01 December 2020: Lab/In Vitro Research
Plasma Metabolomics Analysis Identifies Abnormal Energy, Lipid, and Amino Acid Metabolism in Abdominal Aortic Aneurysms
Yaming Guo1EF, Shuwei Wan1EF, Mingli Han1C, Yubo Zhao1B, Chuang Li1B, Gaopo Cai1C, Shuai Zhang1D, Zhi Sun2D, Xinhua Hu3F, Hui Cao1AG*, Zhen Li1ADOI: 10.12659/MSM.926766
Med Sci Monit 2020; 26:e926766
Abstract
BACKGROUND: Abdominal aortic aneurysm (AAA) is a complicated aortic dilatation disease. Metabolomics is an emerging system biology method. This aim of this study was to identify abnormal metabolites and metabolic pathways associated with AAA and to discover potential biomarkers that could affect the size of AAAs.
MATERIAL AND METHODS: An untargeted metabolomic method was used to analyze the plasma metabolic profiles of 39 patients with AAAs and 30 controls. Multivariate analysis methods were used to perform differential metabolite screening and metabolic pathway analysis. Cluster analysis and univariate analysis were performed to identify potential metabolites that could affect the size of an AAA.
RESULTS: Forty-five different metabolites were identified with an orthogonal projection to latent squares-discriminant analysis model and the differences between them in the patients with AAAs and the control group were compared. A variable importance in the projection score >1 and P<0.05 were considered statistically significant. In patients with AAAs, the pathways involving metabolism of alanine, aspartate, glutamate, D-glutamine, D-glutamic acid, arginine, and proline; tricarboxylic acid cycling; and biosynthesis of arginine are abnormal. The progression of an AAA may be related to 13 metabolites: citric acid, 2-oxoglutarate, succinic acid, coenzyme Q1, pyruvic acid, sphingosine-1-phosphate, platelet-activating factor, LysoPC (16: 00), lysophosphatidylcholine (18: 2(9Z,12Z)/0: 0), arginine, D-aspartic acid, and L- and D-glutamine.
CONCLUSIONS: An untargeted metabolomic analysis using ultraperformance liquid chromatography-tandem mass spectrometry identified metabolites that indicate disordered metabolism of energy, lipids, and amino acids in AAAs.
Keywords: Chromatography, Liquid, Metabolism, Plasma, Energy Metabolism, Amino Acids, Case-Control Studies, Cluster Analysis, Discriminant Analysis, Lipid Metabolism, Metabolome, Metabolomics, Principal Component Analysis
Background
Abdominal aortic aneurysms (AAAs) are characterized by permanent localized dilation of the aorta [1]. An abdominal aorta diameter >3 cm typically is diagnostic of the condition [2]. Moreover, the rate of mortality associated with AAA rupture is extremely high at almost 70% [3]. Surgical treatment of AAAs has become increasingly sophisticated and less invasive, but it can only be used for aneurysms ≥5.5 cm [4]. There remains an urgent need to identify pathways and potential biomarkers that predispose patients to aneurysmal formation and that can be used to focus attention from surgery to medical approaches that can help limit the progression of small AAAs.
AAAs are characterized by progressive luminal dilation accompanied by aortic wall inflammation, decreased medial smooth muscle cells, and disruption of the extracellular matrix. Although studies have been conducted on a variety of biologically plausible markers for diagnosis and prediction of disease activity in AAA [5–7], there is still a need for a systematic understanding of biological disturbances associated with the condition. Metabolomics is a high-throughput omics technology that follows on the heels of genomics, transcriptomics, and proteomics, and which can be used to simultaneously measure and study a large number of small molecules (<1000 Da) [8]. The systematic identification and quantification of metabolites in biological systems with metabolomics has been extensively studied in clinical and preclinical studies as a way of identifying predictors of disease [9]. This study aimed to identify differences in plasma metabolites between patients with AAAs and a control group, and to identify potential biomarkers that could influence the size of AAAs.
Material and Methods
CHEMICALS AND REAGENTS:
The 2-chloro-L-phenylalanine used in the present study was obtained from J&K Chemical (Beijing, China), the ketoprofen was purchased from Sigma-Aldrich (St. Louis, Missouri, U.S.A.), and the acetonitrile and methanol (HPLC-grade) were supplied by Fisher Scientific (Pittsburgh, Pennsylvania, U.S.A.). The HPLC-grade formic acid was purchased from Sigma-Aldrich (St. Louis, Missouri, U.S.A.).
SAMPLES:
We collected 69 plasma samples in this study, from 39 patients with AAAs that were identified on ultrasound at the First Affiliated Hospital of Zhengzhou University (AAA group) and from 30 volunteers who were recruited for the research (control group). The maximum diameter of the infrarenal AAAs in all patients was measured by 2 experienced ultrasonographers. Because many risk factors are associated with AAA [10,11], baseline data from all of the participants were extracted from their medical records. Factors analyzed included age; sex; smoking status; history of chronic obstructive pulmonary disease (COPD), diabetes, or hypertension; serum lipid levels; and drug exposure.
All subjects fasted beginning at 10 p.m. the day before their plasma was collected. Patients were excluded if they had thoracic aortic aneurysms, aortic dissection, an AAA combined with inflammatory aortic disease, or a systemic connective tissue disorder. EDTA anticoagulant tubes were used to collect blood samples from all patients, which were immediately centrifuged at 3000 rpm for 15 min and then at 4000 rpm for 10 min. All of the samples were stored at −80°C before analysis.
UPLC-MS ANALYSIS:
An untargeted metabolomics analysis was performed with an Ultimate 3000 Ultra Performance Liquid Chromatography system (Dionex, U.S.A.) coupled with a Thermo Q-Exactive Oribitrap mass spectrometer and autosampler. Extraction of metabolites was performed by sampling 100 μL of plasma into 300 μL of a methanol solution created following existing standards, which was then subjected to vortex mixing for 2 min and centrifugation at 13 000 rpm for 10 min to pellet the proteins.
