24 November 2020: Animal Study
Ethanol Extract of Chinese Hawthorn () Fruit Reduces Inflammation and Oxidative Stress in Rats with Doxorubicin-Induced Chronic Heart Failure
Fangzhou Cheng1AE, Wenlong Jiang2AB, Xiaoshuan Xiong2CD, Juan Chen2BC, Yunzhi Xiong2CF, Yinghong Li3FG*DOI: 10.12659/MSM.926654
Med Sci Monit 2020; 26:e926654
Abstract
BACKGROUND: Chinese hawthorn (Crataegus pinnatifida) fruit is a traditional Chinese medicine for treatment of digestive system and cardiovascular diseases. The fruit contains polyphenol compounds, such as epicatechin, that have anti-inflammatory activity. This study aimed to investigate the effects of an alcohol extract of hawthorn fruit (HAE) on inflammation and oxidative stress in rats with doxorubicin-induced chronic heart failure (CHF).
MATERIAL AND METHODS: Rats were intraperitoneally injected with doxorubicin to induce CHF and subsequently treated with HAE intragastrically once daily for 6 weeks. At the end of the experiment, echocardiographic and hemodynamic parameters were assessed, and enzyme-linked immunoassays were used to detect the levels of cardiac injury markers (brain natriuretic peptide, creatine kinase-MB, aspartate aminotransferase, lactate dehydrogenase, copeptin, and adrenomedullin), oxidative stress markers (glutathione peroxidase and malondialdehyde), and inflammatory cytokines (interleukin [IL]-6, IL-8, IL-1β, and tumor necrosis factor-a). The IL-1β, IL-6, glutathione peroxidase-1, and catalase mRNA levels were also measured by quantitative real-time polymerase chain reaction.
RESULTS: Our findings indicated that HAE exerts a cardioprotective effect, as shown by improved echocardiographic and hemodynamic parameters, decreased activity of serum myocardial enzymes, reduced serum levels of CHF markers, and inhibited inflammatory response in cardiac tissue. In addition, HAE treatment downregulated the mRNA expression of IL-1β and tumor necrosis factor-α and upregulated the mRNA expression of glutathione peroxidase-1 and catalase compared with untreated doxorubicin-induced CHF rats.
CONCLUSIONS: HAE shows promise for the prevention and treatment of CHF. The cardioprotective effect of HAE appears to be related to inhibition of both the inflammatory response and oxidative stress in vivo.
Keywords: Inflammation Mediators, Adrenomedullin, Aspartate Aminotransferases, Chromatography, High Pressure Liquid, Chronic Disease, Crataegus, creatine kinase, Cytokines, Electrocardiography, Ethanol, Fruit, Glycopeptides, Heart Function Tests, L-Lactate Dehydrogenase, Malondialdehyde, Natriuretic Peptide, Brain, Plant Extracts, polyphenols, RNA, Messenger, Rats, Wistar
Background
Chronic heart failure (CHF) is a common cardiovascular disease caused by myocardial infarction, and it has high mortality and morbidity [1]. Clinically, the major manifestations of CHF include decreased cardiac output, increased atrial natriuretic peptide gene expression, and ventricular dilation [2,3]. Doxorubicin (DOX) is an antitumor drug that is widely used in the treatment of various cancers, including malignant lymphomas, acute leukemia, ovarian cancer, and lung cancer [4]. However, the clinical utility of DOX is restricted because the drug is associated with a high incidence of heart failure and cumulative cardiotoxicity [5]. Irreversible heart failure and cardiomyopathy are the main manifestations of DOX-induced cardiotoxicity, although the pathophysiology of DOX-induced cardiotoxicity is multifactorial and has not been completely clarified [6]. However, oxidative stress is a predominantly suspected mechanism underlying DOX-induced heart failure, and various other mechanisms, including myofibril degeneration, mitochondrial dysfunction, inflammatory response, and cardiomyocyte apoptosis, are also considered to be important [7,8]. Among these, inflammatory responses play a vital role in myocardial damage. Although inflammatory reactions are indispensable for normal tissue reconstruction, ongoing inflammation can exacerbate left ventricular dysfunction, cell proliferation, and cardiac hypertrophy [9,10]. Therefore, it is necessary to better understand the etiopathogenesis of CHF and to explore novel therapeutic strategies that are safe and effective. Some reports have demonstrated that anti-inflammatory and antioxidant active ingredients could help achieve the latter purpose [11,12].
Traditional Chinese medicines may be safe and effective in treating CHF. One possibility is Chinese hawthorn (
Material and Methods
REAGENTS AND MATERIALS:
Enzyme-linked immunosorbent assay (ELISA) kits for measurement of inflammatory cytokines and antioxidant enzymes were purchased from Nanjing Jiancheng Bioengineering (Nanjing, China). Chlorogenic acid, neochlorogenic acid, caffeic acid, epicatechin, quercetin, and DOX were purchased from Sigma-Aldrich (St. Louis, MO, USA). Other reagents were obtained from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).
PLANT MATERIAL AND EXTRACTION:
Hawthorn fruits were collected in October 2019, in Xi’an (Shanxi Province, China). After being air-dried, the fruits were milled into a powder. The extraction process was conducted according to a previous study with minor modifications [21]. Briefly, hawthorn powder (500 g) was extracted with 70% ethanol (2×300 mL) for 30 min. The extract was filtered, combined, and concentrated under vacuum at 40°C to obtain 100 mL of the aqueous phase. After the aqueous phase was extracted with petroleum ether (2×100 mL), the petroleum ether layer was discarded, and the water layer was collected and concentrated under vacuum at 40°C to obtain HAE.
HIGH-PERFORMANCE LIQUID CHROMATOGRAPHY (HPLC) ANALYSIS OF HAE:
The polyphenolic compounds of HAE were quantified using an Agilent 1260 system. Polyphenolic constituents were separated on a Welch ultimate XB-C18 column (150×4.6 mm, 5 μm). Gradient elution was carried out by a mixture of 0.1% formic acid in acetonitrile (A) and 0.1% formic acid in water (B), and the following linear gradient was applied: 0% A (0 min) to 40% A (25 min). The detection wavelength was set to 254 nm, and the flow rate was 0.85 mL/min.
ANIMALS:
All experimental protocols involving animals were approved by the Shenzhen University and performed in accordance with the guidelines of the Care and Use of Laboratory Animals. Male Wistar rats, 8–10 weeks of age and weighing 180–210 g, were obtained from the Experimental Animal Center of Guangdong Province. The animals were housed in a temperature-controlled animal laboratory (22–24°C) with a 12-h dark-light cycle. All rats had ad libitum access to tap water and a standard chow diet.
INDUCTION OF HEART FAILURE AND EXPERIMENTAL PROTOCOL:
All rats were allowed to acclimatize for 7 days before the start of the experiments. Animals were randomly divided into 4 experimental groups (n=8 per group): the control group received tap water every day via gavage; the CHF group received DOX (2.5 mg/kg body weight every second day for 6 doses) by intraperitoneal injection to establish the heart failure model and received tap water every day via gavage; the CHF+LHAE group received DOX (2.5 mg/kg body weight every second day for 6 doses) by intraperitoneal injection and received low HAE (100 mg/kg body weight) every day via gavage; and the CHF+HHAE group received DOX (2.5 mg/kg body weight every second day for 6 doses) by intraperitoneal injection and received high HAE (200 mg/kg body weight) every day via gavage. All treatments were intragastrically administered for 6 weeks. HAE dosages were based on a previous study [22].
