13 July 2021: Clinical Research
Risk Factors for Fentanyl-Induced Cough Following General Anesthesia in Adults: A Retrospective Study from a Single Center in China
Xiao Zhao1ABCDEF, Hong Wang1ABCDEF, Hui-Juan Wang1BCDEF, Yun Wang1BCDEF, Yi-An Xing1BCD, Shi-Tong Li1ABC*, Lian-Hua Chen1ABCDOI: 10.12659/MSM.930369
Med Sci Monit 2021; 27:e930369
Abstract
BACKGROUND: Fentanyl-induced cough (FIC) during general anesthesia induction and postoperative nausea and vomiting are common complications, yet the risk factors for FIC remain controversial. This retrospective study was conducted at a single center in China and aimed to investigate the risk factors for fentanyl-induced cough following general anesthesia in adults.
MATERIAL AND METHODS: A total of 601 adult patients undergoing elective surgery were enrolled, and the incidence of FIC during general anesthesia induction and postoperative adverse events were recorded. The risk factors for FIC during general anesthesia induction and postoperative nausea and vomiting were assessed using multivariate logistic regression analysis.
RESULTS: The incidence of FIC, nausea, and vomiting were 21.8%, 6.3%, and 4.5%, respectively. The results of multivariate logistic regression analysis indicated that pharyngitis history was associated with an increased risk of FIC during general anesthesia induction (odds ratio [OR]: 2.852; 95% confidence interval [CI]: 1.698-4.792; P<0.001), whereas use of lidocaine could protect against FIC risk (OR: 0.649; 95% CI: 0.557-0.757; P<0.001). However, the characteristics of patients were not associated with the risk of postoperative nausea and vomiting.
CONCLUSIONS: The findings from this study showed that a history of pharyngitis increased the risk of FIC, while the use of lidocaine was associated with a reduced risk of FIC. The risk of postoperative nausea and vomiting was not affected by fentanyl use or patient characteristics.
Keywords: Anesthesia, General, Cough, Postoperative Complications, Adjuvants, Anesthesia, Administration, Intravenous, Anesthetics, Intravenous, Elective Surgical Procedures, Fentanyl, Incidence, Lidocaine, Odds Ratio, Postoperative Nausea and Vomiting, Risk Factors, young adult
Background
There remain gaps in the assessment, prevention, and treatment of pain in critically ill adults [1–4]. Opioids are widely used for analgesia and premedication. The advantages of opioids include perioperative analgesia, attenuation of the hemodynamic response, and reduction in the requirement of inhalational agents during general anesthesia. Currently, several short-acting opioids, including alfentanil, fentanyl, and sufentanil, are often used as intravenous boluses, which is associated with faster onset and recovery and high potency, with few adverse effects. Fentanyl is one of the widely used opioids for perioperative analgesia due to its rapid onset, short duration, intense analgesia, and low histamine release [5–8]. However, fentanyl-induced coughing (FIC) is a common adverse reaction occurring in 28–65% of patients, which always occurs within 2 min after fentanyl injection [9]. Although FIC in most patients is benign, transient, and self-limiting, it can be spasmodic or explosive and life-threatening, needing immediate intervention, especially for neurosurgical and ophthalmic procedures [10,11]. A previous study indicated FIC and postoperative nausea and vomiting share some common mechanisms, and FIC is associated with an increased risk of postoperative nausea and vomiting [12].
Numerous studies have already illustrated the role of pharmacological and nonpharmacological treatment strategies in the progression of FIC [13–25]. However, whether the incidence of FIC during general anesthesia induction and postoperative adverse events are associated with patient characteristics of remains controversial. Clarifying the risk factors for preventing FIC and postoperative adverse events is particularly important in patients undergoing general anesthesia induction, as it has not been definitively determined. Therefore, this retrospective study aimed to investigate the risk factors for FIC following general anesthesia in adults at a single center in China.
Material and Methods
PATIENTS:
This study was approved by the Ethics Committee of our hospital. The purpose and procedures of the study were carefully explained and written informed consent was obtained from all participants. A total of 601 patients undergoing general anesthesia induction were enrolled in this study between January 2015 and October 2018. All the included patients underwent general anesthesia induction and were ≥18.0 years of age. The exclusion criteria of this study were age <18.0 years, absence of preanesthetic anxiolytic drugs, administration of lidocaine or antitussive agents within the previous week, and persistent symptomatic coughing that would make it difficult to identify FIC. All patients were fasting for 8 h before the operation, and no anesthesia was used before the operation. The venous access of the upper limb was opened in the pre-anesthesia room, and the sodium lactate Ringer’s solution was supplemented according to the preoperative loss. The monitor was connected, and the electrocardiogram, noninvasive blood pressure, and pulse oxygen saturation of upper limb were routinely monitored.
