13 December 2021: Clinical Research
Comparison of Outcomes Following Neuronavigation-Assisted Aspiration and Thrombolysis Using Single and Multiple Catheter Insertion for Moderate-Volume Supratentorial Spontaneous Intracerebral Hemorrhage: A Single-Center Retrospective Study of 102 Patients
In-Hyoung LeeDOI: 10.12659/MSM.934935
Med Sci Monit 2021; 27:e934935
Abstract
BACKGROUND: This retrospective study from a single center aimed to investigate 102 patients with isolated moderate-volume (30-60 mL) supratentorial spontaneous intracerebral hemorrhage (sICH) treated with neuronavigation-assisted aspiration and thrombolysis to compare outcomes using single and multiple catheter insertion.
MATERIAL AND METHODS: We retrospectively enrolled 102 patients (58 single-catheter insertion recipients and 44 multi-catheter insertion recipients) diagnosed with isolated moderate-volume supratentorial sICH who underwent neuronavigation-assisted aspiration and thrombolysis surgery in a single center between March 2017 and December 2019. The impact of multi-catheter insertion on the radiologic and clinical outcomes and complications were compared with those of single-catheter insertion.
RESULTS: The baseline characteristics, clinical status, and outcomes of both groups were not significantly different, except for the number of inserted catheters and surgical time. The single-catheter group had a significantly shorter surgical time than the multi-catheter group (39.52±8.76 min vs 61.39±16.6 min; P<0.001). The surgery-related complication catheter tract hemorrhage (CTH) occurred significantly more frequently in the multi-catheter group than in the single-catheter group (8.6% vs 27.3%; P=0.019). In the regression analysis, international normalized ratio prolongation and multi-catheter insertion were independent risk factors for CTH.
CONCLUSIONS: Single-catheter insertion is not inferior to multi-catheter insertion for isolated moderate-volume (30-60 mL) supratentorial sICH in terms of radiologic and clinical outcomes and significantly shortened the surgical time and reduced the incidence of CTH.
Keywords: catheters, Cerebral Hemorrhage, Neuronavigation, Tissue Plasminogen Activator, Biopsy, Fine-Needle, Female, Humans, Male, Radiography, Interventional, Thrombolytic Therapy, Tomography, X-Ray Computed
Background
Spontaneous intracerebral hemorrhage (sICH) accounts for approximately 10–15% of all cases of stroke, with a high mortality rate of approximately 40%, and can cause serious neurologic deficits [1,2]. Currently, sICH management mainly consists of conservative medical treatment and surgical intervention [3]. In general, conservative care is usually performed in patients with small hematomas and absence of neurologic deficits, while surgical evacuation tends to be used in those with large-volume hemorrhage and progressive neurological deterioration [4].
Conventional open craniotomy is generally preferred in treating large hematomas because it allows a wide surgical field, resulting in a dramatic reduction of mass effects [5,6]. However, a recent well-performed trial of craniotomy for supratentorial sICH failed to prove significant benefits in functional outcomes [7].
In recent years, new surgical techniques, collectively referred to as minimally-invasive surgery (MIS), such as computed tomography (CT)-guided stereotactic aspiration, neuronavigation-assisted aspiration, and endoscopic hematoma evacuation, have emerged as an alternative approach for treating sICH by sparing the brain parenchyma from secondary damage induced by surgical procedures; they have shown satisfactory outcomes, unlike open craniotomy [8–10].
In detail, these techniques showed great potential for the treatment of sICH and replacement of craniotomy for patients with moderate hematoma volume (30–50 mL) and mildly impaired consciousness [11,12]. Among them, MIS plus recombinant tissue plasminogen activator (tPA) for intracerebral hemorrhage (ICH) evacuation (MISTIE) was recently completed in a phase III trial, and its safety was verified [13]. Furthermore, it was shown to have benefits in mortality, with no significant increase in severe disability. The amount of hematoma to be removed that could have functional benefits was specified and presented in a recent clinical trial [14].
However, there has been no study on the classification according to the number of inserted catheters required in relation to the hematoma burden. When performing neuronavigation-assisted aspiration and thrombolysis surgery for sICH, neurosurgeons may be unconsciously inclined to use multiple catheters to evacuate more hematoma or be concerned regarding catheter obstruction. The present study was conducted on the basis of the assumption that multi-catheter insertion may not be necessary for isolated moderate-volume (30–60 mL) hematomas. By assessing and comparing the surgical impact of single and multiple catheter insertions on hematoma evacuation, functional outcomes, and associated complications, we attempted to determine the proper number of catheters inserted for isolated moderate-volume supratentorial sICH. Therefore, this retrospective study from a single center investigated 102 patients with isolated moderate-volume supratentorial sICH treated with neuronavigation-assisted aspiration and thrombolysis to compare outcomes using single and multiple catheter insertion.
Material and Methods
STUDY DESIGN AND PATIENT ENROLLMENT:
After obtaining approval from our Institutional Review Board (IRB No. HKS 2020-04-023), we conducted this retrospective study based on electronic medical records and imaging data of 160 patients who underwent neuronavigation-assisted aspiration with thrombolysis surgery in the Department of Neurosurgery at our institution after being diagnosed with moderate-volume ICH (30–60 mL) on CT scans from March 2017 to December 2019.
The exclusion criteria were as follows: (1) hemorrhage induced by clear pre-existing causes, such as arteriovenous malformation, intracranial tumor bleeding, moyamoya disease, ruptured intracranial aneurysm, hemorrhagic transformation of cerebral infarction, or trauma; (2) hemorrhage originating from the infratentorial region or brainstem; (3) hematoma extension into the ventricles on CT scans derived from ventricular rupture, especially thalamic ICH; (4) loss to follow-up; and (5) brain CT follow-up not meeting the required criteria.
The patient selection process is depicted schematically in Figure 1.
DATA COLLECTION AND CLINICAL OUTCOME ASSESSMENT:
The following clinical information of the patients was collected: age and sex; initial systolic blood pressure measured at the emergency room; coagulation time in the blood test; presence of pre-existing diseases, such as history of hypertension, diabetes mellitus, chronic kidney disease, cardiovascular disease, liver disease, and stroke; history of antiplatelet or anticoagulant use; preoperative consciousness level (Glasgow Coma Scale); surgical time; number of inserted catheters; and catheter duration.
The clinical outcomes included the Intensive Care Unit stay duration, in-hospital mortality, and modified Rankin scale (mRS) score at discharge and after 3 and 6 months. An mRS score at 6 months of 0–3 was considered to indicate a favorable outcome. Pneumonia, urinary tract infection, and venous thromboembolism during the postoperative period were defined as non-surgical complications.
NEURORADIOLOGIC ASSESSMENT:
The following radiologic data of the patients were obtained using INFINITT PACS M6 (INFINITT Healthcare, Seoul, Korea): ICH location, preoperative ICH volume, immediate postoperative ICH volume, postoperative day 2 ICH volume, and ICH volume at the endpoint of treatment (EOT) defined as the time of catheter removal. The proportion of patients who achieved the target values was identified on the basis of ICH removal to ≤15 mL (or of ≥70%) at the EOT. In addition, surgery-related complications, such as postoperative re-bleeding, catheter tract hemorrhage (CTH), and intracranial infection, were also evaluated.
All examinations included in this study were performed as part of standard clinical care using a dual-source 64-slice CT scanner (SOMATOM Definition Flash; Siemens Healthcare Sector, Forchheim, Germany), including non-contrast CT (120 kV, 380 mA, and contiguous 5-mm axial slices) and CT angiography to evaluate vascular pathologies.