HEMODYNAMIC MEASUREMENTS:
After the last HAE treatment, all rats were anesthetized with sodium pentobarbital (30 mg/kg) by intraperitoneal injection. A catheter with a micro pressure sensor (Aerospace Medical Engineering Research Institute, Beijing, China) was inserted into the right carotid artery and advanced to the left ventricle to measure left ventricular pressure changes. After 5 min, left ventricular end-systolic pressure (LVESP), left ventricular end-diastolic pressure (LVEDP), and left ventricular pressure (±dP/dtmax) were analyzed by Spectrum software.
ECHOCARDIOGRAPHIC MEASUREMENTS:
All rats were anesthetized with sodium pentobarbital (30 mg/kg) by intraperitoneal injections. Then, a Siemens ACUSON Sequoia 512 (Siemens, Munich, Germany) was used to assess echocardiographic parameters. The left ventricular end-diastolic diameter (LVEDD) and the left ventricular end-systolic diameter (LVESD) were measured, and the results were calculated by the ultrasound system.
MEASUREMENT OF CHF MARKERS:
After echocardiographic measurements, blood samples were collected from the abdominal aorta and centrifuged at 1500×
HISTOPATHOLOGICAL ANALYSIS:
All rats were euthanized by an intravenous injection of potassium chloride. The heart tissues were immediately removed, cleaned, and weighed. Left ventricle specimens were fixed in 10% formalin and embedded in paraffin. The paraffin-embedded tissues were cut into 5-μm thickness, and sections were stained with hematoxylin and eosin and examined under a light microscope (Olympus America Inc, Melville, NY, USA).
MEASUREMENT OF CARDIAC INFLAMMATORY CYTOKINES AND OXIDATIVE STRESS:
Heart tissues were homogenized using a homogenizer, and the homogenate was centrifuged at 4000×
QUANTITATIVE REAL-TIME POLYMERASE CHAIN REACTION:
Total RNA was extracted and purified from the heart tissues using the TRIzol reagent (Invitrogen, USA) according to the manufacturer’s guidelines. cDNA synthesis was carried out using SuperScript II Reverse Transcriptase (Invitrogen, USA). Then, quantitative real-time polymerase chain reaction (qRT-PCR) was carried out with an ABI ViiA 7 Dx instrument (Applied Biosystems, USA) using the SYBR Green qPCR Master Mix (Thermo Fisher, USA). The qRT-PCR was conducted in duplicate, and the results were quantified using the 2−ΔΔCt method and normalized to GAPDH. Primers are shown in Table 1.
DATA STATISTICS AND ANALYSIS:
Results are expressed as the means±standard deviation (SD). Statistical comparisons between groups were conducted using 1-way analysis of variance followed by Tukey’s multiple comparison test. Statistical analysis was carried out with GraphPad Prism software (GraphPad Software, Inc., CA, USA), and
Results
HPLC ANALYSIS OF POLYPHENOLIC COMPOUNDS IN HAE:
Major polyphenolic compounds were first quantified to standardize the HAE. As shown in Table 2, 5 polyphenolic compounds (chlorogenic acid, neochlorogenic acid, caffeic acid, epicatechin, and quercetin) in HAE were measured by HPLC. In addition, the amounts of chlorogenic acid, neochlorogenic acid, caffeic acid, epicatechin, and quercetin were 16.77, 12.97, 0.46, 32.71, and 2.3 mg/g, respectively.
HAE IMPROVED DOX-INDUCED LOSS OF CARDIAC FUNCTION IN CHF RATS:
As shown in Figure 1, DOX treatment decreased LVESP and ±dP/dtmax level and increased the LVEDP level compared with the control group (P<0.01). However, these hemodynamic parameters were improved in HAE-treated CHF rats compared with CHF rats (P<0.01).
As shown in Figure 2, DOX treatment significantly increased LVEDD and LVESD levels compared with the control group (P<0.01). However, these echocardiographic parameters were decreased in HAE-treated CHF rats compared with the CHF rats (P<0.01).
HAE SUPPRESSED DOX-INDUCED CARDIOTOXICITY IN CHF RATS:
As shown in Figure 3, DOX treatment significantly increased the BNP, CK-MB, AST, and LDH levels compared with the control group (P<0.01). However, HAE treatment decreased these cardiac injury markers relative to the CHF group (P<0.01). As shown in Figure 4A and 4B, DOX treatment significantly increased the copeptin and adrenomedullin levels compared with the control group (P<0.01). However, HAE treatment decreased these cardiac injury markers compared with the CHF group (P<0.01).
HISTOPATHOLOGICAL STUDY:
Histopathological assessment of cardiac tissue was performed to further verify the cardiotoxicity caused by DOX. As shown in Figure 4C, cardiac muscle in the control group had normal myocardium architecture and regular cell distribution. However, cardiac muscle in the DOX group had obvious myocardial degeneration in the form of infiltration of inflammatory cells and disordered muscle fibers in the myocardial structure. Interestingly, treatment with HHAE attenuated infiltration of inflammatory cells and the disrupted myocardial structure in DOX-induced CHF.
HAE TREATMENT INHIBITED THE DOX-INDUCED INFLAMMATORY RESPONSE:
As shown in Figure 5, DOX treatment was associated with increased IL-6, IL-8, IL-1β, and TNF-α in the myocardial tissue relative to the control group (P<0.01). However, HAE treatment decreased those pro-inflammatory markers compared with the CHF group (P<0.01). These findings indicate that HAE inhibited the DOX-induced inflammatory response in CHF rats.
HAE INHIBITED DOX-INDUCED OXIDATIVE STRESS:
As shown in Figure 6, DOX treatment decreased cardiac GSH-Px activity and increased cardiac MDA content compared with the control group (P<0.01). HAE treatment improved GSH-Px activity and decreased MDA content compared with the CHF group (P<0.01). These findings indicate that HAE inhibited DOX-induced oxidative stress in CHF rats.
EFFECT OF HAE ON INTERLEUKIN AND ANTIOXIDANT ENZYME MRNA LEVELS:
As shown in Figure 7, DOX treatment upregulated mRNA expression levels of IL-6 and IL-1β and downregulated mRNA expression levels of catalase (CAT) and glutathione peroxidase-1 (GPX-1) compared with the control group (P<0.01). However, HAE treatment downregulated mRNA expression levels of IL-6 and IL-1β and upregulated mRNA expression levels of CAT and GPX-1 compared with the CHF group (P<0.01).
Discussion
In the present study, our findings indicated that HAE has cardioprotective effects in DOX-induced CHF rats through alleviation of oxidative stress and inhibition of inflammatory responses. DOX-induced CHF was evaluated by hemodynamic parameters, as well as echocardiographic parameters, cardiac injury markers, and histological studies of cardiac tissue. Abnormal hemodynamic parameters and echocardiography parameters reflected the attenuation of left ventricular function and the presence of arrhythmias, and these changes were consistent with previous reports [12]. Additionally, DOX-induced myocardial damage was further confirmed by increased levels of cardiac injury markers. Such markers, including CK-MB, BNP, LDH, and AST, are used for monitoring and diagnosing CHF [12,23]. Previous reports indicated that the copeptin level is a predictor of mortality and cardiac decompensation in outpatients with CHF [24]. Adrenomedullin is another biomarker with similar prognostic potential for CHF, and patients with CHF have been found to exhibit higher adrenomedullin levels in the blood compared with healthy people [25].