To ensure the reliability of the experimental results, quality control (QC) samples were interpolated in the sequence to monitor acquisition of data. In the first run sequence, 6 QC samples were used to balance the instruments. A QC sample then was interspersed after every 7 samples from the participants. All samples were separated using an ACQUITY UPLC BEH C18 column (particle sizes 100 mm, 2.1 mm, and 1.7 μm) (Waters, Milford, Massachusetts, U.S.A.). The column temperature was maintained at 40°C and the flow rate at 0.3 mL/min (mobile phase A: water/0.1% formic acid; mobile phase B: acetonitrile). The gradient conditions were as follows: 0–0.5 min, 5% B; 0.5–1.0 min, 5% B; 1.0–9.0 min, 5–100% B; 9.0–12.0 min, 100% B; 12.0–15.0 min, 5% B. The sample injection volume was 5 μL.
Ion trapping was performed in both cation and anion modes, with a scanning range of 80 to 1200 m/z, a primary resolution of 70 000, and a secondary resolution of 17 500. The operating parameters in the positive mode were set as follows. The ion source temperature was 300°C, the ion transfer tube temperature was 320°C, the sheath gas flow rate was 40 arb, the auxiliary gas flow rate was 10 arb, and the spray voltage was 3.50 kV. In the negative mode, the conditions were an ion source temperature of 300°C, ion transfer tube temperature of 320°C, sheath gas flow rate of 38 arb, auxiliary gas flow rate of 10 arb, and spray voltage of 3.00 kV.
DATA PROCESSING AND STATISTICAL ANALYSIS:
Univariate statistical analysis was performed using SPSS Statistics 21 software (IBM, U.S.A.) Clinical information about the group with AAAs and the control group was expressed as a mean±SD, median (minimum-maximum), or percentage. The
The raw data collected through mass spectrometry analysis were processed with Compound Discoverer 3.0 software (Thermo Fisher Scientific, Waltham, Massachusetts, U.S.A.) to determine peak extraction, to compare peaks, and to perform peak analysis. SPSS Statistics 21 software was used to obtain P values for the processed dataset by doing an independent
MetaboAnalyst (
Results
PATIENT CHARACTERISTICS:
Table 1 summarizes patient demographics, including age, gender, body mass index, history of hypertension, alcohol consumption, smoking status, history of COPD, medication history (statins, aspirin, fibrates), and levels of triglycerides, total cholesterol, high-density lipoprotein-cholesterol (HDL-c), and low-density lipoprotein-cholesterol (LDL-c). The differences between these characteristics in the patients and the controls were not statistically significant. We conducted a PCA analysis for differential ions and the results showed that sex is not the main cause of metabolomic differences (Supplementary Figure 1).
METABOLIC PROFILES OF AAA:
A total of 2605 ion peaks were extracted in the cation mode and 2344 ion peaks were extracted in the anion mode (Figure 1). In the score plot of the PCA model, the metabolic profile of AAA was distinctly different between controls, with the majority of samples presenting within a 95% Hotelling T2 ellipse (Figure 2). The first 2 principal components (PCs) accounted for 16.5% and 5.3% of the total variability in the positive ion mode and the first 2 PCs accounted for 16.5% and 7.6% of the total variability in the negative ion mode. The QC samples were well aggregated, reflecting good stability of and repeatability with the instruments and methods used in this study. The OPLS-DA model was performed between the 2 groups to further identify the metabolites that differed between the AAA and control groups (Figure 3). The reliability of the model was identified by 7-fold cross-validation with 200-time permutation tests. The R2Y and Q2Y values were 0.993 and 0.977 in the electrospray ionization (ESI)+ mode and the R2Y and Q2Y values were 0.979 and 0.96 in the ESI- mode, which demonstrated acceptable goodness of fit and high-quality predictability. Given the importance of projection (VIP) value >1 and P<0.05, a total of 121 ion peaks (65 in positive mode and 56 in negative mode) differed between the 2 groups (Figure 4). Using molecular weight and mass spectrometry information, a search was conducted of the HMDB to identify metabolites with significant differences. Forty-five compounds that differed most between groups, including amino acids, phospholipids, and fatty acids, were selected based on their clinical and biological significance (Table 2). With pathway analysis, 5 vital metabolic pathways were identified: metabolism of alanine, aspartate, and glutamate; metabolism of D-glutamine and D-glutamic acid; cycling of tricarboxylic acid (TCA); metabolism of arginine and proline; and biosynthesis of arginine (Figure 5). The cluster analysis of 45 metabolites showed a distinction between patients with large (>5.5 cm) and small (<5.5 cm) AAAs (Figure 6). With univariate analysis, another 13 metabolites were identified that may affect the size of AAAs (P<0.05) (Figure 7).
Discussion
ENERGY METABOLISM:
Three key metabolites of citric, 2-oxoglutaric, and succinic acids were downregulated in AAAs and their levels were lower in large AAAs, which indicates that the production of energy (adenosine triphosphate [ATP]) in the TCA cycle was disturbed [14]. Succinate is a substrate of succinate dehydrogenase that can promote the dehydrogenation of succinate to fumarate. Succinate dehydrogenase (also called complex II) couples 2 major pathways in mitochondria, the TCA cycle and the respiratory chain. Succinic acid exhibits 2-sidedness in blood vessels. A low concentration of succinic acid can induce vasodilation, whereas a high concentration can induce vasoconstriction [15]. Coenzyme Q (CoQ) plays a crucial role in the production of mitochondrial energy and reactive oxygen. CoQ is considered to be an antioxidant, and lack of it can lead to an increase in mitochondrial reactive oxygen species [16]. This could suggest that AAAs have mitochondrial dysfunction.