DATA COLLECTION:
The baseline characteristics of the enrolled patients were collected using a pre-structured questionnaire and medical records, and all patients received physical examinations and systemic examination for chronic disease. The collected information included gender, age, type of surgery, operative time, body mass index (BMI), intraoperative blood loss, smoking status, alcohol intake, pharyngitis, history of gastroesophageal reflux, other digestive tract diseases, hypertension, diabetes mellitus, history of asthma, psychiatric history, history of respiratory infection (within 2 months), history of surgery, lidocaine, and use of midazolam, dexmedetomidine, sufentanil, propofol, and rocuronium bromide.
ANESTHESIA INDUCTION:
After 3 min of pure oxygen mask ventilation, patients received intravenous injection of lidocaine 0.04 ml/kg (2% concentration), midazolam 0.02–0.03 mg/kg, intravenous infusion of sufentanil 18 μg/kg/h for 1 min, propofol 120 mg/kg/h for 1 min, and injection of rocuronium 0.8 mg/kg after the loss of consciousness. Then, endotracheal intubation was applied after 120 s, and mechanical ventilation was used for inhalation of 60% concentration of oxygen. The respiratory parameters were adjusted as follows: tidal volume: 8–10 ml/kg, respiratory rate: 10–15 times/min, inhalation/respiration ratio: 1: 1.5. End-tidal carbon dioxide partial pressure was maintained at 35–45 mmhg during the whole course. Sevoflurane (0.8–1.2 mac) was inhaled and rocuronium and sufentanil were injected as needed to maintain anesthesia. The above ventilation mode was maintained until the end of the operation.
OUTCOMES:
The primary outcome of this study was the incidence of FIC during general anesthesia induction, and the secondary outcomes were postoperative nausea, vomiting, tongue falling back, laryngospasm, and anoxia. An episode of coughing within 2 min after fentanyl administration was defined as FIC, and the severity of FIC was divided into mild (1 or 2 coughs), moderate (3 or 4 coughs), and severe (≥5 coughs) [21]. The timing of the cough from drug administration was recorded in each enrolled patient. Postoperative adverse events were assessed, and the incidence of specific adverse events were recorded by a trained nurse. Specific adverse events were defined as any degree of nausea, vomiting, tongue falling back, laryngospasm, or anoxia within 24 h after surgery.
STATISTICAL ANALYSIS:
The characteristics of patients are presented as medians and quartiles or as event rates. Comparisons of continuous variables between FIC and non-FIC patients were calculated using Kruskal-Wallis tests due to the non-normal distributions, while the frequencies of data between these 2 groups were calculated using chi-squared tests. Multivariate logistic regression analyses were used to explore the potential association between patient and clinical characteristics and FIC during general anesthesia induction and postoperative nausea, vomiting, tongue falling back, laryngospasm, and anoxia. All reported
Results
The baseline characteristics of the recruited patients are shown in Table 1. Overall, 601 patients (131 FIC and 470 non-FIC patients) undergoing general anesthesia induction were enrolled in this retrospective cohort study. There were significant differences between the FIC and non-FIC groups in pharyngitis history (
Table 2 summarizes the potential association of patient and clinical characteristics with the incidence of FIC during general anesthesia induction. The results of multivariate logistic regression analysis suggested that patients with pharyngitis had an increased risk of FIC during general anesthesia induction (odds ratio [OR]: 2.852; 95% confidence interval [CI]: 1.698–4.792;
Table 3 summarizes the potential association of patient and clinical characteristics with the incidence of postoperative nausea. Overall, no significant differences were observed in gender (
Table 4 summarizes the potential association of patient and clinical characteristics with the incidence of postoperative vomiting. We found that gender (
The incidences of tongue falling back, laryngospasm, and anoxia within 24 h after surgery were 13/601, 2/601, and 3/601, respectively. Overall, BMI (OR: 2.271; 95% CI: 1.557–3.312;
Discussion
FIC and postoperative adverse events can affect patient mobility, prolong discharge from the postanesthesia care unit, and increase care cost, which are the most common postoperative problems [26–29]. The present retrospective cohort study enrolled 601 patients undergoing general anesthesia induction across wide range of patient characteristics. The multiple adjusted results indicated patients with pharyngitis are more likely to have FIC, whereas use of lidocaine was associated with a reduced risk of FIC during general anesthesia induction. Moreover, the risk of postoperative tongue falling back was associated with BMI, psychiatric history, history of respiratory infection (within 2 months), and the use of sufentanil. No other risk factors were identified for FIC during general anesthesia induction and postoperative nausea, vomiting, tongue falling back, laryngospasm, and anoxia.
