15 January 2021: Review Articles
Alterations in Cerebral Hemodynamics During Microgravity: A Literature Review
Jichen Du1ADE*, Jiangbo Cui2BC, Jing Yang1D, Peifu Wang1B, Lvming Zhang1D, Bin Luo1BDF, Bailin Han1EDOI: 10.12659/MSM.928108
Med Sci Monit 2021; 27:e928108
Abstract
ABSTRACT: Most reported neurological symptoms that happen after exposure to microgravity could be originated from alterations in cerebral hemodynamics. The complicated mechanisms involved in the process of hemodynamics and the disparate experimental protocols designed to study the process may have contributed to the discrepancies in results between studies and the lack of consensus among researchers. This literature review examines spaceflight and ground-based studies of cerebral hemodynamics and aims to summarize the underlying physiological mechanisms that are altered in cerebral hemodynamics during microgravity. We reviewed studies that were published before July 2020 and sought to provide a comprehensive summary of the physiological or pathological theories of hemodynamics and to arrive at firm conclusions from incongruous results that were reported in those related articles. We give plausible explanations of inconsistent results on factors including intracranial pressure, cerebral blood flow, and cerebrovascular autoregulation. Although there are no definitive data to confirm how cerebral hemodynamics changes during microgravity, every discrepancy in results was interpreted by existing theories, which were derived from physiological and pathological processes. We conclude that microgravity-induced alterations of hemodynamics at the brain level are multifaceted. Factors including duration, partial pressures of carbon dioxide, and individual adaptability contribute to this process and are unpredictable. With a growing understanding of this hemodynamics model, additional factors will likely be considered. Aiming for a full understanding of the physiological and/or pathological changes of hemodynamics will enable researchers to investigate its cellular and molecular mechanisms in future studies, which are desperately needed.
Keywords: cerebral hemodynamics, Intracranial Pressure, Neurology, Cerebrovascular Circulation, Hemodynamics, Weightlessness
Background
It is an increasingly accepted concept that spaceflight negatively impacts human physiology with adverse effects on every organ or system, which are mainly due to the effects of microgravity, space radiation, lack of movement, and circadian rhythm disturbance [1]. In particular, microgravity might lead to the disruption of hydrostatic pressure gradients which normally exist on Earth due to the effects of gravity, and consequently, head-ward fluid shift is deemed as a primary contributor to altered hemodynamics [2]. These phenomena might formally represent the prime initiating factors that account for observed neurologic symptoms including post-flight headache, dizziness, syncope, impaired ability to execute cognitive functions, and the appearance of visual impairment and intracranial pressure (VIIP) syndrome [3–6]. With the booming development of manned spaceflight projects, the resolution of these problems in the has rapidly become an urgent priority. In this article, we review studies that were published before July 2020 to provide a comprehensive summary of the physiological or pathological theories of hemodynamics and to arrive at firm conclusions from the incongruous results that were reported in those related articles.
Ground-based Analogues for Microgravity
HEAD-DOWN BED REST:
The head-down bed rest (HDBR) position has been used as a ground-based spaceflight analogue for decades to simulate the effects of microgravity on various physiological systems [7]. During HDBR, a participant lies in a bed that is inclined with the head down at various tilt angles including −6°, −12°, and −18°; an angle of −6° has been applied in most cases. This analogue can reproduce changes seen in spaceflight, such as a cephalic fluid shift, confinement, and feelings of isolation. However, since gravitational and vestibular input remains, it cannot reproduce actual microgravity. In addition, the different tilt angles might have different physiological effects; for example, in a study with long-duration HDBR, bed rest at an angle of −6° failed to reproduce ophthalmic findings similar to those seen in VIIP syndrome [8].
DRY IMMERSION:
Dry immersion is an experimental method in which a participant equipped with a waterproof fabric is immersed in thermoneutral water, while making no direct contact with the water. This is an adequate spaceflight alternative since it mimics several spaceflight features, including confinement and body fluid shift. Dry immersion also removes afferent signals from the support zones and triggers a drop in the activity of the postural muscle system. As an adequate analogue for microgravity, this model has been well described [9].
PARABOLIC FLIGHT:
During a parabolic flight, a specific flight trajectory is carried out in an airplane, in which the normo-, hyper-, and microgravity phases are experienced. In this manually operated process, the acceleration speed can be adjusted accordingly. The duration of microgravity in each parabola is controlled for around 20 s, and the aircraft usually flies 15 to 30 parabolas during a single mission, which makes it the only ground-based analogue for actual microgravity. However, during each parabola, there is only approximately 20 s of microgravity, and prior to experiencing microgravity, there is always a period of hypergravity that must be met, which might disrupt the quality and integrity of the data. Further information about parabolic flight has been previously described [10].
Because each analogue has its own advantages, the fidelity of the simulated effect on specific organs or physiological processes varies from one analogue to the next [11]. It is necessary to choose an appropriate analogue to simulate microgravity based on the specific objectives an experiment is trying to achieve. However, although a well-founded ground-based simulation is a key factor worthy of investigation, we admit that we cannot fully simulate spaceflight with ground-based simulation, particularly when the laws of physics and principles of sociology are taken into consideration.
