25 April 2021: Clinical Research
Predicting the Risk of Acute Kidney Injury in Patients After Percutaneous Coronary Intervention (PCI) or Cardiopulmonary Bypass (CPB) Surgery: Development and Assessment of a Nomogram Prediction Model
Yi Du1ABCDEFG, Xiu-Zhe Wang1ABFG, Wei-Dong Wu1ABCDE*, Hai-Peng Shi1ABC, Xiao-Jing Yang1CD, Wen-Jing Wu1BC, Shu-Xian Chen1EFDOI: 10.12659/MSM.929791
Med Sci Monit 2021; 27:e929791
Abstract
BACKGROUND: We sought to create a model that incorporated ultrasound examinations to predict the risk of acute kidney injury (AKI) after percutaneous coronary intervention (PCI) or cardiopulmonary bypass (CPB) surgery.
MATERIAL AND METHODS: A total of 292 patients with AKI after PCI or CPB surgery were enrolled for the study. Afterwards, treatment-related information, including data pertaining to ultrasound examination, was collected. A random forest model and multivariate logistic regression analysis were then used to establish a predictive model for the risk of AKI. Finally, the predictive quality and clinical utility of the model were assessed using calibration plots, receiver-operating characteristic curve, C-index, and decision curve analysis.
RESULTS: Predictive factors were screened and the model was established with a C-index of 0.955 in the overall sample set. Additionally, an area under the curve of 0.967 was obtained in the training group. Moreover, decision curve analysis also revealed that the prediction model had good clinical applicability.
CONCLUSIONS: The prediction model was efficient in predicting the risk of AKI by incorporating ultrasound examinations and a number of factors. Such included operation methods, age, congestive heart failure, body mass index, heart rate, white blood cell count, platelet count, hemoglobin, uric acid, and peak intensity (kidney cortex as well as kidney medulla).
Keywords: Abdominal Injuries, Acute Kidney Injury, Polycystic Kidney, Autosomal Dominant, Cardiopulmonary Bypass, Models, Statistical, nomograms, percutaneous coronary intervention, Postoperative Complications, Risk, Ultrasonography
Background
Acute kidney injury (AKI) is one of the most serious and life-threatening complications after percutaneous coronary intervention (PCI) and cardiopulmonary bypass (CPB) operations [1–3]. Current studies suggest that abnormal renal blood perfusion in the early stages of the disease plays a key role in the development and progression of AKI with complicated pathophysiological properties [4]. Therefore, early diagnosis of AKI and identification of risk factors should be the focus of clinical intensive care.
Early detection of AKI can promote positive treatment outcomes and reduce the progression of kidney injury. Therefore, extensive research has been done to find risk stratification models for early prediction of AKI [5–8]. However, these AKI diagnostic models mainly rely on urine output and biochemical indicators that are affected by factors such as severity of disease, duration, time of detection, and etiology [9]. As a consequence of the shortcomings of the currently existing methods, a gap remains in the early diagnosis and treatment of AKI. Additionally, there is no model that can predict the risk of AKI following PCI or CPB surgery.
The diagnosis of AKI currently relies on biochemical and early disease indicators [10,11]. Blood biochemical indicators and markers cannot fully reflect the functional status of a unilateral kidney. However, evaluation through functional imaging can compensate for this deficiency and can be of value in clinical practice. Functional imaging using a nuclide nephrogram, computed tomography, and magnetic resonance imaging can provide important information regarding the flow of blood and functional status of the kidney. However, they have the disadvantages of a high cost, radioactive contamination, and nephrotoxicity. Fortunately, ultrasound contrast agents are safe, are not nephrotoxic, and have no contraindications for renal insufficiency [12]. Studies have shown that contrast-enhanced renal ultrasound has a good correlation with the plasma filtration rate of the kidney. Moreover, contrast-enhanced ultrasound assessment of the overall and local flow of blood in the kidney during AKI is helpful in both diagnosis and prognosis [12,13]. Previous studies found that the peak intensity (PI) of contrast-enhanced renal ultrasound could change in patients with AKI at an early stage [12,14].
In this study, the characteristics of contrast-enhanced renal ultrasound and a variety of possible risk factors for AKI after PCI and CPB surgery were systematically analyzed. The study included disease-related factors (eg, congestive heart failure, high blood pressure, diabetes), treatment-related factors (eg, surgery and other related medications), and patient-related factors (eg, age, body mass index [BMI], sex, smoking history), as well as routine blood biochemical examination indicators. Based on these factors, it was possible to establish an effective nomogram model for early prediction of the risk of AKI after PCI or CPB surgery.
In summary, this study developed an effective and simple predictive tool that can assess the risk of AKI after PCI or CPB surgery simply by using available information. The prediction model is expected to enable improving the prognosis of patients with AKI and enhancing their quality of life.
Material and Methods
STUDY POPULATION AND DATA COLLECTION:
This retrospective study involved postoperative AKI in patients that had undergone either PCI or CPB. The study was approved by the Ethics Committee of the Shanxi Bethune Hospital (Shanxi Academy of Medical Sciences; approval no. YXLL-2019–021), and it met the requirements of the Declaration of Helsinki. All methods were performed in accordance with relevant guidelines and regulations. The patients were admitted to the Shanxi Bethune Hospital between June 2017 and July 2020 and underwent PCI or CPB surgery in the same facility. All patients lived in China and provided written informed consent to participate in the study. Patients who received hemodialysis, metformin, or nonsteroidal anti-inflammatory drugs were excluded from the study. Additionally, patients who died during surgery, refused further treatment, or were discharged for various reasons were excluded. Moreover, patients with a history of surgery or trauma within the previous month, known malignant tumors, fever, autoimmune diseases, acute or chronic inflammatory diseases, or recent infection history were excluded. If a patient had a fever (>37.5°C) in the course of treatment, they were also excluded. After careful examination of the data, 292 patients were included in the analysis. Finally, the patients were divided into 2 groups: AKI (−) and AKI (+). There were no significant differences between the groups with regard to the volume of contrast media administered during PCI, duration of cardiac surgery, and number of treated coronary vessels.
