22 January 2022: Clinical Research
A Questionnaire-Based Study to Obtain a Consensus from 5 Polish Burns Centers on Eschar Removal by Bromelain-Based Enzymatic Debridement (Nexobrid) in Burns Following the 2020 Updated European Consensus Guidelines
Tomasz Korzeniowski12ABCDEFG*, Jerzy Strużyna13ACDEFG, Anna M. Chrapusta4ABCDE, Andrzej Krajewski5ABDE, Marek Kucharzewski6ABDEF, Krzysztof PiorunDOI: 10.12659/MSM.935632
Med Sci Monit 2022; 28:e935632
Abstract
BACKGROUND: The supplementary treatment of burns with enzymatic debridement with Nexobrid® was approved in Europe in 2013. The 2017 European consensus guidelines on the removal of eschar in burns by bromelain-based enzymatic debridement were updated in 2020. This questionnaire-based study aimed to obtain a consensus from 5 Polish burns centers on eschar removal by Nexobrid® in burns following the 2020 updated European consensus guidelines.
MATERIAL AND METHODS: A panel of 5 experts representing the leading burn treatment centers in Poland (Cracow, Gryfice, Siemanowice Śląskie, Poznań, and Łęczna) was convened. A modified Delphi process was implemented with panel member selection, literature review, 2 rounds of voting in which panelists were asked to evaluate the European consensus and Polish consensus building by data analysis, statements preparation, final voting, and manuscript drafting.
RESULTS: The knowledge and experience of experts from Poland’s leading burn centers resulted in the development of guidelines, formulated as 24 statements representing the following areas: indications and usage, pain management, application principles, post-enzymatic debridement wound dressing, and early and long-term outcomes. An analysis of the 7-point Likert scale polls revealed that 23 of the 24 statements achieved 100% consensus.
CONCLUSIONS: The findings from this survey from 5 major centers in Poland supported the main recommendations from the 2020 updated European consensus guidelines on the removal of eschar in burns by Nexobrid® and may serve as a practical guide for surgeons who care for patients with burns in this country.
Keywords: Burns, Consensus, Debridement, Bromelains, Burn Units, Europe, Humans, Poland, Practice Guidelines as Topic, Surveys and Questionnaires, Wound Healing
Background
The standard operating procedure in the treatment of deep burns involves early removal of necrotic tissue and application of skin grafts [1,2]. Even though surgical excision of necrotic tissue is regarded as the method of choice, enzymatic debridement of burn wounds with the use of Nexobrid® is becoming an increasingly popular approach [3]. Nexobrid® is a concentrate of proteolytic enzymes enriched in bromelain derived from stems of pineapple plants and indicated for the removal of dead tissue in thermal burns [4]. Bromelain-based enzymatic debridement was approved in Europe in 2013 [5]. The growing number of articles in the literature, as well as proof confirming the efficacy of this method and the advantages of selective removal of necrotic tissue in burns of varying thickness, result in the enzymatic approach becoming one of the fundamental tools that surgeons have at their disposal [6,7].
In 2017, based on data from more than 500 cases, an initial set of European guidelines were published [8]. In 2020, based on the experience of 1232 treated patients, an updated European consensus document was published, containing 43 statements to address the following topics: indications; pain management and anesthesia; large surface indication; timing of application for various indications; preparation and application; post-interventional wound management; skin grafting outcomes; scar and revision management; cost-effectiveness; patient perspectives; logistic aspects; and training strategies [9].
A panel of experts representing the leading burn treatment centers in Poland has been established to determine the clinical purpose of enzymatic debridement, as well as to structure the current state of knowledge and experience with the use of the Nexobrid® product, thus creating a consensus that is going to consist of guidelines for enzymatic debridement of burn wounds. The European recommendations were the basis for the development of Polish consensus. We decided to create guidelines to better capture trends and variations that are often country and region specific.
Therefore, this questionnaire-based study aimed to obtain a consensus from 5 Polish burns centers on eschar removal by Nexobrid® in burns following the 2020 updated European consensus guidelines.
