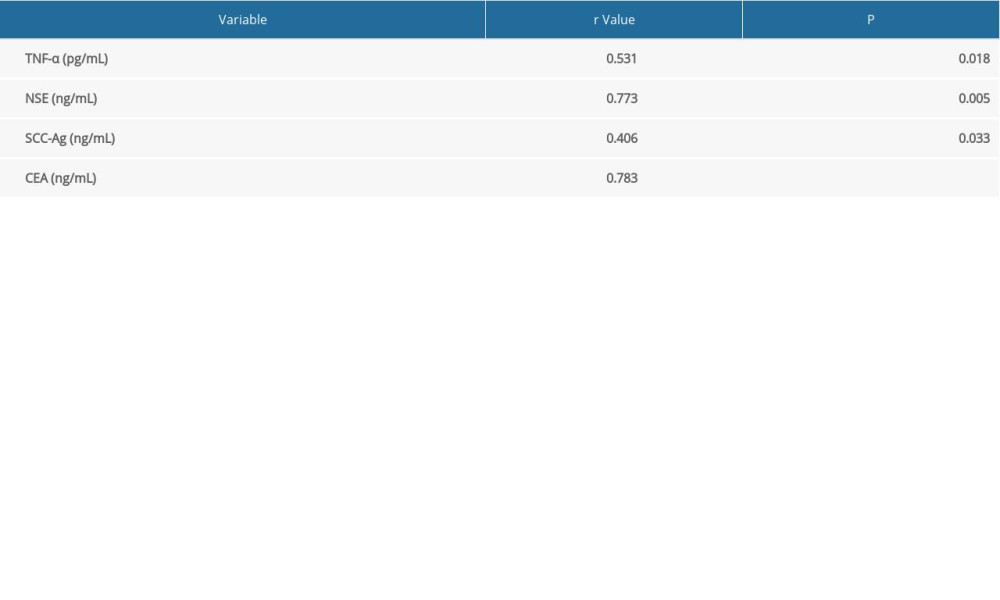 Table 3. Correlation between miR-21 level and tumor markers.
Table 3. Correlation between miR-21 level and tumor markers. Table 1. Comparison of tumor efficacy.
Table 1. Comparison of tumor efficacy. Table 2. Serum levels of miR-21, TNF-α, NSE, SCC-Ag and CEA.
Table 2. Serum levels of miR-21, TNF-α, NSE, SCC-Ag and CEA. Table 3. Correlation between miR-21 level and tumor markers.
Table 3. Correlation between miR-21 level and tumor markers. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952









![Comparison of overall survival (OS) in patients with brain metastasis (BM) from lung cancer treated with whole-brain radiotherapy (WBRT) or intensity-modulated radiotherapy (IMRT). (A) Higher OS rates were seen in patients with BM from lung cancer treated with IMRT than in those treated with WBRT (hazard ratio [HR]=5.550, P=0.0185). (B) Higher OS rates also were seen in patients with BM from lung cancer that expressed low levels of miR-21 (HR=8.471, P=0.0372).](https://jours.isi-science.com/imageXml.php?i=medscimonit-26-e924640-g001.jpg&idArt=924640&w=1000)