22 April 2023: Clinical Research
Measurement of Serum Ultra-Sensitive Thyroid-Stimulating Hormone Levels to Determine the Risk for Recurrence of Atrial Fibrillation Following Catheter Ablation in 575 Patients from a Single Center
Zhizhao Li1ABCDEFG*, Xiao-Xia Liu2AF, Qiong Huang3F, Yu-Qing Song1F, Xue-Yuan Guo2AF, Chang-Sheng Ma2ADOI: 10.12659/MSM.937958
Med Sci Monit 2023; 29:e937958
Abstract
BACKGROUND: Thyroid dysfunction has been proved to contribute to the occurrence of atrial fibrillation (AF), leading to the development of AF in animal models and clinical populations. This single-center study investigated the relationship between ultra-sensitive thyroid-stimulating hormone (uTSH) levels and the recurrence of atrial fibrillation (AF) in 575 hospitalized patients who had undergone catheter ablation.
MATERIAL AND METHODS: The study enrolled 575 hospitalized patients with AF who needed catheter ablation, 105 were non-first catheter ablation patients, and 470 were first catheter ablation (CA) patients. Before ablation, fasting biochemical indexes, including uTSH, were detected. Patients were classified according to uTSH quartile. The presence of AF was confirmed by 12-lead electrocardiogram or 24-h ambulatory electrocardiogram.
RESULTS: A total of 105 (18.44%) patients had undergone catheter ablation of AF twice or more. Univariate logistic regression analysis showed no significant relationship between uTSH and AF recurrence (HR, 1.047; 95% CI 0.986-1.111; P=1.127). Multivariate logistic regression analysis indicated that compared with low quartiles (Q1 OR, 0.71, 95% CI: 0.35-1.46; P=0.36; Q2 OR 0.71, 95% CI 0.36-1.39; P=0.31;Q3 OR 0.22, 95% CI 0.09-0.53; P=0.001), high quartiles of uTSH had a higher risk of AF recurrence. After adjusting for sex, the risk of AF recurrence in the high quartile uTSH was higher in males than in the low quartile (Q1 OR, 0.60, 95% CI: 0.29-1.26; P=0.18;Q2 OR, 0.52, 95% CI, 0.24-1.13; P=0.09;Q3 OR, 0.42, 95% CI, 0.18-0.94; P=0.03), but not in women.
CONCLUSIONS: Serum TSH levels in male patients treated for AF with cardiac ablation were significantly associated with AF recurrence.
Keywords: Atrial Fibrillation, Catheter Ablation, Hypothyroidism, Neoplasm Recurrence, Local, Male, Female, Humans, Treatment Outcome, Risk Factors, thyrotropin, Recurrence
Background
With the increased incidence of atrial fibrillation (AF), its risk factors have gradually been discovered. Studies have shown that thyroid hormones (TH) significantly affect cardiovascular and atrial fibrillation [1]. Thyroid dysfunction, including hyperthyroidism and hypothyroidism, were associated with increased risk of atrial fibrillation [2,3]. Clinically, a more than 10-year history of hypothyroidism in patients is closely related to the occurrence of AF [4]. An animal study also showed that hypothyroidism could lead to increased susceptibility to AF because of increasing atrial interstitial fibrosis [5]. Significant progress has been made in treating atrial fibrillation with catheter ablation (CA), but the high relapse rate of postoperative AF and its prognostic factors have drawn attention [6]. Furthermore, thyroid hormone (TH) levels have been significantly related to the success of CA of atrial fibrillation [7,8], and patients with free tetraiodothyronine (FT4) levels higher than normal have an increased risk of relapse of atrial fibrillation after CA. Although FT4 levels were normal, the high levels of FT4 were related to high recurrence of AF CA. Considering the relationship between the occurrence of AF and thyroid function, thyroid function might also adversely influence the result of CA of AF. However, there are few data on the effect of thyroid function on the outcome of catheter ablation of AF, and even less on uTSH, and data on the differences between men and women were lacking in 2020 [9]. Guidelines for the European Society of Cardiology (ESC) for the identification and management of risk factors and concomitant diseases are recommended as an integral part of treatment in AF patients.
Therefore, this study from a single center investigated the association between ultra-sensitive thyroid-stimulating hormone (uTSH) levels and recurrence of atrial fibrillation (AF) in 575 hospitalized patients following cardiac ablation.
