15 September 2021: Clinical Research
Development and Validation of a Prevalence Model for Latent Autoimmune Diabetes in Adults (LADA) Among Patients First Diagnosed with Type 2 Diabetes Mellitus (T2DM)
Zhida Wang1ABCDEF, Jie Zhang2ABCDEF, Hui Xu3CDEF, Liming Chen1ADEFG*, Abigail Dove4ADEFDOI: 10.12659/MSM.932725
Med Sci Monit 2021; 27:e932725
Abstract
BACKGROUND: We designed this study to develop and validate a prevalence model for latent autoimmune diabetes in adults (LADA) among people initially diagnosed with type 2 diabetes mellitus (T2DM).
MATERIAL AND METHODS: The study recruited 930 patients aged ≥18 years who were diagnosed with T2DM within the past year. Demographic information, medical history, and clinical biochemistry records were collected. Logistic regression was used to develop a regression model to distinguish LADA from T2DM. Predictors of LADA were identified in a subgroup of patients (n=632) by univariate logistic regression analysis. From this we developed a prediction model using multivariate logistic regression analysis and tested its sensitivity and specificity among the remaining patients (n=298).
RESULTS: Among 930 recruited patients, 880 had T2DM (96.4%) and 50 had LADA (5.4%). Compared to T2DM patients, LADA patients had fewer surviving b cells and reduced insulin production. We identified age, ketosis, history of tobacco smoking, 1-hour plasma glucose (1hPG-AUC), and 2-hour C-peptide (2hCP-AUC) as the main predictive factors for LADA (P<0.05). Based on this, we developed a multivariable logistic regression model: Y=-8.249-0.035(X1)+1.755(X2)+1.008(X3)+0.321(X4)-0.126(X5), where Y is diabetes status (0=T2DM, 1=LADA), X1 is age, X2 is ketosis (1=no, 2=yes), X3 is history of tobacco smoking (1=no, 2=yes), X4 is 1hPG-AUC, and X5 is 2hCP-AUC. The model has high sensitivity (78.57%) and selectivity (67.96%).
CONCLUSIONS: This model can be applied to people newly diagnosed with T2DM. When Y ≥0.0472, total autoantibody screening is recommended to assess LADA.
Keywords: Diabetes Mellitus, Type 2, Latent Autoimmune Diabetes in Adults, Predictive Value of Tests, Age Factors, Blood Glucose, C-peptide, Female, Humans, ketosis, Prevalence, Reproducibility of Results, Sensitivity and Specificity, tobacco smoking, young adult
Background
The global prevalence of diabetes mellitus (DM) is on the rise. According to the International Diabetes Federation (IDF) 2019 Diabetes Atlas [1], diabetes is one of the fastest growing health challenges of the 21st century, with prevalence more than tripling over the past 20 years. In 2019, an estimated 9.3% of adults aged 20–79 years were living with diabetes (463 million adults). Furthermore, it is estimated that 1 in 2 adults with diabetes are undiagnosed (232 million people). In China, the state of DM is characterized by high prevalence and low rates of diagnosis, making disease management very challenging. In 2019, China had the largest number of adults with diabetes in the world, at 116.4 million, and this is projected to reach 147.2 million by 2045.
Tuomi et al first identified latent autoimmune diabetes in adults (LADA) in 1993 [2]. The Immunology of Diabetes Society (IDS) established diagnostic standards for LADA in 2005, and these have been recognized internationally [3]. In 2012, the Chinese Diabetes Society (CDS) published a consensus for LADA diagnosis and treatment in the Chinese population, thus establishing the diagnostic standards for LADA in China. According to these standards, LADA is defined by: 1) diabetes diagnosis at age ≥18 years; 2) islet autoantibody positivity (first with serum glutamic acid decarboxylase antibody [GADA], followed by insulinoma-associated antigen-2 antibody [IA-2A], and zinc transporter 8 antibody [ZnT8A] to increase the detection rate); 3) no insulin requirements for at least 6 months after diagnosis [4]. LADA is a slowly progressing form of T1D with onset in adulthood [5]. Moreover, according to the WHO Classification of Diabetes Mellitus 2019, LADA is defined as a new type of diabetes, and is a slowly evolving, immune-mediated diabetes of adults [6]. It is associated with many T1DM and T2DM susceptibility gene variants because of shared pathogenic mechanisms between these different forms of diabetes [5].
It is estimated that 7–15% of diabetes cases are initially misdiagnosed as the wrong diabetes subtype [7]. Few clinics perform antibody testing on every single T2DM patient given the high cost and limited availability of autoantibody tests, combined with the fact that only approximately 5–10% of people newly diagnosed with diabetes test positive for GADA [8,9]. For this reason, it is particularly important to develop predictive models to calculate an individual’s likelihood of having LADA as opposed to T2DM, thereby targeting a subset of likely LADA patients for autoantibody testing to confirm LADA diagnosis, and, if possible, initiate earlier insulin treatment. A study that followed LADA patients for 3 years showed that treatment with low doses of insulin can preserve residual β cell function, thus slowing disease progression [10]. Therefore, without the correct DM diagnosis, LADA patients are unlikely to receive the most effective treatment. However, due to differences in professional and testing abilities between hospitals and differences in patients’ ability to afford testing and treatment, it is nearly impossible to perform autoantibody testing in all newly diagnosed DM patients. Therefore, the aim of the present study was to develop a predictive model for LADA prevalence among patients diagnosed with T2DM to support better diagnostics and clinical decision-making.
Material and Methods
STUDY SUBJECTS:
Patients at the Center for Special Diagnosis at the Diabetes Clinic of Tianjin Medical University Hospital for Metabolic Syndromes were recruited into the present study between May 2015 and April 2018. The study enrolled a total of 930 patients aged ≥18 years and with a ≤1 year history of diabetes. Among them, 880 had T2DM and 50 had LADA. Following a 2: 1 ratio, 632 patients enrolled from June 2015 to December 2016 were assigned to the model development group and 298 patients enrolled from January 2017 to April 2018 were assigned to the model validation group. A LADA prevalence model was developed based on the data from the model development group and was then validated using data from the model validation group.