ASSESSED PARAMETERS:
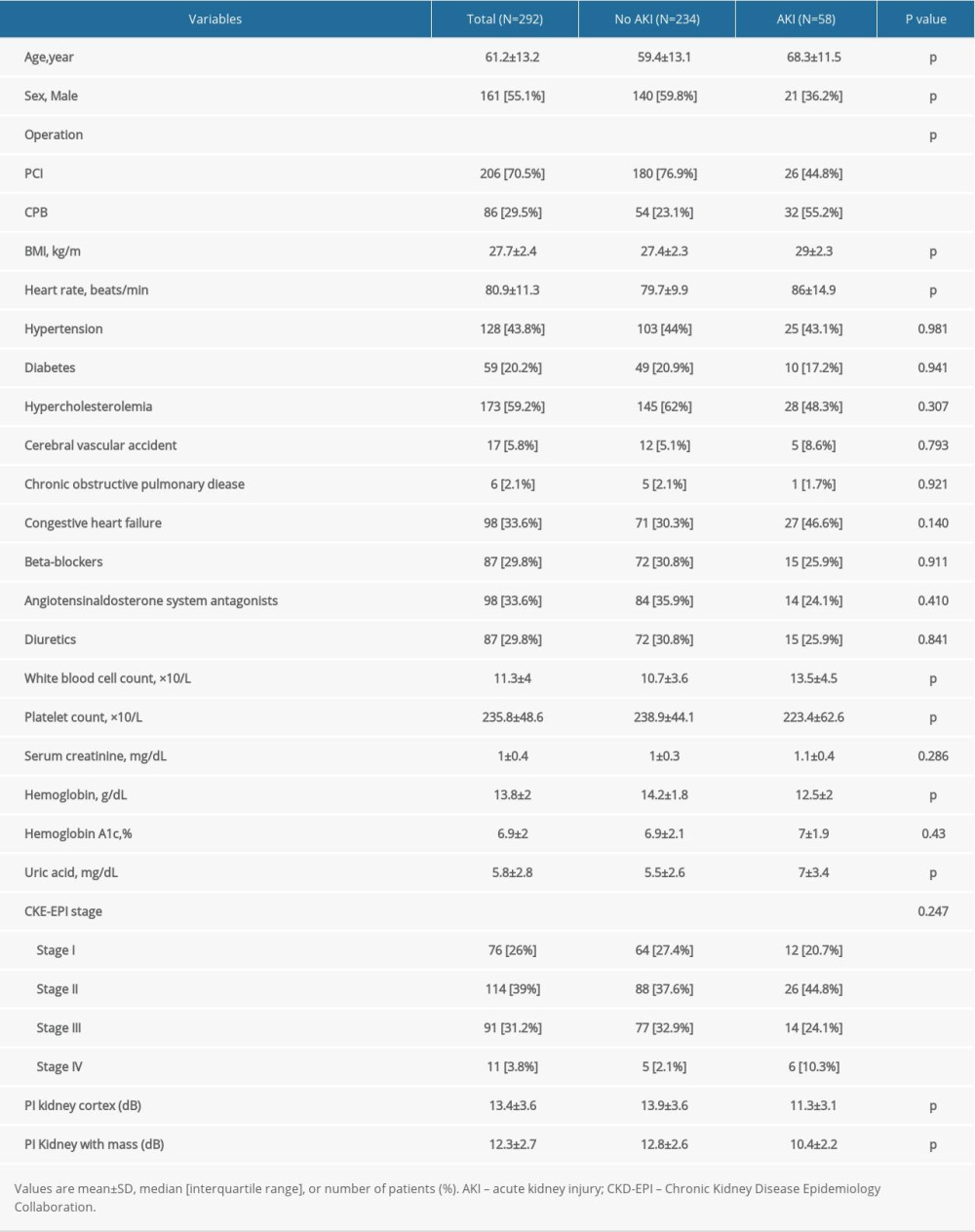
Patients’ basic information included sex, age, BMI, heart rate, history of diabetes, high blood pressure, hyper cholesterolemia, history of cerebrovascular accident, and history of smoking. Additionally, estimated glomerular filtration rate (eGFR) was derived from serum creatinine 1 to 2 days before surgery, according to the guidelines of the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [15]. Moreover, treatment features such as type of surgery and the use of diuretics, beta-blockers, and angiotensin-aldosterone system antagonists were included in the current analysis. Additional data such as white blood cell (WBC) and platelet counts, serum creatinine, hemoglobin, hemoglobin A1c, uric acid, PI of kidney cortex, and the PI of kidney medulla were also collected. Finally, postoperative AKI was diagnosed using the Acute Kidney Injury Network criteria (absolute increase in the serum creatinine concentration 0.3 mg/dL) within 48 h of operation [16]. Serum creatinine and urine output are the 2 defining criteria for AKI. However, as described in previous studies, urine volume has considerable limitations in the perioperative diagnosis of AKI [11]. Urine output is a nonspecific marker that is influenced by a combination of several clinical conditions, and therefore, we used only creatinine criteria.
INSTRUMENTS AND CONTRAST AGENTS:
The study used the Philips iU Elite ultrasound system, a C5-1 probe, a probe frequency of 1.0–5.0 MHz, and the ultrasound system’s built-in image analysis software QLAB. The mechanical index (MI) was equal to 0.08 and the gain adjustment was 65–75%. The contrast agent used was Sono Vue, a lyophilized powder of sulfur hexafluoride microbubbles coated with phospholipids. First, a suspension was prepared with 5 mL of normal saline. Thereafter, a cubital intravenous bolus injection at a dose of 0.02 mL/kg body weight was administered to each kidney.
CONTRAST ULTRASOUND:
The coronal sections of both kidneys in the left and right lateral positions were subjected to ultrasound imaging. Briefly, a switch was made to contrast-enhanced ultrasound conditions and the prepared SonoVue suspension was bolused through the cubital vein at a dose of 0.02 mL/kg body weight. This was followed by a bolus injection of 5 mL of normal saline for flushing. The bolusing step was timed, and the following aspects were maintained: the mechanical index, gain, depth, and other conditions. Additionally, the probe position was fixed and the patient was asked to breathe calmly to ensure that the scanning plane remained stable. The dynamic images were recorded within 3 min of the contrast agent bolus injection. The exact procedure was then repeated for the contralateral kidney 15 min after the previous one. After the contrast-enhanced ultrasound, the QLAB software was used for analysis. Briefly, the time-intensity curve (TIC) program was booted, and then the region of interest (ROI) was set to the default rectangular frame with a side length of 5 mm. Thereafter, the ultrasound contrast was combined with 2-dimensional images, and then the ROI between the middle of the kidney and the sound beam was selected. The cortex and medulla were sampled 3 times at the same depth. The software automatically drew the original TIC and used the local density random walk (LDRW) wash-in/wash-out (WIWO) fitting mode for curve fitting. Finally, the best fit for obtaining the PI of the cortex and medulla was chosen.
STATISTICAL ANALYSIS:
Statistical analysis was conducted using R software (version 3.5.3). A random forest model can quantify the significance of each feature in a data set and balance errors, and it is particularly well suited to handling high-latitude data [17]. First, we selected 24 features using the randomForestSRC software package. Additionally, the randomSurvivalForest algorithm was used to rank the prognosis-related genes (nrep=100, which indicates that the number of iterations in the Monte Carlo simulation was 100; nstep=5, which indicates that the number of steps forward was 5). Eventually, 22 features were identified as AKI-related signatures. Afterwards, the receiver-operating characteristic (ROC) curve from the univariate logistic regression analysis of continuous variables was obtained. The ROC value was then used to find the best cutoff point for the continuous variables. Afterwards, the continuous variables were divided into 2 groups based on the determined cutoff point, to facilitate clinical evaluation. Risk factor analysis was then performed using univariate and multivariate logistic regression analyses as previously reported [18]. To prevent overfitting of the prediction model, we performed dimensionality reduction via least absolute shrinkage and selection operator (LASSO) analysis and repeated it 1000 times to filter out the most suitable predictors [19]. The above 22 factors were incorporated into LASSO analysis in order to minimize data dimensionality and screen out predictors [19–22]. LASSO regression is similar to Ridge regression, but LASSO regression has more advantages in terms of variable selection. In this step, our aim was to filter the core variables to obtain a more clinically useful predictive model; therefore, we chose LASSO to filter the core variables.