Material and Methods
EXPERT SELECTION:
The first stage involved appointing a body of experts by means of selecting Poland’s most experienced burn surgeons from Cracow, Gryfice, Siemianowice Śląskie, Poznań, and Łęczna who had been using the enzymatic debridement method for many years. The total number of burn patients in whom the enzymatic debridement method was applied at the above-referenced centers exceeds 500. A national consensus panel was assembled with 5 burn surgeons (1 of each center) who were selected for their established clinical and scientific expertise in enzymatic treatment.
LITERATURE REVIEW:
After reviewing the core bibliography, panel members were asked to list studies that they believed, on the basis of their knowledge and clinical experience, were useful in creating enzymatic debridement guidelines.
EUROPEAN CONSENSUS EVALUATION:
The subsequent step in building the Polish consensus involved evaluation of the European guidelines. These guidelines were prepared by expert panels with representatives of Europe’s leading burn centers (in 2017 and 2019), which resulted in 2 publications consisting of 108 statements: 65 from the first European consensus and 43 from the second one [8,9]. Statement-evaluation questionnaires were distributed among all members of the Polish expert panel, who evaluated the statements on a Likert-type scale and provided commentary. In these survey rounds, panel members also prioritized statements for use in the Polish consensus, and statements that failed to receive a priority were eliminated.
POLISH CONSENSUS PROCESS:
Based on a qualitative elaboration of the information obtained, the 2 principal investigators drew up a list of Polish consensus statements. An in-depth analysis of the resulting data allowed preparation of Polish guidelines that were subjected to further modification during the general meeting of the expert panel. In this round, definitions were refined according to voting and comments. After the in-person meeting, the document with conclusions reached by the experts underwent a final review and approval through a last round of the Delphi process. In particular, experts were asked to express their degree of agreement using a 7-point Likert scale. After completion of this round, the results were regarded as valid for publication as a consensus.
Results
Indications and Usage
COMMENT: Early removal of necrotic tissue can be achieved in numerous ways: mechanically, chemically, or biologically. Nexobrid®-based enzymatic debridement involves the use of the active substance bromelain, which removes eschar enzymatically. This is another effective tool in the surgeon’s repository of early burn treatment methods [6].
COMMENT: Nexobrid® removes necrotic tissue without interfering with healthy tissue. It is a safe method. However, given its specialized nature, it requires a considerable degree of practical experience and know-how from the surgeon, especially in terms of determining eligibility, evaluation, and post-debridement management [3].
COMMENT: Enzymatic debridement removes necrotic tissue subjected to thermal damage. There is an insufficient number of reports unequivocally indicating that this method is fully effective for treatment of chemical or electrical burns [11].
COMMENT: Early debridement of the facial area, hands, or groin is a controversial approach due to the specific anatomical conditions and the functional aspect of these parts of the body. Being a safe and selective method, enzymatic debridement is particularly useful in these areas [12–17]. Full conformity was achieved for this statement. However, 2 out of 5 participating centers had not had any experience with using this method in the groin area.
COMMENT: The use of enzymatic debridement on extensive surfaces is possible and has been described in the literature as effective and safe [18]. However, the manufacturer recommends using Nexobrid® on up to 15% of the body surface area per session. For extensive burns, it is recommended to carry out enzymatic debridement in several stages [19]. The majority of the centers participating in the consensus panel had experiences with enzymatic debridement of extensive burns covering >15% of the body surface area.
COMMENT: Burns are seldom homogenous in terms of depth. A non-selective tangential excision removes both necrotic and healthy tissue. An excision up to the fascia creates a lesion in the treated site. In treatment of mixed deep dermal and full-thickness burns, Nexobrid® allows selective removal of necrotic tissue [20,21].
COMMENT: By removal of eschar, early enzymatic debridement prevents compartment syndrome, thus avoiding the need for eschar incision [22–24]. Some of the experts mentioned the ineffectiveness of Nexobrid® when used with dry eschar. If this method fails to remove the necrotic tissue causing circumferential pressure, escharotomy needs to be performed.