Cerebral Hemodynamics
INTRACRANIAL PRESSURE:
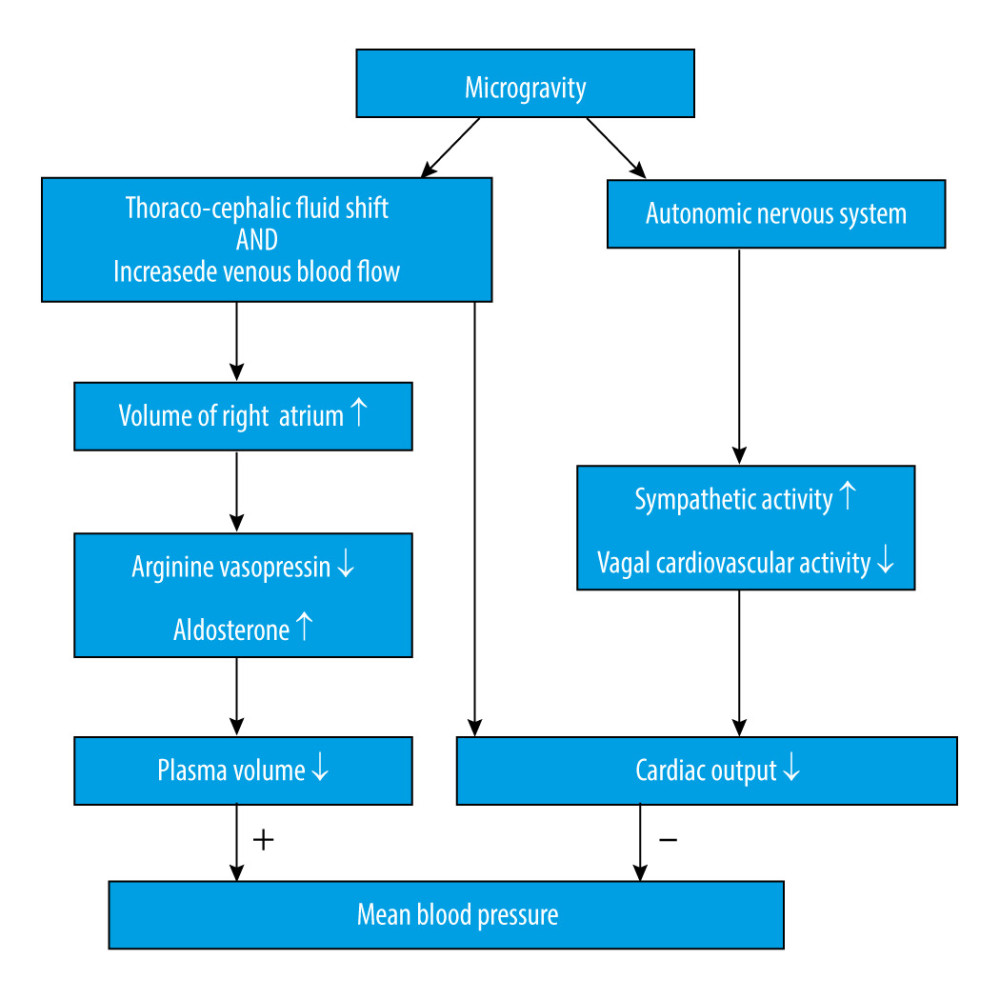
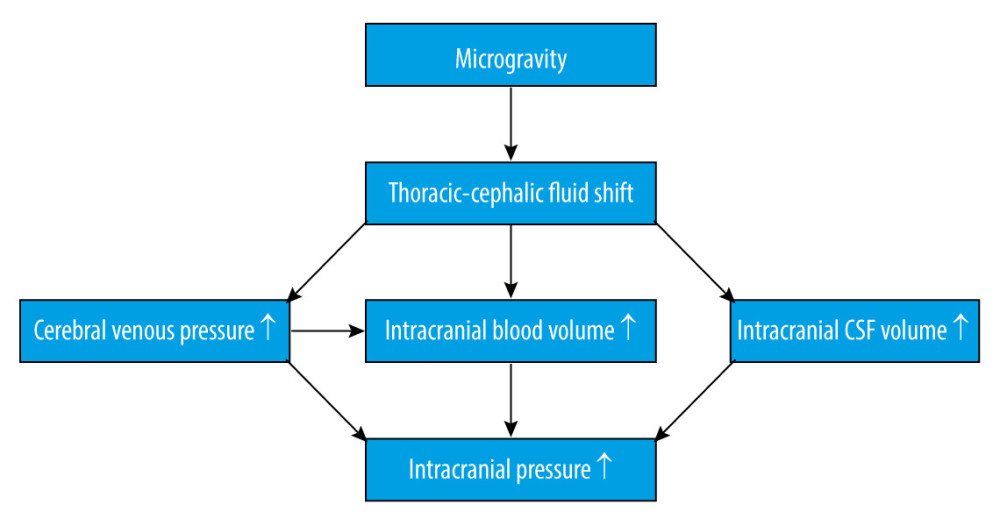
It is widely known that cephalic fluid redistribution causes blood pooling within the brain during microgravity, which most likely causes an increase in intracranial pressure (ICP). Theoretically, the shift of fluid to the upper part of the body leads to increased intracellular and interstitial fluid volume. The subsequent lack of hydrostatic gradients associated with gravity might increase the permeability of the blood-brain barrier [12], combining increased blood flow in the cerebral arteries, disturbance in cerebrospinal fluid circulation, and missing drainage from the blood (Figure 1). Although microgravity induces a mild decrement in plasma volume [13], which could counteract the factors increasing ICP, one study showed that mildly decreased plasma volume caused by the administration of 0.2 mg/kg of furosemide did not change ICP [14]. Collectively, these factors will eventually lead to an increased ICP. An elevated ICP after exposure to microgravity has been proposed as a mechanism for the initiation of VIIP syndrome [15]. Moreover, perineural transmission of increased ICP pulsatility might be important in the pathogenesis of orbital abnormalities [16]. Considering ICP as a pertinent element, it is important for us to understand the homeostatic and post-flight physiology associated with ICP.