DATA COLLECTION:
Participants completed standard questionnaires and underwent a physical examination at the Diabetes Clinic. General information, diabetes status, other disease history, family history of chronic diseases, and personal history were collected from the participants through questionnaires. We measured and recorded height, weight, waist circumference, and hip circumference and used these to calculate body mass index (BMI) and waist-hip ratio (WHR). We additionally measured glycosylated hemoglobin A1c (HbA1c) and performed a 75-g oral glucose tolerance test (OGTT), an insulin releasing test (IRT), and a C-peptide releasing test (CRT) at different time points (1-hour and 2-hour). The area under the curve (AUC) for plasma glucose (PG), insulin (INS), and C-peptide (CP) at the first and second hours were calculated according to the approximate trapezoid area formula. The HOMA2 calculator was used to calculate the homeostasis model assessment of insulin resistance index (HOMA2-IR) and pancreatic beta cell function (HOMA2-β). Pancreas autoantibody testing was performed by trained professionals using an enzyme-linked immunosorbent assay (ELISA) testing kit from RSR Company. Three autoantibodies were analyzed: serum glutamic acid decarboxylase antibody (GADA), insulinoma-associated antigen-2 antibody (IA-2A), and zinc transporter 8 antibody (ZnT8A).
STATISTICAL METHODS:
Statistical analysis was performed using SPSS 25.0 software. Data that satisfied the normal distribution and the uniform variance were compared with the 2 independent-samples t test; otherwise, the Wilcoxon rank sum test was used. Categorical data were compared using the χ2 test or Wilcoxon rank sum test. The predictors related to LADA diagnosis in T2DM were identified by univariate logistic regression analysis, and then the regression model was established by multivariate logistic regression analysis. The Hosmer-Lemeshow test was used to assess the degree of fit for the evaluation model. The area under the receiver operating characteristic (ROC) curve of prediction probability was used to evaluate the discrimination of the model in the model validation group. The Youden index [12] was calculated according to the sensitivity and specificity of the ROC curve coordinate points, and the cut-off point of the model predictive value was determined by combining the statistical index with the clinical situation. P values<0.05 were considered statistically significant. Statistical figures were generated using GraphPad Prism 8.0.1 software.
Results
COMPARISON BETWEEN PATIENTS WITH T2DM AND LADA:
We excluded 123 people in this study, including 112 people who did not meet standard diagnostic criteria for DM and patients with possible or certain diagnosis of T1DM, gestational diabetes mellitus (GDM), or secondary DM; 3 patients who tested positive for pancreas autoantibodies including GADA, IA-2A, and ZnT8A but who had not been treated with insulin and had a ≤6-month history of DM; and 8 patients with incomplete medical records or clinical testing records. A total of 930 patients were enrolled in the present study, of whom 880 had T2DM (94.6%) and 50 had LADA (5.4%). As shown in Table 1, between the T2DM and LADA patient groups, no statistically significant differences were detected with respect to sex, ethnicity, education level, typical symptoms of DM (ie, polydipsia, polyphagia, polyuria, emaciation), eye floaters, arm and leg numbness, personal disease history (including high blood pressure, coronary heart disease, and high blood lipids), family disease history, and history of alcohol drinking. Compared with T2DM patients, LADA patients were younger and more likely to have a history of tobacco smoking and ketosis. No statistically significant differences were found between LADA and T2DM patients with respect to BMI, WHR, and BMI class. However, LADA patients had a lower rate of abdominal obesity. Patients with LADA had higher HbA1c, FPG, 1hPG, and 2hPG than T2DM patients, but lower 1hINS, 2hINS, FCP, 1hCP, and 2hCP. No differences in fasting insulin (FINS) were detected between the 2 groups. Compared with the T2DM group, the LADA group had lower CP-AUC, 1hCP-AUC, 2hCP-AUC, INS-AUC, 2hINS-AUC, and HOMA2-β and higher PG-AUC, 1hPG-AUC, and 2hPG-AUC.
COMPARISON BETWEEN THE MODEL DEVELOPMENT AND MODEL VALIDATION GROUPS:
In the study there were 32 LADA patients in the model development group and 18 LADA patients in the model validation group. Age, rate of ketosis, rate of abdominal obesity, and rate of history of tobacco smoking did not differ significantly between the model development group and the model validation group (P>0.05). Compared to the model validation group, the model development group had higher HbA1c and 1hCP and lower 2hINS (P<0.05). However, no statistically significant differences were observed between the 2 groups with respect to FPG, 2hPG, FINS, 1hINS, FCP, and 2hCP; 1hPG-AUC and 1hCP-AUC were higher in the model development group than the model validation group. There were no significant differences between the groups for PG-AUC, 2hPG-AUC, INS-AUC, 1hINS-AUC, 2hINS-AUC, CP-AUC, 2hCP-AUC, and HOMA2-β (Table 2).
SINGLE-VARIABLE LOGISTIC REGRESSION ANALYSIS FOR THE MODEL DEVELOPMENT GROUP:
Single-variable logistic regression analysis in the model development group indicated that age, ketosis, a history of tobacco smoking, HbA1c, FPG, 1hPG, 2hPG, 1hCP, 2hCP, PG-AUC, 1hPG-AUC, 2hPG-AUC, CP-AUC, 1hCP-AUC, 2hCP-AUC, and HOMA2-β were correlated with diagnosis of LADA (P<0.05) (Table 3).