In the present study, the levels of CK-MB, BNP, LDH, AST, adrenomedullin, and copeptin were higher in the CHF group than in the control group, indicating the progression of the pathological process. These findings are consistent with previous reports [12,26]. DOX induced infiltration of inflammatory cells and disordered muscle fibers in the myocardial structure in the current study, and similar histopathological changes were reported in a previous study [27]. Treatment with HAE ameliorated changes in DOX-induced hemodynamic parameters and echocardiography parameters and suppressed elevations of cardiac injury markers. Furthermore, hematoxylin and eosin staining of tissue sections showed that HAE ameliorated DOX-induced lesions. These results indicate that HAE exerted a cardioprotective effect in DOX-induced CHF.
DOX and other anthracycline compounds are known to induce cardiac dysfunction. DOX is efficacious and widely used in chemotherapy; however, heart failure and cardiac toxicity associated with its use remain major limiting factors in its clinical application [28]. Therefore, developing a novel therapeutic strategy is urgently needed. Both lipid peroxidation products and oxidative stress are hallmarks of DOX-induced cardiomyopathy [29]. In addition, because cardiolipin has a high affinity for DOX, treatment with the drug leads to reactive oxygen species being generated in the cardiac mitochondria [30]. To address these problems, treatment strategies should include administering products with antioxidant activities. Our findings indicated that DOX induced an increase in MDA content and a decrease in GSH-Px enzyme activity, which was also demonstrated in a previous report showing that DOX provoked cardiac toxicity via disruption of the balance between antioxidant enzyme activity and reactive oxygen species [31]. A previous study showed that HAE had antioxidant activity in the context of high-salt-induced hypertension [32]. Consistent with these results, our findings showed an obvious decline in MDA content and enhanced GSH-Px enzyme activity after treatment with HAE. Our findings also imply that administering HAE might exert an antioxidant activity that would be of significant value in the treatment of DOX-induced cardiac toxicity.
Inflammatory cytokines were also assessed in DOX-induced CHF rats because inflammation is another primary factor in the etiopathogenesis of CHF. As suggested in previous studies, the inhibition of inflammation may alleviate DOX-related cardiotoxicity [11,33]. Furthermore, some studies have indicated that inflammatory factors are involved in DOX-induced cardiotoxicity [34,35]. Clinical findings have revealed that the overexpression of both IL-6 and TNF-α contribute to the progression of CHF [36]. In addition, increased expression and secretion of inflammatory factors, including IL-6, IL-10, and TNF-α, have been observed in failing myocardium and in plasma [37]. Moreover, previous reports have indicated the importance of inhibiting the expression of pro-inflammatory factors, including IL-1β and TNF-α, in alleviating DOX-induced CHF [33,38]. In the current study, DOX-induced cardiotoxicity was associated with increased IL-1β, TNF-α, IL-6, and IL-8 levels as well as increased IL-1β and IL-6 mRNA expression, indicating increased inflammatory responses. Interestingly, HAE treatment inhibited the inflammatory responses as shown by reduced levels of IL-1β, TNF-α, IL-6, and IL-8 as well as decreased expression of IL-1β and IL-6. We suggest that these findings support the hypothesis that HAE exerts satisfactory anti-inflammatory effects. Our findings are also consistent with a previous report that an alcohol extract of hawthorn fruit reduced isoproterenol-induced inflammation in rats [39].
Epicatechin and other polyphenolic compounds are widely present in nature. They are major components of various medicinal plants and have been demonstrated to have cardioprotective effects [40–42]. In our study, chemical characterization by HPLC indicated the presence of polyphenolic compounds in HAE, with the main ones being epicatechin and chlorogenic acid (Table 2). We therefore speculate that these polyphenolics are, to a certain degree, responsible for the cardioprotective effects of HAE.
Conclusions
Our findings indicated for the first time that HAE alleviates the progression of DOX-induced CHF in rats. The results imply that the cardioprotective activity of HAE may be associated with its antioxidant and anti-inflammatory effects. HAE could be a potential therapeutic agent for the prevention and treatment of CHF, but its cardioprotective activity needs to be validated in clinical trials.
Figures
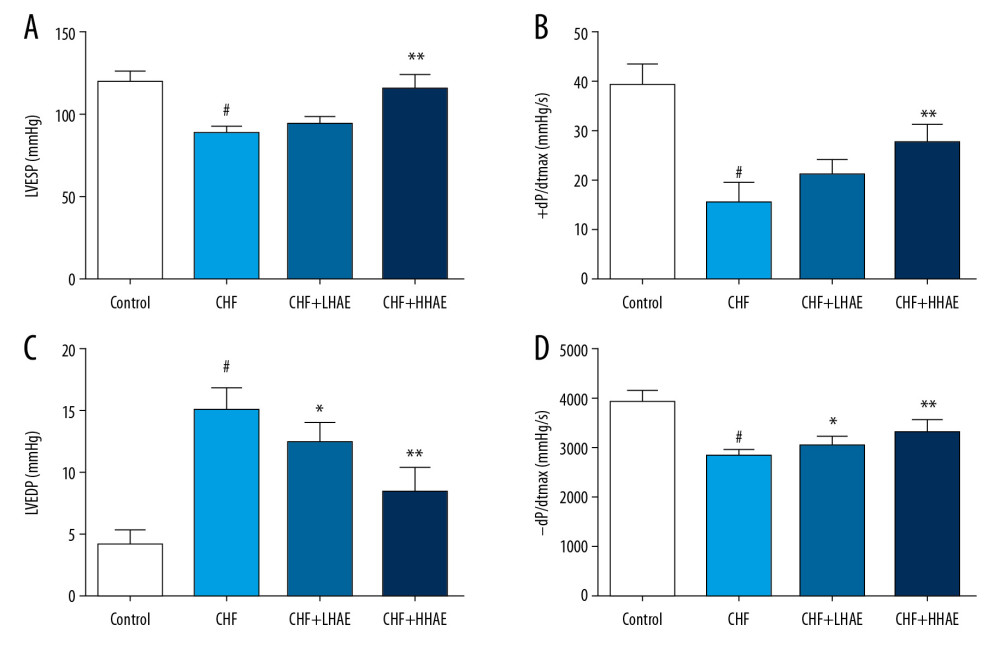 Figure 1. (A–D) The effects of HAE on hemodynamic parameters (LVESP, ±dP/dtmax, and LVEDP) in doxorubicin-evoked CHF in rats. Results are expressed as the means±SD (n=8). # P<0.01 versus the control group; * P<0.05 and ** P<0.01 versus the CHF group. CHF – chronic heart failure; HAE – alcohol extract of hawthorn (Crataegus pinnatifida) fruit; LVEDP – left ventricular end-diastolic pressure; LVESP – left ventricular end-systolic pressure.
