11 May 2021: Clinical Research
Efficacy and Safety of Sea Salt-Derived Physiological Saline Nasal Spray as Add-On Therapy in Patients with Acute Upper Respiratory Infection: A Multicenter Retrospective Cohort Study
Min Jiang1ABDEF, Junwen Chen2B, Yuanhua Ding3B, Chenxi Gan4BDF, Ya Hou5CD, Junge Lei1BE, Mengzhi Wan1B, Xing Li1B, Zuke Xiao1ACG*DOI: 10.12659/MSM.929714
Med Sci Monit 2021; 27:e929714
Abstract
BACKGROUND: The purpose of this study was to assess the effects of seawater on nasal congestion and runny nose symptoms in adults with an acute upper respiratory infection (URI).
MATERIAL AND METHODS: This was a multicenter retrospective cohort trial of patients with acute URI and symptoms of nasal congestion and runny nose. The patients were assigned to 2 groups and were administered regular non-drug supportive treatment or supportive treatment with nasal irrigation with sea salt-derived physiological saline. The primary efficacy endpoint was the effective rate (percentage of patients with ≥30% symptom score reduction from baseline for nasal congestion and runny nose).
RESULTS: In total, 144 patients were enrolled, including 72 in each group, and 143 patients completed the study. Both groups had similar demographics and vital signs. The effective rates for nasal congestion and runny nose were significantly increased in the seawater group compared with patients in the control group (87.3% vs 59.7% for nasal congestion; 85.9% vs 61.1% for runny nose; both P<0.001). In addition, the 2 groups showed markedly different degrees of patient symptom score improvement in sleep quality and appetite (both P<0.01), but not in cough and fatigue (both P>0.05). There were no adverse events in either group.
CONCLUSIONS: The sea salt-derived physiological saline nasal spray device satisfactorily improved nasal congestion, runny nose, sleep quality, and appetite in adults with URI, with no adverse effects.
Keywords: Clinical Trial, Nasal lavage, Respiratory Tract Infections, Administration, Intranasal, Nasal Obstruction, Nasal Sprays, saline solution, Salts, Seawater, young adult
Background
Acute upper respiratory infections (URIs) are the most common diseases affecting adults, who usually have from 2 to 5 acute URIs yearly [1–5]. Acute URIs include acute infections involving the nose, sinus, pharynx, middle ear, larynx and epiglottis, airway, and bronchus. The common cold, the URI with the highest incidence, constitutes an acute, self-limiting disease affecting the upper respiratory tract, causing different levels of sneezing, nasal congestion, runny nose, sore throat, cough, mild fever, headache, and weakness [1,3–7]. Acute URIs may be caused by various pathogenic viruses [1]. Acute URIs generally requires only symptomatic treatment, with no need for antibiotics [5,8]. The usual treatment for an acute URI is supportive, and high fluid intake and rest are recommended [1,2].
Nasal irrigation is considered to alleviate the symptoms of URI by clearing mucus, decreasing congestion, and improving breathing [9]. In addition, mucus clearance could be improved through increased frequency of the ciliary beats, and infection materials could be washed away [10].
Nasal irrigation is mainly used for sinusitis and nasal diseases such as allergic rhinitis [11,12], but is used less often in acute URIs. There are only a few reports utilizing seawater or saline in children with URI and influenza [12,13]. Consequently, whether seawater effectively relieves URI symptoms in adults remains unknown. A meta-analysis of studies conducted before 2015 suggested that nasal saline irrigation might not hasten symptom improvement in adults with acute URI [14]. A previous trial showed that the use of hypertonic saline results in no nasal symptom improvement or shortened disease duration in adult URI [15].
The present trial aimed to assess the relieving effects of seawater on nasal congestion and runny nose symptoms in adults with acute URI in the context of supportive treatment.
Material and Methods
STUDY DESIGN:
The current trial was approved by the Medical Ethics Committee of the Jiangxi Provincial People’s Hospital (No. 2017-clinical inspection 14). Informed consent was not required due to the retrospective nature of this study.
This was a multicenter retrospective cohort trial. The study complied with the Good Clinical Practices, the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use Guidelines, and all applicable laws and regulations. The trial was carried out at the Jiangxi Provincial People’s Hospital. It was registered under No. ChiCTR2000031171.
Exclusion criteria were: 1) pregnancy or breastfeeding; 2) known hypersensitivity to seawater; 3) use of alcohol or illicit drugs; 4) other respiratory diseases such as herpangina, suppurative tonsillitis, bronchitis, and chronic rhinitis; 5) present acute pathology or uncontrolled chronic ailment; 6) serious systemic diseases; 7) antiviral treatment required for influenza A or B; 8) antibiotic treatment required for acute respiratory infection; 9) drug treatment of conditions prior to enrollment; 10) participation in another clinical trial less than 3 months before enrollment; and 11) likelihood of poor compliance or unlikeliness to complete the trial, as determined by the investigators.
TREATMENT:
Patients with acute URI underwent routine basic supportive treatment (control group) or routine basic supportive treatment and nasal irrigation with sea salt-derived physiological saline (seawater group). The routine basic supportive treatment included drinking warm or boiled water and getting good rest, without specific drugs or medication. The amount and the temperature of the drinking water and the resting time depended on the specific needs of the patients.