DEVELOPMENT OF A MULTIVARIABLE LOGISTIC REGRESSION ANALYSIS AND PREDICTION MODEL IN THE MODEL DEVELOPMENT GROUP:
According to single-variable logistic regression analysis, 16 factors are correlated with the diagnosis of LADA. However, considering the collinear relationship between independent variables and the clinical significance of each independent variable, it was not appropriate to include all 16 factors in the logistic regression model at the same time. The corresponding degrees of fit and differentiation were evaluated in the optimal model containing 5 independent variables was finally selected after the combination analysis of a variety of different variables. We selected 5 single variables after considering the clinical significance of these factors and the correlations between them. These were age, ketosis, history of tobacco smoking, 1hPG-AUC, and 2hCP-AUC (Table 4).
EVALUATION OF THE PREDICTIVE MODEL:
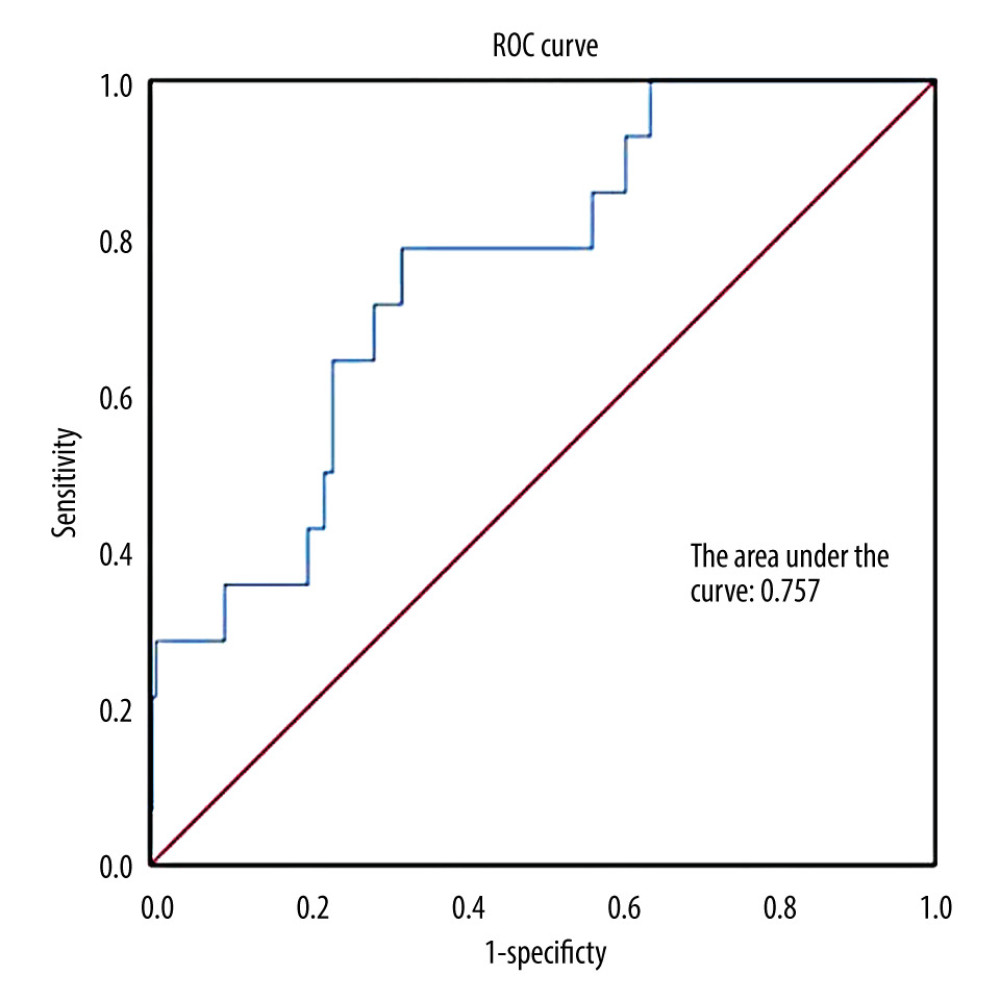
Moreover, Hosmer-Lemeshow testing showed χ2=12.687 and P=0.123, indicating that the model fits the data well. The area under the ROC curve generated by the probability prediction in the validation group was 0.757 (P<0.05) (Figure 1). The Youden index was calculated according to the sensitivity and specificity at the origin. When Y=0.0472, sensitivity is 78.57% and selectivity is 67.96%. The Youden index reached its maximum value of 0.47 under these conditions, so 0.0472 was set as the model intercept.
Discussion
This study identified people with LADA among a population of patients recently diagnosed with T2DM and we developed a prediction model for LADA based on clinical characteristics.
In our study, 5.4% of the population had LADA. For comparison, a 2011 study from Tianjin reported that 9.2% of people first diagnosed with T2DM had LADA [13]. This discrepancy may be due to different study populations and sample sizes. The 2011 study included 8109 patients aged ≥15 years from 27 communities in 3 districts in Tianjin, while our study included 930 patients aged ≥18 years exclusively from the Center for Special Diagnosis, Diabetes Clinic of Tianjin Medical University Hospital for Metabolic Syndromes. In a 2013 multi-center study from China [8], the LADA prevalence among people aged ≥30 years first diagnosed with T2DM was 5.9%, which is comparable with the present study. Discrepancies in the literature could reflect differences in study populations. Furthermore, among people with ≥6-months history of diabetes who test positive for pancreas autoantibodies and have not received insulin treatment, it can be difficult to distinguish between LADA and typical T1DM due to the recency of diagnosis. For this reason, patients with these characteristics were not included in the present study. This may have affected the LADA prevalence calculation in the present study, as some of the excluded patients may have had LADA.
In the present study, age emerged as an important risk factor for T2DM and LADA. This is in line with a previous report from Carlson et al [14]. A review [15] compared the clinical characteristics of LADA and T2DM, indicating that LADA is typically diagnosed in people aged ≥30 years, while T2DM usually occurs in adulthood and rarely in childhood or adolescence. Cross-sectional studies from China [13] and Nigeria [16] show that LADA frequently occurs at ages 50–59 years.