Numerous studies have assessed the incidence of FIC, whereas the potential association of patient characteristics and anesthetic technique with the incidence of FIC has only been reported in 1 study [30]. This study suggested aging, cigarette smoking, prior epidural injection of lidocaine, or a priming dose of vecuronium are significantly associated with the risk of FIC in patients undergoing elective surgery under general anesthesia. First, they point out aging was associated with a reduced risk of FIC, which could be due to the decreased number of rapidly adapting stretch receptors in aging [31]. However, this significant difference was not observed in this retrospective cohort study, and is consistent with another previous study, which suggested the capsaicin cough thresholds are similar among various age groups in the same sex [32]. Second, a previous study indicated absence of smoking was associated with an increased risk of FIC, which could be due to an increase capsaicin cough threshold in smokers [33]. However, the present study indicated current smoker status was associated with a non-significant increased incidence of FIC, which could be because lung function is affected by smoking [34]. Third, prior epidural injection of lidocaine was not associated with a reduced risk of FIC, and this antitussive effect could be attributed to the systemic effects of this local anesthetic [35,36]. However, our study found the use of lidocaine was associated with a reduced risk of FIC owing to the arrhythmogenic effects of lidocaine. An appropriate dose of lidocaine can reduce the risk of FIC [37], and the prophylactic effect of intravenous lidocaine on the risk of opioid-induced cough has already demonstrated [38,39]. Fourth, the use of vecuronium can affect histamine release, which is involved in the onset of FIC [40]. The lack of a significant association in the present study could be because the number of patients in each category of vecuronium was not balanced, which affected the power to detect the association of vecuronium and the onset of FIC. Finally, we found that patients with pharyngitis history had an increased risk of FIC during general anesthesia induction. The reason for this could be that pharyngitis-induced cough can cause overestimation of FIC.
The results of the present study showed no association of patient and clinical characteristics with postoperative nausea and vomiting. The reason for this could be the lower incidence of postoperative nausea and vomiting, which always has broad confidence intervals, meaning there is no statistically significant difference. A similar reason could explain the lack of significant risk factors for the onset of postoperative laryngospasm and anoxia. Finally, although the incidence of postoperative tongue falling back was lower, BMI, psychiatric history, history of respiratory infection (within 2 months), and the use of sufentanil were significantly associated with the risk of postoperative tongue falling back. However, these results were not stable and need to be verified by a larger prospective study.
This study has several limitations. First, stratified results were not conducted due to all of patients’ characteristics entered the logistic model, which prevented us from exploring the risk factor on FIC during general anesthesia induction and postoperative nausea, vomiting, tongue falling back, laryngospasm, and anoxia in patients with specific characteristics. Second, this was a retrospective cohort study, with inherent risk of bias due to lack of control of confounding variables. Third, the sequence for drugs administration, and timing for the use of fentanyl could affect the progression of FIC [41,42], which were not addressed in our study owing to this study designed as retrospective cohort. Fourth, the analysis of this study based on the frequent of FIC incidence, while the frequency and severity scoring system for FIC were not combined [43]. Fifth, the severity of FIC during general anesthesia induction and postoperative nausea, vomiting, tongue falling back, laryngospasm, and anoxia were not addressed in this study. Finally, other limitations of this study, including small sample size, the use of a single center, and any study bias.
Conclusions
The results of this study indicated that a history of pharyngitis increased the risk of FIC, while the use of lidocaine was associated with a reduced risk of FIC. However, fentanyl use and the characteristics of patients were not associated with the risk of postoperative nausea and vomiting. However, these results are based on a retrospective cohort analysis, and further large-scale prospective cohort studies are needed to identify potential risk factors for FIC during general anesthesia induction, as well as to explore the possible associations with postoperative nausea, vomiting, tongue falling back, laryngospasm, and anoxia in patients with specific characteristics.