Sea salt-derived physiological saline (0.9%) nasal spray was provided by Jiangsu-Aipeng Medical Technology Co., Ltd, and included 5 parts: a bottle, manual pump, nozzle, sea salt-derived physiological saline, and dust cover. The clinical product registration of the device has been filed with the State Food and Drug Administration (SFDA; No. 2013-2640258). The SFDA approval of the device covers uses for nasal dryness, stuffy nose, nasal itching, runny nose, and nasal bleeding.
All patients were instructed on how to operate the device. First, the patient needed to remove the dust cover (Figure 1A). Second, the patient tilted their head backward, put the nozzle in the nostrils one at a time, and gently pressed the manual pump (Figure 1B) 4 to 8 times per nostril. Third, the patient wiped nasal secretions and excess sea salt-derived physiological saline with a paper towel (Figure 1C). Finally, the patient cleaned the nozzle and replaced the dust cover (Figure 1D). The patients used 4 to 8 sprays (0.12 mL/spray) in each nostril each time, 2 to 6 times per day, depending upon the severity of nasal congestion.
CLINICAL TRIAL DURATION AND ENDPOINTS:
The duration of the clinical trial was 5 days: screening and enrollment lasted for 2 days, and the treatment period was 3 days. The visual analog scale of symptom severity was used for various symptoms, including runny nose, nasal congestion, cough, sleep quality, appetite, and fatigue. A 10-cm line was marked with 0 (no symptoms at all) on the left side and 10 (worst imaginable symptoms) on the right side. The patients marked the line for each symptom accordingly and completed a diary of the use of the nasal spray.
The primary efficacy endpoint was the effective rate, defined as the percentage of patients with ≥30% symptom score reduction from baseline for nasal congestion and runny nose. The secondary efficacy endpoints were cough, sleep quality, appetite, and fatigue. Adverse events were recorded throughout the study, by the investigator or in the patient diary. The patients were advised that a slight tingling sensation might be felt in the nasal cavity after using the spray, and that the fluid flowing out of the nasal cavity can cause some discomfort. Nevertheless, they were told to note such discomforts if they occurred. Vital sign assessment and physical examinations were conducted at screening and on day 3.
STATISTICAL ANALYSIS:
Categorical variables were expressed as frequencies and percentages and compared by the chi-square (intergroup comparisons) and McNemar’s (intragroup comparisons) tests. Continuous data were described by mean±standard deviation or ranges and were analyzed by the
Results
CHARACTERISTICS OF THE PATIENTS:
In total, 144 patients were examined, including 72 each in the seawater and control groups, and 143 patients completed the study (Figure 2). One patient in the seawater group dropped out on day 2. The groups were comparable in baseline sex, age, body temperature, heart and respiratory rates, and systolic and diastolic blood pressure levels (Table 1).
EFFICACY:
The effective rates for nasal congestion and runny nose were significantly elevated in the seawater group compared with that of the control group (87.3% vs 59.7% for nasal congestion; 85.9% vs 61.1% for runny nose; both P<0.001) (Figures 3–5). The 2 groups did not differ significantly in symptom score improvement for cough (Figure 6) (P>0.05); they differed significantly for sleep quality (Figure 7) and appetite (Figure 8) (both P<0.01); they did not differ significantly for fatigue (Figure 9) (P>0.05).
SAFETY:
The 2 groups were comparable in body temperature, respiration rate, pulse rate, and systolic and diastolic blood pressure (all P>0.05) (Table 1). No intragroup differences from day 0 to day 3 were observed (all P>0.05). There were no device-related accidents, nasal hemorrhage, or other adverse events in either group.
Discussion
The effectiveness of nasal irrigation in adults with URI is not yet established [14,15]. Therefore, this multicenter retrospective cohort trial investigated the alleviating effects of sea salt-derived physiological saline on nasal congestion and runny nose in adult acute URI. The results indicated that the sea salt-derived physiological saline nasal spray device had satisfactory effective rates for nasal congestion, runny nose, sleep quality, and appetite in adult patients with URI. In addition, its safety profile did not differ from traditional supportive treatment.
Across the United States, acute URIs constitute 1 of the 3 most diagnosed diseases in outpatients, accounting for 10 million yearly outpatient visits [3]. Non-influenza viral URIs cost approximately $22 billion yearly [16]. Symptom alleviation is the major reason for outpatient visits in adult individuals in the first days following disease onset, with most appointments to the doctor resulting in the administration of prescribed drugs. Decongestants, either combined with antihistamines or not, alleviate congestion, cough, and other symptoms in adult patients [17]. In addition, topical (eg, oxymetazoline) and oral (eg, pseudoephedrine) nasal decongestants are moderately beneficial to adults and adolescents in decreasing nasal airway resistance [6,18]. Owing to the risk of increased blood pressure with decongestant use, patients with hypertension should be cautious while using decongestants [19,20]. H1-receptor antagonists modestly reduce rhinorrhea and sneezing in the initial 2 days after cold onset in adult patients [18]. First-generation antihistamines present adverse effects such as drowsiness, to which patients should pay attention [21]. Acetaminophen and nonsteroidal anti-inflammatory drugs could reduce discomfort and pain but are also associated with adverse effects such as gastric irritation [22,23]. Therefore, considering the possible harm that can be caused by the available drugs, the development of novel, safe, and effective drugs or devices for relieving acute URI symptoms is needed.