LIPID METABOLISM:
Sphingosine 1-phosphate (S1P) is secreted by different blood cells and is taken up by HDL [17]. S1P interacts with various S1P receptors on the cell membrane. As a sensor to balance the inflammatory signal in the blood vessel wall, S1P stimulates S1P receptor-1 (S1PR1) in endothelial cells, which activates the endothelial chaperone downstream of Akt and promotes the production of NO [18,19]. On the other hand, S1PR1 activation inhibits the inflammatory activation of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1, which reduces monocyte recruitment [20]. The anti-inflammatory effect of S1P may have contributed to atheroprotection. The downregulation of S1P receptors in aortic tissues also promotes the formation of AAAs [21]. PAF is an important phospholipid mediator that can cause various platelet aggregation and inflammatory reactions and impair vascular endothelial function [22]. PAF is also related to the activity of metalloproteinases [23]. Platelet-activating factor-acetylhydrolase (PAF-AH) is a member of the phospholipase A2 enzyme superfamily and circulates in the blood together with plasma lipoprotein particles such as LDL and HDL, which can hydrolyze the acetyl group of PAF by converting PAF into hemolytic PAF [24]. We suspect that downregulation of PAF may be due to the higher level of PAF-AH activity in plasma of patients with AAAs than in healthy people [25]. In addition, the results of our study demonstrated that levels of LysoPC (16: 0/0: 0) and LysoPC (18: 2(9Z,12Z)/0: 0) decreased with the development of AAAs. LysoPC (16: 0) has been confirmed to be related to the inflammation and vulnerability of atherosclerotic plaque, which could affect the progression of AAAs [26,27].
AMINO ACID METABOLISM:
Abnormal glutamate metabolism and dysfunctional metabolism of arginine metabolism also may play a role in the development of AAAs. Arginine is the source of nitric oxide (NO). Arginine deficiency can lead to the development of atherosclerosis, based on the fact that it leads to impairment of NO synthesis and consequently affects the function of vascular endothelial cells [28]. However, the formation of AAAs may be related to excessive NO produced by vascular smooth muscle cells, which promotes the degradation of elastin and the destruction of the extracellular matrix [29,30]. Glutamate and glutamine are precursors of l-arginine and a substrate for ATP and protein synthesis. Dysfunction of glutamine metabolism can promote the development of cardiovascular diseases by facilitating unrestricted growth and migration of vascular cells and deposition of the extracellular matrix [31]. As a derivative of arginine, creatine levels increase, which is reflected in the reduced energy requirements of the tissue. High concentrations of phenylalanine may increase the risk of aortic disease because phenylalanine levels may be relevant to the activation of immunity and inflammation [32,33]. Proline is a component of collagen metabolites, and an increase in proline level may be related to the activation of collagen breakdown. The reduced amount of collagen makes the tissue more prone to rupture and dissection, which may accelerate AAA formation [33,34].
STUDY LIMITATIONS:
This article focused on identifying metabolites that differ between patients with AAAs and healthy individuals, based on untargeted plasma metabolomics, and discovering potential biomarkers that could affect the size of AAAs. Although we identified metabolites that differed in patients with large versus small AAAs, confirmation of our results in a small sample at a single center is required in an analysis of a large sample. Moreover, the precise relationship between these metabolites and AAAs is not yet clear. In future research, proteomics and genomics should be used in combination to evaluate the specific significance of each of the metabolites.
Conclusions
We used untargeted metabolomics of UPLC-MS to identify different metabolites associated with AAAs and which are related to different metabolic aspects of the condition. The metabolites we found may indicate that metabolism of energy, lipids, and amino acids in AAAs is disordered.
Figures
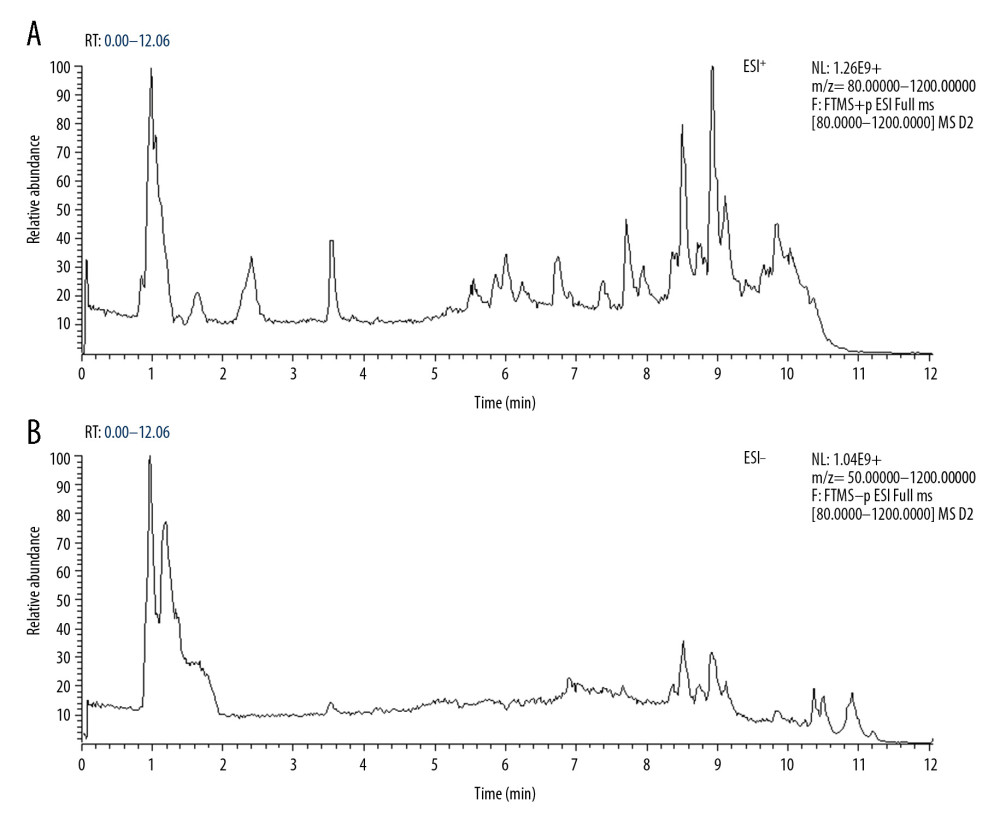 Figure 1. Total ion chromatography of plasma samples. (A) Electrospray ionization (ESI)+ mode. (B) ESI− mode.