ICP has both pulsatile and static components. Pulsatile ICP is created by oscillation of the cerebral blood flow (CBF) and is modulated by passive downstream venous and cerebrospinal fluid volume outflow. Meanwhile, static ICP represents a low-pass-filtered pressure, which is actively modulated by the cerebrospinal fluid formation rate. Together, the passive and active modulation of ICP are components of the compensatory reserve system. With progressive reduction of the compensatory reserve capacity, craniospinal compliance, which is defined as the ratio of volume change to pressure change, could become insufficient, resulting in exponential increases in ICP with additional volume.
Kermorgant et al. used dry immersion as an approach to mimic microgravity and showed that it could significantly induce optic nerve sheath diameter enlargement during an experiment in days 1 and 3 and in recovery period day 1 [17], which indicated that a short-term exposure to microgravity could lead to increased ICP. Mader et al. found 4 astronauts who presented with optic disc edema after a 6-month spaceflight with higher ICP [3]. Another study was performed on the Russian Kosmos-2229 biosatellite with a Macaque monkey on a 9-day spaceflight [18], which showed a circadian pattern with observed changes in ICP or the ICP wave. In addition, ICP may achieve physiological homeostasis during a flight. According to Marshall-Goebel et al. [19], this result could also be specifically interpreted as follows: reduced system compliance in the first days of spaceflight appeared to magnify the effect of the fluid shift, which resulted in elevated ICP; however, starting on day 5, system compliance normalized, which resulted in a reduced ICP that approximated baseline values (although still slightly elevated).
Lawley et al. directly measured ICP in human participants during parabolic flights that simulated microgravity for the first time [20]. Their findings revealed that the complete removal of gravity does not pathologically elevate ICP, but it does prevent the normal lowering of ICP. From this they proposed that the human brain is protected by daily circadian cycles in regional ICPs, without which, pathology may occur.
In most studies (Table 1), regardless of the model that was used to create microgravity or the measurement that was taken, we conclude that ICP in short-term exposure to microgravity remained unchanged, partially owing to limited sample size and individual variability. However, ICP in long-term exposure to microgravity conditions tends to be somewhat elevated. One of the elementary factors that might account for elevated ICP could be the predominant influence of the cephalic fluid shift. We noticed that another pivotal role for regulating ICP could be in the context of the circadian rhythm, which remains a missing part in all simulated microgravity analogues. Circadian rhythm is always taken into account when considering elevated intraocular pressure (IOP), and it is our current understanding that IOP might provoke some impairment in distant or near visual acuity [3,21]. However, an increase in ICP during microgravity could occur through several mechanisms, including differences in arterial blood flow, venous drainage, vasculature, and cerebrovascular autoregulation. Next, we will introduce some recent researches to delve into these phenomena.
CEREBRAL CIRCULATION AND VASCULATURE:
Numerous factors are involved in the regulation of CBF or perfusion [22], such as arterial blood pressure, cerebrovascular autoregulation, endothelial function, and regional metabolic status. And many of these factors might be altered in a microgravity environment. While this is also related to fluid flow, Ohm’s law can be used to estimate the effects of microgravity on brain blood flow by the following equation: CBF=(Pa–Pv)/CVR, in which CBF is proportional to the difference between arterial pressure (Pa) and venous pressure (Pv) across the brain, and CBF is inversely related to the cerebral vascular resistance (CVR), which is also referred to as the reciprocal of cerebral vascular conductance (CVC). The loss of gravity vector induces a cephalic fluid shift that influences hydrostatic pressure at the level of the head, which is believed to increase from 70 mmHg to 100 mmHg [23]. It is thought that the Pa would theoretically increase by approximately 30 mmHg; however, since there are multiple physiological mechanisms involved in blood pressure (Figure 2) [24], which, as a whole, impose a bidirectional influence, it is still unknown whether Pa would increase or decrease [25]. Well-rounded reviews of cardiovascular physiology during microgravity have been previously described [23,25].
Next, we focused on alterations in CBF and cerebral vasculature that were found in recent studies, hoping to elucidate the interpretation of outcomes of cerebral hemodynamics.
CEREBRAL BLOOD FLOW:
As one of the most elementary factors, CBF has been frequently tested and used as a representation of cerebral circulation during or after microgravity. In addition, CBF accounts for the metabolism in the brain since it contains and traffics most of the oxygen and nutrition the brain needs. To some extent, an altered CBF might explain the cognitive impairment or orthostatic intolerance that is known to occur after spaceflight. However, study results from spaceflight or ground-based analogues of spaceflight have revealed increased [26], decreased [27,28], or unaltered [29] CBF velocity (CBFV). In addition, some studies showed that CBFV is a dynamic value during microgravity, which would increase initially, and then return to baseline as the mission was prolonged [30]. These data are mostly measured by transcranial Doppler ultrasonography on a single side (usually the right side) of the middle cerebral artery (MCA), thereby leaving out the entire posterior circulation and representing only partial anterior cerebral circulation. Furthermore, considering each cerebral hemisphere preferentially controls specific physiological function, it is conceivable that there is a different status of cerebral circulation between the right and left hemispheres. Moreover, CBFV could only partially interpret CBF, owing to the differential vessel diameters in the vasculature. Therefore, we should be cautious when explaining these results.