The ABC/2 formula was used to estimate the hematoma volume on CT scans, where A is the maximal transverse diameter on the maximal hematoma slice, B is the largest diameter perpendicular to A, and C is the number of CT slices in which hematoma is visibly multiplied by the slice thickness in centimeters [15].
Postoperative re-bleeding was defined as a hematoma volume of any CT scan after aspiration that is larger than that of the preoperative CT scan or any previous CT scan after aspiration or a <5-mL difference between the preoperative and immediate postoperative CT hematoma volume measurements [16].
CTH was defined as newly identified bleeding that was visually observed, which is usually easily recognized in the catheterized tract on the CT scan taken after surgery (Figure 2). All imaging data were analyzed and evaluated independently by 2 neurosurgeons and 1 neuro-radiologist blinded to clinical information.
SURGICAL PLANNING:
All surgeries were performed using the StealthStation® S7 AxiEM™ navigation system (Medtronic, Minneapolis, MN, USA). Preoperative 1-mm slice high-resolution CT was performed to determine the entry point, optimal trajectory, and most efficient target point. After tracer registration (surface matching), the entry point, usually Kocher’s point or that above the subcortical hematoma, was set up, and 1 or multiple target points were established. The number of the catheters to be inserted was determined by preference, based on the clinical experience of each of the 3 attending neurosurgeons at our institution.
The entry point and catheter trajectory were set in consideration of the shortest path that can minimize secondary injury to the eloquent brain cortex and blood vessels.
In the case of single-catheter insertion, the target points were located in the dependent portion and center of the hematoma to maximize the area exposed to the catheter and thrombolytic agent. In the case of 2-catheter insertion, the long axis of the hematoma in the axial CT plane was evenly divided, and the target points were set at each middle point. With this method, the target points were located in the lower 2 quadrants when the longest transverse axis and perpendicular line to it were drawn in the sagittal CT plane. In the case of 3-catheter insertion, the target point was additionally set between the target points of the 2 catheters described above. Additionally, to prevent brain tissue injury due to invasion of the lower boundary of the hematoma caused by navigation error, we set the target point at approximately 5 mm superficial from the lower margin of the hematoma (Figure 3).
INTRAOPERATIVE PROCEDURES AND POSTOPERATIVE STRATEGIES:
The subsequent surgical procedure followed a generally accepted method. External ventricular drainage (EVD) tubes of 10.5 French (Yushin Medical, Seoul, Korea) were inserted into the target point, with assistance from the navigation stylet. The liquefied hematoma was manually aspirated with low tension using a 10-mL syringe to target confirmation and diminish the mass effect, until resistance was observed.
Postoperative CT was performed to confirm that the catheters were properly located in the hematoma and to measure the amount of residual hematoma. When the catheter location required adjustment, appropriate withdrawal was performed. Thereafter, intra-clot administration of tPA (Actilyse®, Boehringer Ingelheim, Seoul, Korea) was performed. The dose regimen for tPA was based on the MISTIE clinical trial [17]. However, tPA was not used when the amount of hematoma volume immediately after surgery was less than 10 mL or when catheter malposition or re-bleeding occurred.
When the residual hematoma volume was less than 10 mL or significant re-bleeding occurred on the follow-up CT scans obtained 2 days after surgery, tPA administration was stopped and the hematoma naturally drained. When the ICH volume did not increase, and the amount of drainage significantly decreased, the catheters were removed. We termed these 2 prerequisites for catheter removal as hematoma stability. The postoperative strategies are outlined in Figure 4. Figure 5 shows an example of the treatment process for patients in our cohort following this postoperative strategy.
The medical management of these patients followed the American Heart Recommendations for the treatment of sICH [18], including correction of coagulopathy, control of blood pressure and glucose, and prevention of medical complications. Additionally, in accordance with our institutional routine practice, patients received prophylactic antibiotics until 2 days after the surgical procedure, and no prophylactic anti-epileptic drugs were administered.
STATISTICAL ANALYSIS:
The data collected in this study were analyzed using the SPSS 27.0 software (SPSS, Inc., Chicago, IL, USA). Continuous variables were expressed as means±standard deviations (ranges) and categorical variables as numbers of patients (percentages). The
Results
PATIENT CHARACTERISTICS:
A total of 58 patients were excluded from the study. Thus, a total of 102 patients with isolated moderate-volume (30–60 mL) supratentorial sICH who underwent neuronavigation-assisted aspiration with thrombolysis surgery were enrolled in this study: 58 patients who underwent single-catheter insertion and 44 patients who underwent multi-catheter insertion.
There were no significant differences in demographics, severity, and clinical features between the 2 groups, except for the number of inserted catheters and surgical time. The average number of catheters inserted in the multi-catheter group was 2.27. The single-catheter group had significantly shorter surgical time than the multi-catheter group (39.52±8.76 min vs 61.39±16.6 min; P<0.001) (Table 1).
CLINICAL OUTCOMES AND COMPLICATIONS:
Table 2 shows the differences in the clinical outcomes and complications between the single-catheter and multi-catheter groups. Irrespective of the group, the clinical status improved in terms of the mRS score from the time of discharge and up to the 6-month postoperative period, with no significant difference. In addition, the achievement of an mRS score of <4 at 6 months after surgery (favorable outcome) and in-hospital mortality were not significantly different.
The incidence of CTH was significantly higher in the multi-catheter group than in the single-catheter group (8.6% vs 27.3%; P =0.019). Multivariate analyses revealed that prothrombin time (PT) (international normalized ratio [INR]) prolongation and multi-catheter insertion were the independent risk factors associated with CTH (OR=4.386, 95% CI=1.202–16.01, P=0.025; OR=5.115, 95% CI=1.467–17.836, P=0.01, respectively) (Table 3).
RADIOLOGIC OUTCOMES:
The mean preoperative and immediate postoperative ICH volumes were 47.2±9.5 mL and 28.3±8.9 mL, respectively. As a result, the mean immediate postoperative ICH clearance rate was approximately 40%. The differences between the 2 groups, including the values on postoperative day 2 and at the EOT, are summarized in Table 4 and Figure 6.
There were no significant differences in the ICH volume and clearance rate immediately after surgery, on postoperative day 2, and at the EOT. Similarly, the daily average hematoma volume removed and the proportion of patients who achieved the target values (ICH removal to ≤15 mL [or of ≥70%]) were similar in both groups.
Discussion
LIMITATIONS:
This study had several limitations. First, this was a retrospective, single-center study with a small number of enrolled patients. Second, the hematoma removal rate may depend on the competence of the neurosurgeon and the variability of the neuronavigation accuracy. We believe that a prospective, randomized, multicenter study with a large cohort that subdivides the operator factor, neuronavigation accuracy, and hematoma volume is likely to provide more reliable, objective reference values and surgical guidelines.
Conclusions
In conclusion, the comprehensive comparison of radiologic and clinical outcomes showed that single-catheter insertion was not inferior to multi-catheter insertion. Instead, single-catheter insertion was advantageous in complications, especially CTH, and could significantly shorten the surgical time. Therefore, we recommend single-catheter insertion in neuronavigation-assisted stereotactic aspiration and thrombolysis surgery for isolated moderate-volume (30–60 mL) supratentorial sICH.