HOMA2-β (%) is used to evaluate an individual’s pancreatic β cell function, with lower values indicating greater β cell dysfunction. In the present study, HOMA2-β values were significantly lower among LADA patients than T2DM patients. Additionally, 1- and 2-hour C-peptide and 1- and 2-hour insulin levels were lower among LADA patients, indicating that pancreatic β cell function had already started to decrease even at an early stage of disease. Consistent with other studies [17,18], the number of remaining pancreatic β cells and insulin secretion levels were also lower among LADA patients. Compared to T2DM patients, LADA patients require insulin to control blood sugar early in the disease [19]. A cross-sectional study from Nigeria [16] reported that the expression of GAD autoantibodies in T2DM patients is corelated with the use of insulin. A higher percentage of LADA patients use insulin to control blood glucose, while only 19% in the GADA-negative population use insulin as a treatment. GADA is the most common biomarker used to distinguish people with LADA from those with T2DM. However, recent transcriptomics analysis has pointed to the possibility of novel LADA biomarkers [20,21].
Tobacco smoking is linked with LADA. Consistent with the present study, past studies have indicated that smokers with higher levels of GAD autoantibody and lower C-peptide levels have a higher risk of developing LADA than non-smokers [22]. However, in a 22-year follow-up study from Norway, tobacco smoking reduced the risk of LADA, and the reduced risk was positively associated with the number of pack-years [23]. Some studies [24,25] suggest that the nicotine from tobacco could participate in the immune response and inflammation reaction that underlies LADA, but the underlying mechanisms are still under debate. In a LADA case-control study on smoking in the general population [26], no protective effect of smoking was observed for autoimmune and LADA risk. In contrast, heavy smoking increased the risk of LADA. Compared with never-smokers, HOMA-IR and HOMA2-β levels are higher among heavy smokers, while GADA levels are lower. This could indicate more severe insulin resistance caused by tobacco smoking [27].
Buzzetti et al [15] compared genetic, metabolic, and clinical characteristics of LADA and T2DM in a review of autoimmune diabetes in adults. The prevalence of ketosis was low in both the LADA and T2DM populations. However, in the present study, ketosis emerged as a predictive factor that increases the risk of LADA onset. Studies from China, Ghana, Switzerland, and Australia [15,28–31] showed that LADA patients had higher rates of risk factors, including overweight/obesity, high blood pressure, abnormal blood lipids, smoking, and alcohol, use compared to non-LADA patients. A study from Ghana [29] found that T2DM patients who were autoantibody-negative had a higher rate of abdominal-type obesity. Furthermore, their clinical and metabolic biomarkers could not be used to distinguish LADA patients from T2DM patients.
As the prevalence of chronic disease grows, clinical predictive models have become a popular subject in clinical research. The development of diagnostic or post-treatment predictive models based on the clinical characteristics of an individual enables the calculation of the probability of developing a certain disease or predicting an individual’s clinical status after treatment. This approach has great significance for the screening of high-risk populations, personalized disease prevention, communication between physicians and patients, and early interventions.
To date, several studies have developed predictive models for the prevalence of diabetes or its prognosis after treatment, but few studies have brought predictive models to bear on the different subtypes of diabetes [32–35]. As a subtype of T1DM, LADA is significantly different from T2DM on a population basis. However, LADA and T2DM have similar clinical symptoms and metabolic characteristics on an individual basis. LADA is often misdiagnosed as T2DM if pancreatic autoantibody testing cannot be performed in a timely manner. GAD antibody testing could provide important information regarding appropriate therapy and would save costs related to inappropriate initial diabetes treatment and the development diabetic complications. However, it is highly impractical to perform antibody testing on every T2DM patient given the limited availability of autoantibody tests. For this reason, it is particularly important to develop predictive models to calculate an individual’s likelihood of having LADA as opposed to T2DM, thereby targeting individual patients for autoantibody testing to confirm LADA diagnosis, and, if possible, initiate earlier insulin treatment. In a study by Brophy et al [36], the median time to receiving insulin treatment was earlier in the clinics where GAD antibody testing was performed than in those where it was not. To distinguish LADA patients from T2DM patients, a “clinical risk score for LADA” was developed after analyzing significant differences in clinical parameters between the 2 groups [28]. In this retrospective study, 5 factors were identified as components of the clinical risk score for LADA: age of onset <50 years, the typical symptoms of DM (polydipsia, polyphagia, polyuria, emaciation), BMI <25 kg/m2, and personal and family history of autoimmune disease. Cases with at least 2 of the 5 parameters had 90% sensitivity and 71% selectivity, and the predictive value was 99% for clinical risk scores ≤1.
LADA patients are a heterogeneous group, making the standardization of treatment very difficult [15]. Individualized treatment plans are developed to improve blood glucose control and insulin sensitivity according to each patient’s clinical characteristics. This cross-sectional study compared clinical characteristics and differences in pancreatic β cell function between T2DM and LADA patients to establish a clinical predictive model. It calculates the probability of LADA in patients initially diagnosed with T2DM based on clinical information and laboratory testing results and is therefore well-suited to a local hospital setting where it is not feasible to test autoantibodies in every T2DM patient. Our model provides a quantitative tool for clinical recognition of LADA, and has important clinical significance for accurate diagnosis and the timely initiation of individualized treatment.