A study using parabolic flight to simulate microgravity found that MCA velocity remained unchanged throughout the experiment; however, the CVC index (the quotient of MCA velocity and mean arterial pressure) was increased. This observation could be explained by an increased cardiac output and a decreased mean arterial pressure, which were recorded during the experiment [31]. Because of the structural and functional differences in each cerebral artery, researchers recently began focusing on CBF in the different cerebral arterial branches, such as the common carotid artery, internal carotid artery (ICA), external carotid artery (ECA), and vertebral artery (VA). A study by Ogoh et al. using dry immersion showed that after short-term weightlessness exposure, the CBF in each cerebral artery (ICA, ECA, and VA) was unchanged during the dry immersion [32]. To prevent ICP or excessive cerebral perfusion pressure, self-protection reserves successfully controlled the CBF by decreasing cerebral conductance, perhaps by way of cerebrovascular autoregulation. Another intriguing finding is the decreased cardiac output associated with changes in ICA and VA blood flow but not with changes in ECA blood flow [32], indicating that the contributions of those mechanisms involved in CBF regulation vary with different cerebral vascularity. However, a study conducted by Marshall-Goebel et al. using magnetic resonance imaging with the HDT model found that arterial blood flow velocity decreased in ICA but remained unchanged in VA. Changes in cross-sectional area revealed that arterial vasodilation occurred in the ICAs but not in the VAs [33]. Furthermore, a 2-month HDBR study demonstrated that the posterior arterial blood flow was well maintained; although that of the anterior cerebral circulation was reduced on day 30, and then recovered to the baseline level [34].
These findings (Table 2) indicated that irrespective of a short- or long-term exposure to microgravity, the major arteries of the brain would have a heterogeneous response. This might be attributable to different physiological roles between different areas of the brain, further suggesting that results from the anterior circulation do not reflect the entire cerebral circulation. Considering that both known and unknown factors could easily change CBF, an unpredictable result for CBF is acceptable. Thus, only by taking full control of all known factors, can we derive a more plausible result.
CEREBRAL VASCULATURE:
Prolonged exposure to microgravity removes the head-to-foot gravitational vector, causing cephalic fluid shift, and cerebrovascular resistance must increase to compensate for the acute elevation of perfusion pressure in microgravity. These factors make it easy to speculate that the property of the vasculature above the heart would be changed [23]. As an important myogenic mechanism involved in cerebrovascular autoregulation, alterations in cerebral vasculature would affect cerebral circulation, leading to neurological symptoms. However, because there are a myriad of determinants taking part in the regulation of cerebral vasculature, confirmed, like endothelial nitric oxide mechanism [35], and unconfirmed, we will next look at those mechanisms involved in the adaptation or maladaptation of cerebral vasculature.
Elevated cerebral arterial pressure results in the increased intrinsic vasoconstrictor responsiveness of the cerebral arteries [36] and the remodelling of the cerebral arterial vasculature, which is characterized by the thickening of the medial smooth muscle cell layer, and, in some cerebral arteries, results in a decreased maximal intraluminal diameter [35,37]. It is noteworthy that it has not yet been determined whether stiffness, myogenic tone, and vasoconstriction of the cerebral artery increases or decreases, or whether its structural property changes. Hindlimb unweighting (HU) in a rat model resulted in an increase in the myogenic tone of the cerebral middle artery, and its cerebral arteries were less distensible due to the nitric oxide-dependent and -independent mechanisms modulating the myogenic reactivity of the cerebral arteries in the HU-induced animals. In addition, maximum passive diameters were unchanged [38]. Another study showed completely different results with a reduction in both myogenic vasoconstriction and stiffness and an increase in the maximal diameter of cerebral arteries after 13 days of spaceflight [39]. These studies used different animals (rats vs. mice), different conditions (HU vs. spaceflight) and different cerebral arteries (cerebral middle artery vs. basilar and posterior communicating arteries), which may explain the discrepancy.
To delve further into the above scenarios, a previous study showed that the myogenic stretch-sensing mechanism and non-receptor voltage-gated Ca2+-channel mechanism impaired cerebral arterial smooth muscle contraction, both functioning in part via the RhoA/Rho-kinase signalling pathway [40]. Many cardiovascular functions exhibit circadian rhythm, and Li et al. found that 28 days of HU disrupted intrinsic diurnal oscillation in rat cerebrovascular contractility by altering circadian regulation of the BMAL1/miR-103/CaV1.2 signalling pathway [41]. To gain a better understanding of these cerebrovascular properties, it is imperative to clarify these complex mechanisms.
CEREBROVASCULAR AUTOREGULATION:
Cerebrovascular autoregulation, with the assistance of a variety of intrinsic mechanisms and neural reflexes, is a regulative process in which vascular resistance is controlled with the aim of maintaining CBF to stabilize fluctuating cerebral perfusion pressure [42,43]. This indicates that the brain is capable of sustaining an appropriate blood flow to maintain a homeostatic environment [44,45]. Moreover, cerebrovascular autoregulation is a combination of 2 different situations: static cerebrovascular autoregulation and dynamic cerebrovascular autoregulation, where static cerebrovascular autoregulation is the quantification of the steady-state change in CBF in relation to steady-state changes in arterial blood pressure, and dynamic cerebrovascular autoregulation characterizes the CBF response to abrupt changes in arterial blood pressure or the interplay between spontaneous arterial blood pressure oscillations and CBF velocity oscillations [42]. The static one reflects the global efficiency of the cerebrovascular autoregulation but does not take into account the latency, while the dynamic one reflects the latency and efficiency of cerebrovascular autoregulation [46]. With the development of transcranial Doppler ultrasonography recording instant changes in CBF, more experiments tend to focus on dynamic cerebrovascular autoregulation.