Material and Methods
PATIENTS:
From January 2017 to December 2017, 575 patients who planned to undergo circumferential pulmonary vein ablation (CPVA) were enrolled in this case-control study. Paroxysmal atrial fibrillation (PAF) was ablated by circumferential pulmonary vein, and persistent atrial fibrillation (CAF) was ablated by circumferential pulmonary vein plus left top line, mitral isthmus line, and tricuspid isthmus line. After ablation, antiarrhythmic drugs were routinely taken for 3 months. Patients with percutaneous coronary intervention (PCI), New York Heart Association (NYHA) functional class III or IV, severe mitral stenosis, left atrial thrombosis within 6 months before ablation, malignant tumors, inflammatory illnesses or autoimmune, serious liver, and kidney dysfunction, hyperthyroidism, or hypothyroidism were excluded from this study. Before ablation, we performed clinical assessment, 12-lead echocardiography, and 24-h ambulatory electrocardiography. The recurrence of AF was confirmed by 24-h ambulatory electrocardiogram or 12-lead echocardiography 3 months after catheter ablation, which lasted ≥30 s. This research was supported by the Ethics Committee of Beijing Anzhen Hospital, and each patient signed a written informed consent form.
DATA COLLECTION:
The baseline characteristics of patients at admission were collected, including age, sex, smoking, drinking, body mass index (BMI), atrial fibrillation type, diabetes, hypertension, coronary heart disease, and lipid-lowering drugs. Non-paroxysmal atrial fibrillation was used to describe continuous and long-term continuous atrial fibrillation.
ULTRA-SENSITIVE THYROID-STIMULATING HORMONE DETERMINATION:
Fasting blood was collected at the time of admission to measure a series of biochemical indicators, and uTSH was determined using the chemoluminescence technique [10]. We used the German Siemens Centaur CP automatic chemiluminescence points analysis instrument and detection reagent. The analytical sensitivity of uTSH was detected by the Siemens Centaur CP system, consistent with the value provided by manufacturers [11], which is the normal value of uTSH: 0.34–5.60 mIU/L (male and female). According to the distribution of uTSH values, the quartiles of uTSH were identified as: quartile (Q), Q1 <1.18 mIU/L, Q2 1.18–1.84 mIU/L, Q3 1.84–2.94 mIU/L, and Q4 >2.94 mIU/L.
STATISTICAL ANALYSIS:
Data with normal distribution were expressed as mean±standard deviation (SD) and were compared using a one-way analysis of variance (ANOVA). Data that did not conform to normal distribution were expressed as the median and interquartile range (IQR) and compared using the Mann-Whitney U test. Categorical variables were expressed as percentages and compared using the χ2 test. Patients were divided into quartiles according to uTSH level, and differences in AF recurrence between quartiles were calculated using the χ2 test. Odds ratio (OR) and 95% confidence intervals (95% CI) were assessed using logistic hazard models to determine the association between uTSH levels and atrial fibrillation relapse. Two models were used for logistic regression adjustment. Model 1 was adjusted for sex and age. Model 2 was adjusted for BMI, diabetes, coronary heart disease(CAD), PAF, CAF, hypertension, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), total cholesterol, left atrial diameter (LAD), triglyceride (TG), C-reactive protein (CRP), systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting blood glucose (FPG), and white blood cells (WBC). Data analyses were performed using SPSS software, version 17.0 (SPSS, Inc., Chicago, IL).
Results
PATIENT DEMOGRAPHICS:
Table 1 presents details of baseline characteristics for each uTSH quartile. The following parameters were not significantly different between uTSH quartiles: age, number of females, BMI, history of alcohol drinking, CAD, DM, relapse of atrial fibrillation after catheter ablation, hypertension, lipid-lowering drugs, LAD, TG, HDL-C, LDL-C, CRP, SBP, DBP, FPG, and WBC.
UNIVARIATE LOGISTIC REGRESSION ANALYSIS OF AF RECURRENCE AND RISK FACTORS:
Table 2 depicts a univariate logistic regression analysis, showing that uTSH (HR 1.047; 95% CI, 0.986–1.111, P=1.13) were not significantly correlated with AF recurrence. The analysis showed that age, sex, BMI, smoking and drinking history, lipid-lowering drugs, DM, hypertension, CAD, PAF, CAF, CRP, and WBC were not significantly associated with recurrence of AF.