Figures
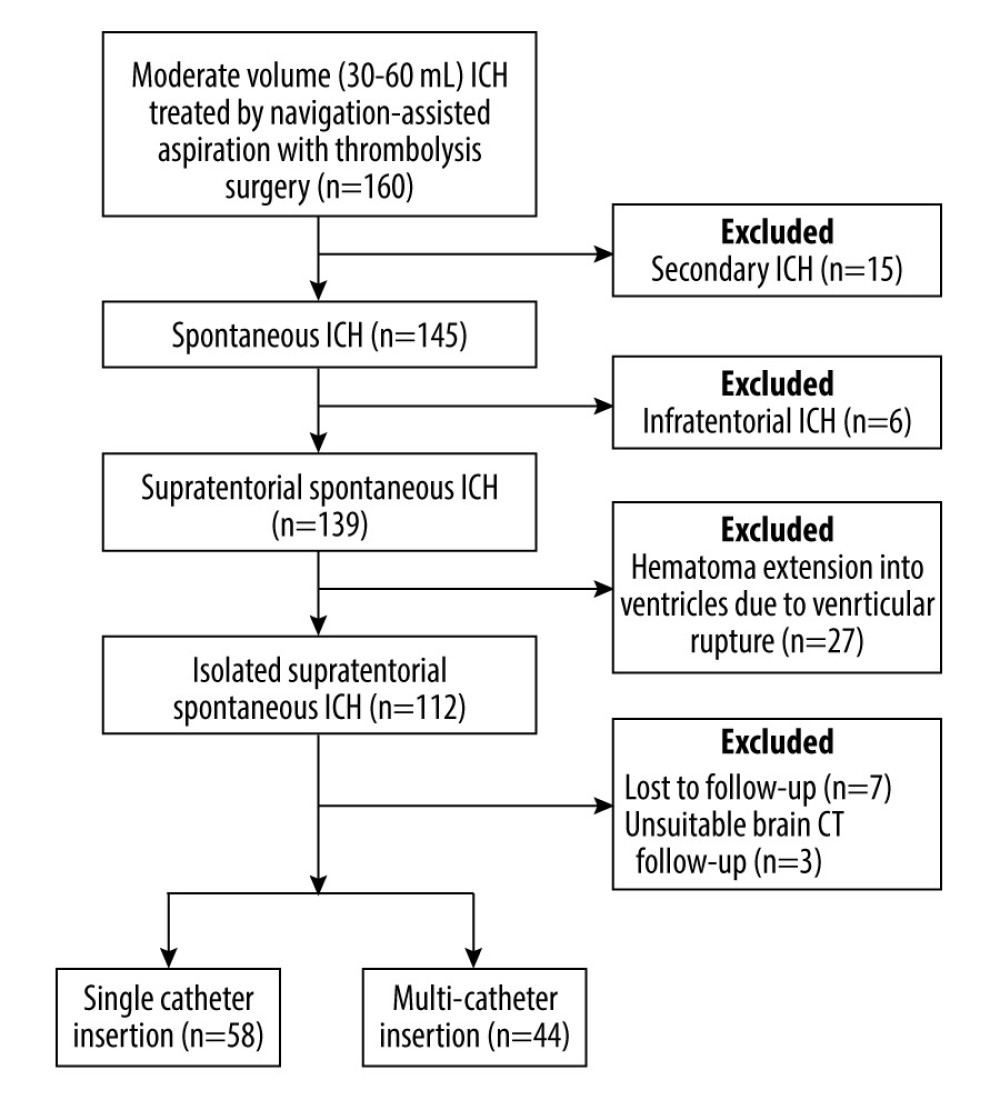 Figure 1. Flow diagram demonstrating the patient selection process in this studyICH – intracerebral hemorrhage; CT – computed tomography. The figure was generated using Microsoft Word 2010 (Microsoft, Redmond, WA, USA).
Figure 1. Flow diagram demonstrating the patient selection process in this studyICH – intracerebral hemorrhage; CT – computed tomography. The figure was generated using Microsoft Word 2010 (Microsoft, Redmond, WA, USA). 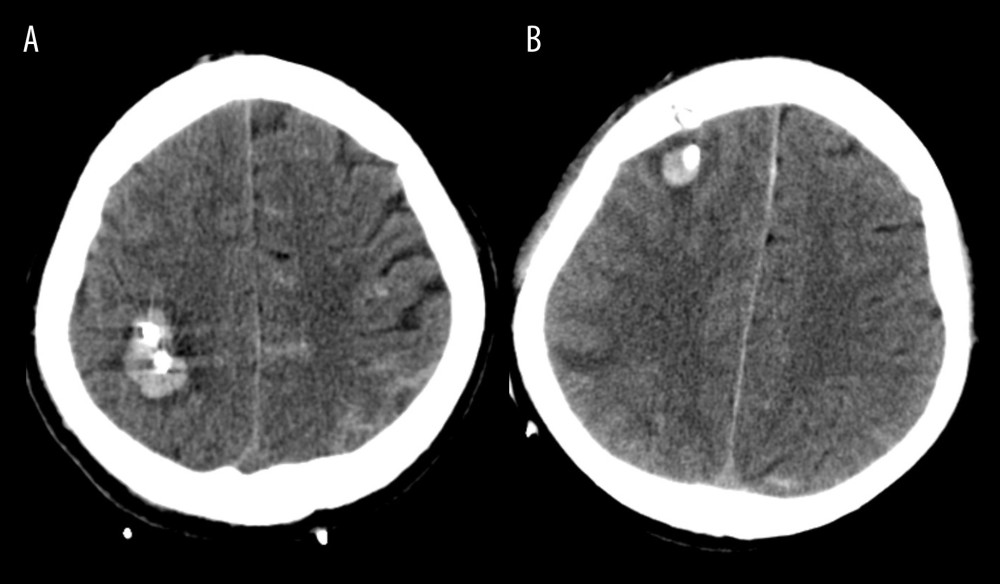 Figure 2. (A, B) Illustration of catheter tract hemorrhage on axial computed tomography scan obtained after surgery.The figure was created by the author from the PACS database of our institution using FastStone capture 9.0 (FastStone Soft).