Tables
Table 1. Baseline characteristics of included patients.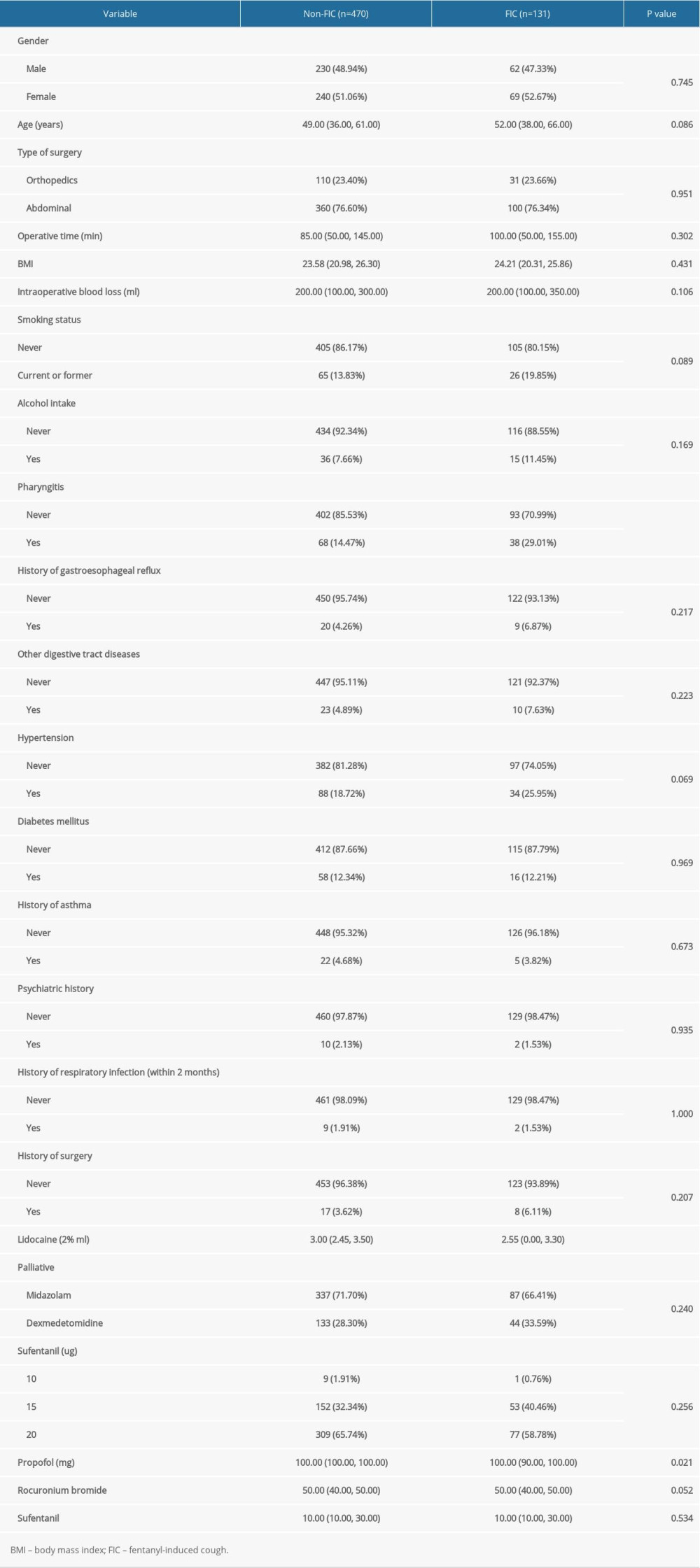 Table 2. The risk factors for the incidence of fentanyl-induced cough by multivariate logistic regression analysis.
Table 2. The risk factors for the incidence of fentanyl-induced cough by multivariate logistic regression analysis.