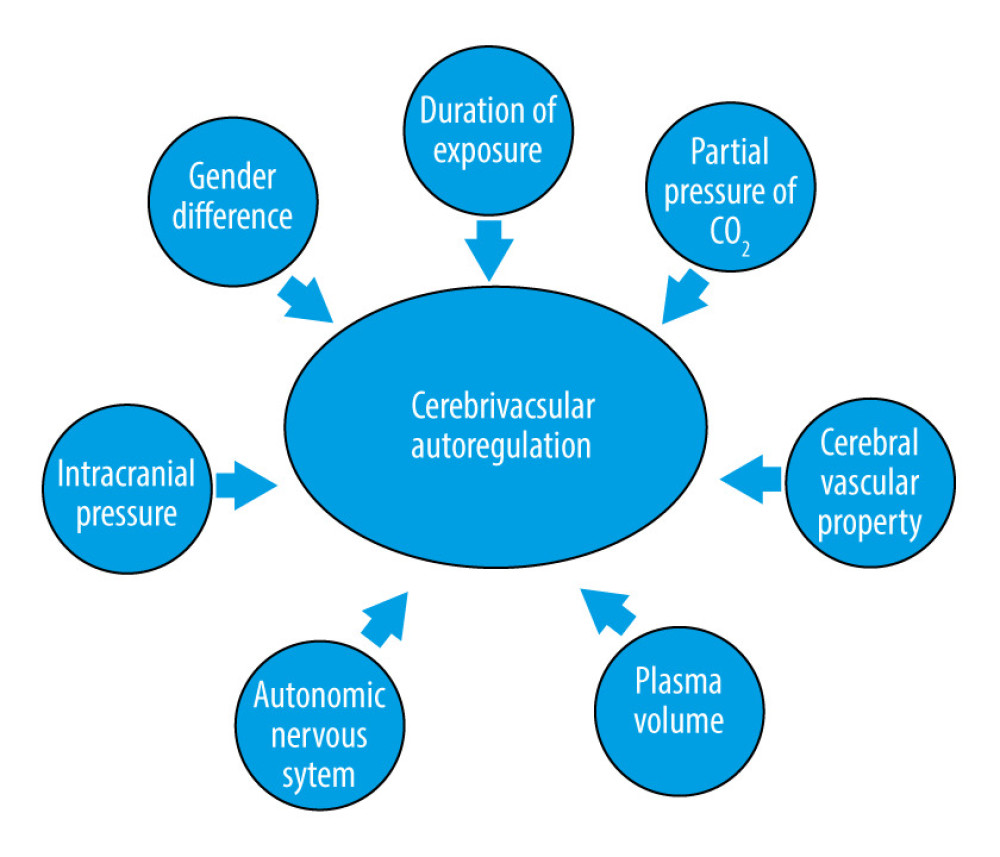
Similar to CBF, there are also many factors that might affect cerebrovascular autoregulation (Figure 3), for example, duration of exposure to microgravity, intracranial pressure [17,47], autonomic nervous system [48], plasma volume [49,50], cerebral vascular tone and property [29], partial pressure of CO2 [51], and even sex differences [52]; although, there is no conclusive evidence showing that the duration of exposure to microgravity would affect cerebrovascular autoregulation. However, people tend to suffer orthostatic intolerance during long-term microgravity, and an association between orthostatic intolerance and impaired cerebrovascular autoregulation has been found [53,54].
Currently, no concurring results of cerebrovascular autoregulation have been reached (Table 3). Dynamic cerebrovascular autoregulation was improved after 21 days of HDBR by reducing plasma volume [55], which was observed during a short-term study [49]. A study of optic nerve sheath diameter and cerebral autoregulation revealed a strong negative correlation between these factors, suggesting that a persistently elevated ICP can be attributed to poor cerebral autoregulation recovery [17]. Therefore, to achieve a convincing result, the above factors should be closely monitored in future studies.
Current Difficulties
Because exposure to microgravity is a prolonged experience, a subsequent altered vasculature, due to either cerebrovascular autoregulation or physiological adaptation, gradually develops to an irreversible and deep-rooted state, which would eventually reach a state of space-type equilibrium. Physiological alterations due to microgravity are more complicated than is apparent from this discussion and include cerebral metabolic demands, partial pressures of O2 and CO2, and the interplay of the autonomic nervous system; all of which were not fully discussed in this review [56]. In addition, although previous studies that were based on simulated or actual microgravity focused partially on the relationship between cells and blood flow in the brain, we have recognized another major part of the nervous system, which is the extracellular space and glymphatic pathway. The importance of extracellular space and the glymphatic pathway has been gradually acknowledged in recent years [57], and they could play pivotal roles in the pathways that regulate hydrodynamics, hemodynamics, and metabolic homeostasis.
Conclusions
To conclude, microgravity-induced alterations of hemodynamics at the brain level are multifaceted. In addition, other factors including duration, partial pressures of carbon dioxide, and individual adaptability contribute to this process, which could be metaphorically described as an unpredictable see-saw model. With a growing understanding of this model, additional factors will likely be considered and added to this equation. Aiming for a full understanding of the physiological and/or pathological changes of hemodynamics will enable researchers to investigate its related cellular and molecular mechanisms in future studies, which are desperately needed.