Multiple reports assessing nasal irrigation have demonstrated its effectiveness in treating seasonal allergic rhinitis in adult and pediatric patients [24–26]. Seawater nasal irrigation, as utilized in the present trial, represents a simple method with high reproducibility and facile execution. Therefore, the study participants performed nasal irrigation without reserve. The current trial assessed the effects of nasal irrigation on acute URI signs in adults. We found a significant improvement on day 3 in the seawater group after administration of nasal irrigation in comparison with the control group, which was administered routine non-drug supportive treatment alone. These findings indicate that nasal seawater irrigation provides a safe and cost-effective method for treating acute URI, although the precise mechanism of the treatment remains undefined and may involve multiple parameters.
Nasal irrigation exerts several physiological effects, which might help the nasal mucosa decrease the pathological activities of inflammatory factors and other inducers of allergic rhinitis [12]. It might increase mucus displacement toward the nasopharynx [9]. The mucosal lining of the nasal cavity represents an important barrier to pathogens and comprises multiple inflammatory factors, including histamine, prostaglandins, and leukotrienes [27].
Koksal et al [13] reported relief in nasal congestion, rhinorrhea, weakness, sleep quality, diet, and cough after seawater administration in pediatric patients with a common cold. Symptom alleviation was similarly observed in nasal congestion, rhinorrhea, sleep quality, and diet in the present study. Nevertheless, no relief was observed in cough and fatigue. This discrepancy might be related to factors including patient age, work, and social responsibilities. The patients recruited in this study were adults, while Koksal et al [13] assessed individuals below 2 years of age. This suggests that the use of seawater as a nasal drop may be more suitable for young children.
The strengths of this study rely its design. This was a multicenter study, and patients with common URI were recruited, while those with chronic conditions that could influence the results were excluded. Nevertheless, this study had limitations. The current spray was designed to relieve the general symptoms of all upper respiratory infections, not focusing on any specific type. Therefore, specific upper respiratory infections were not recorded. Excluding some patients with specific conditions may have helped us observe the effect of nasal irrigation more precisely but limited the generalizability of the results. In addition, although the sample size was adequate, as per the calculation, larger studies may pinpoint the exact benefits of nasal irrigation in adults with URI. Furthermore, whether sea salt derived-physiological saline causes respiratory tract injury (even transiently) is unknown and deserves further investigation. Finally, the retrospective nature of the study carries inherent shortcomings.
Conclusions
The present findings indicated that nasal irrigation constitutes an efficient, cost-effective adjunct therapy for relieving acute URI in adults, with no adverse effects. Significant improvements were observed in mean symptom scores for nasal obstruction, runny nose, sleep quality, and appetite after irrigation.
Figures
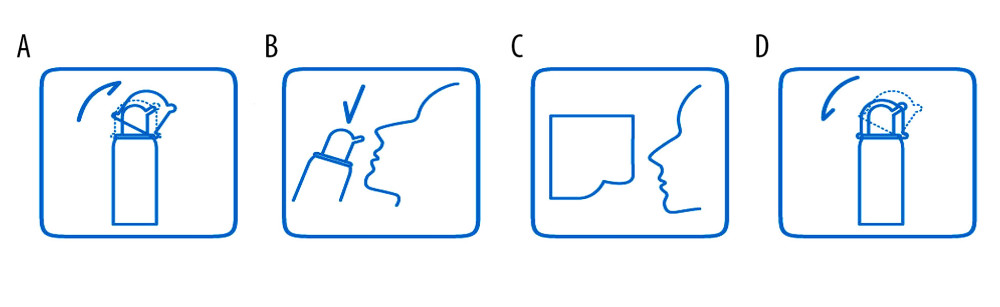 Figure 1. Instructions for sea salt-derived physiological saline nasal spray use. The spray device consisted of a bottle (up to 60 mL), a hand pump, a nozzle, a dust cover, and sea salt-derived physiological saline. Instructions: (A) remove the dust cover; (B) tilt the head backward, put the nozzle in the nostrils, and gently press the manual pump 4 to 8 times per nostril (spray distance ≥200 mm); (C) wipe the nasal secretions and excess sea salt-derived physiological saline with a paper towel; (d) clean the nozzle and replace the dust cover.
Figure 1. Instructions for sea salt-derived physiological saline nasal spray use. The spray device consisted of a bottle (up to 60 mL), a hand pump, a nozzle, a dust cover, and sea salt-derived physiological saline. Instructions: (A) remove the dust cover; (B) tilt the head backward, put the nozzle in the nostrils, and gently press the manual pump 4 to 8 times per nostril (spray distance ≥200 mm); (C) wipe the nasal secretions and excess sea salt-derived physiological saline with a paper towel; (d) clean the nozzle and replace the dust cover. 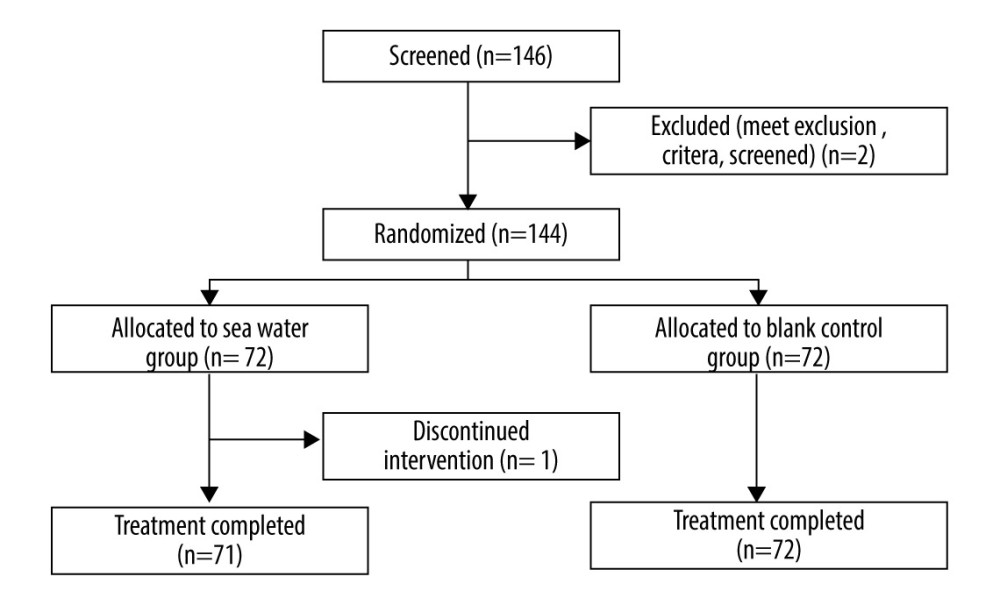 Figure 2. Study flowchart.