UTSH QUARTILES AND RECURRENCE OF ATRIAL FIBRILLATION (CONSIDERING TOTAL):
Table 3 presents uTSH levels as a categorical variable, and univariate logistic regression analysis showed that subjects in the lower quartile of uTSH, Q1–Q3 had a lower risk of AF recurrence compared with subjects in the highest quartile, and Q3 (OR, 0.49, 95% CI 0.26–0.92, P=0.03). After adjusting for sex and age, the results of the multivariate model (model 1) were consistent with those of univariate logistic regression. In the multivariate model (model 2), after adjusting for BMI, DM, CAD, PAF, CAF, LAD, TC, LDL-C, HDL-C, TG, CRP, TG, HDL-C, and TG, the effect of uTSH on the risk of AF recurrence remained significant. The low quartile had a lower risk of AF recurrence compared with the highest quartile, and the Q3 uTSH (OR, 0.22, 95% CI, 0.09–0.53, P=0.001) was significantly different.
LIPID QUARTILES AND RECURRENCE OF ATRIAL FIBRILLATION DISAGGREGATED BY SEX:
Patients were divided based on sex differences in endocrine hormones (Tables 4, 5). The highest quartile was associated with a high risk of atrial fibrillation relapse in the multivariable model (model 2) (Tables 4, 5), but only in males (Table 5). Table 4 compares different quartile OR and 95% CIs to the highest quartile of uTSH in females. After adjustment for the multivariate model (model 2), the OR and 95% CIs for the lower and highest quartiles for males were as follows: uTSH Q1 (OR 0.60, 95% CI, 0.29–1.26, P=0.18); Q2 (OR 0.52, 95% CI, 0.24–1.13, P=0.09); Q3 (OR, 0.42; 95% CI, 0.18–0.94, P=0.03). A 1.0 mmol/L increase in Q1 uTSH was related to a 3% increase in the risk of AF recurrence.
PERSISTENT UTSH LEVELS AND ATRIAL FIBRILLATION RELAPSE:
Using uTSH level as a continuous variable (Table 6), multivariate adjusted logistic analysis (models 1 and 2) showed that elevated uTSH levels were not significantly associated with a reduced risk of AF recurrence. Logistic regression analysis showed increased uTSH levels were not significantly associated with an increased risk of AF recurrence in female and male patients.
Discussion
The important finding of this study is that the recurrence rate of atrial fibrillation after catheter ablation in the highest quartile of uTSH was higher than that in the low quartile, especially in males. These findings suggest serum TSH levels in male patients treated for AF with cardiac ablation were significantly associated with AF recurrence.
Previous studies on the correlation between AF, AF recurrence, and hypothyroidism or hyperthyroidism are inconsistent. In a community-based study, there was no obvious relation between TSH >10 mIU/L and the risk of atrial fibrillation in 10 years [12]. A recent large population-based systematic review showed that in individuals with normal thyroid function, only higher circulating free thyroxine levels were related to a rising danger of atrial fibrillation [13]. In 2018, Shao-bin Wei et al [14] found that high or low FT3 levels were related to atrial fibrillation relapse after catheter ablation. Higher than normal levels of FT4 were associated with atrial fibrillation relapse, but no relation was shown in normal TSH levels and atrial fibrillation relapse, neither of which seem to support our study results. In contrast, Itsuro Morishima et al [15] found that, in addition to hypothyroidism, a normal high TSH value may be an independent risk factor for recurrence of atrial tachyarrhythmias after catheter ablation of atrial fibrillation. Previous studies did not include uTSH; neither the effect of uTSH level on AF ablation outcomes nor its difference in males and females was clear.