Figure 1. Total ion chromatography of plasma samples. (A) Electrospray ionization (ESI)+ mode. (B) ESI− mode. 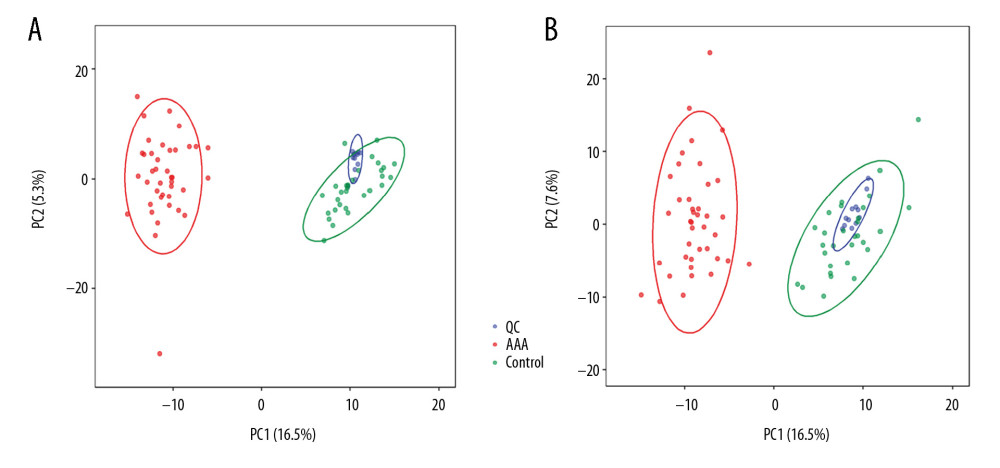 Figure 2. Principal component analysis score plot. (A) Electrospray ionization (ESI)+ mode. (B) ESI− mode.
Figure 2. Principal component analysis score plot. (A) Electrospray ionization (ESI)+ mode. (B) ESI− mode. 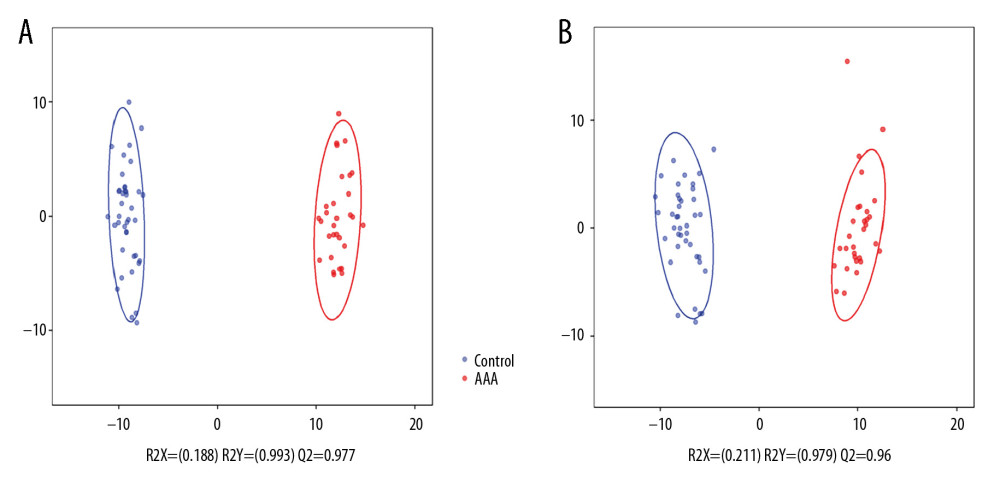 Figure 3. Orthogonal projection to latent squares-discriminant analysis model. (A) Electrospray ionization (ESI)+ mode. (B) ESI− mode.
Figure 3. Orthogonal projection to latent squares-discriminant analysis model. (A) Electrospray ionization (ESI)+ mode. (B) ESI− mode. 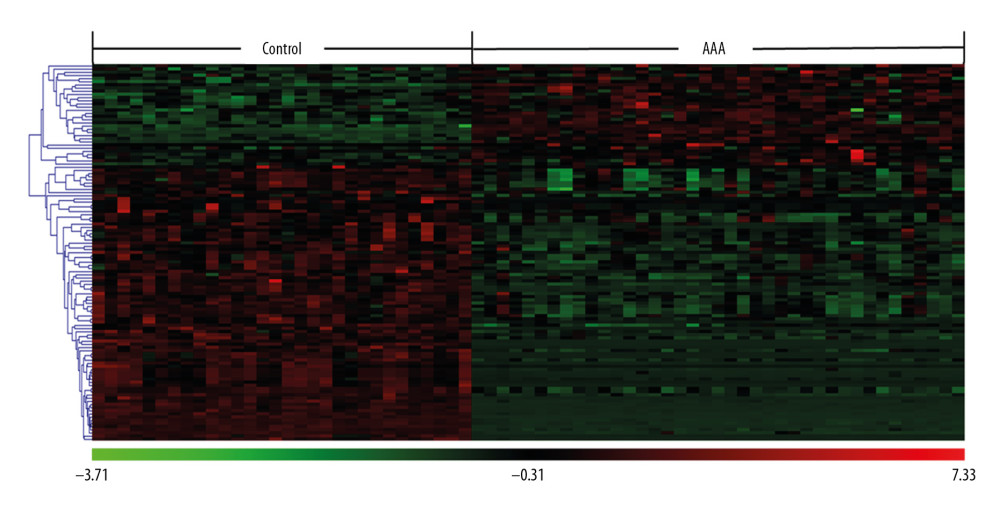 Figure 4. Clustering analysis of 121 differential ions. Rows represent differential ions and columns represent samples. Green denotes low intensity and red denotes high intensity.