Figure 2. Study flowchart. 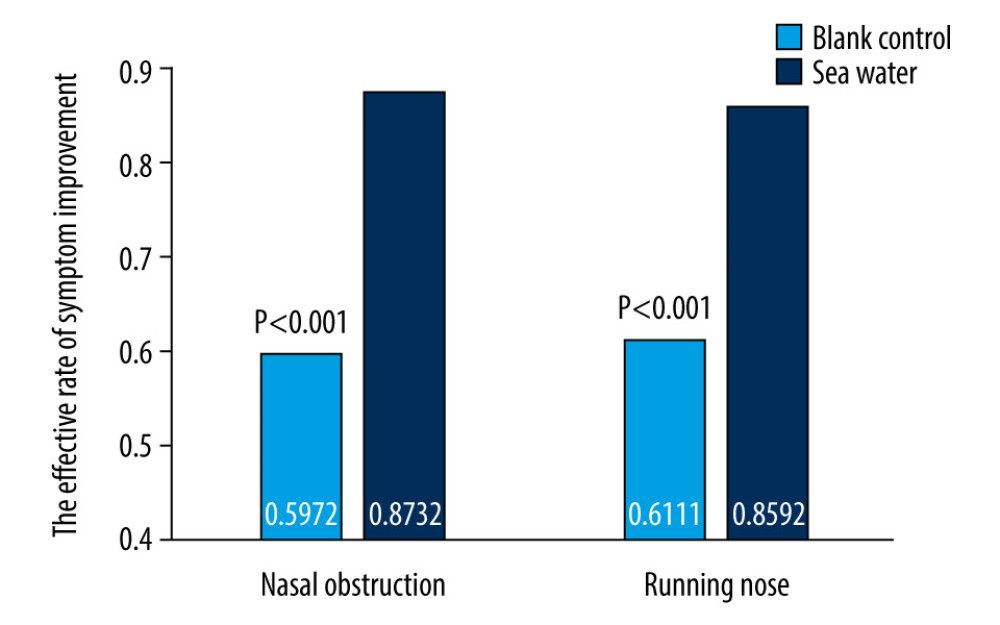 Figure 3. Effective rates of seawater in relieving nasal congestion and runny nose.
Figure 3. Effective rates of seawater in relieving nasal congestion and runny nose. 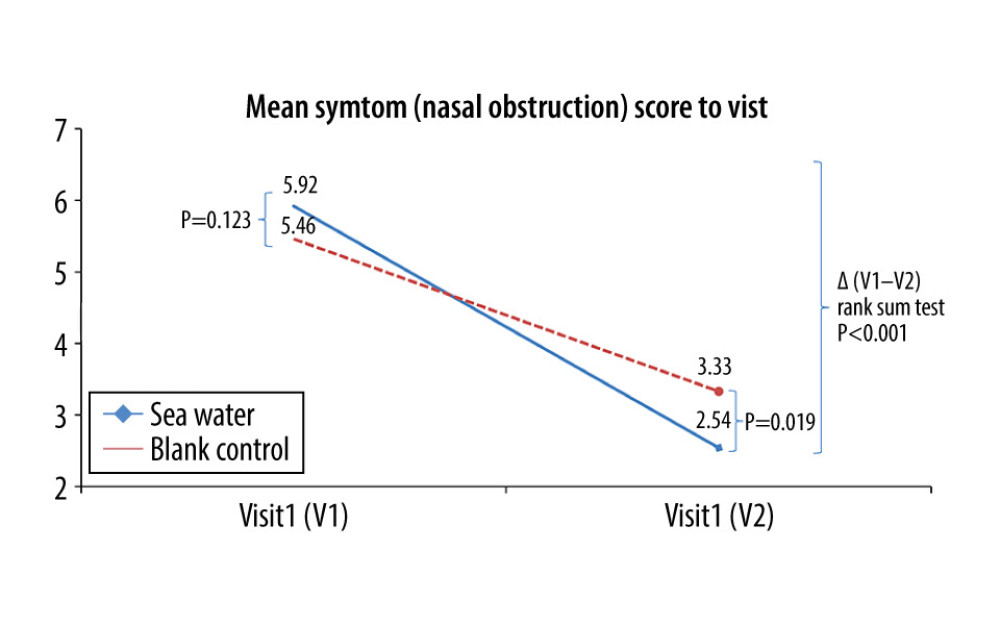 Figure 4. Mean symptom (nasal obstruction) scores before and after treatment. * Comparison between mean symptom scores by paired t test for independent samples; ** Mean score variations between the 2 groups (V1–V2) assessed by the rank-sum test.