There are 2 perspectives in the ablation of AF: (1) Eliminate the trigger points primarily originating from PV; or (2) Alter the matrix of atrial fibrillation, like the left atrial line and the after the wall of isolation. Patients with paroxysmal atrial fibrillation usually have only pulmonary vein trigger points, whereas patients with non-paroxysmal atrial fibrillation are more likely to have abnormal atrial substrates besides the trigger points of PV [16]. However, other studies highlighted the heterogeneity of paroxysmal atrial fibrillation patients, demonstrating that the paroxysmal AF subgroup has the same arrhythmic substrate as non-paroxysmal AF, making it resistant to pulmonary vein isolation (PVI) [17,18]. The recurrence of AF supports both views. The occurrence of AF is related to hypothyroidism or hyperthyroidism. Researchers found that changes in the structure and function of the atrium are linked to the occurrence and progression of AF [19]. Many risk factors for atrial fibrillation are also associated with AF relapse after catheter ablation [20]. Researchers confirmed that high or low thyroxine levels could all induce atrial vagus nerve rebuilding and uncontrolled protein expression of NGF (nerve growth factor), which is essential for the relapse and maintenance of atrial fibrillation [3]. Zhang et al demonstrated that hypothyroidism promotes myocardial fibrosis [2] and long-term hyperthyroidism can also cause increased myocardial fibrosis and decreased cardiac function [21]. Atrial fibrosis may be critical in the development and recurrence of AF following ablation. A multicenter prospective study (DECAAF) showed the relations between the number of atrial fibrotic changes and relapse after ablation of atrial fibrillation, demonstrating that for every 1.06-fold rise in left atrial fibrosis, the total heart rate rose by 1% [22]. The difference in long-term prognosis between patients in TSH quartiles 1–3 and quartile 4 may be related to the degree of arrhythmia substrate, and are closely associated with atrial fibrillation fibrosis [23], which partially supports the results of the present study; however, they did not conduct a sex-disaggregated study. Females had a lower PV reconnection rate and a more significant increase in left atrial pressure after catheter ablation of AF, and there were additional PV triggers, resulting in adverse cardiac rhythm results, which can be attributed to a different recurrence mechanism in females [24]. The high quartile of uTSH and the high recurrence of AF were observed primarily in males.
This study has several limitations. First, this was a cross-sectional study with a limited number of patients. Second, this study did not measure myocardial fibrosis, and T3 and T4 data were not collected. Furthermore, the retrospective non-randomized design has its inherent limitations. Third, this was a single-center study, and recurrent atrial fibrillation cases from other hospitals need to be assessed. Thus, further prospective studies with larger samples are required to strengthen the results.
Conclusions
Serum uTSH levels in male patients treated for AF with cardiac ablation were significantly associated with AF recurrence. Baseline uTSH should be assessed in patients with AF undergoing CA. Whether we can reduce the recurrence rate of atrial fibrillation by maintaining an appropriate uTSH level needs further study.
Tables
Table 1. Characteristics based on quartiles of uTSH level.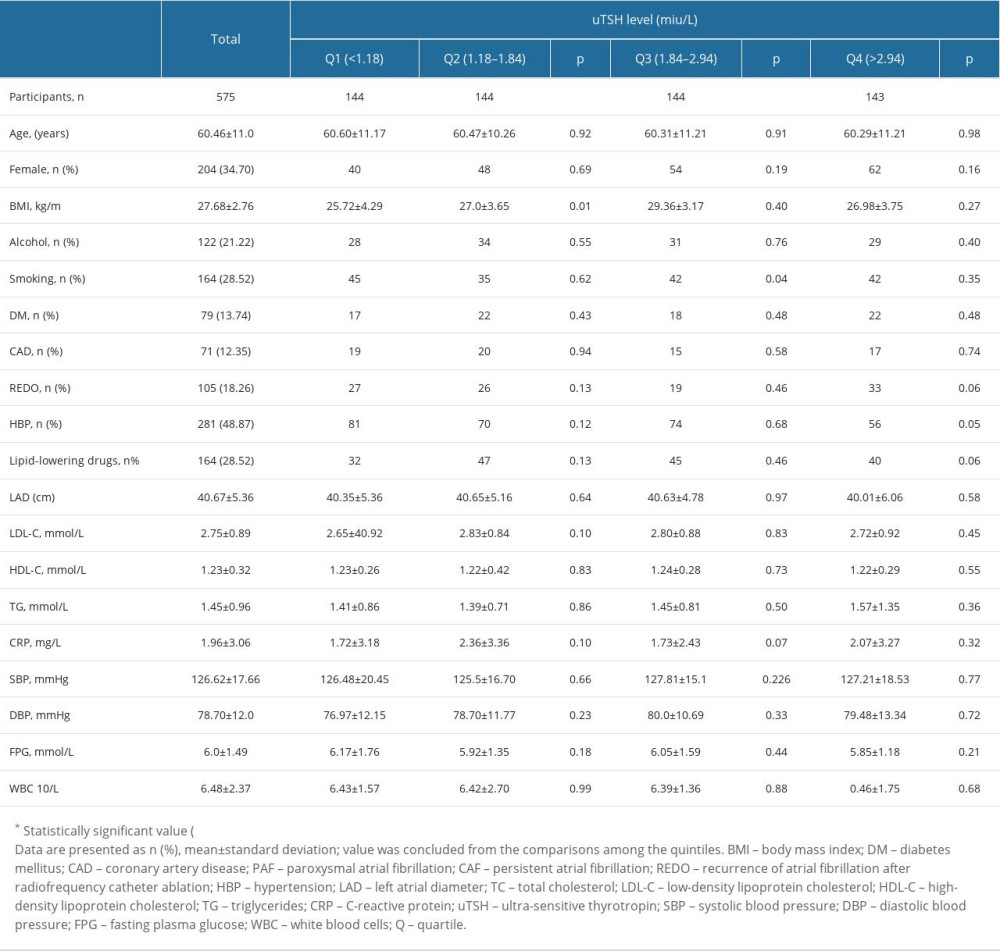 Table 2. Univariable analysis of AF redo.