Given the present study’s cross-sectional design, it is possible that selection bias and information bias may have impacted the results. To minimize this, patients were recruited by strict selection criteria and a sufficiently large research population was collected to truly reflect the conditions of patients with LADA and T2DM. However, the predictive model developed in the present study is not perfect. First, the samples for the present study were from a single medical center. Although patients from both urban and suburban areas of Tianjin were recruited, the model development and validation groups were derived from different time periods. The model can therefore be considered a validation of internal samples, and external validation with data from outside medical centers is required. Second, blood glucose, insulin, and C-peptide levels from the OGTT were only tested at baseline (fasting) and at 1- and 2-hour timepoints. These parameters were not collected at 30 minutes and 3 hours. As a result, the OGTT-blood glucose and C-peptide AUCs collected here were not as accurate and precise as the standard 5-point curve. Third, evidence from studies of human pancreata indicate that beta cell mass is more decreased in LADA than in T2DM [37]. However, beta cell mass could not be assessed in this study and was therefore not included in the model. Fourth, the study had a limited sample size (including only 50 participants with LADA), so future studies should include patients from additional centers in order to improve the predictive aL1 ccuracy of the model.
Conclusions
Age, ketosis, a history of tobacco smoking, 1hPG-AUC, and 2hCP-AUC are predictive of LADA among people first diagnosed with T2DM. For newly-diagnosed T2DM patients, especially young patients with history of ketosis, history of tobacco smoking, decreased β cell function, and poor blood glucose control, a comprehensive antibody screening is recommended for earlier detection of LADA. Among people newly diagnosed with T2DM, the probability of LADA should be calculated according to the model presented here. When Y ≥0.0472, a comprehensive antibody screening is recommended; when Y <0.0472, antibody screening depends on the patient’s preferences and ability to afford the procedure.
Tables
Table 1. Comparison of the clinical features among patients with T2DM vs LADA.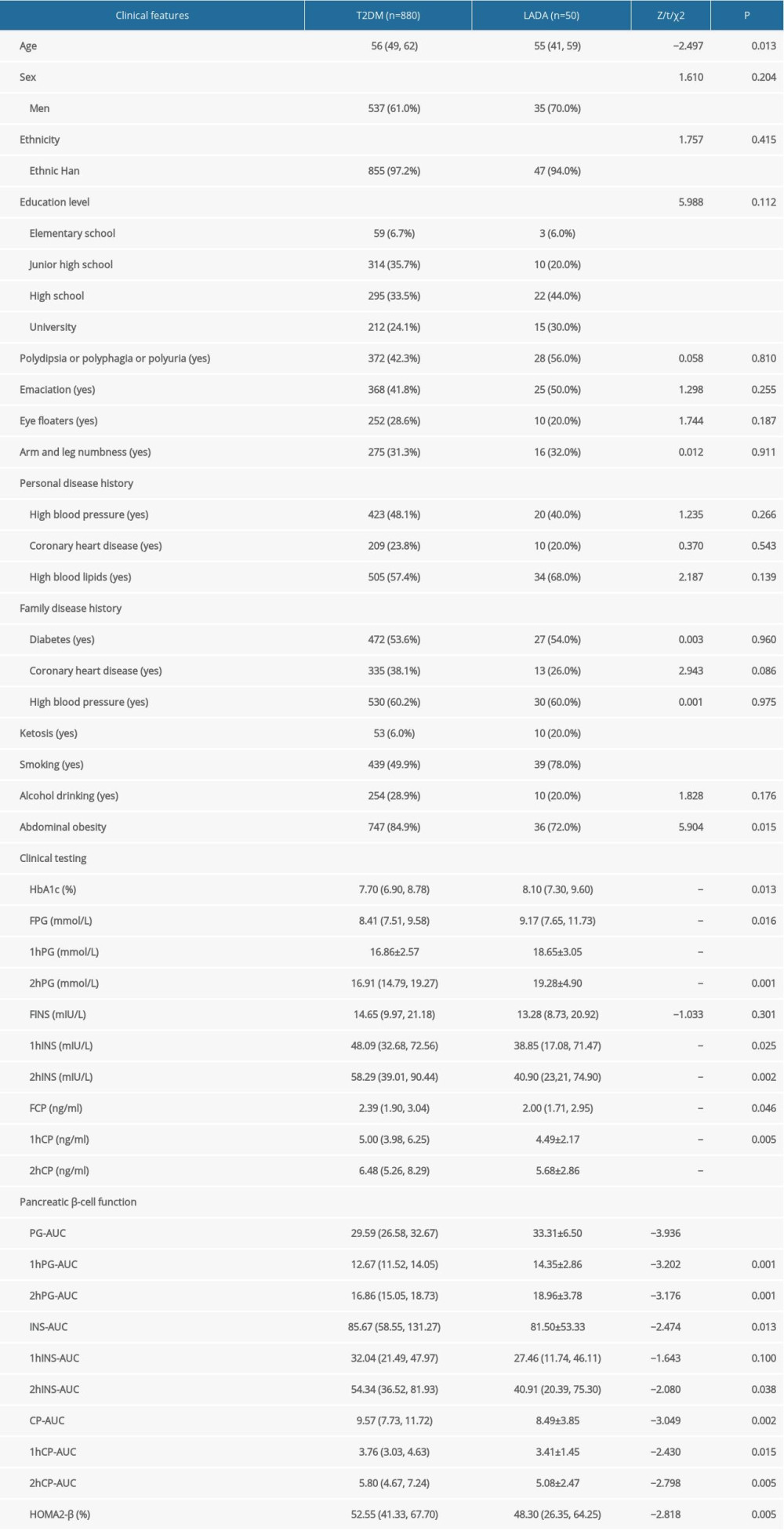 Table 2. Comparison of the clinical features between model development and validation groups.