Figure 4. Mean symptom (nasal obstruction) scores before and after treatment. * Comparison between mean symptom scores by paired t test for independent samples; ** Mean score variations between the 2 groups (V1–V2) assessed by the rank-sum test. 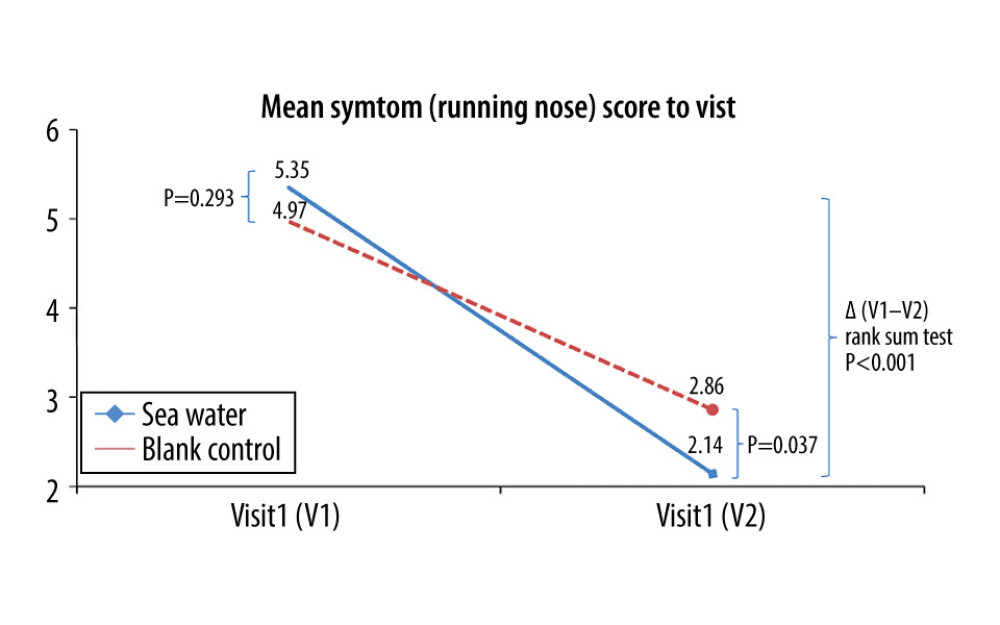 Figure 5. Mean symptom (runny nose) scores before and after treatment. * Comparison between mean symptom scores by paired t test for independent samples; ** Mean score variations between the 2 groups (V1–V2) assessed by the rank-sum test.
Figure 5. Mean symptom (runny nose) scores before and after treatment. * Comparison between mean symptom scores by paired t test for independent samples; ** Mean score variations between the 2 groups (V1–V2) assessed by the rank-sum test. 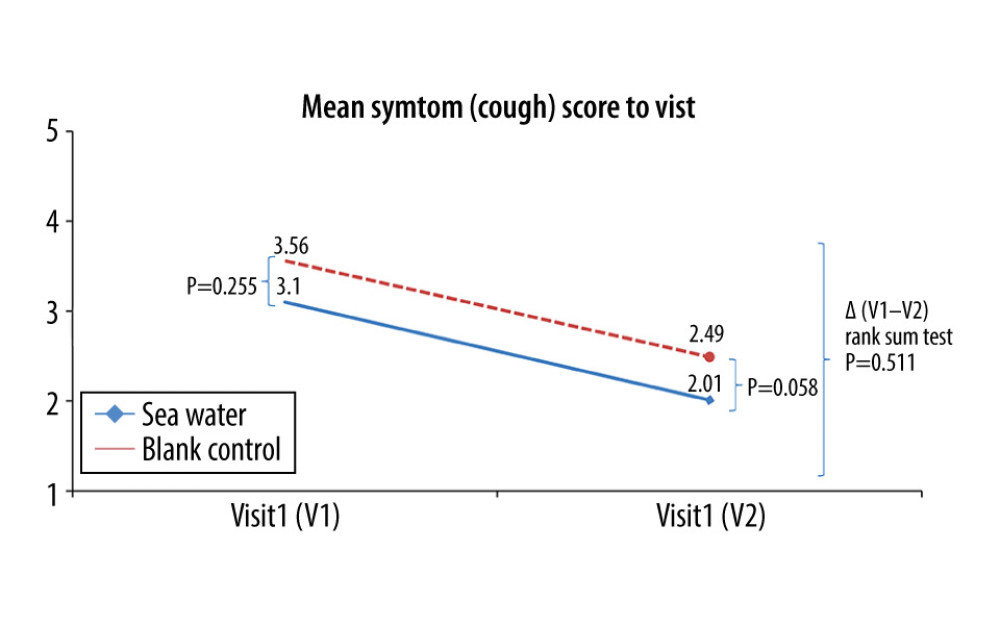 Figure 6. Mean symptom (cough) scores before and after treatment. * Comparison between mean symptom scores by paired t test for independent samples; ** Mean score variations between the 2 groups (V1–V2) assessed by the rank-sum test.