Figure 1. (A–D) The effects of HAE on hemodynamic parameters (LVESP, ±dP/dtmax, and LVEDP) in doxorubicin-evoked CHF in rats. Results are expressed as the means±SD (n=8). # P<0.01 versus the control group; * P<0.05 and ** P<0.01 versus the CHF group. CHF – chronic heart failure; HAE – alcohol extract of hawthorn (Crataegus pinnatifida) fruit; LVEDP – left ventricular end-diastolic pressure; LVESP – left ventricular end-systolic pressure. 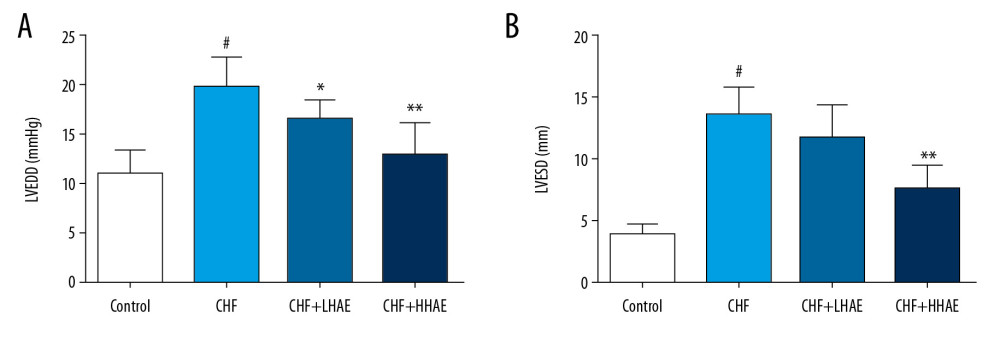 Figure 2. (A, B) The effects of HAE on echocardiographic parameters (LVEDD and LVESD) in doxorubicin-evoked CHF in rats. Results are expressed as the means±SD (n=8). # P<0.01 versus the control group; * P<0.05 and ** P<0.01 versus the CHF group. CHF – chronic heart failure; HAE, alcohol extract of hawthorn (Crataegus pinnatifida) fruit; LVEDD – left ventricular end-diastolic diameter; LVESD – left ventricular end-systolic diameter.
Figure 2. (A, B) The effects of HAE on echocardiographic parameters (LVEDD and LVESD) in doxorubicin-evoked CHF in rats. Results are expressed as the means±SD (n=8). # P<0.01 versus the control group; * P<0.05 and ** P<0.01 versus the CHF group. CHF – chronic heart failure; HAE, alcohol extract of hawthorn (Crataegus pinnatifida) fruit; LVEDD – left ventricular end-diastolic diameter; LVESD – left ventricular end-systolic diameter. 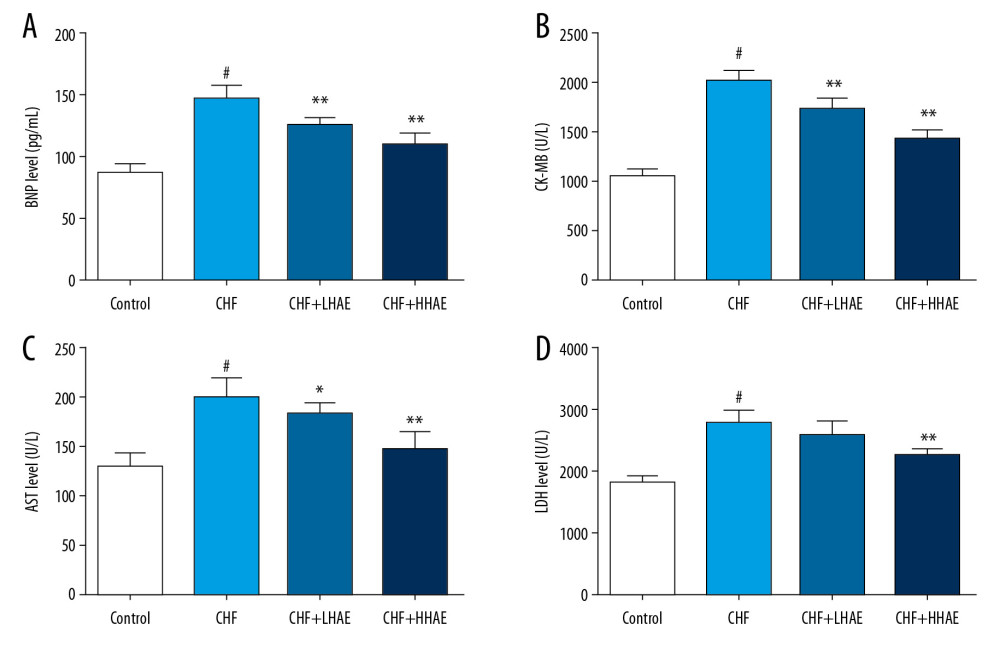 Figure 3. The effects of HAE on serum BNP (A), CK-MB (B), AST (C), and LDH (D) levels in doxorubicin-evoked CHF in rats. Results are expressed as the means±SD (n=8). # P<0.01 versus the control group; * P<0.05 and ** P<0.01 versus the CHF group. AST – aspartate aminotransferase; BNP – brain natriuretic peptide; CHF – chronic heart failure; CK-MB – creatine kinase-MB; HAE – alcohol extract of hawthorn (Crataegus pinnatifida) fruit; LDH – lactate dehydrogenase.
Figure 3. The effects of HAE on serum BNP (A), CK-MB (B), AST (C), and LDH (D) levels in doxorubicin-evoked CHF in rats. Results are expressed as the means±SD (n=8). # P<0.01 versus the control group; * P<0.05 and ** P<0.01 versus the CHF group. AST – aspartate aminotransferase; BNP – brain natriuretic peptide; CHF – chronic heart failure; CK-MB – creatine kinase-MB; HAE – alcohol extract of hawthorn (Crataegus pinnatifida) fruit; LDH – lactate dehydrogenase. 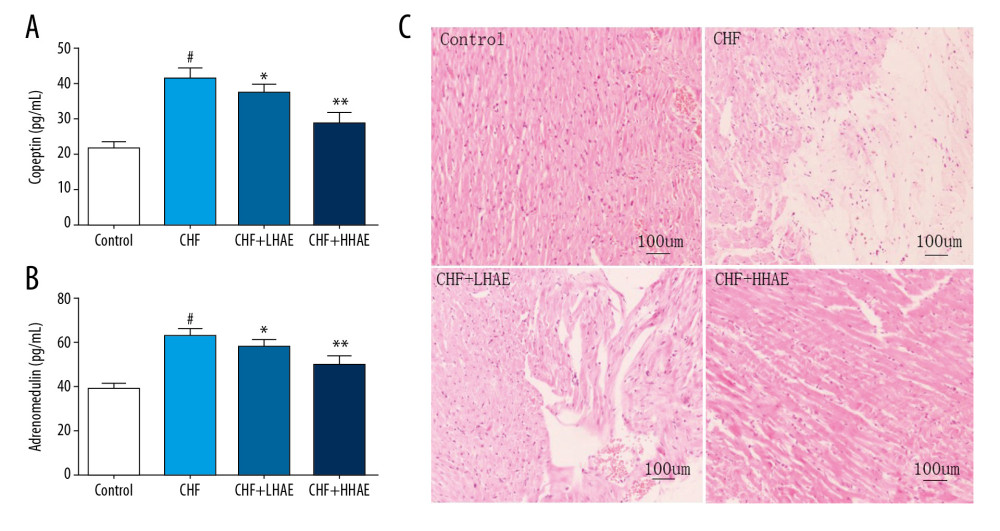 Figure 4. The effects of HAE on serum copeptin (A), adrenomedullin (B) levels, and histopathological changes in heart tissues (C) of rats in doxorubicin-induced CHF. Heart tissues were examined by hematoxylin and eosin staining using light microscopy (×100). Scale bar=100 μm. Results are expressed as the means±SD (n=8). # P<0.01 versus the control group; * P<0.05 and ** P<0.01 versus the CHF group. CHF – chronic heart failure; HAE – alcohol extract of hawthorn (Crataegus pinnatifida) fruit.