As previously reported [23], the study cohort was randomly divided into the training and validation (7: 3) groups for diagnostic and prognostic analysis. This process was then used to develop and evaluate the models. Thereafter, the multivariate logistic regression method was used to establish a predictive model for the risk of AKI [19,22,24,25]. Furthermore, the study drew a calibration curve to evaluate the accuracy of the nomogram. Evaluation using the calibration curve alone was not sufficient [26]; therefore, to further quantify the identification performance of the nomogram, the C-index and the AUC curve were used. The R package was used to calculate a more accurate C-index for further verification on the nomogram (10 000 repeated samples) [27]. Moreover, decision curve analysis was used to evaluate the clinical applicability of the nomogram. This was done by quantifying the net income under different threshold probabilities in patient information. All the statistical tests were 2-sided, and P-values of less than 0.05 were considered statistically significant. This study reports a multivariate prediction model for individual prediction or diagnosis guidelines [28].
Results
BASIC CHARACTERISTICS OF PARTICIPANTS:
The study population consisted of 292 patients after PCI or CPB. The average age of the participants was 61±13 years, and 161 were men (55.1%). Out of the total number (n=292), AKI was observed in 58 patients (19.9%). The overall characteristics of the participants are presented in Table 1. The results show significant differences in age, sex, surgical method, BMI value, basal heart rate, and some biochemical indicators (eg, hemoglobin, uric acid, WBC count, and platelet count) between the 2 groups (P<0.05).
SCREENING FOR PREDICTION FACTORS:
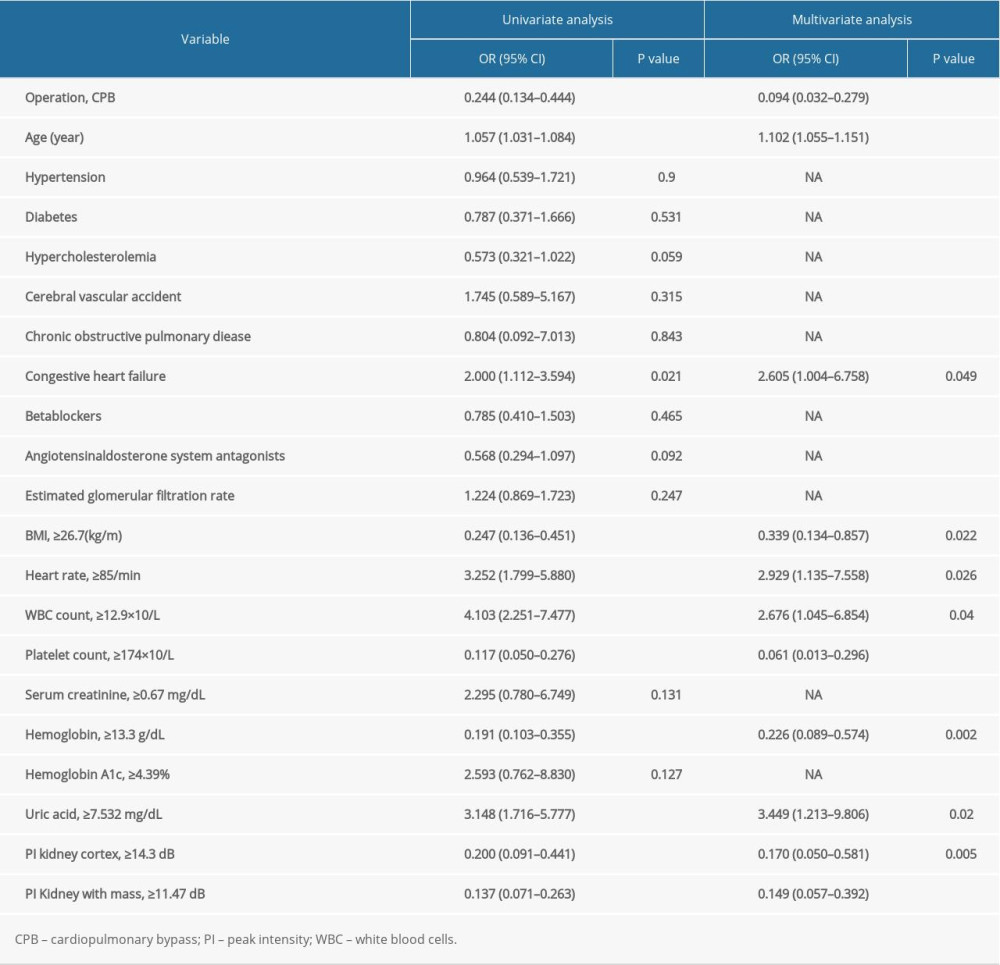
First, the randomForestSRC package was used for feature selection and 22 factors were identified as AKI-related signatures (Figure 1A). Additionally, the AUC index obtained by the random forest model was 0.84 (Figure 1B), indicating that the model composed of these factors had a good predictive ability. The multidimensional scale graph of the neighbor matrix generated by the random forest analysis also showed that the model had a certain layering ability, although it needed further optimization (Figure 1C) [8,18,29]. Moreover, the ROC curve for the univariate logistic regression analysis of continuous variables was obtained. The ROC value was then used to find the best cutoff point for the continuous variables. Eventually, the continuous variables were divided into 2 groups based on the cutoff point to facilitate clinical evaluation (Figure 1D, Table 2).
Overfitting of a model has always been a challenge in machine learning. Therefore, in order to prevent overfitting of the predictive model, the LASSO method was used to further reduce the number of features. The method is highly dependent on “seeds” because LASSO analysis requires cross-validation. However, the cross-validation process is meant for random selection of samples. Therefore, once the seed is changed, the optimal lambda as well as the resulting characteristics also change. In order to obtain the best combination of predictors, the study randomly performed 1000 LASSO regressions. They were then incorporated into the logistic model according to the number of times the predictors occurred and their calculated AUC values. The study found that inclusion of 11 factors could construct the simplest predictive model with a great forecasting ability (Figure 1E, 1F). Moreover, principal component analysis (PCA) revealed that the factors selected by the LASSO method could be divided into 2 (Figure 1G). This indicated that the factors had considerably unique prognostic properties in AKI after cardiac surgery. The blue dots represented patients who died, while the red ones indicated those that survived. The characteristic factors included operation methods, age, congestive heart failure, BMI, heart rate, WBC count, platelet count, hemoglobin, uric acid, and PI (kidney cortex as well as kidney medulla).
BUILDING A PERSONALIZED PREDICTIVE MODEL:
The research cohort was randomly divided into the training and validation sets (7: 3) for diagnosis and prognostic analysis. This was done to develop and evaluate the models. In the training group, the logistic model was used to analyze and determine the 11 predictive factors mentioned above. Thereafter, the nomogramEx package was used to construct a nomogram (Figure 2A, 2B). Notably, age, hemoglobin, uric acid, platelet count, operation methods, and PI (kidney cortex as well as kidney medulla) were identified as the important risk factors for AKI (P<0.01).