Figure 6. Mean symptom (cough) scores before and after treatment. * Comparison between mean symptom scores by paired t test for independent samples; ** Mean score variations between the 2 groups (V1–V2) assessed by the rank-sum test. 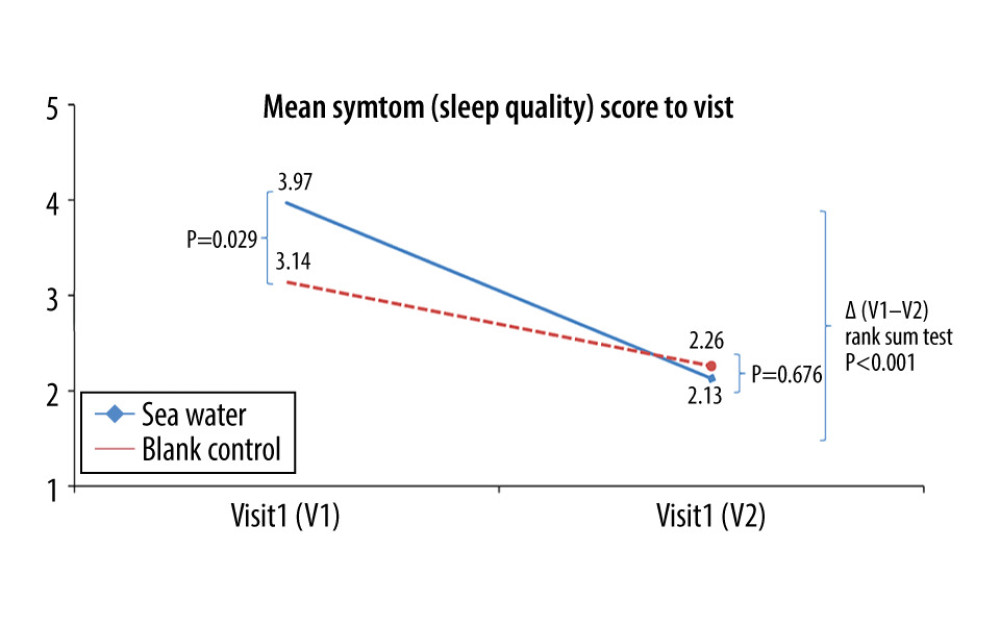 Figure 7. Mean symptom (sleep quality) scores before and after treatment. *Comparison between mean symptom scores by paired t test for independent samples; **Mean score variations between the 2 groups (V1–V2) assessed by the rank-sum test.
Figure 7. Mean symptom (sleep quality) scores before and after treatment. *Comparison between mean symptom scores by paired t test for independent samples; **Mean score variations between the 2 groups (V1–V2) assessed by the rank-sum test. 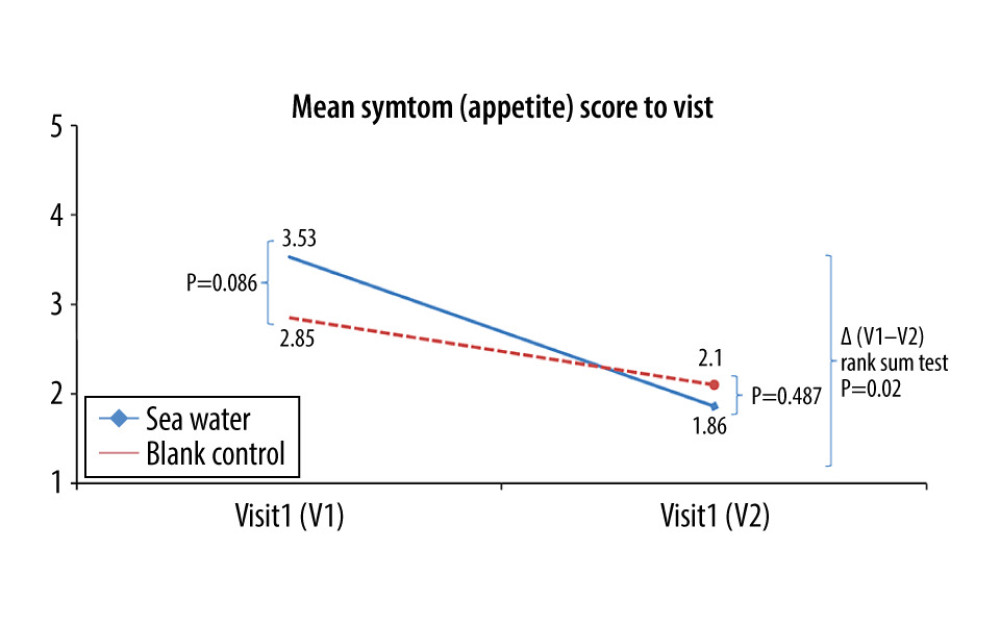 Figure 8. Mean symptom (appetite) scores before and after treatment.* Comparison between mean symptom scores by paired t test for independent samples; ** Mean score variations between the 2 groups (V1–V2) assessed by the rank-sum test.