COMMENT: Human and technical resources are likely to be considerably restricted during mass disasters. Surgical treatment of numerous casualties at the same time is not feasible [25]. Enzymatic debridement can be performed in the procedure room or at the patient’s bed, which makes this method a viable option for effective treatment during crises. However, full conformity has not been achieved for this statement. Some of the experts believed fast and simultaneous debridement of numerous wounds would address staff shortages only to a certain extent. The implied shortage of specialist staff during mass disasters was questioned as well, which also cast doubt on the potential problems with burn patient management after enzymatic debridement. Regardless, creating relevant enzymatic debridement-based treatment algorithms for mass disasters would open extensive opportunities for rapidly assisting numerous patients.
COMMENT: ED is a painful procedure that calls for appropriate pain management. The right anesthetic approach needs to be adopted depending on which area of the body is affected, as well as on the thickness and surface area of the burns [26]. Block anesthesia is recommended for upper- or lower-limb burns. General anesthesia or analgosedation needs to be considered in the remaining cases [27]. Enzymatic debridement can also be applied on small surfaces under local anesthesia.
COMMENT: The efficacy of Nexobrid® depends on the state of the wound. The product shows the highest efficacy shortly after trauma occurs, when there is still a sufficient level of moisture in the wound [5]. With the exception of enzymatic escharotomy, enzymatic debridement does not need to be performed immediately or within hours. Appropriate patient preparation is recommended, whereas the procedure needs to be conducted not later than on the third day after trauma.
COMMENT:
In some cases, performing early enzymatic debridement may not be feasible. This can be due to the general condition of the patient, logistics, product availability, or personnel availability. Enzymatic debridement can be carried out late (>3 days after trauma) if the wound meets the relevant conditions. Nexobrid® is ineffective for dry eschar. In such cases, auxiliary, longer soaking needs to be applied or specialist dressings need to be used to achieve a moist wound and improve wound status.
COMMENT:
Nexobrid® is a mixture of proteolytic enzymes that has the appearance of a white gel in its ready-to-use state. To work effectively, it needs to come into direct contact with the base of the wound. Residual keratin (dead epidermis) prevents the enzymatic reaction from occurring. It is imperative to use great care in cleansing the wound before Nexobrid® is used. A surgical brush can be an effective solution.
COMMENT:
The soaking stage is an important aspect of the procedure and makes enzymatic debridement more effective.
COMMENT: Dressings that maintain moisture balance in the wound (eg, hydrogel or hydrocolloid dressings) can be used at several stages of the enzymatic debridement procedure. They are useful in pre-soaking, as well as in maintaining wound moisture after the procedure is completed [28].
COMMENT:
Post-enzymatic debridement wound evaluation is a key aspect of Nexobrid®-based wound treatment. Whereas the enzymatic debridement procedure itself is defined clearly regarding wound preparation and application, optimal post-enzymatic debridement wound management requires considerable surgeon experience. It affects the outcome of subsequent treatment and reproducibility of results to a considerable extent.
COMMENT: Enzymatic debridement is a tool that not only removes necrotic tissue, but is also a diagnostic tool. Given its selective effect, Nexobrid® allows precise determination of burn depth. Depending on how many layers deep the debridement penetrates, the bottom of the wound will have a particular appearance. Assessment of the color of the wound bed and the bleeding patterns allows determining whether the wound has the potential to spontaneously heal. Only a homogenous pink surface or a white surface with a dense network of bleed sites indicates high potential for epithelization. If the bleed sites are bigger, round or elliptical, or distant from each other, a longer healing time is to be expected, with skin grafts likely to be necessary. Exposure of subcutaneous fat tissue or visible vascularity indicate full-thickness deep burns [29].
COMMENT:
Regardless of post-enzymatic debridement wound depth and whether the preferred course of action is to wait for spontaneous epithelization or to prepare the wound for a skin graft, an appropriate moisture balance needs to be maintained in the wound at all times. If the wound dries, secondary scabbing will occur, which may necessitate additional debridement and longer treatment.