INSPECTION OF THE NOMOGRAM AND CLINICAL APPLICATION:
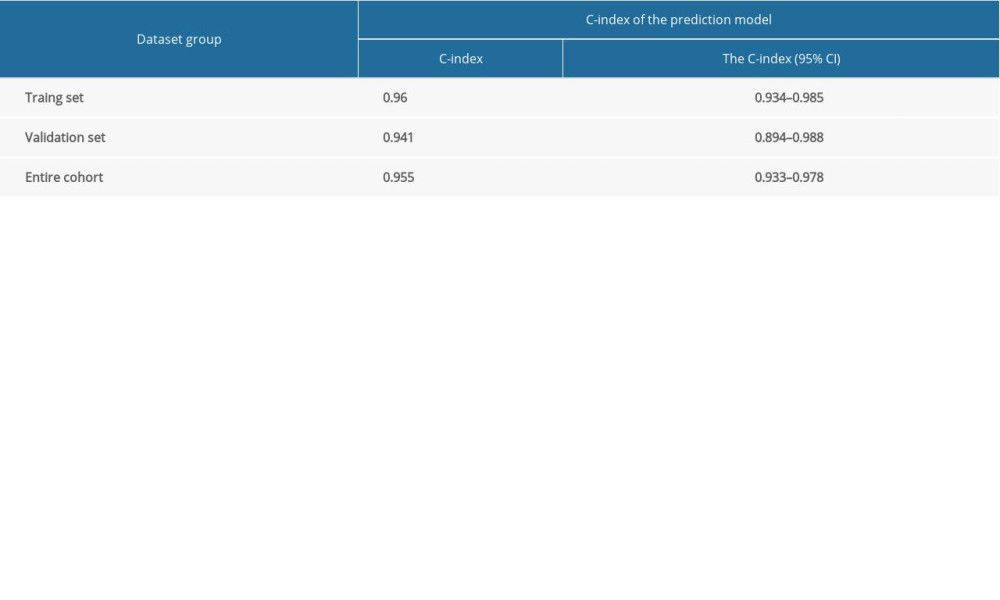
The nomogram calibration curve validated its effectiveness (Figure 3A), indicating that the model could be used to predict the risk of AKI after PCI or CPB. In addition, the AUC of this prediction model was 0.967 in the training set and 0.896 in the validation set (Figure 3B). Moreover, the C-index showed that the model performed well not only in the training and validation sets but also in the entire cohort (Table 3). These results prove that the model has a good predictive ability.
The decision curve analysis for the risk prediction model for AKI after PCI or CPB is shown in Figure 3C. As previously mentioned, decision curves have shown that using this model to predict the efficacy of AKI adds more benefit if the probability of developing AKI is between 2% and 100% in the entire cohort [30]. The decision curve showed that clinical decision making based on the nomogram could be beneficial. This means that the predictive model could effectively guide clinical practice. Additionally, it could facilitate timely prediction of a patient’s postoperative condition, which would ultimately improve postoperative prognosis as well as the patient’s quality of life.
Discussion
This study developed for the first time a new predictive model incorporating ultrasound examinations that can help clinicians predict the risk of AKI after PCI or CPB. Moreover, the nomogram displayed good quantitative indicators that could efficiently evaluate clinical efficacy and prognosis [18,29,31].
The tool combines 11 easily available variables into a user-friendly nomogram that may be helpful in personalized assessment of the risk of AKI after PCI or CPB. The internal sampling verification coupled with the high C-index as well as the AUC index showed that this prediction model can be widely and accurately used to predict the risk of AKI after PCI or CPB.
Previous studies have found that advanced age, diabetes, hypertension, and various chronic diseases are associated with the risk of developing AKI [32,33]. So far, diagnosis of AKI largely relies on biochemical and early disease indicators [11]. However, these markers may be affected by factors such as the severity, duration, time of detection, and etiology of AKI [9]. A number of scoring methods have been developed to assess the probability of AKI in patients in the perioperative period of cardiac surgery [32,34–36]. In previous studies, both the Birnie et al [32] scoring system and the Cleveland Clinic score [34] were constructed (they had comparable AUC values of 0.79 and 0.78 respectively). However, it is not certain that these systems are incorporated into routine clinical practice. Therefore, these methods are still insufficient for the early diagnosis and treatment of AKI. Functional imaging can make up for these deficiencies and give practical clinical value. Notably, ultrasound contrast agent microbubbles have the advantage of safety, lack of nephrotoxicity, and no contraindications for renal insufficiency [12]. Moreover, studies showed that renal contrast-enhanced ultrasound had a good correlation with renal plasma filtration rate. The assessment of overall renal and local blood flow using contrast-enhanced ultrasound is helpful for diagnosis and prognosis of AKI [13,14]. Furthermore, previous studies showed that the PI in contrast-enhanced renal ultrasound could change in patients with early AKI [14,37]. This study similarly found that the PI of the renal cortex and medulla decreased in patients with AKI.
In this study, about 20% of patients developed AKI after PCI or CPB. Additionally, the major risk factors for AKI included PCI, advanced age, congestive heart failure, high BMI, rapid heart rate, high WBC count, low platelet count, low hemoglobin, high uric acid, and high PI index (including PI in kidney cortex as well as kidney medulla). Out of these, age, hemoglobin, uric acid, platelet count, operation methods and PI (kidney cortex as well as kidney medulla) were found to be the most important predisposing factors to AKI.
Insufficient renal perfusion is the main cause of acute renal failure after cardiac surgery [38]. Additionally, heart failure is a recognized risk factor to AKI following cardiac surgery and is closely related to impaired renal perfusion during the operation [39,40]. Moreover, a rapid heart rate often indicates worse cardiac perfusion ability. Interestingly, a previous study showed that the use of dexmedetomidine after cardiac surgery to improve myocardial perfusion by reducing heart rate did not increase the risk of acute renal failure [41]. In addition, risk factors for AKI, including advanced age, have previously been reported in literature [42]. Old age and high BMI are independent risk factors for postoperative AKI in patients with type A acute aortic dissection [43]. This was similar to the results of this study since obesity may increase the risk of AKI through oxidative stress [44].
This research suggests that biochemical indicators such as WBC count, hemoglobin level, platelet count, and uric acid levels may reflect the risk of acute renal failure, corroborating previous studies. Increased WBC counts are independent predictors of AKI and are associated with neutrophil-mediated injury as well as dysfunction [45]. In addition, preoperative anemia in CKD patients was associated with poor outcomes after cardiac surgery. Therefore, having a higher hemoglobin level before heart surgery may lead to a better prognosis [46]. Moreover, low platelet count is a well-known biomarker of organ failure and has been extensively studied. A decrease in the platelet count projects a poor prognosis of AKI [47]. Similarly, this study found that the risk of AKI was also related to the level of hemoglobin. This finding suggests that the level of hemoglobin might be an independent risk factor for AKI after cardiac surgery. Furthermore, previous studies highlighted that there was a strong correlation between serum uric acid levels and AKI in several disease models. Hyperuricemia is related to the presence of crystals in the kidney, appearance of tubular glomeruli, infiltration of macrophages, and increased expression of inflammatory mediators. However, these symptoms can be resolved upon administration of treatments that lower serum uric acid levels [48]. Moreover, serum uric acid has been linked to chronic kidney disease, cardiovascular disease, and hypertension, and their mechanisms were found to have many features in common [48].