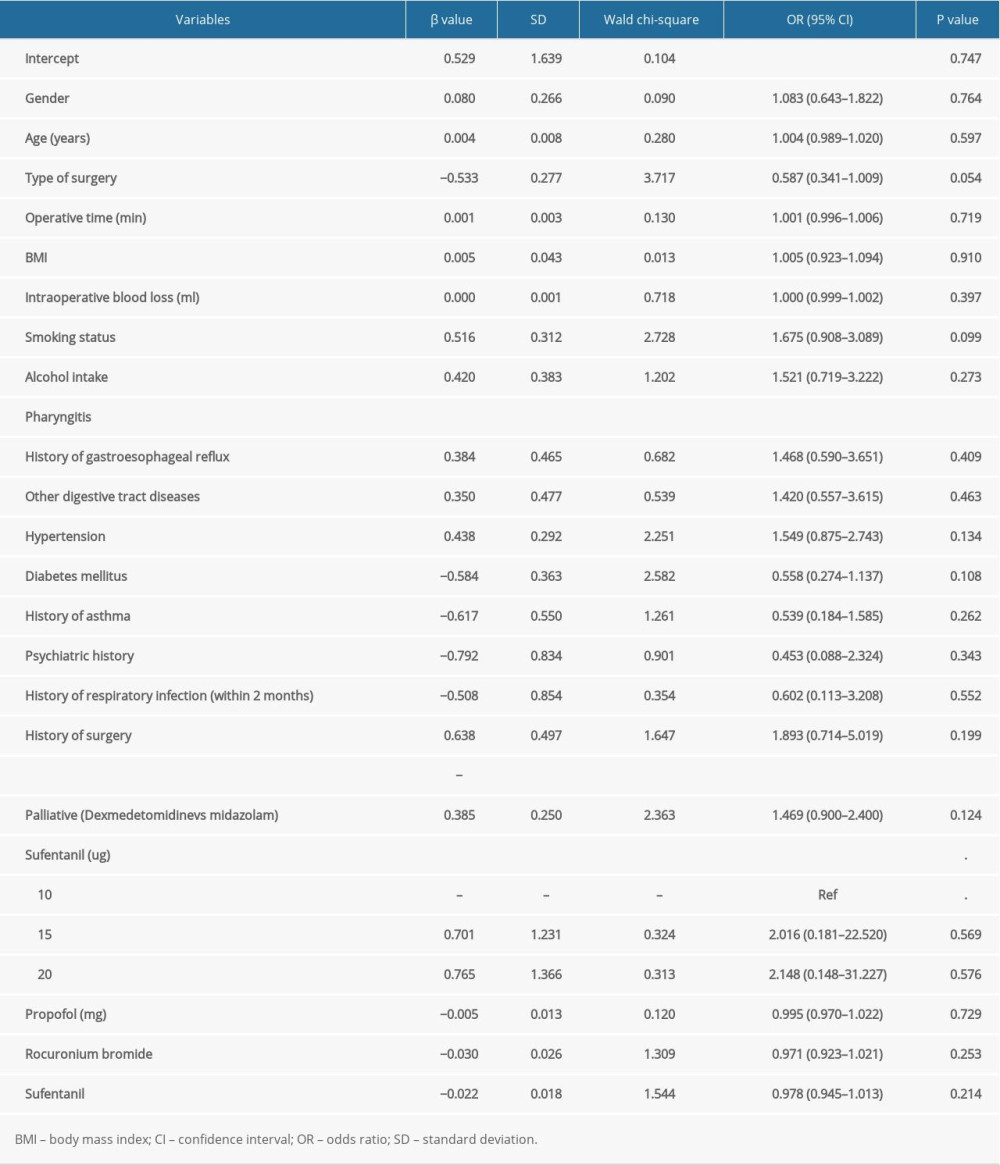 Table 3. The risk factors for the incidence of nausea by multivariate logistic regression analysis.
Table 3. The risk factors for the incidence of nausea by multivariate logistic regression analysis.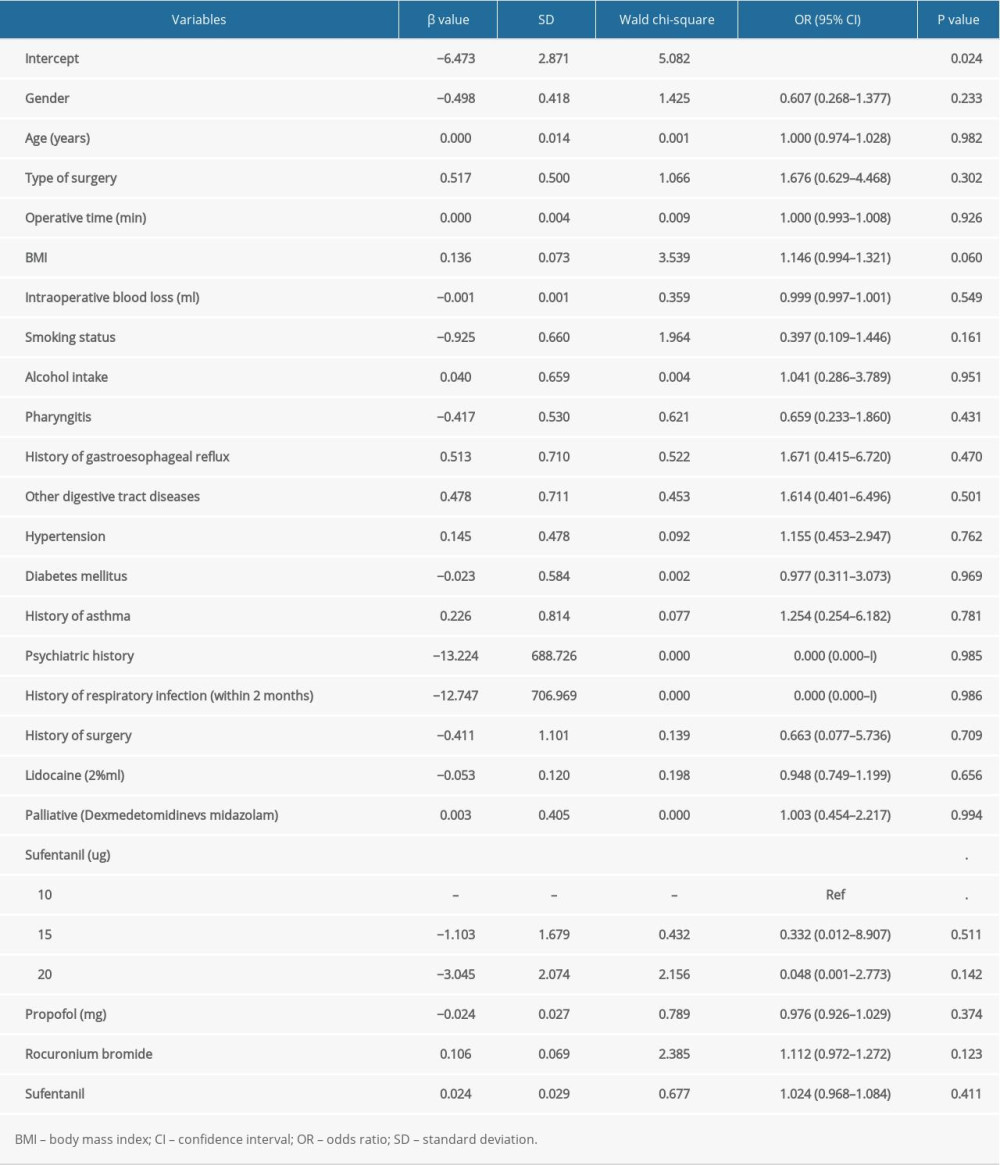 Table 4. The risk factors for the incidence of vomiting by multivariate logistic regression analysis.