Figure 2. (A, B) Illustration of catheter tract hemorrhage on axial computed tomography scan obtained after surgery.The figure was created by the author from the PACS database of our institution using FastStone capture 9.0 (FastStone Soft). 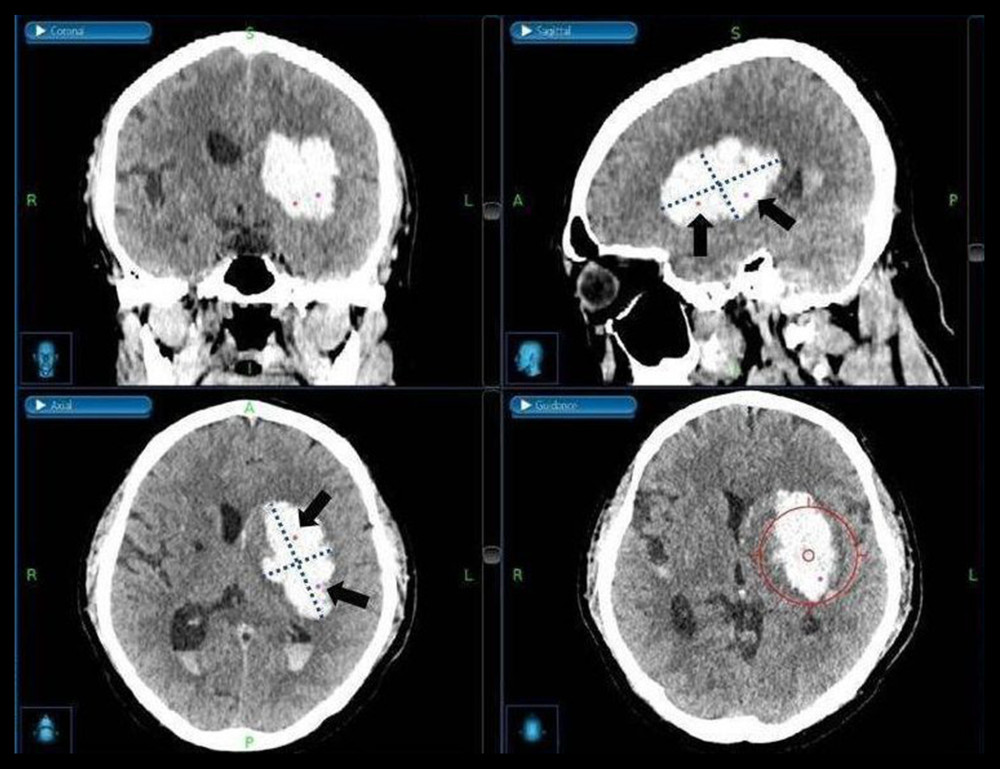 Figure 3. Medtronic AxiEM™ stereotactic neuronavigation targeting screen with target point in multiple planesA virtual line of the largest diameter of the hematoma marked and perpendicular to it on the axial and sagittal planes was drawn (dotted line). After evenly dividing the longest axis, we set the target points around each middle point (black arrow). The planned trajectory can be viewed in 3 planes simultaneously, and the guidance view (bottom right corner) can be used to monitor the position of the stylet in relation to the planned trajectory. The figure was edited using Microsoft PowerPoint 2010 (Microsoft, Redmond, WA, USA) after screenshot from the StealthStation® S7 AxiEM™ navigation system in our institution.
Figure 3. Medtronic AxiEM™ stereotactic neuronavigation targeting screen with target point in multiple planesA virtual line of the largest diameter of the hematoma marked and perpendicular to it on the axial and sagittal planes was drawn (dotted line). After evenly dividing the longest axis, we set the target points around each middle point (black arrow). The planned trajectory can be viewed in 3 planes simultaneously, and the guidance view (bottom right corner) can be used to monitor the position of the stylet in relation to the planned trajectory. The figure was edited using Microsoft PowerPoint 2010 (Microsoft, Redmond, WA, USA) after screenshot from the StealthStation® S7 AxiEM™ navigation system in our institution. 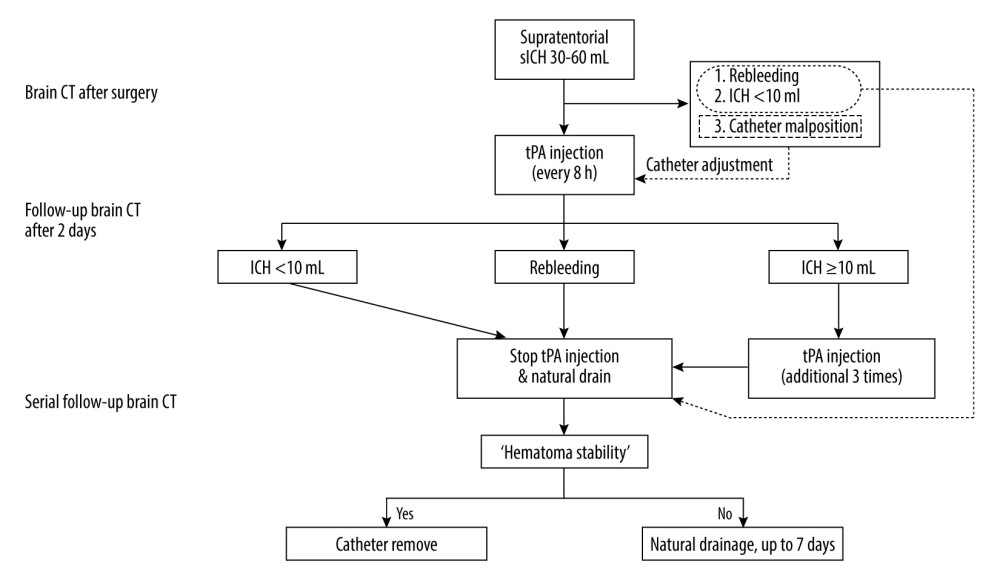 Figure 4. Postoperative strategies and thrombolysis algorithm for moderate-volume (30–60 mL) supratentorial spontaneous intracerebral hemorrhage (sICH) in our institutionICH – intracerebral hemorrhage; CT – computed tomography; tPA – tissue plasminogen activator. The figure was created using Microsoft PowerPoint 2010 (Microsoft, Redmond, WA, USA).
Figure 4. Postoperative strategies and thrombolysis algorithm for moderate-volume (30–60 mL) supratentorial spontaneous intracerebral hemorrhage (sICH) in our institutionICH – intracerebral hemorrhage; CT – computed tomography; tPA – tissue plasminogen activator. The figure was created using Microsoft PowerPoint 2010 (Microsoft, Redmond, WA, USA). 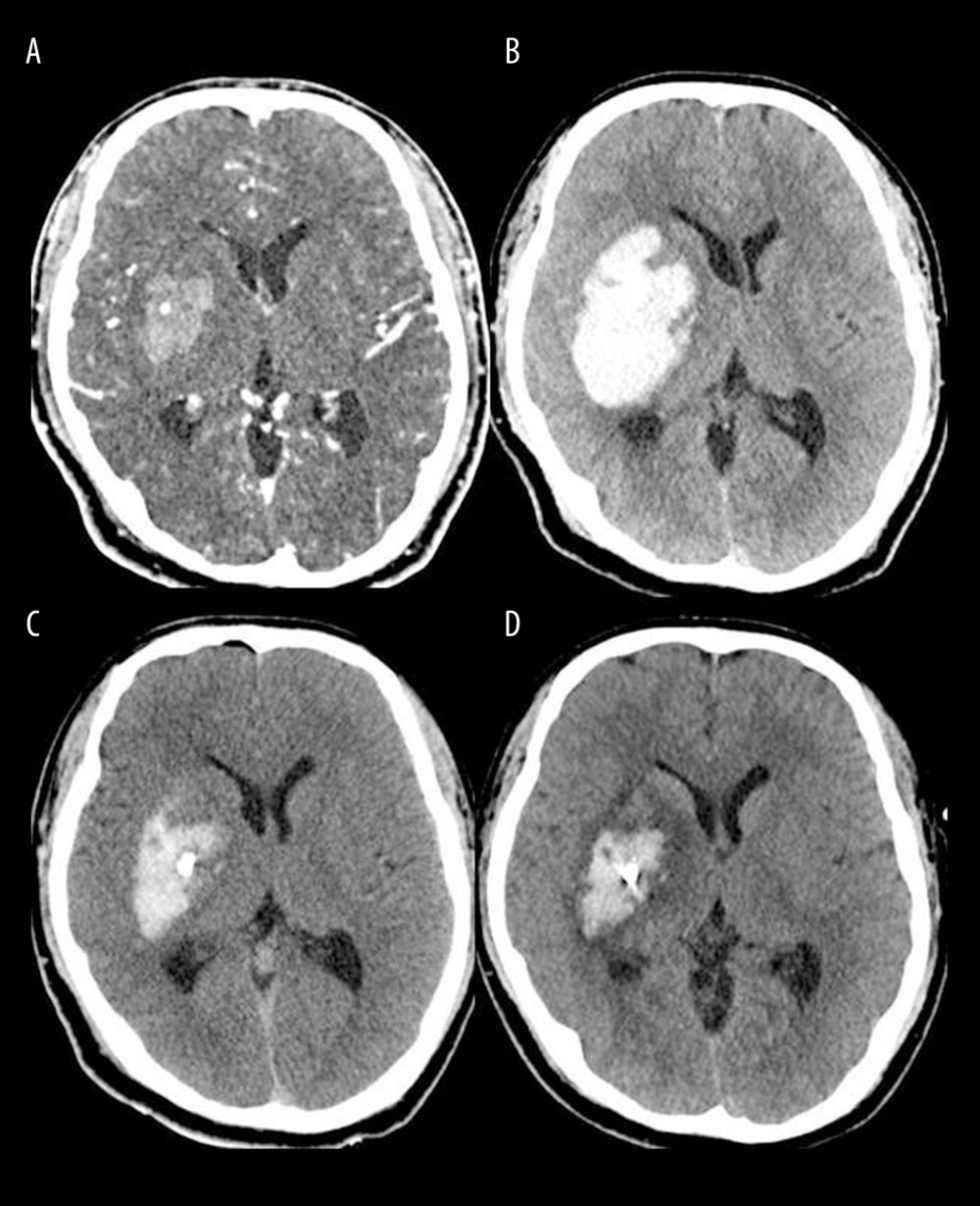 Figure 5. Brain computed tomography (CT) scans of a successful case of hematoma removal using single-catheter insertion in neuronavigation-assisted stereotactic aspiration and thrombolysis surgery(A) Spontaneous intracerebral hemorrhage of 15 mL in the right basal ganglia with the spot sign on the initial CT angiography scan. (B) Marked volume enlargement on the follow-up CT scan (57 mL). (C) Remarkable decrease in the hematoma volume after single-catheter insertion on the postoperative CT scan. (D) Approximately 80% of the hematoma had been removed as a result of tissue plasminogen activator administration on the follow-up CT scan 2 days after surgery. The figure was created by the author from the PACS database of our institution using FastStone capture 9.0 (FastStone Soft).
Figure 5. Brain computed tomography (CT) scans of a successful case of hematoma removal using single-catheter insertion in neuronavigation-assisted stereotactic aspiration and thrombolysis surgery(A) Spontaneous intracerebral hemorrhage of 15 mL in the right basal ganglia with the spot sign on the initial CT angiography scan. (B) Marked volume enlargement on the follow-up CT scan (57 mL). (C) Remarkable decrease in the hematoma volume after single-catheter insertion on the postoperative CT scan. (D) Approximately 80% of the hematoma had been removed as a result of tissue plasminogen activator administration on the follow-up CT scan 2 days after surgery. The figure was created by the author from the PACS database of our institution using FastStone capture 9.0 (FastStone Soft). 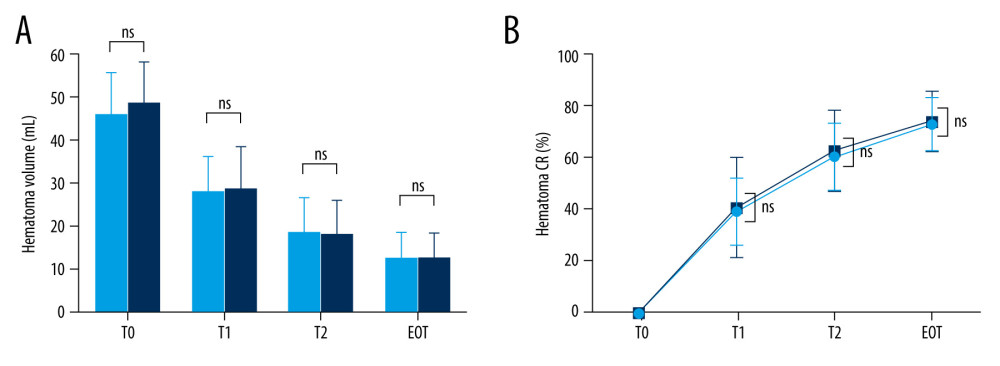 Figure 6. Hematoma volume (A) and chronological change in the hematoma clearance rate (B) at various time points in the single and multiple catheter groups(A) There were no significant differences between the single (light blue bar) and multiple catheter groups (deep blue bar) in hematoma volume (mL) through all chronologies. (B) There were no significant differences between the single (light blue line) and multiple catheter groups (deep blue line) in hematoma clearance rate (%) through all chronologies. T0 – the time at which preoperative computed tomography (CT) scan was carried out; T1 – the time at which immediate postoperative CT scan was taken; T2 – postoperative 2 days; EOT – endpoint of treatment (the time of catheter removal); CR – clearance rate; ns – no significant difference between the 2 values. The figure was created with GraphPad Prism 9.3.0 (GraphPad Software, Inc., San Diego, CA, USA).