After PCI or CPB surgery, doctors in the Intensive Care Department need to take into account the overall condition of patients and prevent complications related to surgery. Although the factors of the prediction model are not modifiable, the ability to predict the risk of developing AKI early is a major advantage of the modified prediction model. Recent studies point to early detection and timely intervention of prevention strategies as key to reducing the incidence of AKI in the perioperative period [11]. For example, the use of bundled interventions has been shown to be a successful method of reducing morbidity and mortality in patients with perioperative AKI [49]. A timely prediction of the risk of AKI will therefore enable doctors to better complete preventive prescriptions before complications occur, thereby improving patient prognosis. In addition, timely prediction of complications helps with communication between doctors and patients and thereby improves compliance to treatment by both patients and their families. Additionally, it can prevent unnecessary interventions and waste of medical resources. The findings of this study show that the assessment of overall renal and local blood flow using contrast-enhanced ultrasound is helpful for the diagnosis and prognosis of AKI. Therefore, the predictive model that we have developed could serve as a tool to provide further theoretical guidance for clinical surgical treatment and research for heart disease patients.
The ultrasound examinations in the current study were performed by experienced doctors. This was done to avoid bias caused by differences in the ultrasound diagnostic capabilities of different physicians. However, despite the insights that it provides, this study had a few limitations. First, all the patients were from China. Therefore, the model may not be generalizable to predict the risk of AKI after PCI or CPB in patients from other regions. Second, complete patient information was not obtained due to departmental and funding restrictions, and objective factors prevented us from including all clinical characteristics (eg, economic status and mental status). Fortunately, our model had relatively strong predictive power because of its higher AUC value and C-index. This study has developed for the first time a new predictive model that incorporates ultrasound examinations, has excellent statistical accuracy, and can help clinicians predict the risk of AKI after PCI or CPB. Our study showed that the model incorporating current factors has the strongest predictive power. However, this does not preclude the existence of other meaningful predictors (such as sex and so forth). Third, although our prediction model had a high prediction accuracy, the model predicted the risk of AKI only as an aid; good prediction accuracy needs to be closely integrated with actual clinical work. Fourth, the data used in this study were obtained from years of clinical history and past clinical work. Thus, although we tried to ensure accuracy and eliminate confounders in this study, it is possible that omissions or errors were made by various personnel. Although our research is highly predictive, more systematic and multi-center prospective studies are still needed. In addition, the future application of ultrasonography in the early assessment of AKI will require standardization of examination techniques and analysis.
Conclusions
This study developed for the first time a new predictive model that incorporates ultrasound examinations, has excellent statistical accuracy, and can help clinicians predict the risk of AKI after PCI or CPB. The predictors include operation methods, age, congestive heart failure, BMI, heart rate, WBC count, platelet count, hemoglobin, uric acid, and PI (kidney cortex as well as kidney medulla). Through this model, clinicians can prevent the occurrence of AKI in patients after surgery. Additionally, it can help provide better prognosis guidance for patients ultimately improving postoperative prognosis and quality of life. However, further research is needed to validate the ability of the model to effectively predict the risk of AKI after PCI or CPB.
Figures