Table 4. The risk factors for the incidence of vomiting by multivariate logistic regression analysis.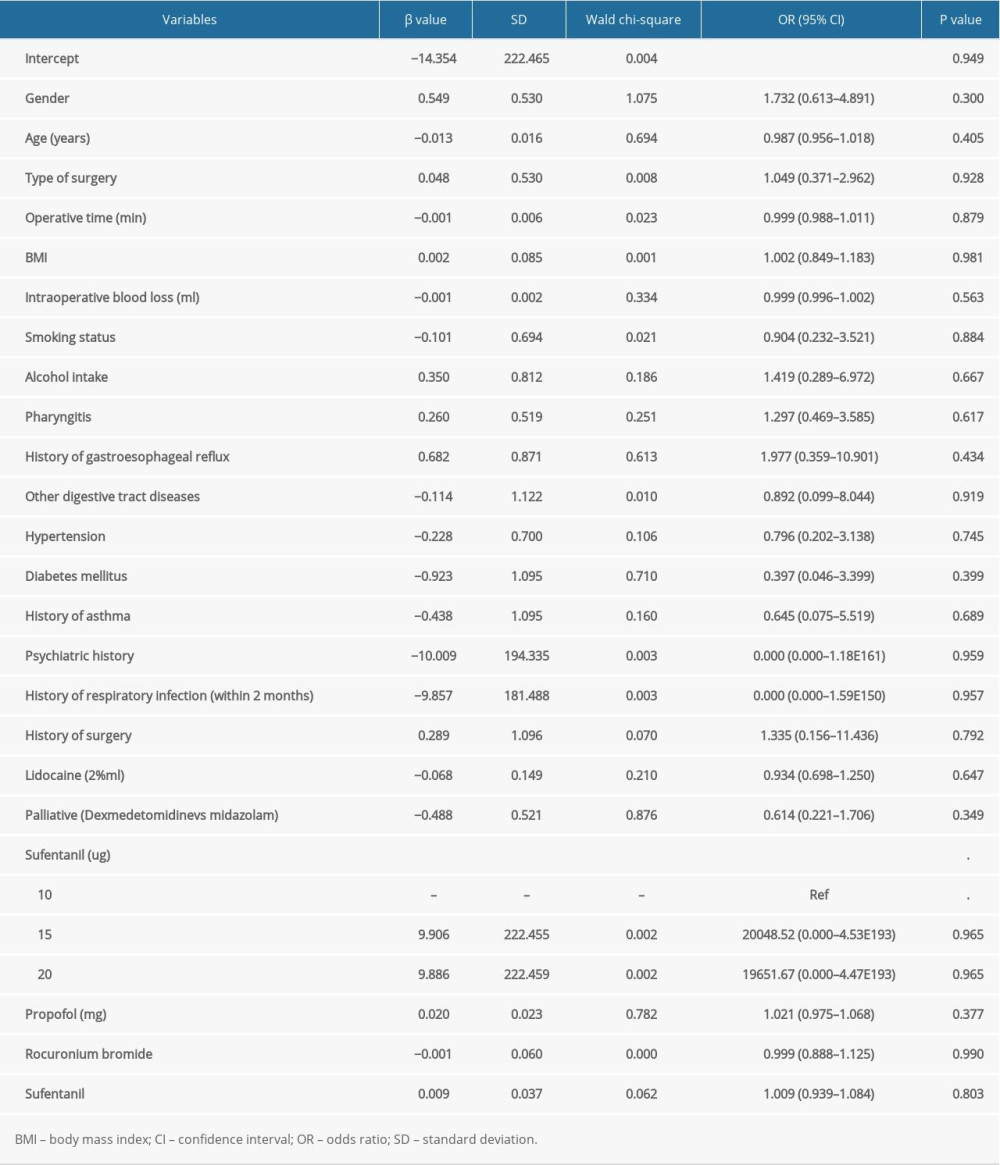
References
1. Barr J, Fraser GL, Puntillo KAmerican College of Critical Care Medicine, Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit: Crit Care Med, 2013; 41; 263-306
2. Chou R, Fanciullo GJ, Fine PG, Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain: J Pain, 2009; 10; 113-30
3. Chou R, Gordon DB, de Leon-Casasola OA, Management of postoperative pain: A clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council: J Pain, 2016; 17; 131-57
4. Devlin JW, Skrobik Y, Gélinas C, Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU: Crit Care Med, 2018; 46; e825-73
5. Li YH, Xu JH, Yang JJ, Predictive performance of ‘Diprifusor’ TCI system in patients during upper abdominal surgery under propofol/fentanyl anesthesia: J Zhejiang Univ Sci B, 2005; 6; 43-48
6. Peter VD, Cough: An unmet clinical need: Br J Pharmacol, 2011; 163; 116-24
7. Faruqi S, Murdoch RD, Allum F, On the definition of chronic cough and current treatment pathways: An international qualitative study: Cough, 2014; 10; 5
8. Karbasy SH, Derakhshan P, The effect of low dose fentanyl as a premedication before induction of general anesthesia on the neonatal Apgar score in cesarean section delivery: Randomized, double-blind controlled trial: Med J Islam Repub Iran, 2016; 30; 361
9. Shrestha SK, Bhattarai B, Shah RS, Preemptive use of small dose fentanyl suppresses fentanyl induced cough: Kathmandu Univ Med J (KUMJ), 2012; 10; 16-19
10. Tweed WA, Dakin D, Explosive coughing after bolus fentanyl injection: Anesth Analg, 2001; 92; 1442-43
11. Lim KJ, Lee SK, Lee HM, Aspiration pneumonia caused by fentanyl-induced cough – a case report: Korean J Anesthesiol, 2013; 65; 251-53
12. Li CC, Chen SS, Huang CH, Fentanyl-induced cough is a risk factor for postoperative nausea and vomiting: Br J Anaesth, 2015; 115; 444-48
13. Mukherjee A, Kundu AK, Ghosh S, Preemptive oral dexmethorphan reduces fentanyl-induced cough as well as immediate postoperative adrenocorticotropic hormone and growth hormone level: J Anaesthesiol Clin Pharmacol, 2011; 27; 489-94
14. Gecaj-Gashi A, Nikolova-Todorova Z, Ismaili-Jaha V, Intravenous lidocaine suppresses fentanyl induced cough in children: Cough, 2013; 9; 20
15. Saleh AJ, Zhang L, Hadi SM, Priming dose of intravenous ketamine-dexmedetomidine suppresses fentanyl-induced coughing: A double-blind, randomized, controlled study: Ups J Med Sci, 2014; 119; 333-37
16. Sedighinejad A, Naderi Nabi B, Haghighi M, Propofol is effective to depress fentanyl-induced cough during induction of anesthesia: Anesth Pain Med, 2013; 2; 170-73