COMMENT:
If post-enzymatic debridement wound bed assessment does not show potential for epithelization, the wound needs to be qualified for an early skin graft. Considering the extent of exudate present following enzymatic debridement, grafting should be planned 2–3 days after the procedure.
COMMENT: Special dressings can be considered as a means of ensuring the optimal level of moisture in the wound following enzymatic debridement. The materials used need to have anti-adhesive properties. Allografts create the most advantageous environment. However, given their limited availability, other materials of different kinds can also be used, according to the experience and capabilities of the burn unit [30].
COMMENT:
In some cases, wounds develop pseudoeschar post-enzymatic debridement. Routine removal of such eschars is not recommended within the first 14 days. Epithelization is still likely to occur despite the pseudoeschar, whereas removal of the eschar may make the wound deeper. If the healing process fails to progress and the pseudoeschar remains, it needs to be removed surgically after 14 days and a skin graft needs to be considered.
COMMENT: If post-enzymatic debridement wound bed evaluation reveals potential for spontaneous healing but epithelialization fails to progress for 21 days, covering the wound with skin grafts may be necessary. In such cases, the procedure must not be postponed, especially if granulation is observed instead of healing [31]. Extended healing time can result in poorer scar quality and impairment of function at the burn site. One of the experts suggested shortening this period to 14 days if no signs of epithelialization are present.
COMMENT: Surgical methods are less selective in terms of eschar removal and preserving healthy skin layers. This applies particularly to burns that are not homogenous in depth. Removing necrotic tissue and keeping live tissues intact, Nexobrid® helps limit the number of necessary surgical procedures and increases the likelihood of spontaneous healing [5].
COMMENT: Despite the advantages of enzymatic debridement and its effectiveness, surgical escharotomy needs to be considered at all times during wound evaluation following Nexobrid®-based debridement. Furthermore, skin grafts need to be applied if the surgeon believes that this is required. The use of enzymatic debridement does not invalidate the general principles of burn wound management [32].
COMMENT: After the enzymatic debridement treatment, the patient needs to receive a standard set of recommended scar management treatments [33]. This may include physiotherapy, silicone products, and compression garments. These recommendations are not affected by the burn wound debridement method used.
Discussion
LIMITATIONS:
While the consensus presented in this paper is based on the extensive experience of experts, the recommendations may be influenced by the subjective opinions and composition of the group that developed them. Not all statements presented in this study could be supported by the available literature, which is due to a lack of research on many aspects of enzymatic debridement.
Conclusions
The findings from this survey from 5 major centers in Poland supported the main recommendations from the 2020 updated European consensus guidelines on the removal of eschar in burns by Nexobrid® and may serve as a practical guide for surgeons who care for patients with burns in this country.
Tables
Table 1. Consensus statements and agreement on indications and usage of enzymatic debridement.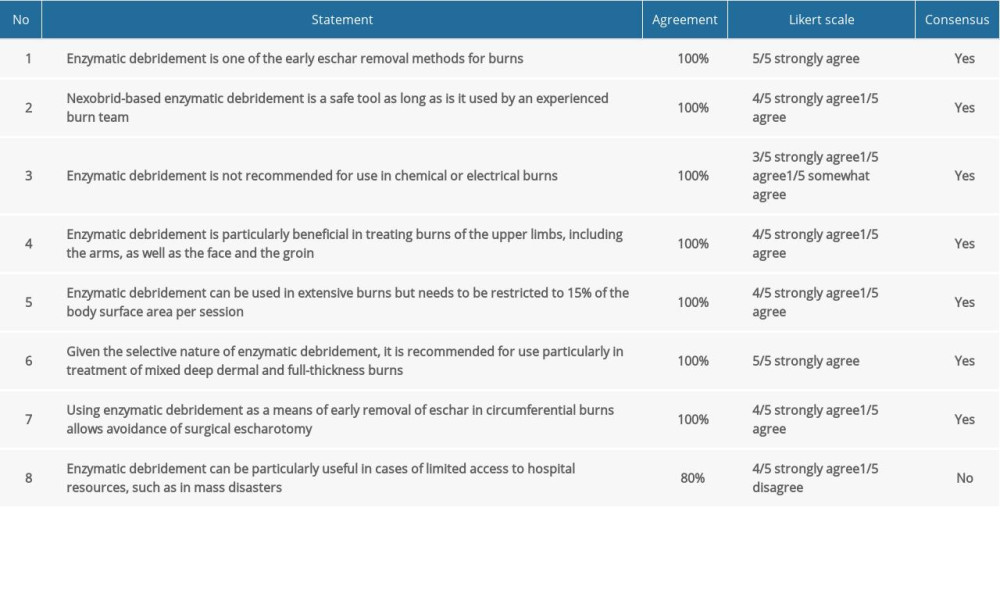 Table 2. Consensus statements and agreement on pain management for enzymatic debridement.