Table 2. Comparison of the clinical features between model development and validation groups.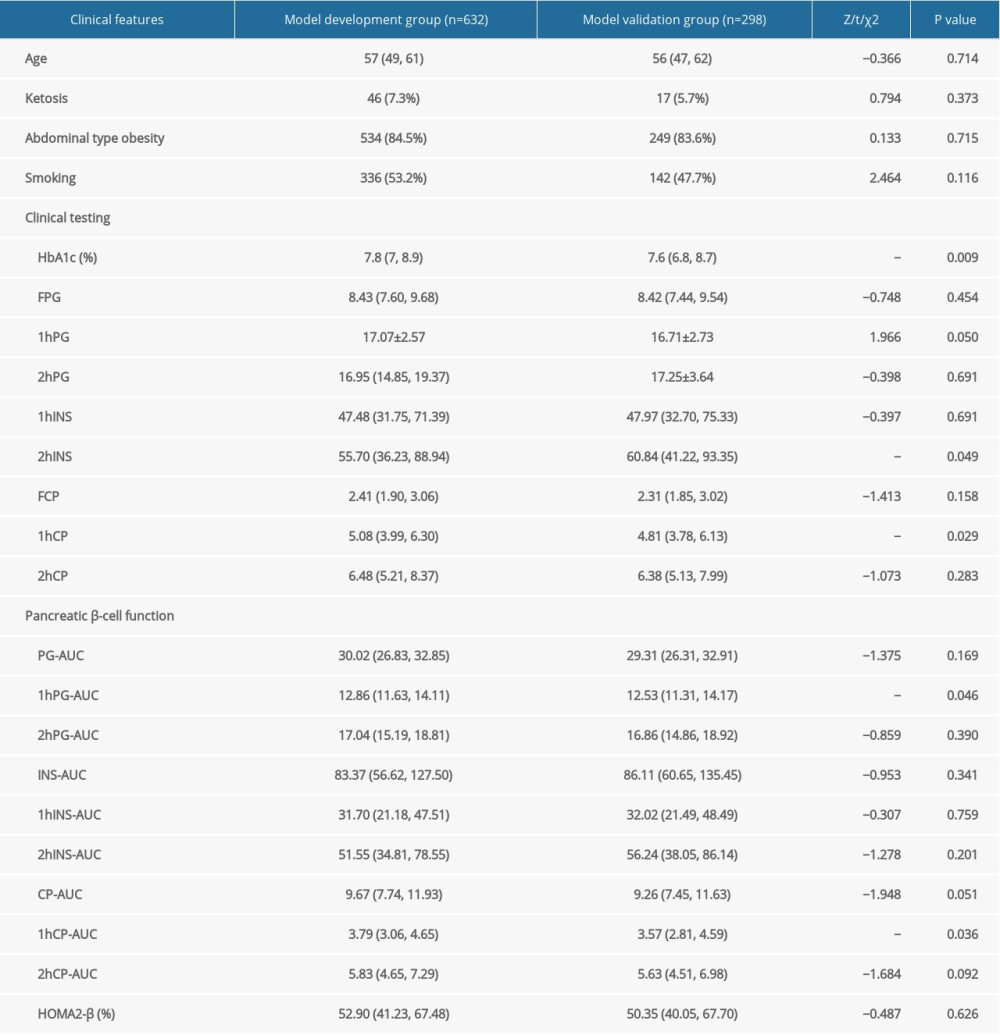 Table 3. Single-variable logistic regression analysis in the model development group.
Table 3. Single-variable logistic regression analysis in the model development group.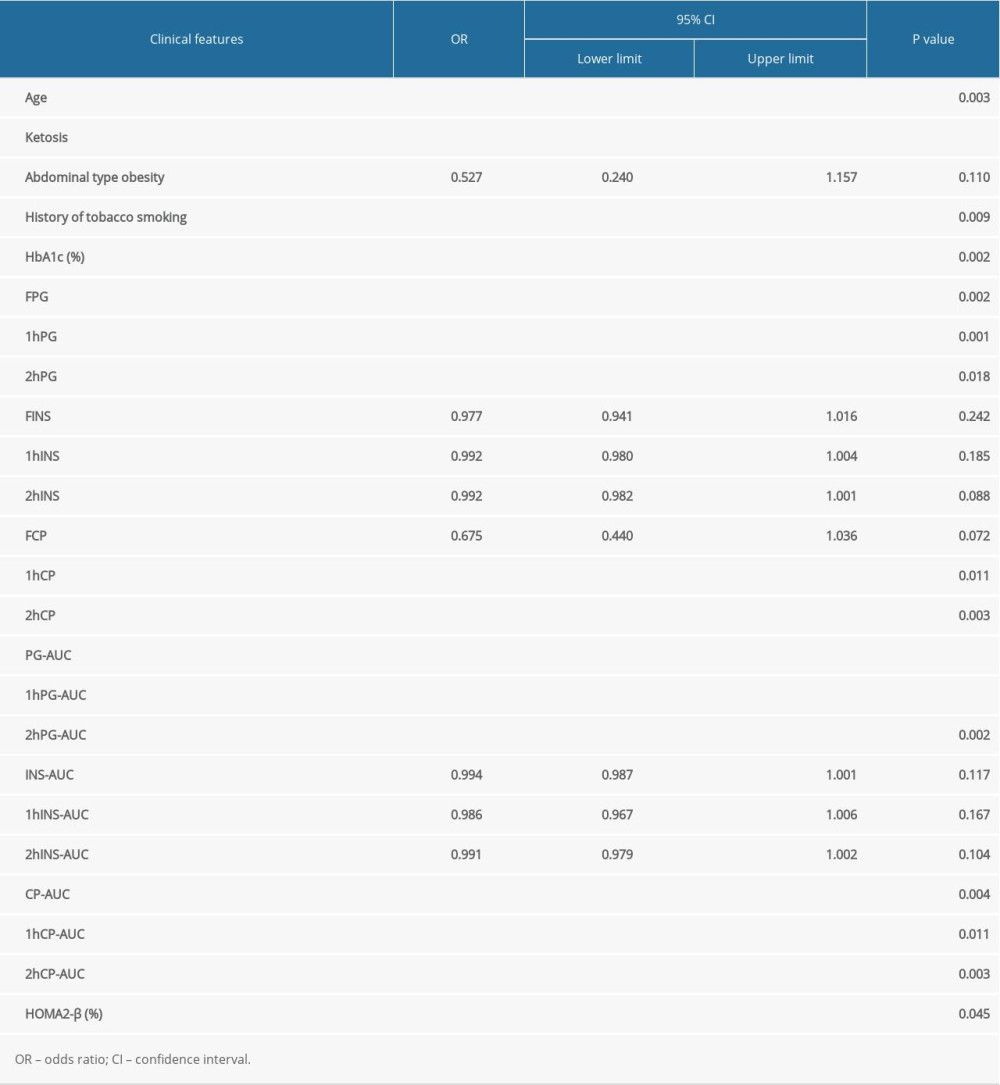 Table 4. Estimated regression coefficient, probability, and OR after the selection of single factors.