17. Firouzian A, Emadi SA, Baradari AG, Can low dose of propofol effectively suppress fentanyl-induced cough during induction of anaesthesia? A double blind randomized controlled trial: J Anaesthesiol Clin Pharmacol, 2015; 31; 522-25
18. Liu HL, An LJ, Su Z, Magnesium sulphate suppresses fentanyl-induced cough during general anesthesia induction: A double-blind, randomized, and placebo – controlled study: Int J Clin Exp Med, 2015; 8; 11332-36
19. Xu Y, Zhu Y, Wang S, Dezocine attenuates fentanyl-induced cough in a dose-dependent manner: A randomized controlled trial: Int J Clin Exp Med, 2015; 8; 6091-96
20. Cheng XY, Lun XQ, Li HB, Butorphanol suppresses fentanyl-induced cough during general anesthesia induction: A randomized, double-blinded, placebo – controlled clinical trial: Medicine (Baltimore), 2016; 95; e3911
21. Lin JA, Yeh CC, Lee MS, Prolonged injection time and light smoking decrease the incidence of fentanyl-induced cough: Anesth Analg, 2005; 101; 670-74
22. Solanki SL, Doctor JR, Kapila SJ, Acupressure versus dilution of fentanyl to reduce incidence of fentanyl-induced cough in female cancer patients: A prospective randomized controlled study: Korean J Anesthesiol, 2016; 69; 234-38
23. Chen R, Tang LH, Sun T, Mechanism and management of fentanyl-induced cough: Front Pharmacol, 2020; 11; 584177
24. El Baissari MCT, Taha SK, Siddik-Sayyid SM, Fentanyl-induced cough-pathophysiology and prevention: Middle East J Anesthesiol, 2014; 22; 449-56
25. Kim JE, Min SK, Chae YJ, Pharmacological and nonpharmacological prevention of fentanyl-induced cough: A meta-analysis: J Anesth, 2014; 28; 257-66
26. Apfel CC, Korttila K, Abdalla M, A factorial trial of six interventions for the prevention of postoperative nausea and vomiting: N Engl J Med, 2004; 350; 2441-45
27. Parra-Sanchez I, Abdallah R, You J, A time-motion economic analysis of postoperative nausea and vomiting in ambulatory surgery: Can J Anaesth, 2012; 59; 366-75
28. Carroll NV, Miederhoff PA, Cox FM, Costs incurred by outpatient surgical centers in managing postoperative nausea and vomiting: J Clin Anesth, 1994; 6; 364-69
29. Lehmann M, Monte K, Barach P, Postoperative patient complaints: A prospective interview study of 12,276 patients: J Clin Anesth, 2010; 22; 13-21
30. Oshima T, Kasuya Y, Okumura Y, Identification of independent risk factors for fentanyl-induced cough: Can J Anaesth, 2006; 53; 753-58
31. Pontoppidan H, Beecher HK, Progressive loss of protective reflexes in the airway with the advance of age: JAMA, 1960; 74; 2209-13