Table 2. Univariable analysis of AF redo.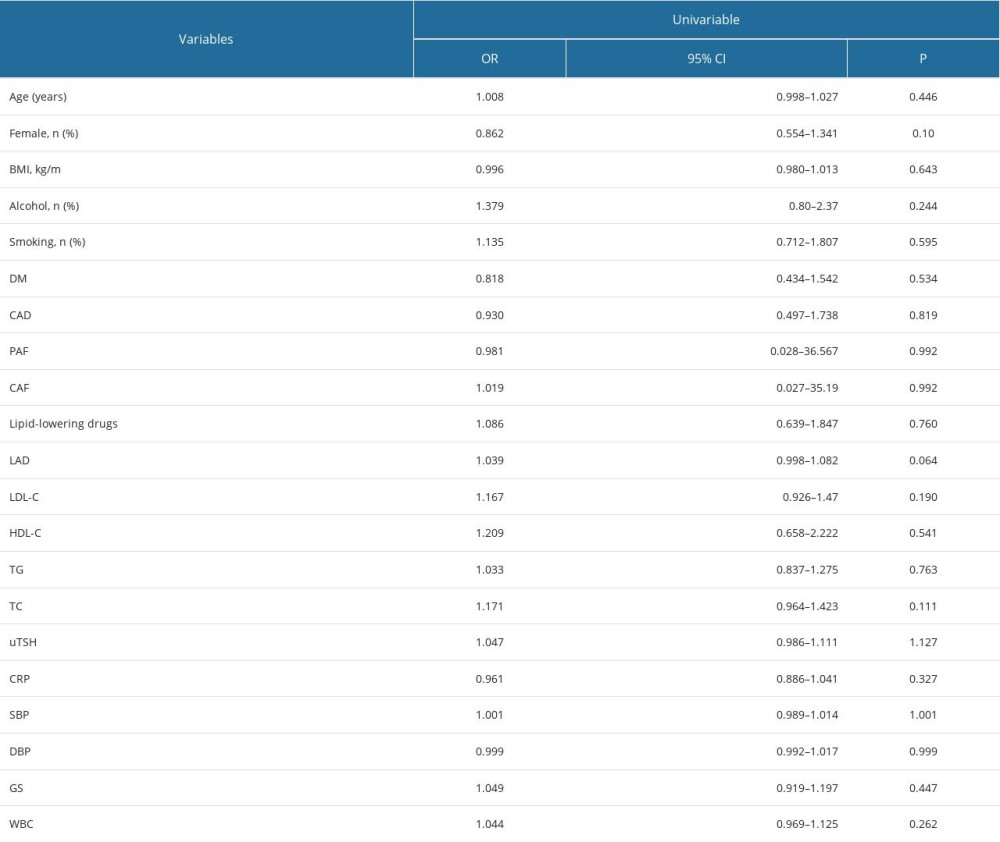 Table 3. Quartiles of uTSH and redo of AF (considering total).