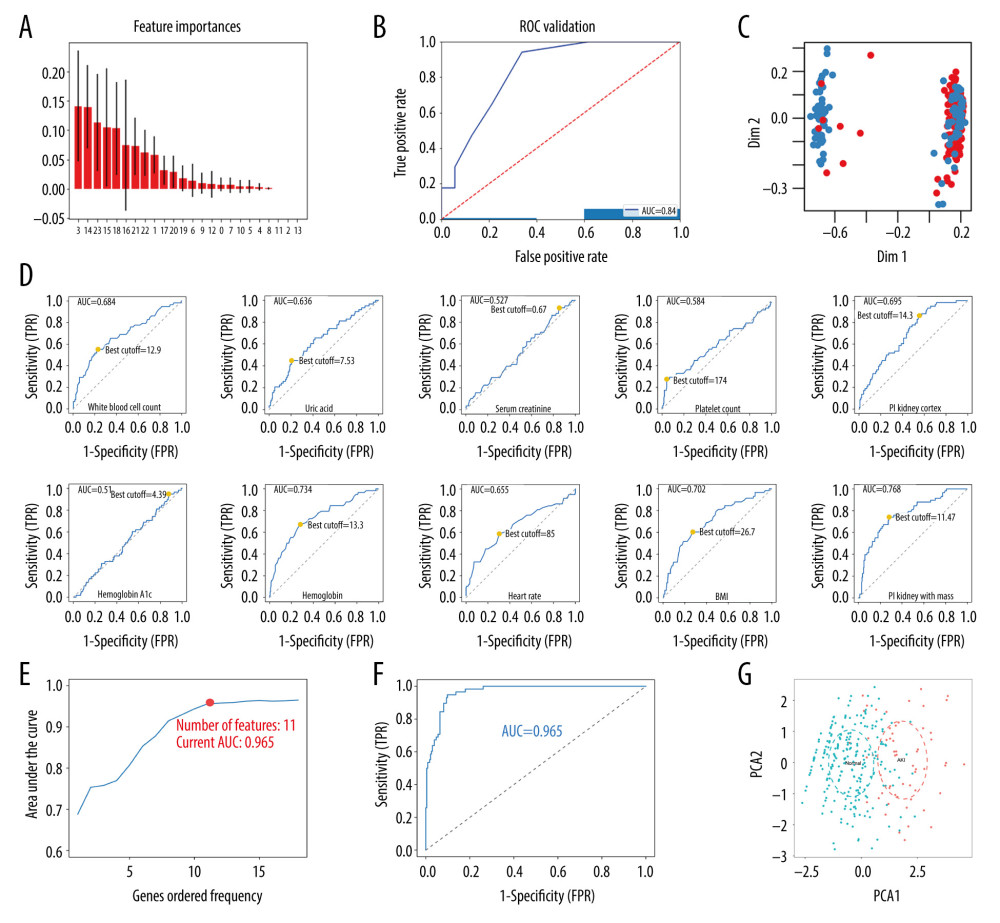 Figure 1. Screening for prediction factors. (A) Importance values of each factor in the random forest model. (B) The ROC curve (AUC=0.84) demonstrated the accuracy of the random forest model. (C) Random forest analysis generated a multidimensional scale map of the matrix. The blue dot represents the normal group and the red dot represents the AKI group. (D) The best cutoff points for the continuous variables. (E) According to the number of times the factors occurred, they were included in the logistic model and the pattern diagram of the AUC value was obtained. Eventually, it was evident that the simplest predictive model with a good predictive ability with only 11 predictive factors could be constructed. (F) The ROC curve (AUC=0.965) demonstrates the accuracy of the logistic model. (G) PCA reveals that those factors selected by the LASSO method could be divided into 2 parts. This indicated that the factors had relatively unique prognostic properties in AKI after cardiac surgery (including CPB and PCI). AKI – acute kidney injury; AUC – area under the curve; CPB – cardiopulmonary bypass; LASSO – least absolute shrinkage and selection operator; PCA – principal component analysis; PCI – percutaneous coronary intervention; ROC – receiver-operating characteristic
Figure 1. Screening for prediction factors. (A) Importance values of each factor in the random forest model. (B) The ROC curve (AUC=0.84) demonstrated the accuracy of the random forest model. (C) Random forest analysis generated a multidimensional scale map of the matrix. The blue dot represents the normal group and the red dot represents the AKI group. (D) The best cutoff points for the continuous variables. (E) According to the number of times the factors occurred, they were included in the logistic model and the pattern diagram of the AUC value was obtained. Eventually, it was evident that the simplest predictive model with a good predictive ability with only 11 predictive factors could be constructed. (F) The ROC curve (AUC=0.965) demonstrates the accuracy of the logistic model. (G) PCA reveals that those factors selected by the LASSO method could be divided into 2 parts. This indicated that the factors had relatively unique prognostic properties in AKI after cardiac surgery (including CPB and PCI). AKI – acute kidney injury; AUC – area under the curve; CPB – cardiopulmonary bypass; LASSO – least absolute shrinkage and selection operator; PCA – principal component analysis; PCI – percutaneous coronary intervention; ROC – receiver-operating characteristic 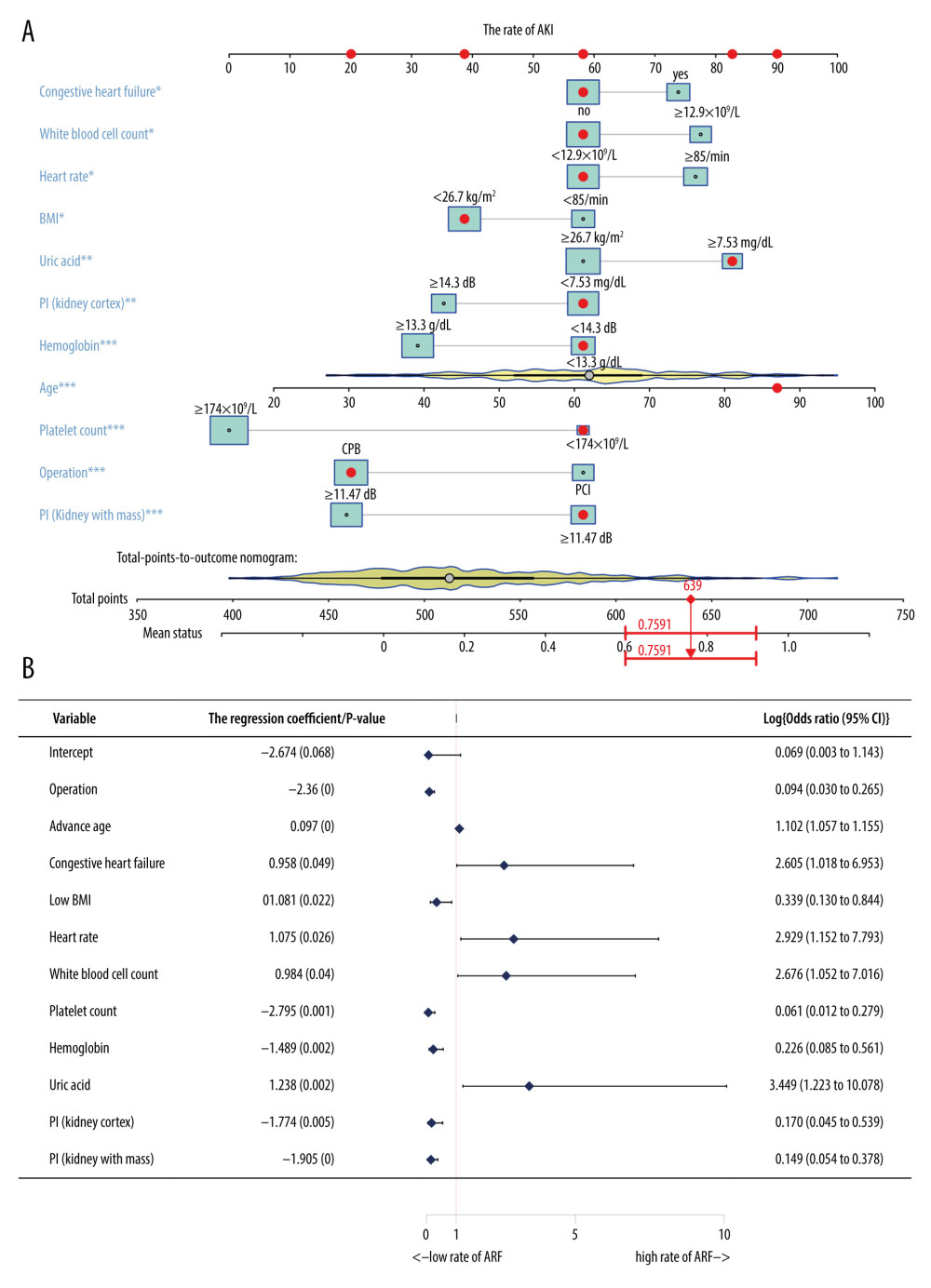 Figure 2. Building a personalized predictive model. (A) A nomogram model predicting the risk of AKI. Note: 11 factors including operation methods, age, congestive heart failure, BMI – heart rate; WBC count – platelet count, hemoglobin, uric acid and PI (kidney cortex as well as kidney medulla) were included in the model. * P<0.05, ** P<0.01, *** P<0.005. (B) A forest chart showing the prediction factors of AKI after PCI or CPB surgery. AKI – acute kidney injury; BMI – body mass index; CPB – cardiopulmonary bypass; PCI – percutaneous coronary intervention; PI – peak intensity; WBC – white blood cell.
Figure 2. Building a personalized predictive model. (A) A nomogram model predicting the risk of AKI. Note: 11 factors including operation methods, age, congestive heart failure, BMI – heart rate; WBC count – platelet count, hemoglobin, uric acid and PI (kidney cortex as well as kidney medulla) were included in the model. * P<0.05, ** P<0.01, *** P<0.005. (B) A forest chart showing the prediction factors of AKI after PCI or CPB surgery. AKI – acute kidney injury; BMI – body mass index; CPB – cardiopulmonary bypass; PCI – percutaneous coronary intervention; PI – peak intensity; WBC – white blood cell. 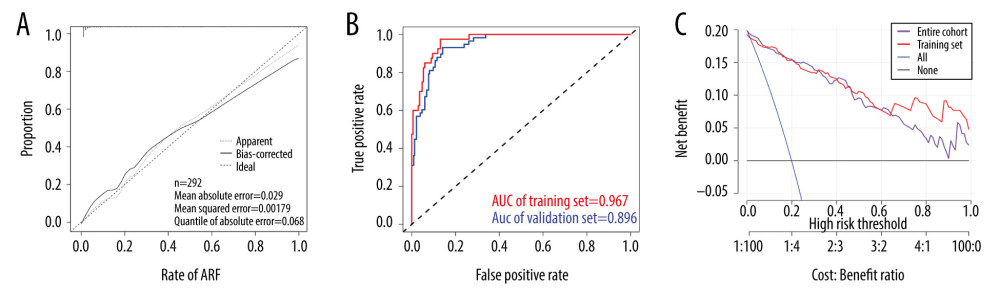 Figure 3. Inspection of the nomogram and clinical applications. (A) A calibration curve for the predictive model of the risk of AKI after PCI or CPB surgery. When the solid line closely fitted with the dotted line, an improved predictive ability was deduced. (B) The AUC indicates the prediction ability of the model. The model exhibited good predictive power, with the AUC values of the training group (red) and the validation group (blue) recorded as 0.967 and 0.896, respectively. (C) Decision curve for the estimation of AKI risk. “None” assumes that no AKI occurred in all patients. “All” assumes that AKI occurred in all patients. AKI – acute kidney injury; AUC – area under the curve; CPB – cardiopulmonary bypass; PCI – percutaneous coronary intervention.