Figure 8. Mean symptom (appetite) scores before and after treatment.* Comparison between mean symptom scores by paired t test for independent samples; ** Mean score variations between the 2 groups (V1–V2) assessed by the rank-sum test. 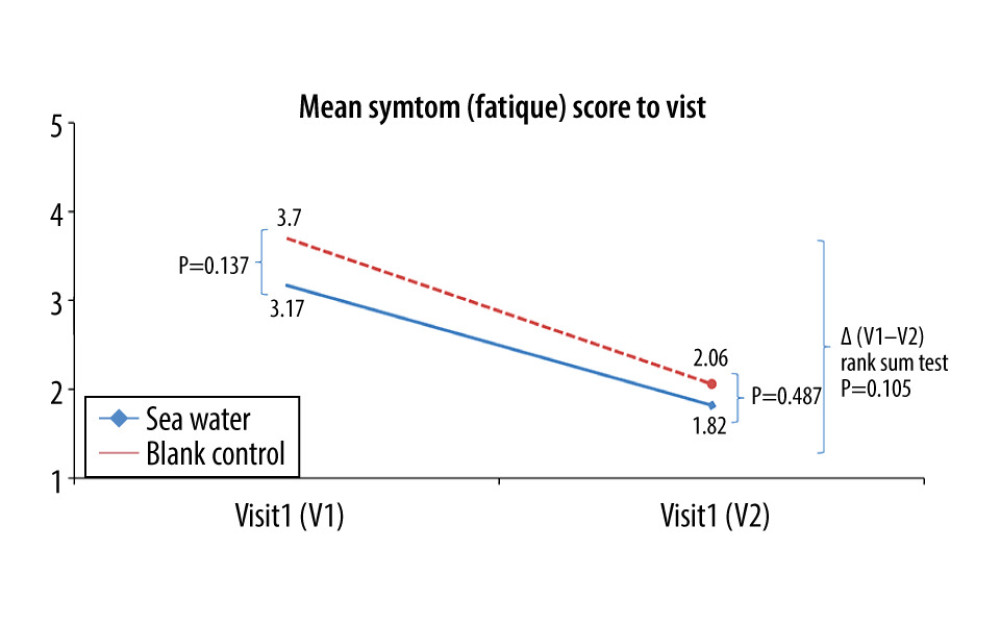 Figure 9. Mean symptom (fatigue) scores before and after treatment. * Comparison between mean symptom scores by paired t test for independent samples; ** Mean score variations between the 2 groups (V1–V2) assessed by the rank-sum test.
Figure 9. Mean symptom (fatigue) scores before and after treatment. * Comparison between mean symptom scores by paired t test for independent samples; ** Mean score variations between the 2 groups (V1–V2) assessed by the rank-sum test. References
1. Harris AM, Hicks LA, Qaseem AHigh Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention, Appropriate antibiotic use for acute respiratory tract infection in adults: Advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention: Ann Intern Med, 2016; 164; 425-34
2. Simasek M, Blandino DA, Treatment of the common cold: Am Fam Physician, 2007; 75; 515-20
3. Thomas M, Bomar PA: Upper respiratory tract infection, 2020, Treasure Island (FL), StatPearls
4. Moriyama M, Hugentobler WJ, Iwasaki A, Seasonality of respiratory viral infections: Annu Rev Virol, 2020; 7; 83-101
5. O’Connor R, O’Doherty J, O’Regan A, Medical management of acute upper respiratory infections in an urban primary care out-of-hours facility: Cross-sectional study of patient presentations and expectations: BMJ Open, 2019; 9; e025396
6. Fashner J, Ericson K, Werner S, Treatment of the common cold in children and adults: Am Fam Physician, 2012; 86; 153-59
7. Malesker MA, Callahan-Lyon P, Ireland B, Pharmacologic and nonpharmacologic treatment for acute cough associated with the common cold: CHEST Expert Panel Report: Chest, 2017; 152; 1021-37
8. Yoon YK, Park CS, Kim JW, Guidelines for the antibiotic use in adults with acute upper respiratory tract infections: Infect Chemother, 2017; 49; 326-52
9. Tomooka LT, Murphy C, Davidson TM, Clinical study and literature review of nasal irrigation: Laryngoscope, 2000; 110; 1189-93
10. Bustamante-Marin XM, Ostrowski LE, Cilia and mucociliary clearance: Cold Spring Harb Perspect Biol, 2017; 9; a028241
11. Sindwani R, Han JK, Soteres DF, NAVIGATE I: randomized, placebo-controlled, double-blind trial of the exhalation delivery system with fluticasone for chronic rhinosinusitis with nasal polyps: Am J Rhinol Allergy, 2019; 33; 69-82
12. Chen JR, Jin L, Li XY, The effectiveness of nasal saline irrigation (seawater) in treatment of allergic rhinitis in children: Int J Pediatr Otorhinolaryngol, 2014; 78; 1115-18
13. Koksal T, Cizmeci MN, Bozkaya D, Comparison between the use of saline and seawater for nasal obstruction in children under 2 years of age with acute upper respiratory infection: Turk J Med Sci, 2016; 46; 1004-13
14. King D, Mitchell B, Williams CP, Spurling GK, Saline nasal irrigation for acute upper respiratory tract infections: Cochrane Database Syst Rev, 2015(4); CD006821
15. Adam P, Stiffman M, Blake RL, A clinical trial of hypertonic saline nasal spray in subjects with the common cold or rhinosinusitis: Arch Fam Med, 1998; 7; 39-43
16. Fendrick AM, Monto AS, Nightengale B, Sarnes M, The economic burden of non-influenza-related viral respiratory tract infection in the United States: Arch Intern Med, 2003; 163; 487-94
17. Irwin RS, Baumann MH, Bolser DC, Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines: Chest, 2006; 129; 1-23S
18. Arroll B, Common cold: Am Fam Physician, 2011; 84; 1390-91
19. Watts AM, Cripps AW, West NP, Cox AJ, Modulation of allergic inflammation in the nasal mucosa of allergic rhinitis sufferers with topical pharmaceutical agents: Front Pharmacol, 2019; 10; 294
20. DeGeorge KC, Ring DJ, Dalrymple SN, Treatment of the common cold: Am Fam Physician, 2019; 100; 281-89
21. Maurer M, Staubach P, Raap U, H1-antihistamine-refractory chronic spontaneous urticaria: it’s worse than we thought – first results of the multicenter real-life AWARE study: Clin Exp Allergy, 2017; 47; 684-92
22. Kim SY, Chang YJ, Cho HM, Non-steroidal anti-inflammatory drugs for the common cold: Cochrane Database Syst Rev, 2015(9); CD006362
23. Li S, Yue J, Dong BR, Yang M, Acetaminophen (paracetamol) for the common cold in adults: Cochrane Database Syst Rev, 2013(7); CD008800
24. Mitsias DI, Dimou MV, Lakoumentas J, Effect of nasal irrigation on allergic rhinitis control in children; Complementarity between CARAT and MASK outcomes: Clin Transl Allergy, 2020; 10; 9