Table 3. Quartiles of uTSH and redo of AF (considering total).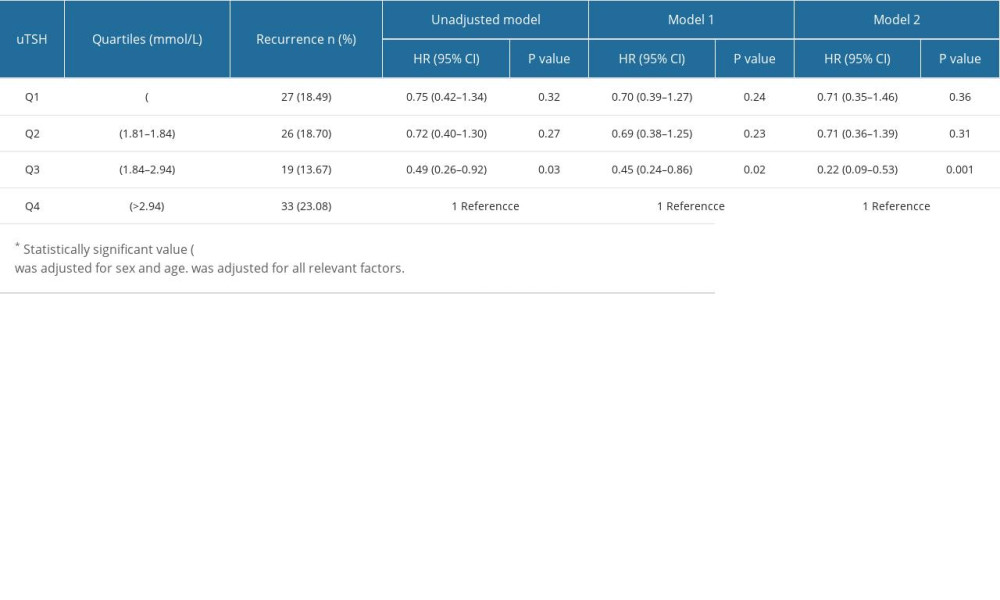 Table 4. Quartiles of uTSH and redo of AF (Women).
Table 4. Quartiles of uTSH and redo of AF (Women).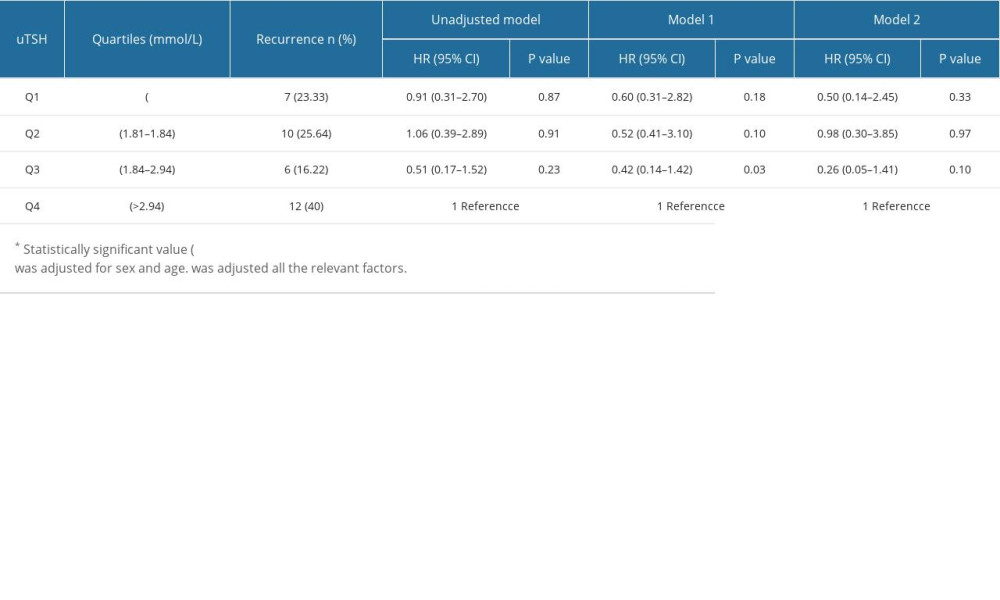 Table 5. Quartiles of uTSH and redo of AF (Men).