Figure 4. Clustering analysis of 121 differential ions. Rows represent differential ions and columns represent samples. Green denotes low intensity and red denotes high intensity. 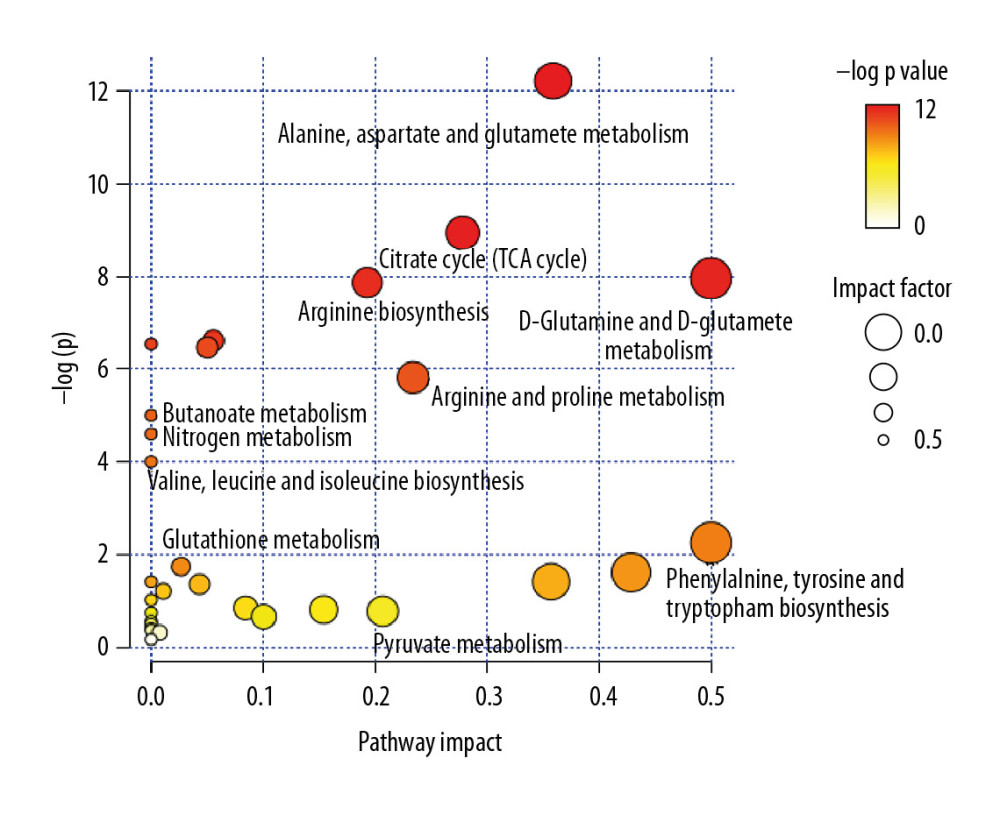 Figure 5. Pathway enrichment analysis of differential metabolites. The X axis represents the impact factor of the pathway topology analysis and the Y axis represents the P value of the pathway enrichment analysis (−log P value).
Figure 5. Pathway enrichment analysis of differential metabolites. The X axis represents the impact factor of the pathway topology analysis and the Y axis represents the P value of the pathway enrichment analysis (−log P value). 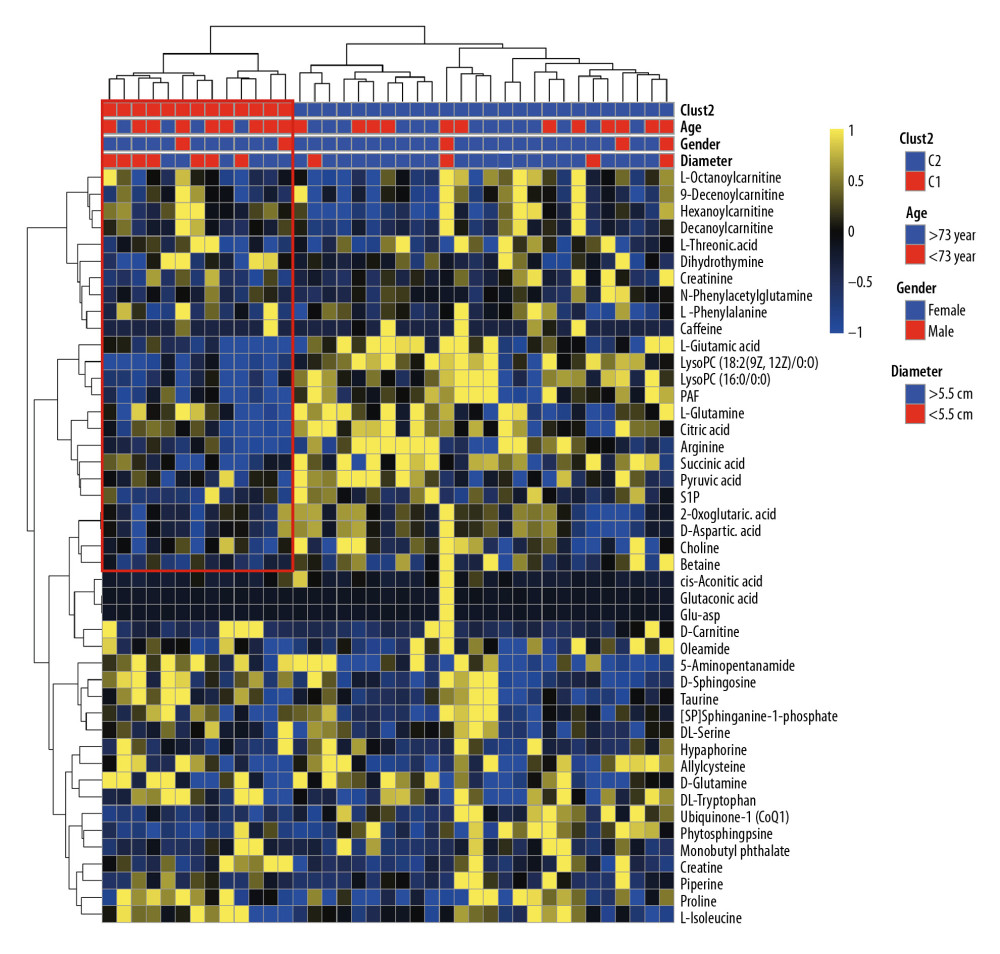 Figure 6. Cluster analysis of 45 differential metabolites in patients with abdominal aortic aneurysms. Rows represent differential metabolites, and columns represent samples.