Figure 4. The effects of HAE on serum copeptin (A), adrenomedullin (B) levels, and histopathological changes in heart tissues (C) of rats in doxorubicin-induced CHF. Heart tissues were examined by hematoxylin and eosin staining using light microscopy (×100). Scale bar=100 μm. Results are expressed as the means±SD (n=8). # P<0.01 versus the control group; * P<0.05 and ** P<0.01 versus the CHF group. CHF – chronic heart failure; HAE – alcohol extract of hawthorn (Crataegus pinnatifida) fruit. 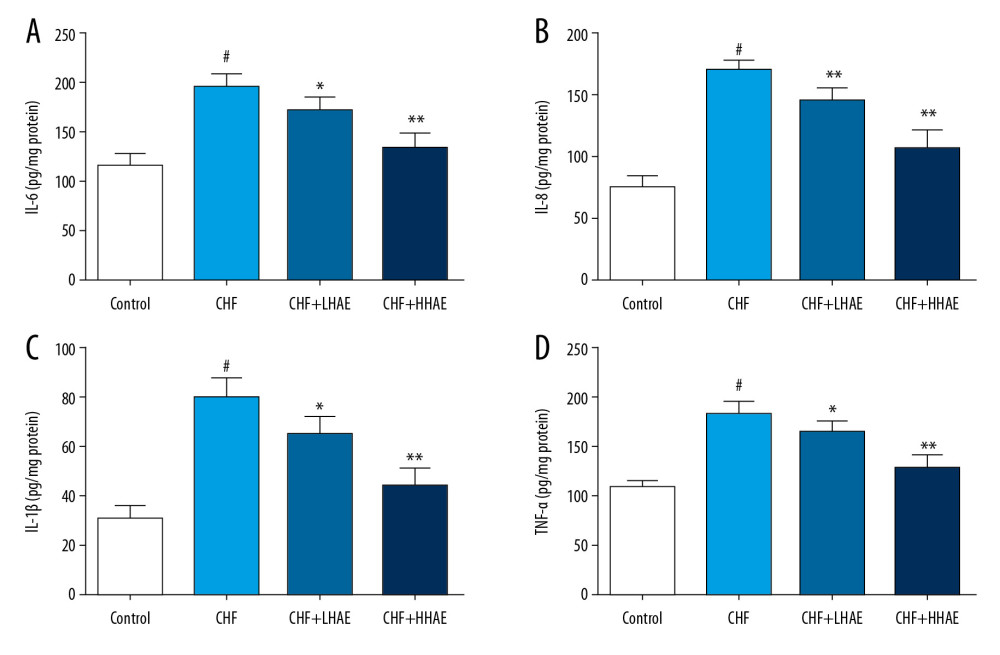 Figure 5. Effects of HAE on heart tissue levels of IL-6 (A), IL-8 (B), IL-1β (C), and TNF-α (D) in doxorubicin-induced chronic heart failure in rats. Results are expressed as the means±SD (n=8). # P<0.01 versus the control group; * P<0.05 and ** P<0.01 versus the CHF group. CHF – chronic heart failure; HAE – alcohol extract of hawthorn (Crataegus pinnatifida) fruit; IL – interleukin; TNF-α – tumor necrosis factor α.
Figure 5. Effects of HAE on heart tissue levels of IL-6 (A), IL-8 (B), IL-1β (C), and TNF-α (D) in doxorubicin-induced chronic heart failure in rats. Results are expressed as the means±SD (n=8). # P<0.01 versus the control group; * P<0.05 and ** P<0.01 versus the CHF group. CHF – chronic heart failure; HAE – alcohol extract of hawthorn (Crataegus pinnatifida) fruit; IL – interleukin; TNF-α – tumor necrosis factor α. 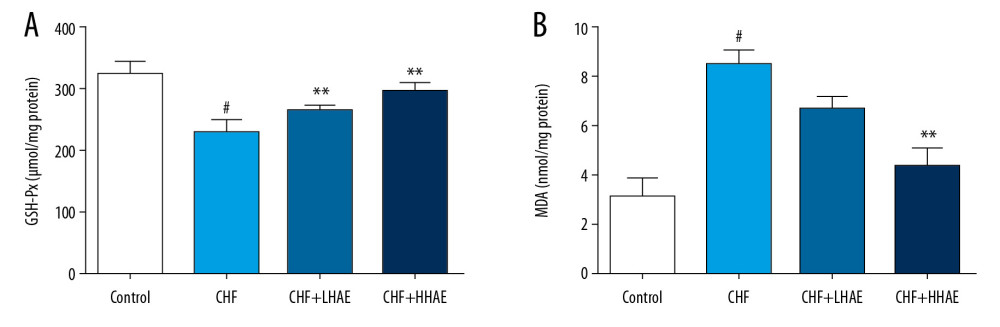 Figure 6. Effects of HAE on heart tissue levels of GSH-Px (A) and MDA (B) in doxorubicin-induced CHF in rats. Results are expressed as the means±SD (n=8). # P<0.01 versus the control group; * P<0.05 and ** P<0.01 versus the CHF group. CHF – chronic heart failure; GSH-Px – glutathione peroxidase; HAE – alcohol extract of hawthorn (Crataegus pinnatifida) fruit; MDA – malondialdehyde.