Table 2. Consensus statements and agreement on pain management for enzymatic debridement.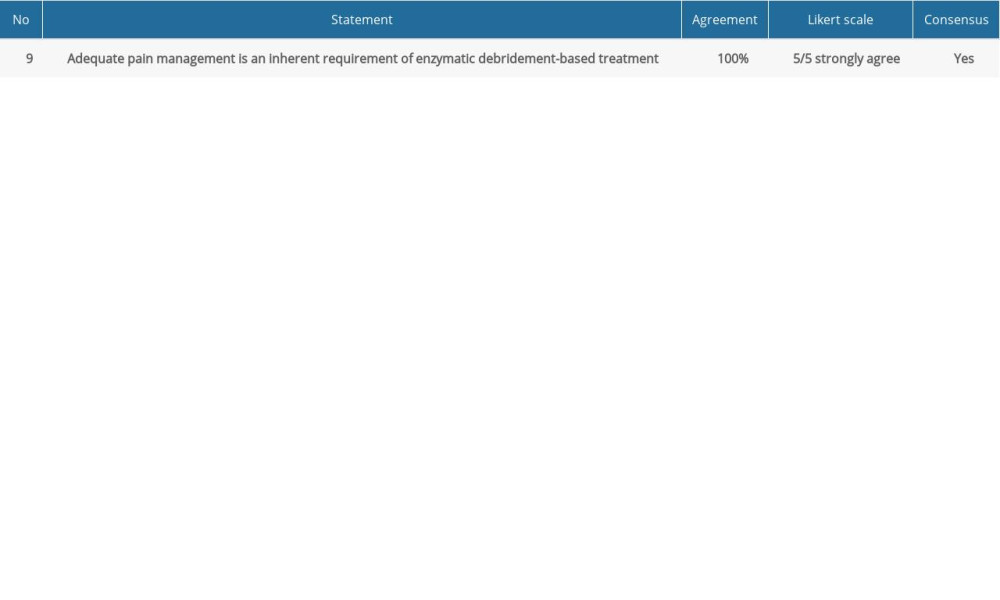 Table 3. Consensus statements and agreement on application of enzymatic debridement.
Table 3. Consensus statements and agreement on application of enzymatic debridement.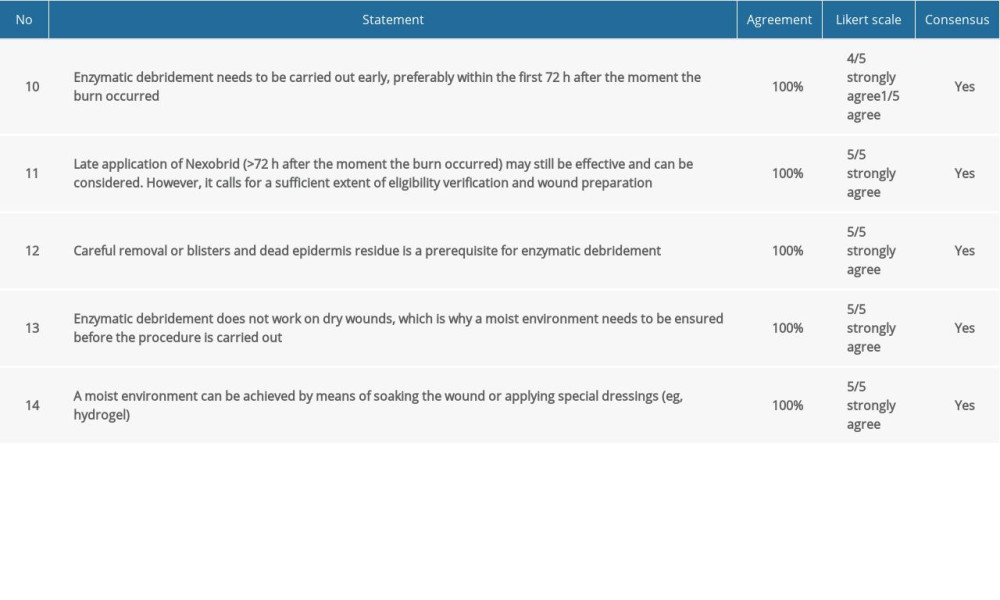 Table 4. Consensus statements and agreement on post-enzymatic debridement wound care.