32. Fujimura M, Kasahara K, Kamio Y, Female gender as a determinant of cough threshold to inhaled capsaicin: Eur Respir J, 1996; 9; 1624-26
33. Dicpinigaitis PV, Cough reflex sensitivity in cigarette smokers: Chest, 2003; 123; 685-88
34. Zhao J, Li M, Chen J, Smoking status and gene susceptibility play important roles in the development of chronic obstructive pulmonary disease and lung function decline: A population-based prospective study: Medicine (Baltimore), 2017; 96; e7283
35. Christensen V, Ladegaard-Pedersen HJ, Skovsted P, Intravenous lidocaine as a suppressant of persistent cough caused by bronchoscopy: Acta Anaesthesiol Scand Suppl, 1978; 67; 84-86
36. Dicpinigaitis PV, Grimm DR, Lesser M, Cough reflex sensitivity in subjects with cervical spinal cord injury: Am J Respir Crit Care Med, 1999; 159; 1660-62
37. Schlimp CJ, Wiedermann FJ: Can J Anaesth, 2005; 52; 207
38. Sun L, Guo R, Sun L, The impact of prophylactic intravenous lidocaine on opioid-induced cough: A meta-analysis of randomized controlled trials: J Anesth, 2014; 28; 325-33
39. Tan W, Li S, Liu X, Prophylactic intravenous lidocaine at different doses for fentanyl-induced cough (FIC): A meta-analysis: Sci Rep, 2018; 8; 9946
40. Agarwal A, Azim A, Ambesh S, Sabutamol, beclomethasone or sodium chromoglycate suppress coughing induced by iv fentanyl: Can J Anesth, 2003; 50; 297-300
41. Kim JE, Min SK, Chae YJ, Pharmacological and nonpharmacological prevention of fentanyl-induced cough: A meta-analysis: J Anesth, 2014; 28; 257-66
42. He J, Zhu L, Zhu H, Does selection of central or peripheral administration of sufentanil affect opioid induced cough?: A prospective, randomized, controlled trial: BMC Anesthesiol, 2018; 18; 38
43. Fisman EZ, Shapira I, Motro M, The combined cough frequency/severity scoring: A new approach to cough evaluation in clinical settings: J Med, 2001; 32; 181-87
Tables
 Table 1. Baseline characteristics of included patients.
Table 1. Baseline characteristics of included patients. Table 2. The risk factors for the incidence of fentanyl-induced cough by multivariate logistic regression analysis.
Table 2. The risk factors for the incidence of fentanyl-induced cough by multivariate logistic regression analysis. Table 3. The risk factors for the incidence of nausea by multivariate logistic regression analysis.
Table 3. The risk factors for the incidence of nausea by multivariate logistic regression analysis. Table 4. The risk factors for the incidence of vomiting by multivariate logistic regression analysis.
Table 4. The risk factors for the incidence of vomiting by multivariate logistic regression analysis. In Press
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