Figure 6. Effects of HAE on heart tissue levels of GSH-Px (A) and MDA (B) in doxorubicin-induced CHF in rats. Results are expressed as the means±SD (n=8). # P<0.01 versus the control group; * P<0.05 and ** P<0.01 versus the CHF group. CHF – chronic heart failure; GSH-Px – glutathione peroxidase; HAE – alcohol extract of hawthorn (Crataegus pinnatifida) fruit; MDA – malondialdehyde. 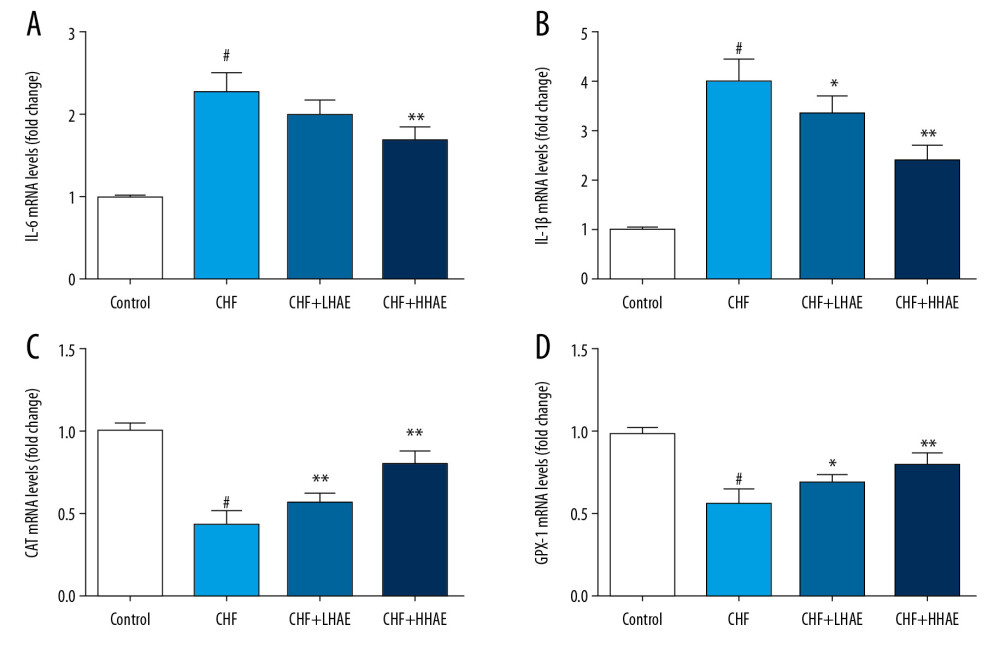 Figure 7. Effects of HAE on mRNA expression of IL-6 (A), IL-1β (B), CAT (C), and GPX-1 (D) in doxorubicin-induced CHF in rats. Results are expressed as the means±SD (n=6). # P<0.01 versus the control group; * P<0.05 and ** P<0.01 versus the CHF group. CAT – catalase; CHF – chronic heart failure; HAE – alcohol extract of hawthorn (Crataegus pinnatifida) fruit; GPX-1 – glutathione peroxidase-1; IL – interleukin.
Figure 7. Effects of HAE on mRNA expression of IL-6 (A), IL-1β (B), CAT (C), and GPX-1 (D) in doxorubicin-induced CHF in rats. Results are expressed as the means±SD (n=6). # P<0.01 versus the control group; * P<0.05 and ** P<0.01 versus the CHF group. CAT – catalase; CHF – chronic heart failure; HAE – alcohol extract of hawthorn (Crataegus pinnatifida) fruit; GPX-1 – glutathione peroxidase-1; IL – interleukin. References
1. Kharchenko EPHeart failure in cardiorenal syndromes: Ter Arkh, 2013; 85(1); 85-91 [in Russian]
2. Rossignol P, Hernandez AF, Solomon SD, Zannad F, Heart failure drug treatment: Lancet, 2019; 393(10175); 1034-44
3. Grote Beverborg N, van Veldhuisen DJ, van der Meer P, Anemia in heart failure: Still relevant?: JACC Heart Fail, 2018; 6(3); 201-8
4. Minotti G, Menna P, Salvatorelli E, Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity: Pharmacol Rev, 2004; 56(2); 185-229
5. Lee KH, Cho H, Lee S, Enhanced-autophagy by exenatide mitigates doxorubicin-induced cardiotoxicity: Int J Cardiol, 2017; 232; 40-47
6. Guo R, Hua Y, Ren J, Cardiomyocyte-specific disruption of Cathepsin K protects against doxorubicin-induced cardiotoxicity: Cell Death Dis, 2018; 9(6); 692
7. Childs AC, Phaneuf SL, Dirks AJ: Cancer Res, 2002; 62(16); 4592-98
8. Briasoulis A, Androulakis E, Christophides T, Tousoulis D, The role of inflammation and cell death in the pathogenesis, progression and treatment of heart failure: Heart Fail Rev, 2016; 21(2); 169-76
9. Van Tassell BW, Raleigh JM, Abbate A, Targeting interleukin-1 in heart failure and inflammatory heart disease: Curr Heart Fail Rep, 2015; 12(1); 33-41
10. O’Brien LC, Mezzaroma E, Van Tassell BW, Interleukin-18 as a therapeutic target in acute myocardial infarction and heart failure: Mol Med, 2014; 20; 221-29
11. Pecoraro M, Del Pizzo M, Marzocco S, Inflammatory mediators in a short-time mouse model of doxorubicin-induced cardiotoxicity: Toxicol Appl Pharmacol, 2016; 293; 44-52
12. Wu Z, Zhao X, Miyamoto A, Effects of steroidal saponins extract from Ophiopogon japonicus root ameliorates doxorubicin-induced chronic heart failure by inhibiting oxidative stress and inflammatory response: Pharm Biol, 2019; 57(1); 176-83
13. Malekinejad H, Shafie-Irannejad V, Hobbenaghi R, Comparative protective effect of hawthorn berry hydroalcoholic extract, atorvastatin, and mesalamine on experimentally induced colitis in rats: J Med Food, 2013; 16(7); 593-601
14. Dehghani S, Mehri S, Hosseinzadeh H: Iran J Basic Med Sci, 2019; 22(5); 460-68
15. Jurikova T, Sochor J, Rop O: Molecules, 2012; 17(12); 14490-509
16. Cui T, Nakamura K, Tian S, Polyphenolic content and physiological activities of Chinese hawthorn extracts: Biosci Biotechnol Biochem, 2006; 70(12); 2948-56
17. Tadic VM, Dobric S, Markovic GM, Anti-inflammatory, gastroprotective, free-radical-scavenging, and antimicrobial activities of hawthorn berries ethanol extract: J Agric Food Chem, 2008; 56(17); 7700-709
18. Kao ES, Wang CJ, Lin WL: J Agric Food Chem, 2005; 53(2); 430-36
19. Jouad H, Lemhadri A, Maghrani M, Hawthorn evokes a potent anti-hyperglycemic capacity in streptozotocin-induced diabetic rats: J Herb Pharmacother, 2003; 3(2); 19-29
20. Holubarsch CJF, Colucci WS, Eha J: Am J Cardiovasc Drugs, 2018; 18(1); 25-36
21. Bujor A, Miron A, Luca SV: J Ethnopharmacol, 2020; 252; 112559
22. Lis M, Szczypka M, Suszko-Pawlowska A: Planta Med, 2020; 86(2); 160-68
23. Gaggin HK, Januzzi JL, Biomarkers and diagnostics in heart failure: Biochim Biophys Acta, 2013; 1832(12); 2442-50
24. Balling L, Kistorp C, Schou M, Plasma copeptin levels and prediction of outcome in heart failure outpatients: Relation to hyponatremia and loop diuretic doses: J Card Fail, 2012; 18(5); 351-58
25. Nishikimi T, Kuwahara K, Nakagawa Y, Adrenomedullin in cardiovascular disease: A useful biomarker, its pathological roles and therapeutic application: Curr Protein Pept Sci, 2013; 14(4); 256-67
26. Latypova GM, Bychenkova MA, Katayev VA: Phytomedicine, 2019; 54; 17-26
27. Fouad AA, Yacoubi MT, Mechanisms underlying the protective effect of eugenol in rats with acute doxorubicin cardiotoxicity: Arch Pharm Res, 2011; 34(5); 821-28
28. Zhang YW, Shi J, Li YJ, Wei L, Cardiomyocyte death in doxorubicin-induced cardiotoxicity: Arch Immunol Ther Exp (Warsz), 2009; 57(6); 435-45
29. Gandhi H, Patel VB, Mistry N, Doxorubicin mediated cardiotoxicity in rats: Protective role of felodipine on cardiac indices: Environ Toxicol Pharmacol, 2013; 36(3); 787-95
30. Giampieri F, Alvarez-Suarez JM, Gasparrini M, Strawberry consumption alleviates doxorubicin-induced toxicity by suppressing oxidative stress: Food Chem Toxicol, 2016; 94; 128-37
31. Tong Y, Wang K, Sheng S, Cui J, Polydatin ameliorates chemotherapy-induced cognitive impairment (chemobrain) by inhibiting oxidative stress, inflammatory response, and apoptosis in rats: Biosci Biotechnol Biochem, 2020; 84(6); 1201-10
32. Zheng X, Li X, Chen M, The protective role of hawthorn fruit extract against high salt-induced hypertension in Dahl salt-sensitive rats: Impact on oxidative stress and metabolic patterns: Food Funct, 2019; 10(2); 849-58