Table 4. Estimated regression coefficient, probability, and OR after the selection of single factors.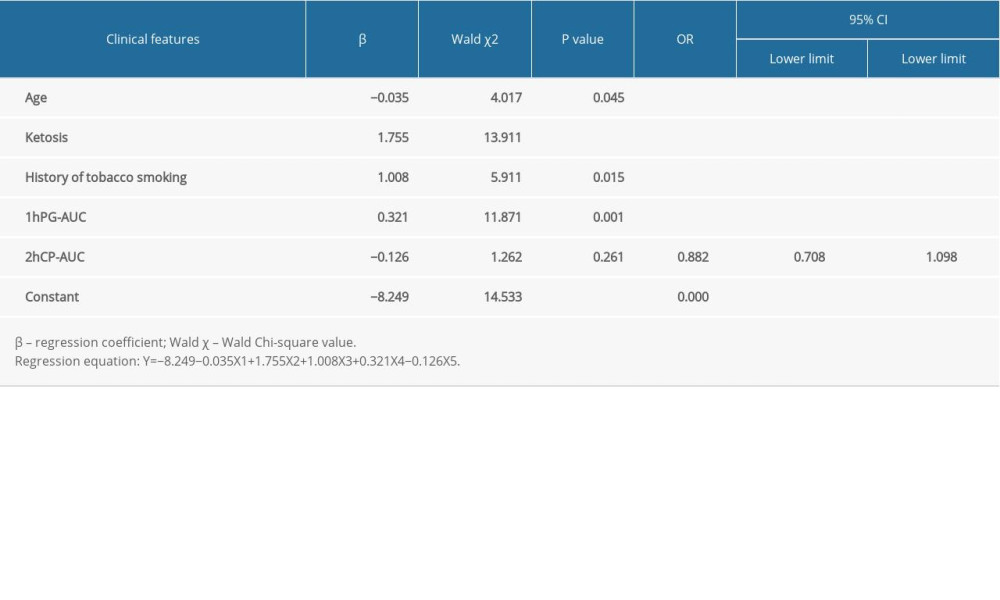
References
1. Saeedi P, Petersohn I, Salpea P: Diabetes Res Clin Pract, 2019; 157; 107843
2. Tuomi T, Groop LC, Zimmet PZ, Antibodies to glutamic acid decarboxylase reveal latent autoimmune diabetes mellitus in adults with a non-insulin-dependent onset of disease: Diabetes, 1993; 42(2); 359-62
3. Fourlanos S, Dotta F, Greenbaum CJ, Latent autoimmune diabetes in adults (LADA) should be less latent: Diabetologia, 2005; 48(11); 2206-12
4. Consensus on the diagnosis and treatment of adult occult autoimmune diabetes mellitus (LADA) by Chinese Diabetes Society: Chin J Diabetes Mellitus, 2012; 4(11); 641-47 [in Chinese]
5. Basile KJ, Guy VC, Schwartz S, Overlap of genetic susceptibility to type 1 diabetes, type 2 diabetes, and latent autoimmune diabetes in adults: Curr Diab Rep, 2014; 14(11); 550
6. World Health Organization (Switzerland): Classification of diabetes mellitus, 2019, Geneva
7. Shields BM, Peters JL, Cooper C, Can clinical features be used to differentiate type 1 from type 2 diabetes? A systematic review of the literature: BMJ Open, 2015; 5(11); e009088
8. Zhou Z, Xiang Y, Ji L, Frequency, immunogenetics, and clinical characteristics of latent autoimmune diabetes in China (LADA China study): A nationwide, multicenter, clinic-based cross-sectional study: Diabetes, 2013; 62(2); 543-50
9. Liu L, Li X, Xiang Y, Latent autoimmune diabetes in adults with low-titer GAD antibodies: similar disease progression with type 2 diabetes: A nationwide, multicenter prospective study (LADA China Study 3): Diabetes Care, 2015; 38(1); 16-21
10. Thunander M, Thorgeirsson H, Torn C, Beta-cell function and metabolic control in latent autoimmune diabetes in adults with early insulin versus conventional treatment: A 3-year follow-up: Eur J Endocrinol, 2011; 164(2); 239-45
11. Coustan DR, Gestational diabetes mellitus: Clin Chem, 2013; 59(9); 1310-21
12. Fluss R, Faraggi D, Reiser B, Estimation of the Youden Index and its associated cutoff point: Biom J, 2005; 47(4); 458-72
13. Qi X, Sun J, Wang J, Prevalence and correlates of latent autoimmune diabetes in adults in Tianjin, China: A population-based cross-sectional study: Diabetes Care, 2011; 34(1); 66-70
14. Carlsson S, Midthjell K, Tesfamarian MY, Age, overweight and physical inactivity increase the risk of latent autoimmune diabetes in adults: results from the Nord-Trondelag health study: Diabetologia, 2007; 50(1); 55-58
15. Buzzetti R, Zampetti S, Maddaloni E, Adult-onset autoimmune diabetes: Current knowledge and implications for management: Nat Rev Endocrinol, 2017; 13(11); 674-86
16. Adeleye OO, Ogbera AO, Fasanmade O, Latent Autoimmune Diabetes Mellitus in Adults (LADA) and its characteristics in a subset of Nigerians initially managed for type 2 diabetes: Int Arch Med, 2012; 5(1); 23
17. Carlsson A, Sundkvist G, Groop L, Insulin and glucagon secretion in patients with slowly progressing autoimmune diabetes (LADA): J Clin Endocrinol Metab, 2000; 85(1); 76-80
18. Hernandez M, Mollo A, Marsal JR, Insulin secretion in patients with latent autoimmune diabetes (LADA): Half way between type 1 and type 2 diabetes: Action LADA 9: BMC Endocr Disord, 2015; 15; 1
19. Zampetti S, Campagna G, Tiberti C, High GADA titer increases the risk of insulin requirement in LADA patients: A 7-year follow-up (NIRAD study 7): Eur J Endocrinol, 2014; 171(6); 697-704
20. Yu K, Huang Z, Zhou J, Transcriptome profiling of microRNAs associated with latent autoimmune diabetes in adults (LADA): Sci Rep, 2019; 9(1); 11347
21. Ji Y, Jiang D, Liu J, Comparative analysis of the transcriptome of Latent Autoimmune Diabetes in Adult (LADA) patients from Eastern China: J Diabetes Res, 2019; 2019; 8616373
22. Carlsson S, Midthjell K, Grill V, Smoking is associated with an increased risk of type 2 diabetes but a decreased risk of autoimmune diabetes in adults: An 11-year follow-up of incidence of diabetes in the Nord-Trondelag study: Diabetologia, 2004; 47(11); 1953-56