Figure 6. Cluster analysis of 45 differential metabolites in patients with abdominal aortic aneurysms. Rows represent differential metabolites, and columns represent samples. 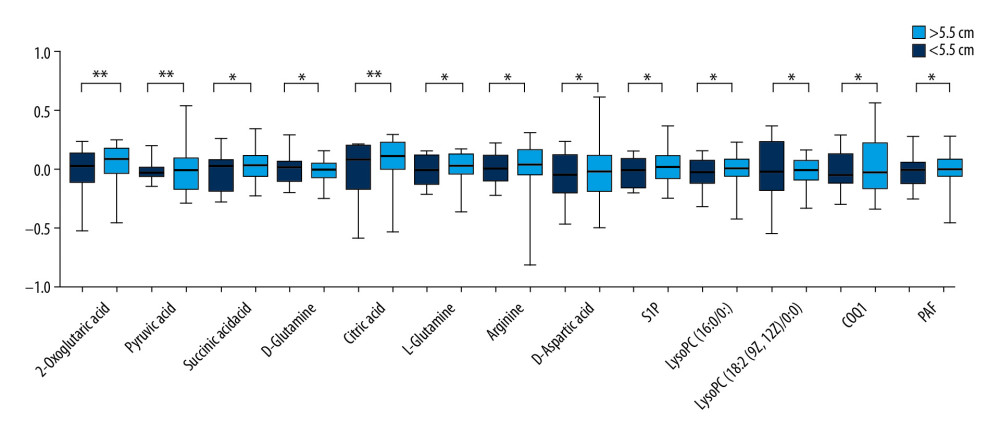 Figure 7. Difference in metabolites between large (>5.5 cm) and small (<5.5 cm) abdominal aortic aneurysms. * P<0.05 and ** P<0.01.
Figure 7. Difference in metabolites between large (>5.5 cm) and small (<5.5 cm) abdominal aortic aneurysms. * P<0.05 and ** P<0.01. References
1. Sakalihasan N, Limet R, Defawe OD, Abdominal aortic aneurysm: Lancet, 2005; 365; 1577-89
2. Weontraub NL, Understanding abdominal aortic aneurysm: N Engl J Med, 2009; 361; 1114-16
3. Tchana-Sato V, Sakalihasan N, Defraigne JORuptured abdominal aortic aneurysm: Rev Med Liege, 2018; 73; 296-99 [in French]
4. Wanhainen A, Verzini F, Van Herzeele I, Editor’s Choice – European Society for Vascular Surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms: Eur J Vasc Endovasc Surg, 2019; 57(1); 8-93
5. Li T, Jiang B, Li X, Serum matrix metalloproteinase-9 is a valuable biomarker for identification of abdominal and thoracic aortic aneurysm: A case-control study: BMC Cardiovasc Disord, 2018; 18; 202
6. Wang Y, Liu CL, Lindholt JS, Plasma Cystatin B association with abdominal aortic aneurysms and need for later surgical repair: A sub-study of the VIVA Trial: Eur J Vasc Endovasc Surg, 2018; 56; 826-32
7. Sundermann AC, Saum K, Conrad KA, Prognostic value of D-dimer and markers of coagulation for stratification of abdominal aortic aneurysm growth: Blood Adv, 2018; 2; 3088-96
8. Clish CB, Metabolomics: An emerging but powerful tool for precision medicine: Cold Spring Harb Mol Case Stud, 2015; 1; a000588
9. Zhang A, Sun H, Yan G, Metabolomics for biomarker discovery: Moving to the clinic: Biomed Res Int, 2015; 2015 354671
10. Pan Z, Cui H, Wu N, Zhang H, Effect of statin therapy on abdominal aortic aneurysm growth rate and mortality: A systematic review and meta-analysis: Ann Vasc Surg, 2020; 67; 503-10
11. Takagi H, Umemoto TALICE (All-Literature Investigation of Cardiovascular Evidence) Group, A Meta-analysis of the association of chronic obstructive pulmonary disease with abdominal aortic aneurysm presence: Ann Vasc Surg, 2016; 34; 84-94
12. Ruperez FJ, Ramos-Mozo P, Teul J, Metabolomic study of plasma of patients with abdominal aortic aneurysm: Anal Bioanal Chem, 2012; 403(6); 1651-60
13. Ciborowski M, Teul J, Martin-Ventura JL, Metabolomics with LC-QTOF-MS permits the prediction of disease stage in aortic abdominal aneurysm based on plasma metabolic fingerprint: PLoS One, 2012; 7(2); e31982
14. Fischer K, Kettunen J, Wurtz P, Biomarker profiling by nuclear magnetic resonance spectroscopy for the prediction of all-cause mortality: An observational study of 17,345 persons: PLoS Med, 2014; 11; e1001606
15. Vale GT, Carballido JM, Alves-filho JC, Tirapelli CR, Pharmacological characterization of the mechanisms underlying the vascular effects of succinate: Eur J Pharmacol, 2016; 789; 334-43
16. Di Lorenzo A, Iannuzzo G, Parlato A, Clinical evidence for Q10 coenzyme supplementation in heart failure: From energetics to functional improvement: J Clin Med, 2020; 9; 1266
17. Schuchardt M, Tolle M, Prufer J, van der Giet M, Pharmacological relevance and potential of sphingosine 1-phosphate in the vascular system: Br J Pharmacol, 2011; 163; 1140-62
18. Krishna SM, Seto SW, Moxon JV, Fenofibrate increases high-density lipoprotein and sphingosine 1 phosphate concentrations limiting abdominal aortic aneurysm progression in a mouse model: Am J Pathol, 2012; 181; 706-18
19. Siedlinski M, Nosalski R, Szczepaniak P, Vascular transcriptome profiling identifies Sphingosine kinase 1 as a modulator of angiotensin II-induced vascular dysfunction: Sci Rep, 2017; 7; 44131
20. Kimura T, Tomura H, Mogi C, Role of scavenger receptor class B type I and sphingosine 1-phosphate receptors in high density lipoprotein-induced inhibition of adhesion molecule expression in endothelial cells: J Biol Chem, 2006; 281(49); 37457-67
21. Qu Z, Cheuk BL, Cheng SW, Differential expression of sphingosine-1-phosphate receptors in abdominal aortic aneurysms: Mediators Inflamm, 2012; 2012 643609
22. Tsoupras A, Lordan R, Zabetakis I, Inflammation, not cholesterol, is a cause of chronic disease: Nutrients, 2018; 10(5); 604