Table 5. Quartiles of uTSH and redo of AF (Men).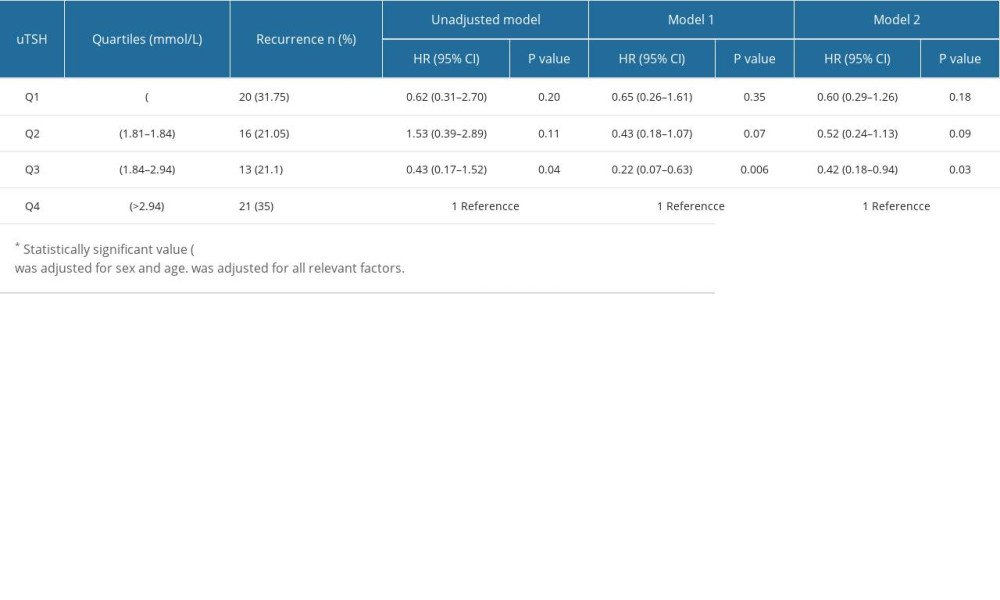 Table 6. uTsh and risk for redo of AF.
Table 6. uTsh and risk for redo of AF.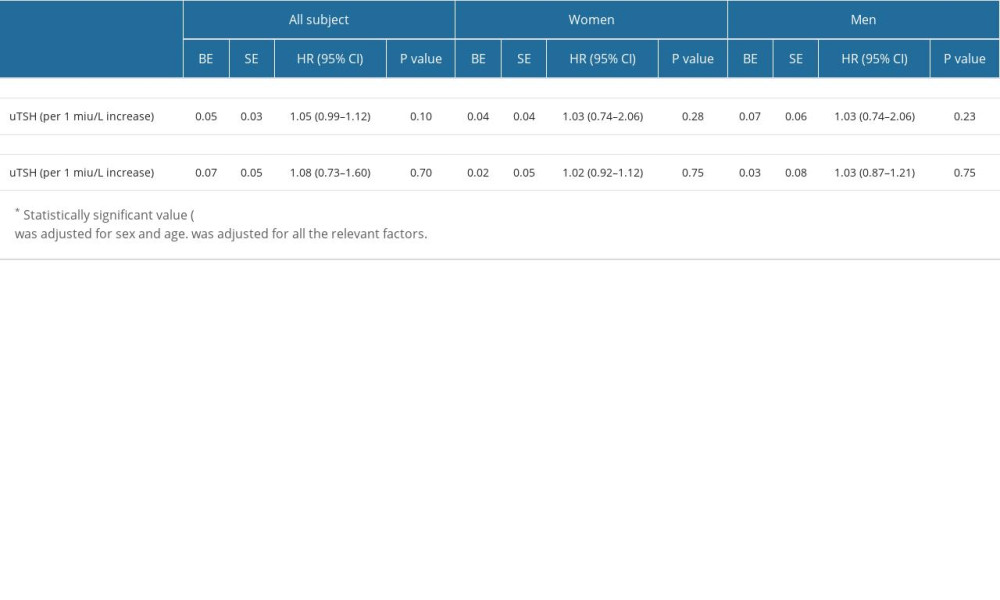
References
1. Jabbar A, Pingitore A, Pearce SH, Thyroid hormones and cardiovascular disease: Nat Rev Cardiol, 2017; 14(1); 39-55
2. Zhang Y, Dedkov EI, Teplitsky D, Both hypothyroidism and hyperthyroidism increase atrial fibrillation inducibility in rats: Circ Arrhythm Electrophysiol, 2013; 6(5); 952-59
3. Liu L, Yun F, Zhao H, Atrial sympathetic remodeling in experimental hyperthyroidism and hypothyroidism rats: Int J Cardiol, 2015; 187; 148-50
4. Bruere H, Fauchier L, Bernard Brunet A, History of thyroid disorders in relation to clinical outcomes in atrial fibrillation: Am J Med, 2015; 128; 30-37
5. Zhang Y, Dedkov EI, Teplitsky D, Both hypothyroidism and hyperthyroidism increase atrial fibrillation inducibility in rats: Circ Arrhythm Electrophysiol, 2013; 6; 952-59
6. Cheema A, Vasamreddy CR, Dalal D, Long-term single procedure efficacy of catheter ablation of atrial fibrillation: J Interv Card Electrophysiol, 2006; 15(3); 145-55
7. Tang RB, Liu DL, Dong JZ, High normal thyroid function and risk of recurrence of atrial fibrillation after catheter ablation: Circ J, 2010; 74(7); 1316-21
8. Sousa PA, Providencia R, Albenque JP, Impact of free thyroxine on the outcomes of left atrial ablation procedures: Am J Cardiol, 2015; 116(12); 1863-68
9. Hindricks G, Potpara T, Dagres NESC Scientific Document Group, 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC: Eur Heart J, 2021; 42(5); 373-498
10. Mirjanic-Azaric B, Jerin A, Radic Z, Thyroid stimulating hormone values of clinical decisions of hypothyroidism measurement by three different automated immunoassays: Scand J Clin Lab Invest, 2020; 80(2); 151-55
11. Zeng L, Ni Y, Zhao Y, Validation of the analytical sensitivity of ultra-sensitive thyroid -stimulating hormone detected by chemiluminescence: Int J Lab Med, 2012; 33(10); 1175-78
12. Kim EJ, Lyass A, Wang N, Relation of hypothyroidism and incident atrial fibrillation (from the Framingham Heart Study): Am Heart J, 2014; 167; 123-26