Figure 6. Hematoma volume (A) and chronological change in the hematoma clearance rate (B) at various time points in the single and multiple catheter groups(A) There were no significant differences between the single (light blue bar) and multiple catheter groups (deep blue bar) in hematoma volume (mL) through all chronologies. (B) There were no significant differences between the single (light blue line) and multiple catheter groups (deep blue line) in hematoma clearance rate (%) through all chronologies. T0 – the time at which preoperative computed tomography (CT) scan was carried out; T1 – the time at which immediate postoperative CT scan was taken; T2 – postoperative 2 days; EOT – endpoint of treatment (the time of catheter removal); CR – clearance rate; ns – no significant difference between the 2 values. The figure was created with GraphPad Prism 9.3.0 (GraphPad Software, Inc., San Diego, CA, USA). References
1. Gokhale S, Caplan LR, James ML, Sex differences in incidence, pathophysiology, and outcome of primary intracerebral hemorrhage: Stroke, 2015; 46(3); 886-92
2. van Asch CJ, Luitse MJ, Rinkel GJ, Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: A systematic review and meta-analysis: Lancet Neurol, 2010; 9(2); 167-76
3. Morgenstern LB, Hemphill JC, Anderson C, Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American Heart Association/American Stroke Association: Stroke, 2010; 41(9); 2108-29
4. Kelly ML, Sulmasy DP, Weil RJ, Spontaneous intracerebral hemorrhage and the challenge of surgical decision making: A review: Neurosurg Focus, 2013; 34(5); E1
5. Zhou H, Zhang Y, Liu L, A prospective controlled study: Minimally invasive stereotactic puncture therapy versus conventional craniotomy in the treatment of acute intracerebral hemorrhage: BMC Neurol, 2011; 11; 76
6. Shi J, Cai Z, Han W, Stereotactic catheter drainage versus conventional craniotomy for severe spontaneous intracerebral hemorrhage in the basal ganglia: Cell Transplant, 2019; 28(8); 1025-32
7. Mendelow AD, Gregson BA, Rowan EN, Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): A randomised trial: Lancet, 2013; 382(9890); 397-408
8. Nam TM, Kim YZ, A meta-analysis for evaluating efficacy of neuroendoscopic surgery versus craniotomy for supratentorial hypertensive intracerebral hemorrhage: J Cerebrovasc Endovasc Neurosurg, 2019; 21(1); 11-17
9. Tang Y, Yin F, Fu D, Efficacy and safety of minimal invasive surgery treatment in hypertensive intracerebral hemorrhage: A systematic review and meta-analysis: BMC Neurol, 2018; 18(1); 136
10. Zhou X, Chen J, Li Q, Minimally invasive surgery for spontaneous supratentorial intracerebral hemorrhage: A meta-analysis of randomized controlled trials: Stroke, 2012; 43(11); 2923-30
11. Ramanan M, Shankar A, Minimally invasive surgery for primary supratentorial intracerebral haemorrhage: J Clin Neurosci, 2013; 20(12); 1650-58
12. Wang WZ, Jiang B, Liu HM, Minimally invasive craniopuncture therapy vs. conservative treatment for spontaneous intracerebral hemorrhage: Results from a randomized clinical trial in China: Int J Stroke, 2009; 4(1); 11-16
13. Hanley DF, Thompson RE, Rosenblum M, Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): A randomised, controlled, open-label, blinded endpoint phase 3 trial: Lancet, 2019; 393(10175); 1021-32
14. Awad IA, Polster SP, Carrión-Penagos J, Surgical performance determines functional outcome benefit in the minimally-invasive surgery plus recombinant tissue plasminogen activator for intracerebral hemorrhage evacuation (MISTIE) procedure: Neurosurgery, 2019; 84(6); 1157-68
15. Kothari RU, Brott T, Broderick JP, The ABCs of measuring intracerebral hemorrhage volumes: Stroke, 1996; 27(8); 1304-5
16. Morgenstern L, Demchuk A, Kim D, Frankowski R, Grotta J, Rebleeding leads to poor outcome in ultra-early craniotomy for intracerebral hemorrhage: Neurology, 2001; 56(10); 1294-99
17. Morgan T, Zuccarello M, Narayan R, Preliminary findings of the minimally-invasive surgery plus rtPA for intracerebral hemorrhage evacuation (MISTIE) clinical trial: Acta Neurochir Suppl, 2008; 105; 147-51
18. Hemphill JC, Greenberg SM, Anderson CS, Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American Heart Association/American Stroke Association: Stroke, 2015; 46(7); 2032-60
19. Broderick JP, Brott TG, Duldner JE, Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality: Stroke, 1993; 24(7); 987-93
20. LoPresti MA, Bruce SS, Camacho E, Hematoma volume as the major determinant of outcomes after intracerebral hemorrhage: J Neurol Sci, 2014; 345(1–2); 3-7