Table 4. Consensus statements and agreement on post-enzymatic debridement wound care.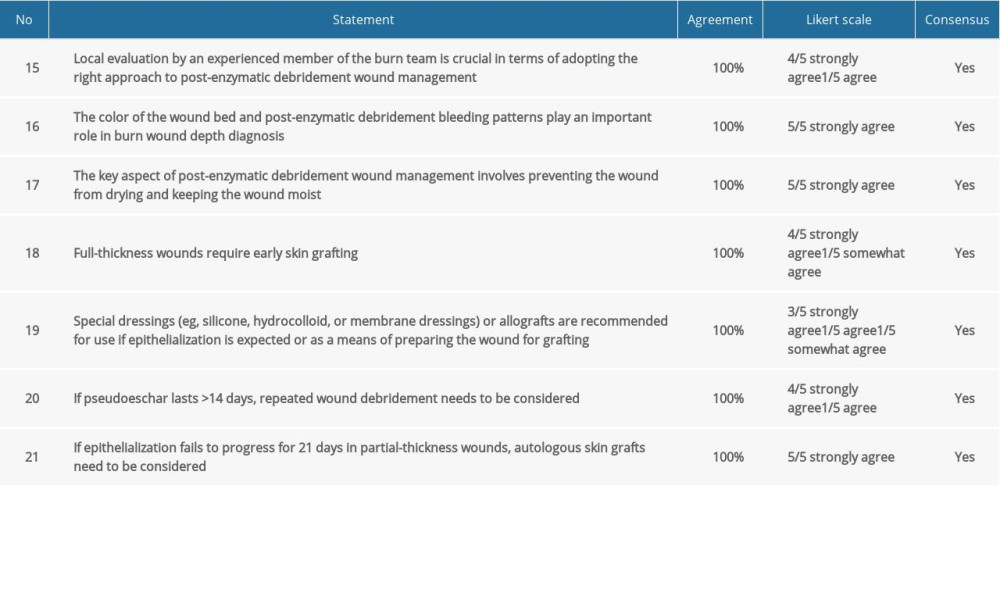 Table 5. Consensus statements and agreement on early and long-term outcomes after enzymatic debridement.