References
1. Williams D, Kuipers A, Mukai C, Thirsk R, Acclimation during space flight: effects on human physiology: CMAJ, 2009; 180(13); 1317-23
2. Kawai Y, Doi M, Setogawa A, Effects of microgravity on cerebral hemodynamics: Yonago Acta Medica, 2003; 46(1); 1-8
3. Mader TH, Gibson CR, Pass AF, Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight: Ophthalmology, 2011; 118(10); 2058-69
4. Blaber AP, Goswami N, Bondar RL, Kassam MS, Impairment of cerebral blood flow regulation in astronauts with orthostatic intolerance after flight: Stroke, 2011; 42(7); 1844-50
5. Goswami N, Batzel JJ, Clément G, Maximizing information from space data resources: A case for expanding integration across research disciplines: Eur J Appl Physiol, 2013; 113(7); 1645-54
6. Lee AG, Mader TH, Gibson CR, Tarver W, Space flight-associated neuro-ocular syndrome: JAMA Ophthalmol, 2017; 135(9); 992-94
7. Pavy-Le Traon A, Heer M, Narici MV, From space to Earth: Advances in human physiology from 20 years of bed rest studies (1986–2006): Eur J Appl Physiol, 2007; 101(2); 143-94
8. Taibbi G, Cromwell RL, Kapoor KG, The effect of microgravity on ocular structures and visual function: A review: Surv Ophthalmol, 2013; 58(2); 155-63
9. Tomilovskaya E, Shigueva T, Sayenko D, Dry immersion as a ground-based model of microgravity physiological effects: Front Physiol, 2019; 10; 284
10. Karmali F, Shelhamer M, The dynamics of parabolic flight: Flight characteristics and passenger percepts: Acta Astronaut, 2008; 63(5–6); 594-602
11. Watenpaugh DE, Analogs of microgravity: Head-down tilt and water immersion: J Appl Physiol (1985), 2016; 120(8); 904-14
12. Lakin WD, Stevens SA, Penar PL, Modeling intracranial pressures in microgravity: The influence of the blood-brain barrier: Aviat Space Environ Med, 2007; 78(10); 932-36
13. Leach CS, Alfrey CP, Suki WN, Regulation of body fluid compartments during short-term spaceflight: J Appl Physiol (1985), 1996; 81(1); 105-16
14. Kurazumi T, Ogawa Y, Morisaki H, Iwasaki KI, The effect of mild decrement in plasma volume simulating short-duration spaceflight on intracranial pressure: NPJ Microgravity, 2018; 4; 19
15. Nelson ES, Mulugeta L, Myers JG, Microgravity-induced fluid shift and ophthalmic changes: Life (Basel), 2014; 4(4); 621-65
16. Liu D, Kahn M, Measurement and relationship of subarachnoid pressure of the optic nerve to intracranial pressures in fresh cadavers: Am J Ophthalmol, 1993; 116(5); 548-56
17. Kermorgant M, Leca F, Nasr N, Impacts of simulated weightlessness by dry immersion on optic nerve sheath diameter and cerebral autoregulation: Front Physiol, 2017; 8; 780
18. Trambovetskii EV, Krotov VP, Korol’kov VIIntracranial pressure in monkeys during the flight of Cosmos-2229: Aviakosm Ekolog Med, 1995; 29(4); 37-41 [in Russian]
19. Marshall-Goebel K, Damani R, Bershad EM, Brain physiological response and adaptation during spaceflight: Neurosurgery, 2019; 85(5); E815-21
20. Lawley JS, Petersen LG, Howden EJ, Effect of gravity and microgravity on intracranial pressure: J Physiol, 2017; 595(6); 2115-27
21. Kramer LA, Sargsyan AE, Hasan KM, Orbital and intracranial effects of microgravity: Findings at 3-T MR imaging: Radiology, 2012; 263(3); 819-27
22. Aaslid R, Cerebral autoregulation and vasomotor reactivity: Front Neurol Neurosci, 2006; 21; 216-28
23. Hargens AR, Watenpaugh DE, Cardiovascular adaptation to spaceflight: Med Sci Sports Exerc, 1996; 28(8); 977-82
24. Mandsager KT, Robertson D, Diedrich A, The function of the autonomic nervous system during spaceflight: Clin Auton Res, 2015; 25(3); 141-51
25. Shen M, Frishman WH, Effects of spaceflight on cardiovascular physiology and health: Cardiol Rev, 2019; 27(3); 122-26
26. Kawai Y, Murthy G, Watenpaugh DE, Cerebral blood flow velocity in humans exposed to 24 h of head-down tilt: J Appl Physiol (1985), 1993; 74(6); 3046-51
27. Sun XQ, Yao YJ, Yang CB, Effect of lower-body negative pressure on cerebral blood flow velocity during 21 days head-down tilt bed rest: Med Sci Monit, 2005; 11(1); CR1-5
28. Arbeille P, Kerbeci P, Mattar L, Insufficient flow reduction during LBNP in both splanchnic and lower limb areas is associated with orthostatic intolerance after bedrest: Am J Physiol Heart Circ Physiol, 2008; 295(5); H1846-54
29. Iwasaki K, Levine BD, Zhang R, Human cerebral autoregulation before, during and after spaceflight: J Physiol, 2007; 579(Pt 3); 799-810
30. Arbeille P, Fomina G, Roumy J, Adaptation of the left heart, cerebral and femoral arteries, and jugular and femoral veins during short- and long-term head-down tilt and spaceflights: Eur J Appl Physiol, 2001; 86(2); 157-68
31. Klein T, Wollseiffen P, Sanders M, The influence of microgravity on cerebral blood flow and electrocortical activity: Exp Brain Res, 2019; 237(4); 1057-62