21. Hall AN, Weaver B, Liotta E, Identifying modifiable predictors of patient outcomes after intracerebral hemorrhage with machine learning: Neurocrit Care, 2021; 34(1); 73-84
22. Hanley DF, Thompson RE, Muschelli J, Safety and efficacy of minimally-invasive surgery plus alteplase in intracerebral haemorrhage evacuation (MISTIE): A randomised, controlled, open-label, phase 2 trial: Lancet Neurol, 2016; 15(12); 1228-37
23. Bernardo F, Rebordão L, Machado S, Salgado V, Pinto AN, In-hospital and long-term prognosis after spontaneous intracerebral hemorrhage among young adults aged 18–65 years: J Stroke Cerebrovasc Dis, 2019; 28(11); 104350
24. Al-Khaled M, Awwad S, Brüning T, Nontraumatic spontaneous intracerebral hemorrhage: Baseline characteristics and early outcomes: Brain Behav, 2020; 10(1); e01512
25. Mendelow AD, Gregson BA, Fernandes HM, Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): A randomised trial: Lancet, 2005; 365(9457); 387-97
26. Umebayashi D, Mandai A, Osaka Y, Effects and complications of stereotactic aspiration for spontaneous intracerebral hemorrhage: Neurol Med Chir (Tokyo), 2010; 50(7); 538-44
27. Dey M, Stadnik A, Riad F, Bleeding and infection with external ventricular drainage: A systematic review in comparison with adjudicated adverse events in the ongoing Clot Lysis Evaluating Accelerated Resolution of Intraventricular Hemorrhage Phase III (CLEAR-III IHV) trial: Neurosurgery, 2015; 76(3); 291-301
28. Wiesmann M, Mayer TE, Intracranial bleeding rates associated with two methods of external ventricular drainage: J Clin Neurosci, 2001; 8(2); 126-28
29. Gardner PA, Engh J, Atteberry D, Moossy JJ, Hemorrhage rates after external ventricular drain placement: J Neurosurg, 2009; 110(5); 1021-25
30. Müller A, Mould WA, Freeman WD, The incidence of catheter tract hemorrhage and catheter placement accuracy in the CLEAR III trial: Neurocrit Care, 2018; 29(1); 23-32
31. Mun JH, Cho KY, Lim BC, Lim JS, Lee RS, Factors related to catheter-induced hemorrhage after brain parenchymal catheterization: Chonnam Med J, 2013; 49(3); 113-17
32. Koivukangas T, Katisko JP, Koivukangas JP, Technical accuracy of optical and the electromagnetic tracking systems: Springerplus, 2013; 2(1); 90
33. Stieglitz LH, Fichtner J, Andres R, The silent loss of neuronavigation accuracy: A systematic retrospective analysis of factors influencing the mismatch of frameless stereotactic systems in cranial neurosurgery: Neurosurgery, 2013; 72(5); 796-807
34. Batista PD, Machado IP, Roios P, Position and orientation errors in a neuronavigation procedure: A stepwise protocol using a cranial phantom: World Neurosurg, 2019; 126; e342-50
35. Chang YH, Hwang SK, Frameless stereotactic aspiration for spontaneous intracerebral hemorrhage and subsequent fibrinolysis using urokinase: J Cerebrovasc Endovasc Neurosurg, 2014; 16(1); 5-10
36. Hinson HE, Melnychuk E, Muschelli J, Drainage efficiency with dual versus single catheters in severe intraventricular hemorrhage: Neurocrit Care, 2012; 16(3); 399-405
37. Hussain SS, Raza A, Shahid S, Asif HH, Postoperative reduction of intraventricular hemorrhage volume: Single-versus dual-catheter drainage: J Neurol Surg A Cent Eur Neurosurg, 2018; 79(4); 279-84
Figures
 Figure 1. Flow diagram demonstrating the patient selection process in this studyICH – intracerebral hemorrhage; CT – computed tomography. The figure was generated using Microsoft Word 2010 (Microsoft, Redmond, WA, USA).
Figure 1. Flow diagram demonstrating the patient selection process in this studyICH – intracerebral hemorrhage; CT – computed tomography. The figure was generated using Microsoft Word 2010 (Microsoft, Redmond, WA, USA). Figure 2. (A, B) Illustration of catheter tract hemorrhage on axial computed tomography scan obtained after surgery.The figure was created by the author from the PACS database of our institution using FastStone capture 9.0 (FastStone Soft).
Figure 2. (A, B) Illustration of catheter tract hemorrhage on axial computed tomography scan obtained after surgery.The figure was created by the author from the PACS database of our institution using FastStone capture 9.0 (FastStone Soft). Figure 3. Medtronic AxiEM™ stereotactic neuronavigation targeting screen with target point in multiple planesA virtual line of the largest diameter of the hematoma marked and perpendicular to it on the axial and sagittal planes was drawn (dotted line). After evenly dividing the longest axis, we set the target points around each middle point (black arrow). The planned trajectory can be viewed in 3 planes simultaneously, and the guidance view (bottom right corner) can be used to monitor the position of the stylet in relation to the planned trajectory. The figure was edited using Microsoft PowerPoint 2010 (Microsoft, Redmond, WA, USA) after screenshot from the StealthStation® S7 AxiEM™ navigation system in our institution.