33. Peng W, Rao D, Zhang M, Teneligliptin prevents doxorubicin-induced inflammation and apoptosis in H9c2 cells: Arch Biochem Biophys, 2019; 683; 108238
34. Akolkar G, Bagchi AK, Ayyappan P, Doxorubicin-induced nitrosative stress is mitigated by vitamin C via the modulation of nitric oxide synthases: Am J Physiol Cell Physiol, 2017; 312(4); C418-27
35. Akolkar G, da Silva Dias D, Ayyappan P, Vitamin C mitigates oxidative/nitrosative stress and inflammation in doxorubicin-induced cardiomyopathy: Am J Physiol Heart Circ Physiol, 2017; 313(4); H795-809
36. Bozkurt B, Activation of cytokines as a mechanism of disease progression in heart failure: Ann Rheum Dis, 2000; 59(Suppl 1); i90-93
37. Gullestad L, Ueland T, Vinge LE, Inflammatory cytokines in heart failure: Mediators and markers: Cardiology, 2012; 122(1); 23-35
38. Mantawy EM, El-Bakly WM, Esmat A, Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis: Eur J Pharmacol, 2014; 728; 107-18
39. Vijayan NA, Thiruchenduran M, Devaraj SN: Mol Cell Biochem, 2012; 367(1–2); 1-8
40. MacRae K, Connolly K, Vella R, Fenning A, Epicatechin’s cardiovascular protective effects are mediated via opioid receptors and nitric oxide: Eur J Nutr, 2019; 58(2); 515-27
41. Carresi C, Musolino V, Gliozzi M, Anti-oxidant effect of bergamot polyphenolic fraction counteracts doxorubicin-induced cardiomyopathy: Role of autophagy and c-kit(pos)CD45(neg)CD31(neg) cardiac stem cell activation: J Mol Cell Cardiol, 2018; 119; 10-18
42. Tian L, Su CP, Wang Q, Chlorogenic acid: A potent molecule that protects cardiomyocytes from TNF-alpha-induced injury via inhibiting NF-kappaB and JNK signals: J Cell Mol Med, 2019; 23(7); 4666-78
Figures
 Figure 1. (A–D) The effects of HAE on hemodynamic parameters (LVESP, ±dP/dtmax, and LVEDP) in doxorubicin-evoked CHF in rats. Results are expressed as the means±SD (n=8). # P<0.01 versus the control group; * P<0.05 and ** P<0.01 versus the CHF group. CHF – chronic heart failure; HAE – alcohol extract of hawthorn (Crataegus pinnatifida) fruit; LVEDP – left ventricular end-diastolic pressure; LVESP – left ventricular end-systolic pressure.
Figure 1. (A–D) The effects of HAE on hemodynamic parameters (LVESP, ±dP/dtmax, and LVEDP) in doxorubicin-evoked CHF in rats. Results are expressed as the means±SD (n=8). # P<0.01 versus the control group; * P<0.05 and ** P<0.01 versus the CHF group. CHF – chronic heart failure; HAE – alcohol extract of hawthorn (Crataegus pinnatifida) fruit; LVEDP – left ventricular end-diastolic pressure; LVESP – left ventricular end-systolic pressure. Figure 2. (A, B) The effects of HAE on echocardiographic parameters (LVEDD and LVESD) in doxorubicin-evoked CHF in rats. Results are expressed as the means±SD (n=8). # P<0.01 versus the control group; * P<0.05 and ** P<0.01 versus the CHF group. CHF – chronic heart failure; HAE, alcohol extract of hawthorn (Crataegus pinnatifida) fruit; LVEDD – left ventricular end-diastolic diameter; LVESD – left ventricular end-systolic diameter.
Figure 2. (A, B) The effects of HAE on echocardiographic parameters (LVEDD and LVESD) in doxorubicin-evoked CHF in rats. Results are expressed as the means±SD (n=8). # P<0.01 versus the control group; * P<0.05 and ** P<0.01 versus the CHF group. CHF – chronic heart failure; HAE, alcohol extract of hawthorn (Crataegus pinnatifida) fruit; LVEDD – left ventricular end-diastolic diameter; LVESD – left ventricular end-systolic diameter. Figure 3. The effects of HAE on serum BNP (A), CK-MB (B), AST (C), and LDH (D) levels in doxorubicin-evoked CHF in rats. Results are expressed as the means±SD (n=8). # P<0.01 versus the control group; * P<0.05 and ** P<0.01 versus the CHF group. AST – aspartate aminotransferase; BNP – brain natriuretic peptide; CHF – chronic heart failure; CK-MB – creatine kinase-MB; HAE – alcohol extract of hawthorn (Crataegus pinnatifida) fruit; LDH – lactate dehydrogenase.
Figure 3. The effects of HAE on serum BNP (A), CK-MB (B), AST (C), and LDH (D) levels in doxorubicin-evoked CHF in rats. Results are expressed as the means±SD (n=8). # P<0.01 versus the control group; * P<0.05 and ** P<0.01 versus the CHF group. AST – aspartate aminotransferase; BNP – brain natriuretic peptide; CHF – chronic heart failure; CK-MB – creatine kinase-MB; HAE – alcohol extract of hawthorn (Crataegus pinnatifida) fruit; LDH – lactate dehydrogenase. Figure 4. The effects of HAE on serum copeptin (A), adrenomedullin (B) levels, and histopathological changes in heart tissues (C) of rats in doxorubicin-induced CHF. Heart tissues were examined by hematoxylin and eosin staining using light microscopy (×100). Scale bar=100 μm. Results are expressed as the means±SD (n=8). # P<0.01 versus the control group; * P<0.05 and ** P<0.01 versus the CHF group. CHF – chronic heart failure; HAE – alcohol extract of hawthorn (Crataegus pinnatifida) fruit.
Figure 4. The effects of HAE on serum copeptin (A), adrenomedullin (B) levels, and histopathological changes in heart tissues (C) of rats in doxorubicin-induced CHF. Heart tissues were examined by hematoxylin and eosin staining using light microscopy (×100). Scale bar=100 μm. Results are expressed as the means±SD (n=8). # P<0.01 versus the control group; * P<0.05 and ** P<0.01 versus the CHF group. CHF – chronic heart failure; HAE – alcohol extract of hawthorn (Crataegus pinnatifida) fruit. Figure 5. Effects of HAE on heart tissue levels of IL-6 (A), IL-8 (B), IL-1β (C), and TNF-α (D) in doxorubicin-induced chronic heart failure in rats. Results are expressed as the means±SD (n=8). # P<0.01 versus the control group; * P<0.05 and ** P<0.01 versus the CHF group. CHF – chronic heart failure; HAE – alcohol extract of hawthorn (Crataegus pinnatifida) fruit; IL – interleukin; TNF-α – tumor necrosis factor α.