Figure 3. Inspection of the nomogram and clinical applications. (A) A calibration curve for the predictive model of the risk of AKI after PCI or CPB surgery. When the solid line closely fitted with the dotted line, an improved predictive ability was deduced. (B) The AUC indicates the prediction ability of the model. The model exhibited good predictive power, with the AUC values of the training group (red) and the validation group (blue) recorded as 0.967 and 0.896, respectively. (C) Decision curve for the estimation of AKI risk. “None” assumes that no AKI occurred in all patients. “All” assumes that AKI occurred in all patients. AKI – acute kidney injury; AUC – area under the curve; CPB – cardiopulmonary bypass; PCI – percutaneous coronary intervention. References
1. Kuitunen A, Vento A, Suojaranta-Ylinen R, Pettilä V, Acute renal failure after cardiac surgery: Evaluation of the RIFLE classification: Ann Thorac Surg, 2006; 81; 542-46
2. Hobson CE, Yavas S, Segal MS, Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery: Circulation, 2009; 119; 2444-53
3. Lok CE, Austin PC, Wang H, Tu JV, Impact of renal insufficiency on short- and long-term outcomes after cardiac surgery: Am Heart J, 2004; 148; 430-38
4. Schneider A, Johnson L, Goodwin M, Bench-to-bedside review: Contrast enhanced ultrasonography – a promising technique to assess renal perfusion in the ICU: Crit Care, 2011; 15; 157
5. Lassnigg A, Schmidlin D, Mouhieddine M, Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: A prospective cohort study: J Am Soc Nephrol, 2004; 15; 1597-605
6. Koyner JL, Garg AX, Coca SG, Biomarkers predict progression of acute kidney injury after cardiac surgery: J Am Soc Nephrol, 2012; 23; 905-14
7. Ho J, Reslerova M, Gali B, Serum creatinine measurement immediately after cardiac surgery and prediction of acute kidney injury: Am J Kidney Dis, 2012; 59; 196-201
8. Song Y, Kim DW, Kwak YL, Urine output during cardiopulmonary bypass predicts acute kidney injury after cardiac surgery: A single-center retrospective analysis: Medicine (Baltimore), 2016; 95; e3757
9. Chen XK, Li WX, Progress and review in acute kidney injury: Chin J Crit Care Med, 2014; 34(2); 111-15
10. Sharma A, Chakraborty R, Sharma K, Development of acute kidney injury following pediatric cardiac surgery: Kidney Res Clin Pract, 2020; 39; 259-68
11. Massoth C, Zarbock A, Meersch M, Risk stratification for targeted AKI prevention after surgery: Biomarkers and bundled interventions: Semin Nephrol, 2019; 39; 454-61
12. Haifang L, Yixing F, Qiang Y, The clinical value in contrast enhanced ultrasonography in evaluation of renal blood perfusion before and after percutaneous transluminal renal angiography with stent: Chin J Ultrasound Med, 2014; 30(10); 908-10
13. Kalantarinia K, Okusa MD, Ultrasound contrast agents in the study of kidney function in health and disease: Drug Discov Today Dis Mech, 2007; 4; 153-58
14. Qiao SStudy on blood perfusion of kidney in rabbits with acute kidney injury caused by cisplatin with contrast enhanced ultrasound: Anhui Medical University [in Chinese]
15. Matsushita K, Mahmoodi BK, Woodward M, Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate: JAMA, 2012; 307; 1941-51
16. Mehta RL, Kellum JA, Shah SV, Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury: Crit Care, 2007; 11; R31
17. Buxton RT, McKenna MF, Clapp M, Efficacy of extracting indices from large-scale acoustic recordings to monitor biodiversity: Conserv Biol, 2018; 32; 1174-84
18. Hu M, Zhong X, Cui X, Development and validation of a risk-prediction nomogram for patients with ureteral calculi associated with urosepsis: A retrospective analysis: PLoS One, 2018; 13; e0201515
19. Kidd AC, McGettrick M, Tsim S, Survival prediction in mesothelioma using a scalable Lasso regression model: Instructions for use and initial performance using clinical predictors: BMJ Open Respir Res, 2018; 5; e000240
20. Friedman J, Hastie T, Tibshirani R, Regularization paths for generalized linear models via coordinate descent: J Stat Softw, 2010; 33; 1-22
21. Sauerbrei W, Royston P, Binder H, Selection of important variables and determination of functional form for continuous predictors in multivariable model building: Stat Med, 2007; 26; 5512-28
22. Sveen A, Ågesen TH, Nesbakken A, ColoGuidePro: A prognostic 7-gene expression signature for stage III colorectal cancer patients: Clin Cancer Res, 2012; 18; 6001-10
23. Chen YS, Cai YX, Kang XR, Predicting the risk of sarcopenia in elderly patients with patellar fracture: Development and assessment of a new predictive nomogram: Peer J, 2020; 8; e8793
24. Iasonos A, Schrag D, Raj GV, Panageas KS, How to build and interpret a nomogram for cancer prognosis: J Clin Oncol, 2008; 26; 1364-70
25. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP, Nomograms in oncology: More than meets the eye: Lancet Oncol, 2015; 16; e173-80
26. Kramer AA, Zimmerman JE, Assessing the calibration of mortality benchmarks in critical care: The Hosmer-Lemeshow test revisited: Crit Care Med, 2007; 35; 2052-56
27. Pencina MJ, D’Agostino RB, Overall C as a measure of discrimination in survival analysis: Model specific population value and confidence interval estimation: Stat Med, 2004; 23; 2109-23
28. Collins GS, Reitsma JB, Altman DG, Moons KG, Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement: BMJ, 2015; 350; g7594
29. Chen YS, Kang XR, Zhou ZH, MiR-1908/EXO1 and MiR-203a/FOS, regulated by scd1, are associated with fracture risk and bone health in postmenopausal diabetic women: Aging (Albany NY), 2020; 12; 9549-84
30. Wang H, Zhang L, Liu Z, Predicting medication nonadherence risk in a Chinese inflammatory rheumatic disease population: Development and assessment of a new predictive nomogram: Patient Prefer Adherence, 2018; 12; 1757-65
31. Wei L, Champman S, Li X, Beliefs about medicines and non-adherence in patients with stroke, diabetes mellitus and rheumatoid arthritis: A cross-sectional study in China: BMJ Open, 2017; 7; e017293
32. Birnie K, Verheyden V, Pagano D, Predictive models for kidney disease: Improving global outcomes (KDIGO) defined acute kidney injury in UK cardiac surgery: Crit Care, 2014; 18; 606
33. Biteker M, Dayan A, Tekkeşin A, Incidence, risk factors, and outcomes of perioperative acute kidney injury in noncardiac and nonvascular surgery: Am J Surg, 2014; 207; 53-59
34. Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP, A clinical score to predict acute renal failure after cardiac surgery: J Am Soc Nephrol, 2005; 16; 162-68
35. Ng SY, Sanagou M, Wolfe R, Prediction of acute kidney injury within 30 days of cardiac surgery: J Thorac Cardiovasc Surg, 2014; 147; 1875-83
36. Mehta RH, Grab JD, O’Brien SM, Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery: Circulation, 2006; 114; 2208-16
37. Qiao S, Jia H, Liang H, Study on blood perfusion of kidney in rabbits with acute kidney injury caused by cisplatin with contrast enhanced ultrasound: Chinese J Ultrasound Med, 2016; 32(11); 1048-51
38. Abu-Omar Y, Ratnatunga C, Cardiopulmonary bypass and renal injury: Perfusion, 2006; 21; 209-13