23. Kim YH, Lee SJ, Seo KW, PAF enhances MMP-2 production in rat aortic VSMCs via a beta-arrestin2-dependent ERK signaling pathway: J Lipid Res, 2013; 54(10); 2678-86
24. Karasawa K, Inoue K, Overview of PAF-degrading enzymes: Enzymes, 2015; 38; 1-22
25. Unno N, Nakamura T, Mitsuoka H, Association of a G994 T Missense mutation in the plasma platelet-activating factor acetylhydrolase gene with risk of abdominal aortic aneurysm in Japanese: Ann Surg, 2001; 235(2); 297-302
26. Gonçalves I, Edsfeldt A, Ko NY, Evidence supporting a key role of Lp-PLA2-generated lysophosphatidylcholine in human atherosclerotic plaque inflammation: Arterioscler Thromb Vasc Biol, 2012; 32(6); 1505-12
27. Han JS, Kim K, Jung Y, Metabolic Alterations associated with atorvastatin/fenofibric acid combination in patients with atherogenic dyslipidaemia: A randomized trial for comparison with escalated-dose atorvastatin: Sci Rep, 2018; 8(1); 14642
28. Wu G, Morris SM, Arginine metabolism: Nitric oxide and beyond: Biochem J, 1998; 336; 1-17
29. Johanning JM, Armstrong PJ, Franklin DP, Nitric oxide in experimental aneurysm formation: Early events and consequences of nitric oxide inhibition: Ann Vasc Surg, 2002; 16; 65-72
30. Farrell K, Simmers P, Mahajan G, Alterations in phenotype and gene expression of adult human aneurysmal smooth muscle cells by exogenous nitric oxide: Exp Cell Res, 2019; 384; 111589
31. Chen J, Zhang S, Wu J, Essential role of nonessential amino acid glutamine in atherosclerotic cardiovascular disease: DNA Cell Biol, 2020; 39; 8-15
32. Welsh P, Rankin N, Li Q, Circulating amino acids and the risk of macrovascular, microvascular and mortality outcomes in individuals with type 2 diabetes: Results from the ADVANCE trial: Diabetologia, 2018; 61; 1581-91
33. Murr C, Grammer TB, Meinitzer A, Immune activation and inflammation in patients with cardiovascular disease are associated with higher phenylalanine to tyrosine ratios: The Ludwigshafen risk and cardiovascular health study: J Amino Acids, 2014; 2014 783730
34. Latorre M, Bersi MR, Humphrey JD, Computational modeling predicts immuno-mechanical mechanisms of maladaptive aortic remodeling in hypertension: Int J Eng Sci, 2019; 141; 35-46
Figures
 Figure 1. Total ion chromatography of plasma samples. (A) Electrospray ionization (ESI)+ mode. (B) ESI− mode.
Figure 1. Total ion chromatography of plasma samples. (A) Electrospray ionization (ESI)+ mode. (B) ESI− mode. Figure 2. Principal component analysis score plot. (A) Electrospray ionization (ESI)+ mode. (B) ESI− mode.
Figure 2. Principal component analysis score plot. (A) Electrospray ionization (ESI)+ mode. (B) ESI− mode. Figure 3. Orthogonal projection to latent squares-discriminant analysis model. (A) Electrospray ionization (ESI)+ mode. (B) ESI− mode.
Figure 3. Orthogonal projection to latent squares-discriminant analysis model. (A) Electrospray ionization (ESI)+ mode. (B) ESI− mode. Figure 4. Clustering analysis of 121 differential ions. Rows represent differential ions and columns represent samples. Green denotes low intensity and red denotes high intensity.
Figure 4. Clustering analysis of 121 differential ions. Rows represent differential ions and columns represent samples. Green denotes low intensity and red denotes high intensity. Figure 5. Pathway enrichment analysis of differential metabolites. The X axis represents the impact factor of the pathway topology analysis and the Y axis represents the P value of the pathway enrichment analysis (−log P value).
Figure 5. Pathway enrichment analysis of differential metabolites. The X axis represents the impact factor of the pathway topology analysis and the Y axis represents the P value of the pathway enrichment analysis (−log P value). Figure 6. Cluster analysis of 45 differential metabolites in patients with abdominal aortic aneurysms. Rows represent differential metabolites, and columns represent samples.
Figure 6. Cluster analysis of 45 differential metabolites in patients with abdominal aortic aneurysms. Rows represent differential metabolites, and columns represent samples. Figure 7. Difference in metabolites between large (>5.5 cm) and small (<5.5 cm) abdominal aortic aneurysms. * P<0.05 and ** P<0.01.