25. Dykewicz MS, Wallace DV, Amrol DJ, Rhinitis 2020: A practice parameter update: J Allergy Clin Immunol, 2020; 146; 721-67
26. Okubo K, Kurono Y, Ichimura K, Japanese guidelines for allergic rhinitis 2020: Allergol Int, 2020; 69; 331-45
27. Kumpitsch C, Koskinen K, Schopf V, Moissl-Eichinger C, The microbiome of the upper respiratory tract in health and disease: BMC Biol, 2019; 17; 87
Figures
 Figure 1. Instructions for sea salt-derived physiological saline nasal spray use. The spray device consisted of a bottle (up to 60 mL), a hand pump, a nozzle, a dust cover, and sea salt-derived physiological saline. Instructions: (A) remove the dust cover; (B) tilt the head backward, put the nozzle in the nostrils, and gently press the manual pump 4 to 8 times per nostril (spray distance ≥200 mm); (C) wipe the nasal secretions and excess sea salt-derived physiological saline with a paper towel; (d) clean the nozzle and replace the dust cover.
Figure 1. Instructions for sea salt-derived physiological saline nasal spray use. The spray device consisted of a bottle (up to 60 mL), a hand pump, a nozzle, a dust cover, and sea salt-derived physiological saline. Instructions: (A) remove the dust cover; (B) tilt the head backward, put the nozzle in the nostrils, and gently press the manual pump 4 to 8 times per nostril (spray distance ≥200 mm); (C) wipe the nasal secretions and excess sea salt-derived physiological saline with a paper towel; (d) clean the nozzle and replace the dust cover. Figure 2. Study flowchart.
Figure 2. Study flowchart. Figure 3. Effective rates of seawater in relieving nasal congestion and runny nose.
Figure 3. Effective rates of seawater in relieving nasal congestion and runny nose. Figure 4. Mean symptom (nasal obstruction) scores before and after treatment. * Comparison between mean symptom scores by paired t test for independent samples; ** Mean score variations between the 2 groups (V1–V2) assessed by the rank-sum test.
Figure 4. Mean symptom (nasal obstruction) scores before and after treatment. * Comparison between mean symptom scores by paired t test for independent samples; ** Mean score variations between the 2 groups (V1–V2) assessed by the rank-sum test. Figure 5. Mean symptom (runny nose) scores before and after treatment. * Comparison between mean symptom scores by paired t test for independent samples; ** Mean score variations between the 2 groups (V1–V2) assessed by the rank-sum test.
Figure 5. Mean symptom (runny nose) scores before and after treatment. * Comparison between mean symptom scores by paired t test for independent samples; ** Mean score variations between the 2 groups (V1–V2) assessed by the rank-sum test. Figure 6. Mean symptom (cough) scores before and after treatment. * Comparison between mean symptom scores by paired t test for independent samples; ** Mean score variations between the 2 groups (V1–V2) assessed by the rank-sum test.
Figure 6. Mean symptom (cough) scores before and after treatment. * Comparison between mean symptom scores by paired t test for independent samples; ** Mean score variations between the 2 groups (V1–V2) assessed by the rank-sum test. Figure 7. Mean symptom (sleep quality) scores before and after treatment. *Comparison between mean symptom scores by paired t test for independent samples; **Mean score variations between the 2 groups (V1–V2) assessed by the rank-sum test.
Figure 7. Mean symptom (sleep quality) scores before and after treatment. *Comparison between mean symptom scores by paired t test for independent samples; **Mean score variations between the 2 groups (V1–V2) assessed by the rank-sum test. Figure 8. Mean symptom (appetite) scores before and after treatment.* Comparison between mean symptom scores by paired t test for independent samples; ** Mean score variations between the 2 groups (V1–V2) assessed by the rank-sum test.
Figure 8. Mean symptom (appetite) scores before and after treatment.* Comparison between mean symptom scores by paired t test for independent samples; ** Mean score variations between the 2 groups (V1–V2) assessed by the rank-sum test. Figure 9. Mean symptom (fatigue) scores before and after treatment. * Comparison between mean symptom scores by paired t test for independent samples; ** Mean score variations between the 2 groups (V1–V2) assessed by the rank-sum test.
Figure 9. Mean symptom (fatigue) scores before and after treatment. * Comparison between mean symptom scores by paired t test for independent samples; ** Mean score variations between the 2 groups (V1–V2) assessed by the rank-sum test. In Press
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952