13. Baumgartner C, da Costa BR, Collet TH, Thyroid function within the normal range, subclinical hypothyroidism, and the risk of atrial fibrillation: Circulation, 2017; 136; 2100-16
14. Wei S, Wei W, Liu N, U-shaped association between serum free triiodothyronine and recurrence of atrial fibrillation after catheter ablation: J Interv Card Electrophysiol, 2018; 51(3); 263-70
15. Morishima I, Okumura K, Morita Y, High-normal thyroid-stimulating hormone shows a potential causal association with arrhythmia recurrence after catheter ablation of atrial fibrillation: J Am Heart Assoc, 2018; 7(19); e02707
16. Kirchhof P, Benussi S, Kotecha D, 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS: Eur Heart J, 2016; 37; 2893-962
17. Charitos EI, Purerfellner H, Glotzer TV, Clinical classifications of atrial fibrillation poorly reflect its temporal persistence: Insights from 1,195 patients continuously monitored with implantable devices: J Am Coll Cardiol, 2014; 63; 2840-48
18. Zaman JAB, Baykaner T, Clopton P, Recurrent postablation paroxysmal atrial fibrillation shares substrates with persistent atrial fibrillation: An 11-center study: JACC Clin Electrophysiol, 2017; 3; 393-402
19. Morin DP, Bernard ML, Madias C, The state of the art: Atrial fibrillation epidemiology, prevention, and treatment: Mayo Clin Proc, 2016; 91(12); 1778-810
20. Link MS, Haissaguerre M, Natale A, Ablation of atrial fibrillation: patient selection, periprocedural anticoagulation, techniques, and preventive measures after ablation: Circulation, 2016; 134(4); 339-52
21. Weltman NY, Wang D, Redetzke RA, Gerdes AM, Longstanding hyperthyroidism is associated with normal or enhanced intrinsic cardiomyocyte function despite decline in global cardiac function: PLoS One, 2012; 7(10); e46655
22. Marrouche NF, Wilber D, Hindricks G, Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study: JAMA, 2014; 311(5); 498-506
23. Dzeshka MS, Lip GY, Snezhitskiy V, Cardiac fibrosis in patients with atrial fibrillation: mechanisms and clinical implications: J Am Coll Cardiol, 2015; 66; 943-59
24. Pak HN, Park JW, Yang SY, Sex differences in mapping and rhythm outcomes of a repeat atrial fibrillation ablation: Heart, 2021; 107(23); 1862-67
Tables
In Press
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