23. Rasouli B, Grill V, Midthjell K, Smoking is associated with reduced risk of autoimmune diabetes in adults contrasting with increased risk in overweight men with type 2 diabetes: A 22-year follow-up of the HUNT study: Diabetes Care, 2013; 36(3); 604-10
24. Sopori M, Effects of cigarette smoke on the immune system: Nat Rev Immunol, 2002; 2(5); 372-77
25. Stampfli MR, Anderson GP, How cigarette smoke skews immune responses to promote infection, lung disease and cancer: Nat Rev Immunol, 2009; 9(5); 377-84
26. Rasouli B, Andersson T, Carlsson PO, Smoking and the risk of LADA: Results from a Swedish population-based case-control study: Diabetes Care, 2016; 39(5); 794-800
27. Bajaj M, Nicotine and insulin resistance: When the smoke clears: Diabetes, 2012; 61(12); 3078-80
28. Fourlanos S, Perry C, Stein MS, A clinical screening tool identifies autoimmune diabetes in adults: Diabetes Care, 2006; 29(5); 970-75
29. Agyei-Frempong MT, Titty FV, Owiredu WK, The prevalence of autoimmune diabetes among diabetes mellitus patients in Kumasi, Ghana: Pak J Biol Sci, 2008; 11(19); 2320-25
30. Hawa MI, Thivolet C, Mauricio D, Metabolic syndrome and autoimmune diabetes: Action LADA 3: Diabetes Care, 2009; 32(1); 160-64
31. Zhou J, Ma X, Bao YStudy on prevalence of latent autoimmune diabetes in adults and its relationship with metabolic syndrome: Zhonghue Yi Xue Za Zi, 2009; 89(18); 1250-54 [in Chinese]
32. Lindstrom J, Tuomilehto J, The diabetes risk score: A practical tool to predict type 2 diabetes risk: Diabetes Care, 2003; 26(3); 725-31
33. Balkau B, Lange C, Fezeu L, Predicting diabetes: clinical, biological, and genetic approaches: Data from the Epidemiological Study on the Insulin Resistance Syndrome (DESIR): Diabetes Care, 2008; 31(10); 2056-61
34. Rahman M, Simmons RK, Harding AH, A simple risk score identifies individuals at high risk of developing Type 2 diabetes: A prospective cohort study: Fam Pract, 2008; 25(3); 191-96
35. Chen L, Magliano DJ, Balkau B, AUSDRISK: An Australian Type 2 Diabetes Risk Assessment Tool based on demographic, lifestyle and simple anthropometric measures: Med J Aust, 2010; 192(4); 197-202
36. Brophy S, Yderstraede K, Mauricio D, Time to insulin initiation cannot be used in defining latent autoimmune diabetes in adults: Diabetes Care, 2008; 31(3); 439-41
37. Jorns A, Wedekind D, Jahne J, Pancreas pathology of Latent Autoimmune Diabetes in Adults (LADA) in patients and in a LADA rat model compared with type 1 diabetes: Diabetes, 2020; 69(4); 624-33
Tables
 Table 1. Comparison of the clinical features among patients with T2DM vs LADA.
Table 1. Comparison of the clinical features among patients with T2DM vs LADA. Table 2. Comparison of the clinical features between model development and validation groups.
Table 2. Comparison of the clinical features between model development and validation groups. Table 3. Single-variable logistic regression analysis in the model development group.
Table 3. Single-variable logistic regression analysis in the model development group. Table 4. Estimated regression coefficient, probability, and OR after the selection of single factors.
Table 4. Estimated regression coefficient, probability, and OR after the selection of single factors. Table 1. Comparison of the clinical features among patients with T2DM vs LADA.
Table 1. Comparison of the clinical features among patients with T2DM vs LADA. Table 2. Comparison of the clinical features between model development and validation groups.
Table 2. Comparison of the clinical features between model development and validation groups. Table 3. Single-variable logistic regression analysis in the model development group.
Table 3. Single-variable logistic regression analysis in the model development group. Table 4. Estimated regression coefficient, probability, and OR after the selection of single factors.
Table 4. Estimated regression coefficient, probability, and OR after the selection of single factors. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952