39. Chertow GMLJ, Christiansen CL, Preoperative renal risk stratification: Circulation, 1997; 95; 878-84
40. Raimundo MCS, Syed Y, Low systemic oxygen delivery and BP and risk of pression of early AKI: Clin J Am Soc Nephrol, 2015; 10; 1340-49
41. Li X, Zhang C, Dai D, Liu H, Ge S, Efficacy of dexmedetomidine in prevention of junctional ectopic tachycardia and acute kidney injury after pediatric cardiac surgery: A meta-analysis: Congenit Heart Dis, 2018; 13; 799-807
42. Brunetti L, Song JH, Suh D, The risk of vancomycin toxicity in patients with liver impairment: Ann Clin Microbiol Antimicrob, 2020; 19(1); 13
43. Wang J, Yu W, Zhai G, Independent risk factors for postoperative AKI and the impact of the AKI on 30-day postoperative outcomes in patients with type A acute aortic dissection: An updated meta-analysis and meta-regression: J Thorac Dis, 2018; 10; 2590-98
44. Billings FT, Pretorius M, Schildcrout JS, Obesity and oxidative stress predict AKI after cardiac surgery: J Am Soc Nephrol, 2012; 23; 1221-28
45. Weylandt KH, Chiu CY, Gomolka B, Omega-3 fatty acids and their lipid mediators: Towards an understanding of resolvin and protectin formation: Prostaglandins Other Lipid Mediat, 2012; 97; 73-82
46. Shavit L, Hitti S, Silberman S, Preoperative hemoglobin and outcomes in patients with CKD undergoing cardiac surgery: Clin J Am Soc Nephrol, 2014; 9; 1536-44
47. Greco E, Lupia E, Bosco O, Platelets and multi-organ failure in sepsis: Int J Mol Sci, 2017; 18(10); 2200
48. Ejaz AA, Johnson RJ, Shimada M, The role of uric acid in acute kidney injury: Nephron, 2019; 142(4); 275-83
49. Stewart JA, Adding insult to injury: Care of patients with acute kidney injury: Br J Hosp Med (Lond), 2009; 70; 372-73
Figures
 Figure 1. Screening for prediction factors. (A) Importance values of each factor in the random forest model. (B) The ROC curve (AUC=0.84) demonstrated the accuracy of the random forest model. (C) Random forest analysis generated a multidimensional scale map of the matrix. The blue dot represents the normal group and the red dot represents the AKI group. (D) The best cutoff points for the continuous variables. (E) According to the number of times the factors occurred, they were included in the logistic model and the pattern diagram of the AUC value was obtained. Eventually, it was evident that the simplest predictive model with a good predictive ability with only 11 predictive factors could be constructed. (F) The ROC curve (AUC=0.965) demonstrates the accuracy of the logistic model. (G) PCA reveals that those factors selected by the LASSO method could be divided into 2 parts. This indicated that the factors had relatively unique prognostic properties in AKI after cardiac surgery (including CPB and PCI). AKI – acute kidney injury; AUC – area under the curve; CPB – cardiopulmonary bypass; LASSO – least absolute shrinkage and selection operator; PCA – principal component analysis; PCI – percutaneous coronary intervention; ROC – receiver-operating characteristic
Figure 1. Screening for prediction factors. (A) Importance values of each factor in the random forest model. (B) The ROC curve (AUC=0.84) demonstrated the accuracy of the random forest model. (C) Random forest analysis generated a multidimensional scale map of the matrix. The blue dot represents the normal group and the red dot represents the AKI group. (D) The best cutoff points for the continuous variables. (E) According to the number of times the factors occurred, they were included in the logistic model and the pattern diagram of the AUC value was obtained. Eventually, it was evident that the simplest predictive model with a good predictive ability with only 11 predictive factors could be constructed. (F) The ROC curve (AUC=0.965) demonstrates the accuracy of the logistic model. (G) PCA reveals that those factors selected by the LASSO method could be divided into 2 parts. This indicated that the factors had relatively unique prognostic properties in AKI after cardiac surgery (including CPB and PCI). AKI – acute kidney injury; AUC – area under the curve; CPB – cardiopulmonary bypass; LASSO – least absolute shrinkage and selection operator; PCA – principal component analysis; PCI – percutaneous coronary intervention; ROC – receiver-operating characteristic Figure 2. Building a personalized predictive model. (A) A nomogram model predicting the risk of AKI. Note: 11 factors including operation methods, age, congestive heart failure, BMI – heart rate; WBC count – platelet count, hemoglobin, uric acid and PI (kidney cortex as well as kidney medulla) were included in the model. * P<0.05, ** P<0.01, *** P<0.005. (B) A forest chart showing the prediction factors of AKI after PCI or CPB surgery. AKI – acute kidney injury; BMI – body mass index; CPB – cardiopulmonary bypass; PCI – percutaneous coronary intervention; PI – peak intensity; WBC – white blood cell.
Figure 2. Building a personalized predictive model. (A) A nomogram model predicting the risk of AKI. Note: 11 factors including operation methods, age, congestive heart failure, BMI – heart rate; WBC count – platelet count, hemoglobin, uric acid and PI (kidney cortex as well as kidney medulla) were included in the model. * P<0.05, ** P<0.01, *** P<0.005. (B) A forest chart showing the prediction factors of AKI after PCI or CPB surgery. AKI – acute kidney injury; BMI – body mass index; CPB – cardiopulmonary bypass; PCI – percutaneous coronary intervention; PI – peak intensity; WBC – white blood cell. Figure 3. Inspection of the nomogram and clinical applications. (A) A calibration curve for the predictive model of the risk of AKI after PCI or CPB surgery. When the solid line closely fitted with the dotted line, an improved predictive ability was deduced. (B) The AUC indicates the prediction ability of the model. The model exhibited good predictive power, with the AUC values of the training group (red) and the validation group (blue) recorded as 0.967 and 0.896, respectively. (C) Decision curve for the estimation of AKI risk. “None” assumes that no AKI occurred in all patients. “All” assumes that AKI occurred in all patients. AKI – acute kidney injury; AUC – area under the curve; CPB – cardiopulmonary bypass; PCI – percutaneous coronary intervention.
Figure 3. Inspection of the nomogram and clinical applications. (A) A calibration curve for the predictive model of the risk of AKI after PCI or CPB surgery. When the solid line closely fitted with the dotted line, an improved predictive ability was deduced. (B) The AUC indicates the prediction ability of the model. The model exhibited good predictive power, with the AUC values of the training group (red) and the validation group (blue) recorded as 0.967 and 0.896, respectively. (C) Decision curve for the estimation of AKI risk. “None” assumes that no AKI occurred in all patients. “All” assumes that AKI occurred in all patients. AKI – acute kidney injury; AUC – area under the curve; CPB – cardiopulmonary bypass; PCI – percutaneous coronary intervention. Tables
In Press
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952