Figure 3. Medtronic AxiEM™ stereotactic neuronavigation targeting screen with target point in multiple planesA virtual line of the largest diameter of the hematoma marked and perpendicular to it on the axial and sagittal planes was drawn (dotted line). After evenly dividing the longest axis, we set the target points around each middle point (black arrow). The planned trajectory can be viewed in 3 planes simultaneously, and the guidance view (bottom right corner) can be used to monitor the position of the stylet in relation to the planned trajectory. The figure was edited using Microsoft PowerPoint 2010 (Microsoft, Redmond, WA, USA) after screenshot from the StealthStation® S7 AxiEM™ navigation system in our institution. Figure 4. Postoperative strategies and thrombolysis algorithm for moderate-volume (30–60 mL) supratentorial spontaneous intracerebral hemorrhage (sICH) in our institutionICH – intracerebral hemorrhage; CT – computed tomography; tPA – tissue plasminogen activator. The figure was created using Microsoft PowerPoint 2010 (Microsoft, Redmond, WA, USA).
Figure 4. Postoperative strategies and thrombolysis algorithm for moderate-volume (30–60 mL) supratentorial spontaneous intracerebral hemorrhage (sICH) in our institutionICH – intracerebral hemorrhage; CT – computed tomography; tPA – tissue plasminogen activator. The figure was created using Microsoft PowerPoint 2010 (Microsoft, Redmond, WA, USA). Figure 5. Brain computed tomography (CT) scans of a successful case of hematoma removal using single-catheter insertion in neuronavigation-assisted stereotactic aspiration and thrombolysis surgery(A) Spontaneous intracerebral hemorrhage of 15 mL in the right basal ganglia with the spot sign on the initial CT angiography scan. (B) Marked volume enlargement on the follow-up CT scan (57 mL). (C) Remarkable decrease in the hematoma volume after single-catheter insertion on the postoperative CT scan. (D) Approximately 80% of the hematoma had been removed as a result of tissue plasminogen activator administration on the follow-up CT scan 2 days after surgery. The figure was created by the author from the PACS database of our institution using FastStone capture 9.0 (FastStone Soft).
Figure 5. Brain computed tomography (CT) scans of a successful case of hematoma removal using single-catheter insertion in neuronavigation-assisted stereotactic aspiration and thrombolysis surgery(A) Spontaneous intracerebral hemorrhage of 15 mL in the right basal ganglia with the spot sign on the initial CT angiography scan. (B) Marked volume enlargement on the follow-up CT scan (57 mL). (C) Remarkable decrease in the hematoma volume after single-catheter insertion on the postoperative CT scan. (D) Approximately 80% of the hematoma had been removed as a result of tissue plasminogen activator administration on the follow-up CT scan 2 days after surgery. The figure was created by the author from the PACS database of our institution using FastStone capture 9.0 (FastStone Soft). Figure 6. Hematoma volume (A) and chronological change in the hematoma clearance rate (B) at various time points in the single and multiple catheter groups(A) There were no significant differences between the single (light blue bar) and multiple catheter groups (deep blue bar) in hematoma volume (mL) through all chronologies. (B) There were no significant differences between the single (light blue line) and multiple catheter groups (deep blue line) in hematoma clearance rate (%) through all chronologies. T0 – the time at which preoperative computed tomography (CT) scan was carried out; T1 – the time at which immediate postoperative CT scan was taken; T2 – postoperative 2 days; EOT – endpoint of treatment (the time of catheter removal); CR – clearance rate; ns – no significant difference between the 2 values. The figure was created with GraphPad Prism 9.3.0 (GraphPad Software, Inc., San Diego, CA, USA).
Figure 6. Hematoma volume (A) and chronological change in the hematoma clearance rate (B) at various time points in the single and multiple catheter groups(A) There were no significant differences between the single (light blue bar) and multiple catheter groups (deep blue bar) in hematoma volume (mL) through all chronologies. (B) There were no significant differences between the single (light blue line) and multiple catheter groups (deep blue line) in hematoma clearance rate (%) through all chronologies. T0 – the time at which preoperative computed tomography (CT) scan was carried out; T1 – the time at which immediate postoperative CT scan was taken; T2 – postoperative 2 days; EOT – endpoint of treatment (the time of catheter removal); CR – clearance rate; ns – no significant difference between the 2 values. The figure was created with GraphPad Prism 9.3.0 (GraphPad Software, Inc., San Diego, CA, USA). Tables
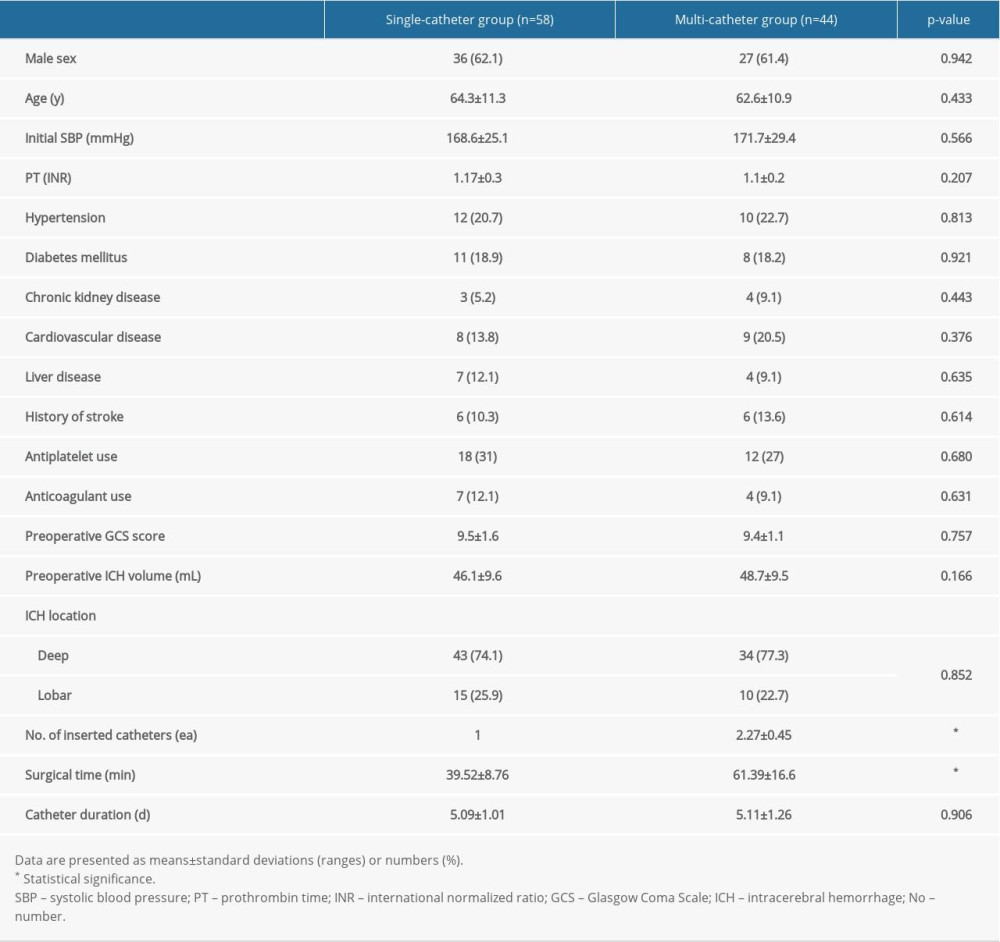 Table 1. Patient characteristics.