32. Ogoh S, Hirasawa A, de Abreu S, Internal carotid, external carotid and vertebral artery blood flow responses to 3 days of head-out dry immersion: Exp Physiol, 2017; 102(10); 1278-87
33. Marshall-Goebel K, Ambarki K, Eklund A, Effects of short-term exposure to head-down tilt on cerebral hemodynamics: A prospective evaluation of a spaceflight analog using phase-contrast MRI: J Appl Physiol (1985), 2016; 120(12); 1466-73
34. Ogoh S, Sato K, de Abreu S, Denise P, Normand H, Arterial and venous cerebral blood flow responses to long-term head-down bed rest in male volunteers: Exp Physiol, 2020; 105(1); 44-52
35. Wilkerson MK, Lesniewski LA, Golding EM, Simulated microgravity enhances cerebral artery vasoconstriction and vascular resistance through endothelial nitric oxide mechanism: Am J Physiol Heart Circ Physiol, 2005; 288(4); H1652-61
36. Xue JH, Chen LH, Zhao HZ, Differential regulation and recovery of intracellular Ca2+ in cerebral and small mesenteric arterial smooth muscle cells of simulated microgravity rat: PLoS One, 2011; 6(5); e19775
37. Prisby RD, Wilkerson MK, Sokoya EM, Endothelium-dependent vasodilation of cerebral arteries is altered with simulated microgravity through nitric oxide synthase and EDHF mechanisms: J Appl Physiol (1985), 2006; 101(1); 348-53
38. Geary GG, Krause DN, Purdy RE, Duckles SP, Simulated microgravity increases myogenic tone in rat cerebral arteries: J Appl Physiol (1985), 1998; 85(5); 1615-21
39. Taylor CR, Hanna M, Behnke BJ, Spaceflight-induced alterations in cerebral artery vasoconstrictor, mechanical, and structural properties: Implications for elevated cerebral perfusion and intracranial pressure: FASEB J, 2013; 27(6); 2282-92
40. Sofronova SI, Tarasova OS, Gaynullina D, Spaceflight on the Bion-M1 biosatellite alters cerebral artery vasomotor and mechanical properties in mice: J Appl Physiol (1985), 2015; 118(7); 830-38
41. Chen L, Zhang B, Yang L, BMAL1 disrupted intrinsic diurnal oscillation in rat cerebrovascular contractility of simulated microgravity rats by altering circadian regulation of miR-103/Ca(V)1.2 signal pathway: Int J Mol Sci, 2019; 20(16); 3947
42. Panerai RB, Assessment of cerebral pressure autoregulation in humans – a review of measurement methods: Physiol Meas, 1998; 19(3); 305-38
43. Paulson OB, Strandgaard S, Edvinsson L, Cerebral autoregulation: Cerebrovasc Brain Metab Rev, 1990; 2(2); 161-92
44. Claassen JA, Meel-van den Abeelen AS, Simpson DM, Panerai RB, Transfer function analysis of dynamic cerebral autoregulation: A white paper from the International Cerebral Autoregulation Research Network: J Cereb Blood Flow Metab, 2016; 36(4); 665-80
45. van Beek AH, Claassen JA, Rikkert MG, Jansen RW, Cerebral autoregulation: An overview of current concepts and methodology with special focus on the elderly: J Cereb Blood Flow Metab, 2008; 28(6); 1071-85
46. Tiecks FP, Lam AM, Aaslid R, Newell DW, Comparison of static and dynamic cerebral autoregulation measurements: Stroke, 1995; 26(6); 1014-19
47. Petersen LG, Ogoh S, Gravity, intracranial pressure, and cerebral autoregulation: Physiol Rep, 2019; 7(6); e14039
48. Nasr N, Czosnyka M, Pavy-Le Traon A, Baroreflex and cerebral autoregulation are inversely correlated: Circ J, 2014; 78(10); 2460-67
49. Jeong SM, Hwang GS, Kim SO, Dynamic cerebral autoregulation after bed rest: Effects of volume loading and exercise countermeasures: J Appl Physiol (1985), 2014; 116(1); 24-31
50. Ogawa Y, Iwasaki K, Aoki K, Central hypervolemia with hemodilution impairs dynamic cerebral autoregulation: Anesth Analg, 2007; 105(5); 1389-96 table of contents
51. Kurazumi T, Ogawa Y, Yanagida R, Dynamic cerebral autoregulation during the combination of mild hypercapnia and cephalad fluid shift: Aerosp Med Hum Perform, 2017; 88(9); 819-26
52. Deegan BM, Devine ER, Geraghty MC, The relationship between cardiac output and dynamic cerebral autoregulation in humans: J Appl Physiol (1985), 2010; 109(5); 1424-31
53. Pavy-Le Trao A, Costes-Salon MC, Vasseur-Clausen P, Changes in kinetics of cerebral auto-regulation with head-down bed rest: Clin Physiol Funct Imaging, 2002; 22(2); 108-14
54. Greaves DK, Arbeille P, Hughson RL, WISE 2005: altered cerebrovascular autoregulation after 60 day head-down bed rest: J Gravit Physiol, 2007; 14(1); P61-62
55. Kermorgant M, Nasr N, Custaud MA, Effects of resistance exercise and nutritional supplementation on dynamic cerebral autoregulation in head-down bed rest: Front Physiol, 2019; 10; 1114
56. Willie CK, Tzeng YC, Fisher JA, Ainslie PN, Integrative regulation of human brain blood flow: J Physiol, 2014; 592(5); 841-59
57. Rasmussen MK, Mestre H, Nedergaard M, The glymphatic pathway in neurological disorders: Lancet Neurol, 2018; 17(11); 1016-24
Figures
Tables
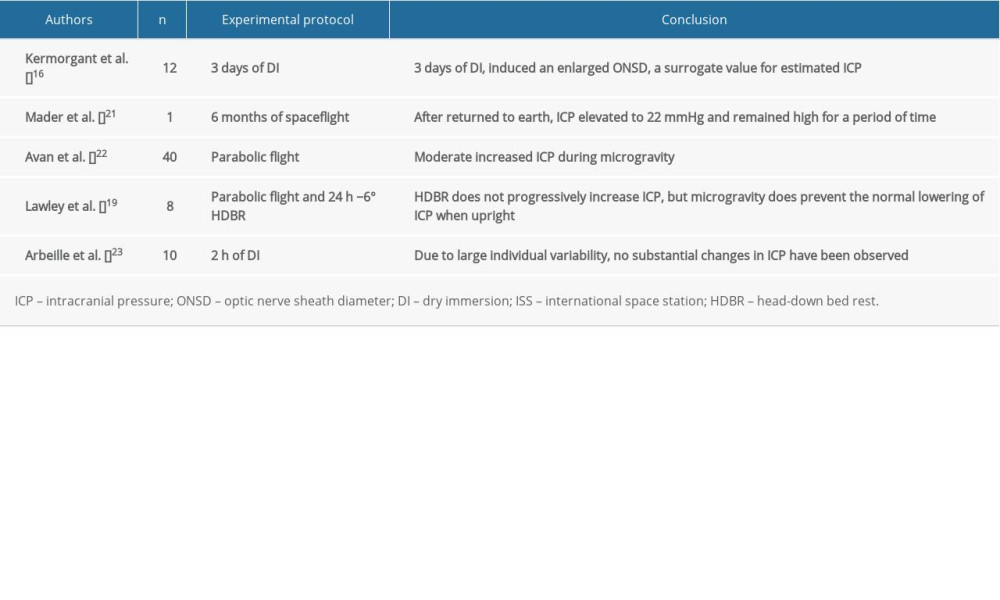 Table 1. Impact of simulated or actual microgravity on intracranial pressure.