Figure 7. Difference in metabolites between large (>5.5 cm) and small (<5.5 cm) abdominal aortic aneurysms. * P<0.05 and ** P<0.01. Tables
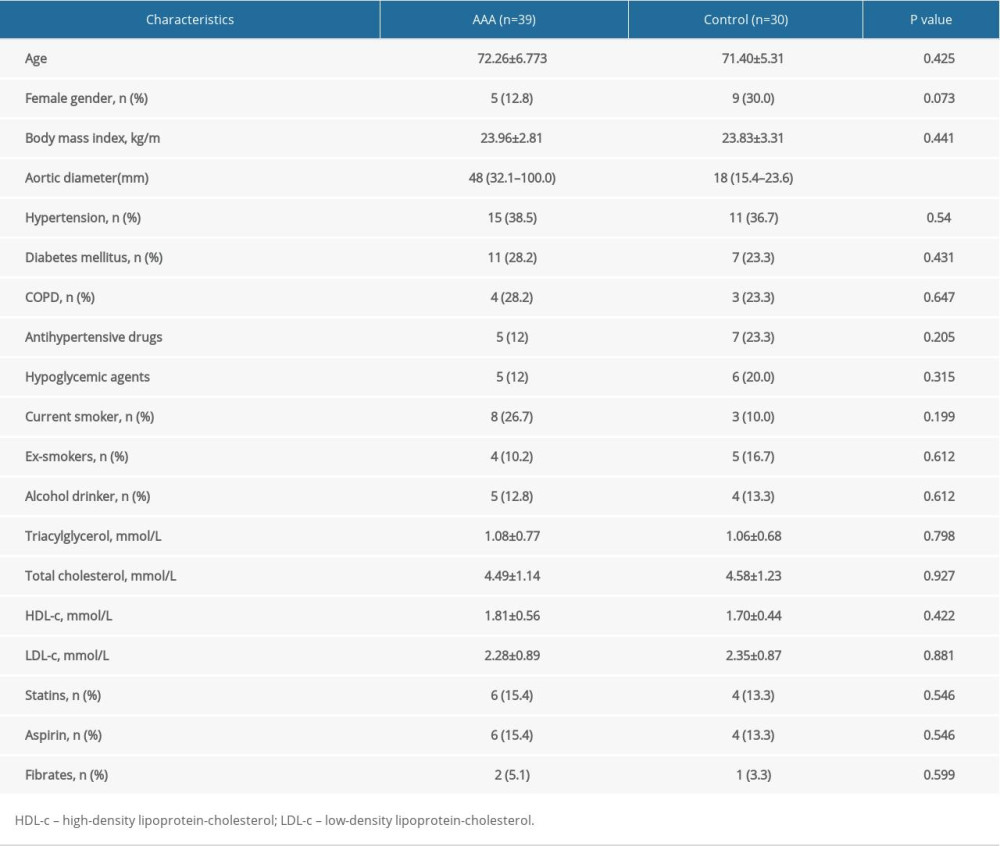 Table 1. Clinical and demographic characteristics of patients with abdominal aortic aneurysms and controls.
Table 1. Clinical and demographic characteristics of patients with abdominal aortic aneurysms and controls.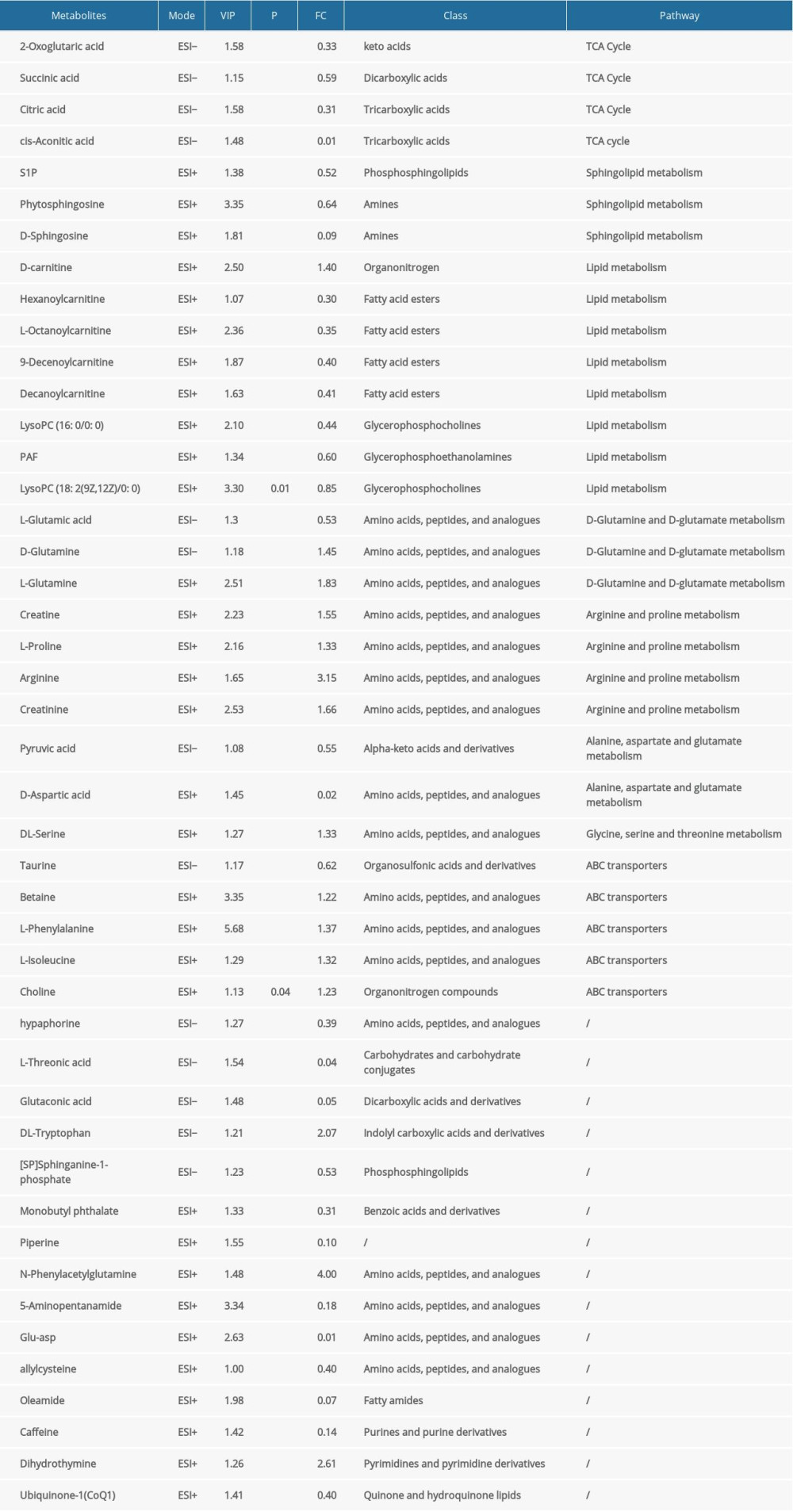 Table 2. Results of identification of differential metabolites.
Table 2. Results of identification of differential metabolites. Table 1. Clinical and demographic characteristics of patients with abdominal aortic aneurysms and controls.
Table 1. Clinical and demographic characteristics of patients with abdominal aortic aneurysms and controls. Table 2. Results of identification of differential metabolites.
Table 2. Results of identification of differential metabolites. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