Figure 5. Effects of HAE on heart tissue levels of IL-6 (A), IL-8 (B), IL-1β (C), and TNF-α (D) in doxorubicin-induced chronic heart failure in rats. Results are expressed as the means±SD (n=8). # P<0.01 versus the control group; * P<0.05 and ** P<0.01 versus the CHF group. CHF – chronic heart failure; HAE – alcohol extract of hawthorn (Crataegus pinnatifida) fruit; IL – interleukin; TNF-α – tumor necrosis factor α. Figure 6. Effects of HAE on heart tissue levels of GSH-Px (A) and MDA (B) in doxorubicin-induced CHF in rats. Results are expressed as the means±SD (n=8). # P<0.01 versus the control group; * P<0.05 and ** P<0.01 versus the CHF group. CHF – chronic heart failure; GSH-Px – glutathione peroxidase; HAE – alcohol extract of hawthorn (Crataegus pinnatifida) fruit; MDA – malondialdehyde.
Figure 6. Effects of HAE on heart tissue levels of GSH-Px (A) and MDA (B) in doxorubicin-induced CHF in rats. Results are expressed as the means±SD (n=8). # P<0.01 versus the control group; * P<0.05 and ** P<0.01 versus the CHF group. CHF – chronic heart failure; GSH-Px – glutathione peroxidase; HAE – alcohol extract of hawthorn (Crataegus pinnatifida) fruit; MDA – malondialdehyde. Figure 7. Effects of HAE on mRNA expression of IL-6 (A), IL-1β (B), CAT (C), and GPX-1 (D) in doxorubicin-induced CHF in rats. Results are expressed as the means±SD (n=6). # P<0.01 versus the control group; * P<0.05 and ** P<0.01 versus the CHF group. CAT – catalase; CHF – chronic heart failure; HAE – alcohol extract of hawthorn (Crataegus pinnatifida) fruit; GPX-1 – glutathione peroxidase-1; IL – interleukin.
Figure 7. Effects of HAE on mRNA expression of IL-6 (A), IL-1β (B), CAT (C), and GPX-1 (D) in doxorubicin-induced CHF in rats. Results are expressed as the means±SD (n=6). # P<0.01 versus the control group; * P<0.05 and ** P<0.01 versus the CHF group. CAT – catalase; CHF – chronic heart failure; HAE – alcohol extract of hawthorn (Crataegus pinnatifida) fruit; GPX-1 – glutathione peroxidase-1; IL – interleukin. Tables
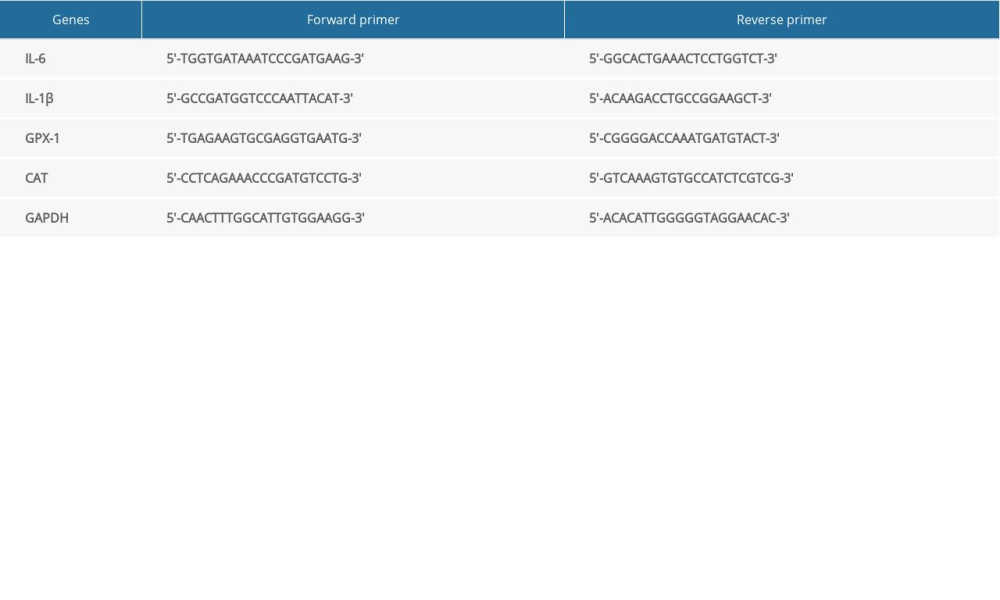 Table 1. Primer sequences for quantitative real-time RNA analysis.
Table 1. Primer sequences for quantitative real-time RNA analysis.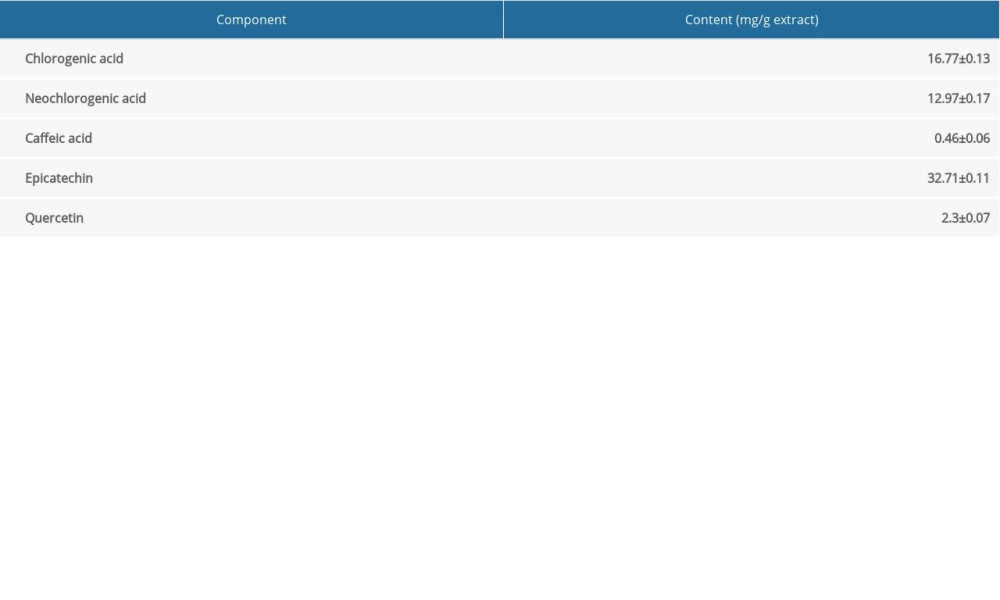 Table 2. Determination of representative polyphenolic compounds in hawthorn extract.
Table 2. Determination of representative polyphenolic compounds in hawthorn extract. Table 1. Primer sequences for quantitative real-time RNA analysis.
Table 1. Primer sequences for quantitative real-time RNA analysis. Table 2. Determination of representative polyphenolic compounds in hawthorn extract.
Table 2. Determination of representative polyphenolic compounds in hawthorn extract. In Press
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