Table 1. Impact of simulated or actual microgravity on intracranial pressure.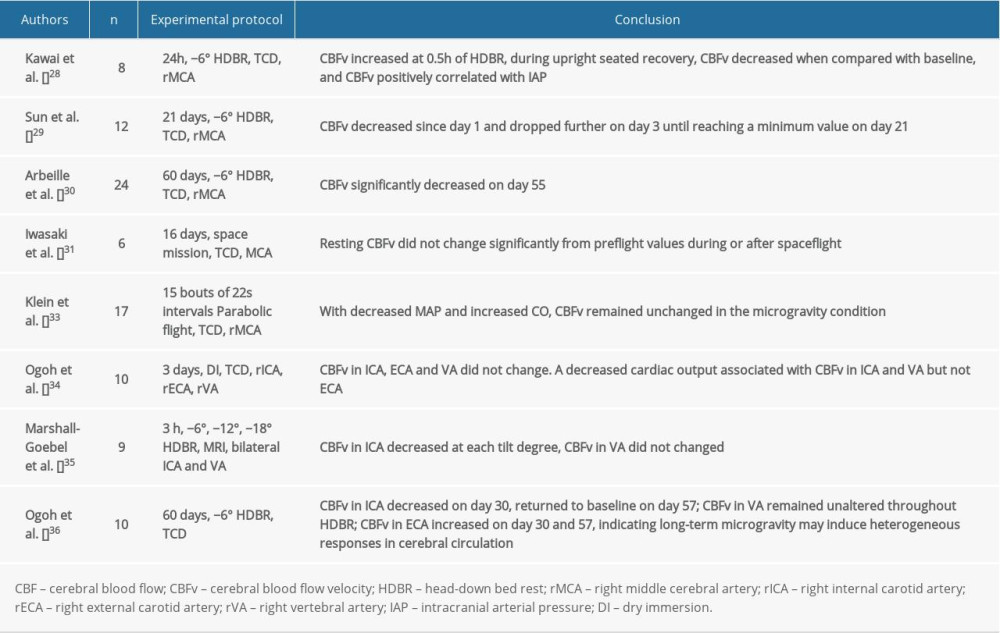 Table 2. Impact of simulated or actual microgravity on cerebral blood flow and cerebral blood flow velocity.
Table 2. Impact of simulated or actual microgravity on cerebral blood flow and cerebral blood flow velocity.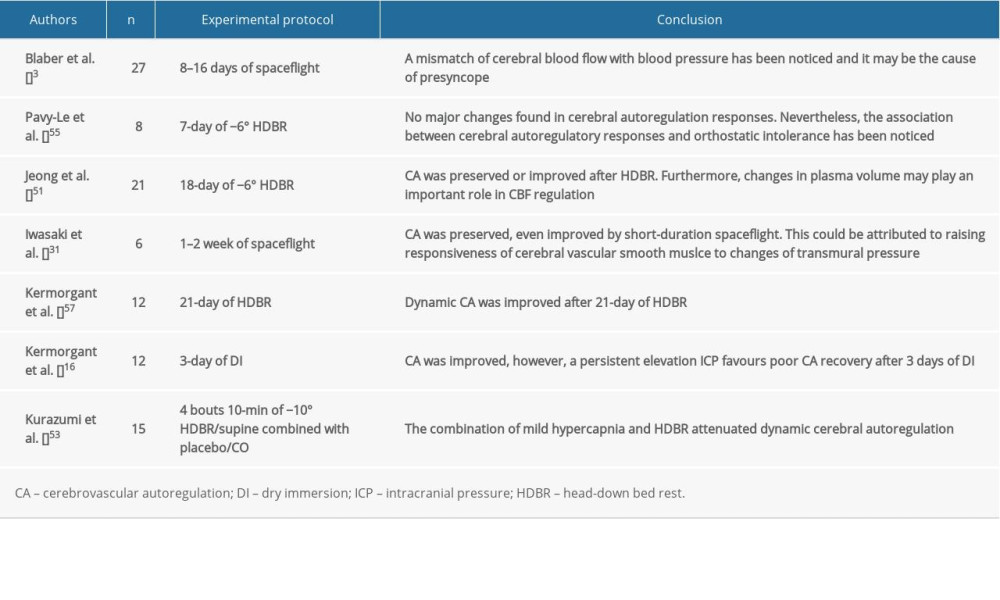 Table 3. Impact of simulated or actual microgravity on cerebrovascular autoregulation.
Table 3. Impact of simulated or actual microgravity on cerebrovascular autoregulation. Table 1. Impact of simulated or actual microgravity on intracranial pressure.
Table 1. Impact of simulated or actual microgravity on intracranial pressure. Table 2. Impact of simulated or actual microgravity on cerebral blood flow and cerebral blood flow velocity.
Table 2. Impact of simulated or actual microgravity on cerebral blood flow and cerebral blood flow velocity. Table 3. Impact of simulated or actual microgravity on cerebrovascular autoregulation.
Table 3. Impact of simulated or actual microgravity on cerebrovascular autoregulation. In Press
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952