Table 5. Consensus statements and agreement on early and long-term outcomes after enzymatic debridement.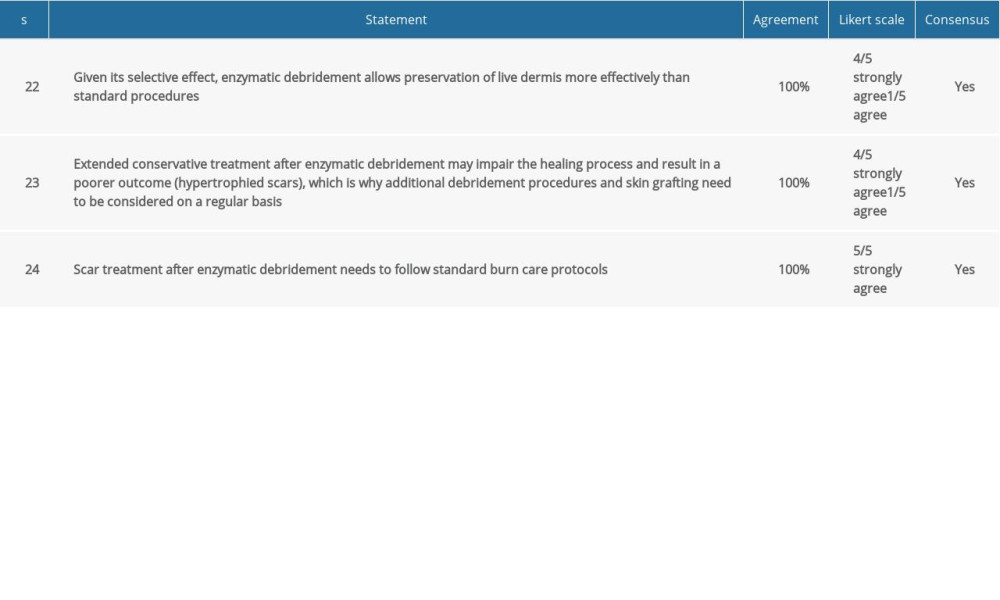
References
1. Ong YS, Samuel M, Song C, Meta-analysis of early excision of burns: Burns, 2006; 32(2); 145-50
2. Gacto-Sanchez P, Surgical treatment and management of the severely burn patient: Review and update: Med Intensiva, 2017; 41(6); 356-64
3. Loo YL, Goh BKL, Jeffery S: J Burn Care Res, 2018; 39; 932-38
4. Rosenberg L, Krieger Y, Silberstein E, Selectivity of a bromelain based enzymatic debridement agent: A porcine study: Burns, 2012; 38; 1035-40
5. Rosenberg L, Krieger Y, Bogdanov-Berezovski A, A novel rapid and selective enzymatic debridement agent for burn wound management: a multi-center RCT: Burns, 2014; 40; 466-74
6. Edmondson SJ, Ali Jumabhoy I, Murray A, Time to start putting down the knife: A systematic review of burns excision tools of randomised and non-randomised trials: Burns, 2018; 44(7); 1721-37
7. Rosenberg L, Shoham Y, Krieger Y: Ann Burns Fire Disasters, 2015; 28(4); 264-74
8. Hirche C, Citterio A, Hoeksema H: Burns, 2017; 43(8); 1640-53
9. Hirche C, Kreken Almeland S: Burns, 2020; 46(4); 782-96
10. Elwyn G, O’Connor A, Stacey DInternational Patient Decision Aids Standards (IPDAS) Collaboration, Developing a quality criteria framework for patient decision aids: Online international Delphi consensus process: BMJ, 2006; 333(7565); 417
11. Claes KEY, Vyncke T, De Wolf E, Enzymatic debridement as an effective treatment for combined flame and chemical burns caused by e-cigarettes: Am J Emerg Med, 2020; 38(6); 1199-202
12. Schulz A, Fuchs PC, Rothermundt I, Enzymatic debridement of deeply burned faces: healing and early scarring based on tissue preservation compared to traditional surgical debridement: Burns, 2017; 43; 1233-43
13. Dadras M, Wagner JM, Wallner C, Enzymatic debridement of hands with deep burns: A single center experience in the treatment of 52 hands: J Plast Surg Hand Surg, 2020; 54(4); 220-24
14. Schulz A, Ribitsch B, Fuchs PC, Treatment of genital burn injuries: Adv Skin Wound Care, 2018; 31; 314-21
15. Krieger Y, Rubin G, Schulz A, Bromelain-based enzymatic debridement and minimal invasive modality (mim) care of deeply burned hands: Ann Burns Fire Disasters, 2017; 30; 198-204
16. Schulz A, Shoham Y, Rosenberg L, Enzymatic versus traditional surgical debridement of severely burned hands: A comparison of selectivity, efficacy, healing time, and three-month scar quality: J Burn Care Res, 2017; 38; e745-55
17. Schulz A, Perbix W, Shoham Y, Our initial learning curve in the enzymatic debridement of severely burned hands – management and pit falls of initial treatments and our development of a post debridement wound treatment algorithm: Burns, 2017; 43; 326-36
18. Bowers C, Randawa A, Sloan B, Enzymatic debridement in critically injured burn patients – our experience in the intensive care setting and during burn resuscitation: Burns, 2021 [Online ahead of print]
19. Krauss S, Bender D, Rothenberger J, Delayed and fractional use of enzymatic debridement with nexobrid for extensive burn injury: A case report: Ann Burns Fire Disasters, 2018; 31(1); 23-30