Table 1. Patient characteristics.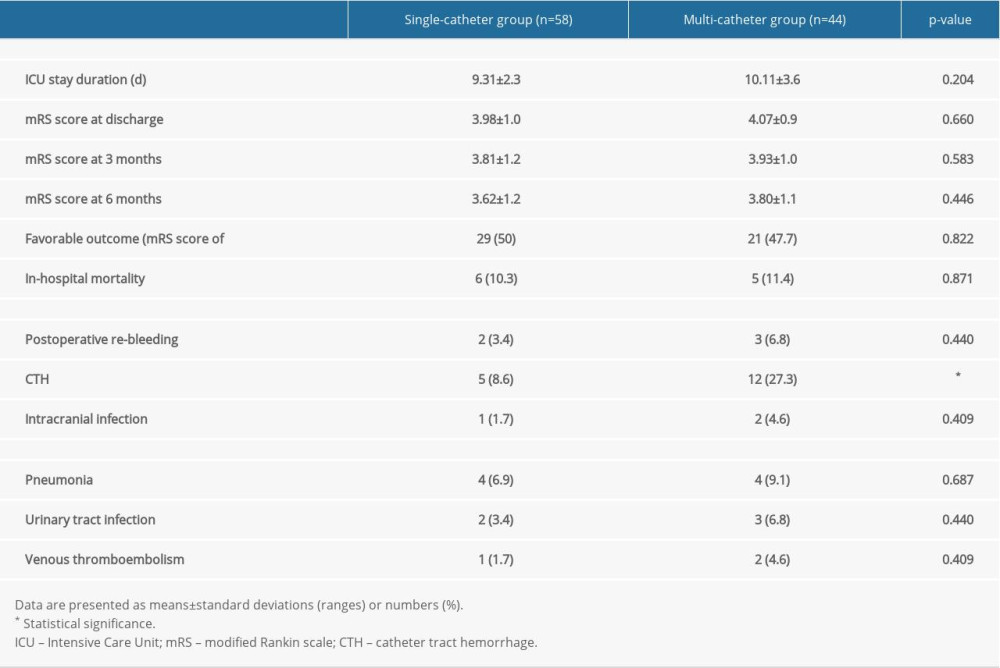 Table 2. Comparison of the clinical outcomes and complications.
Table 2. Comparison of the clinical outcomes and complications.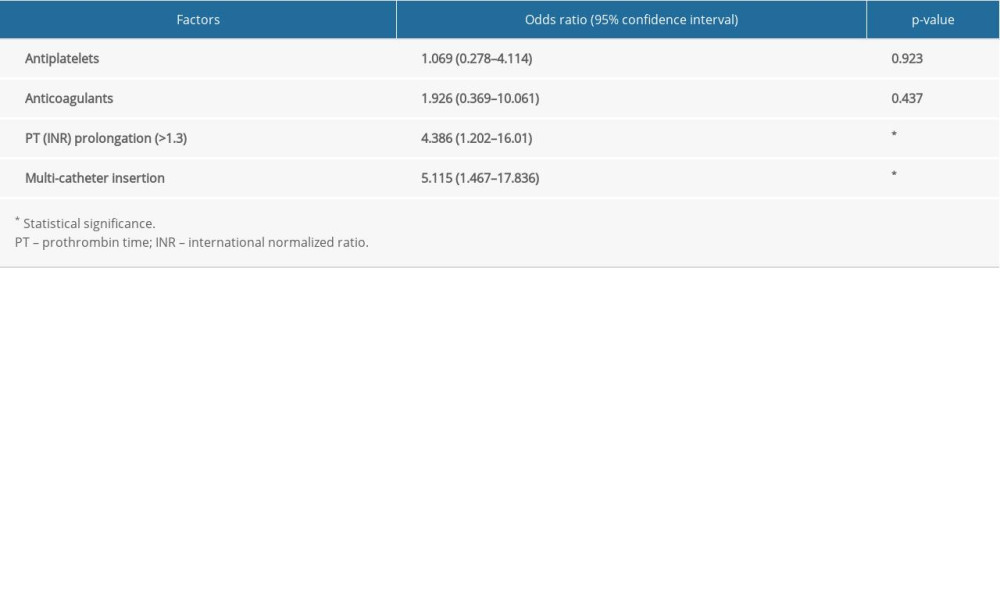 Table 3. Multivariate analyses for the independent risk factors associated with catheter tract hemorrhage.
Table 3. Multivariate analyses for the independent risk factors associated with catheter tract hemorrhage.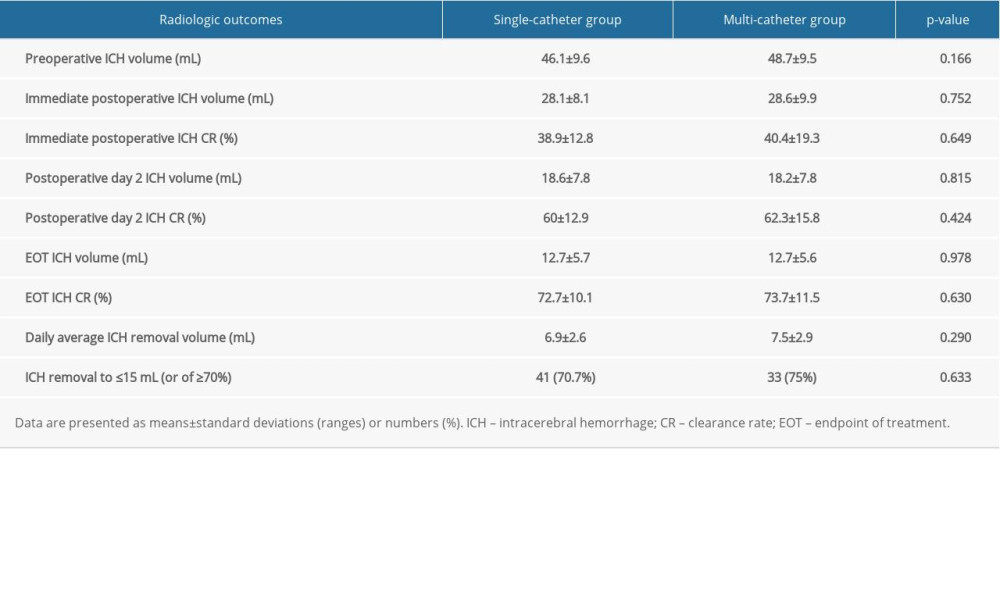 Table 4. Comparison of the radiologic outcomes.
Table 4. Comparison of the radiologic outcomes. Table 1. Patient characteristics.
Table 1. Patient characteristics. Table 2. Comparison of the clinical outcomes and complications.
Table 2. Comparison of the clinical outcomes and complications. Table 3. Multivariate analyses for the independent risk factors associated with catheter tract hemorrhage.
Table 3. Multivariate analyses for the independent risk factors associated with catheter tract hemorrhage. Table 4. Comparison of the radiologic outcomes.
Table 4. Comparison of the radiologic outcomes. In Press
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