20. Bernagozzi F, Orlandi C, Purpura V, The enzymatic debridement for the treatment of burns of indeterminate depth: J Burn Care Res, 2020; 41(5); 1084-91
21. Di Lonardo A, Nardini V, De Rosa M, Enzymatic escharolysis with Nexobrid on partial thickness burn wounds: Pre-and postdebridement histological assessment: Ann Burns Fire Disasters, 2018; 31; 23-27
22. Krieger Y, Rosenberg L, Lapid O, Escharotomy using an enzymatic debridement agent for treating experimental burn-induced compartment syndrome in an animal model: J Trauma, 2005; 58; 1259-64
23. Fischer S, Haug V, Diehm Y, Feasibility and safety of enzymatic debridement for the prevention of operative escharotomy in circumferential deep burns of the distal upper extremity: Surgery, 2019; 165(6); 1100-5
24. Mataro I, Lanza A, Di Franco S, Releasing burn-induced compartment syndrome by enzymatic escharotomy-debridement: A case study: J Burn Care Res, 2020; 41(5); 1097-103
25. Surowiecka-Pastewka A, Witkowski W, Kawecki M, A new triage method for burn disasters: Fast triage in burns (FTB): Med Sci Monit, 2018; 24; 1894-901
26. Galeiras R, Mourelo M, Pértega S, Procedural sedation and analgesia during enzymatic debridement of burn patients: Ann Burns Fire Disasters, 2018; 31(3); 223-27
27. Claes KEY, Amar S, Hoeksema H, Pain management during a bromelain-based selective enzymatic debridement in paediatric and adult burn patients: Burns, 2021 [Online ahead of print]
28. Dhaliwal K, Lopez N, Hydrogel dressings and their application in burn wound care: Br J Community Nurs, 2018; 23(Suppl 9); S24-27
29. Ziegler B, Hundeshagen G, Cordts T, State of the art in enzymatic debridement: Plast Aesthet Res, 2018; 5; 33
30. Rigueros Springford L, Creasy H, Cubison T, Dheansa B, A novel technique of NexoBrid application to burns on the hands: Burns, 2017; 43; 1132-33
31. Liu HF, Zhang F, Lineaweaver WC, History and advancement of burn treatments: Ann Plast Surg, 2017; 78(2 Suppl 1); S2-8
32. Bolton L, Burn debridement: Are we optimizing outcomes?: Wounds, 2019; 31(12); 298-300
33. Mataro I, Delli Santi G, Palombo P, Spontaneous healing and scar control following enzymatic debridement of deep second-degree burns: Ann Burns Fire Disasters, 2017; 30(4); 313-16
Tables
 Table 1. Consensus statements and agreement on indications and usage of enzymatic debridement.
Table 1. Consensus statements and agreement on indications and usage of enzymatic debridement. Table 2. Consensus statements and agreement on pain management for enzymatic debridement.
Table 2. Consensus statements and agreement on pain management for enzymatic debridement. Table 3. Consensus statements and agreement on application of enzymatic debridement.
Table 3. Consensus statements and agreement on application of enzymatic debridement. Table 4. Consensus statements and agreement on post-enzymatic debridement wound care.
Table 4. Consensus statements and agreement on post-enzymatic debridement wound care. Table 5. Consensus statements and agreement on early and long-term outcomes after enzymatic debridement.
Table 5. Consensus statements and agreement on early and long-term outcomes after enzymatic debridement. In Press
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








