09 January 2023: Clinical Research
Effects of Electrical Impedance Tomography-Guided Positive End-Expiratory Pressure on Postoperative Cardiopulmonary Exercise Capacity in Elderly Patients: Study Protocol for a Randomized Controlled Trial
Wentao LiuDOI: 10.12659/MSM.938333
Med Sci Monit 2023; 29:e938333
Abstract
BACKGROUND: Mechanical ventilation can lead to cardiopulmonary complications in elderly patients undergoing abdominal surgery plus general anesthesia. The cardiopulmonary exercise test (CPET) is a dynamic and noninvasive evaluation method for assessing the cardiopulmonary system function under rest and stress. Positive end-expiratory pressure (PEEP) titration guided by electrical impedance tomography (EIT) can individualize lung protection strategies and may be beneficial in postoperative cardiopulmonary exercise capacity for these patients.
MATERIAL AND METHODS: This study is a prospective, single-center, randomized, and controlled trail that will include 80 elderly patients scheduled for major abdominal surgery. The patients will be divided into 2 groups: (1) intervention group: using individualized PEEP ventilation; and (2) control group: using fixed PEEP ventilation (3-5 cmH₂O).
RESULTS: The primary outcome is the change of postoperative cardiopulmonary exercise capacity.
CONCLUSIONS: In this study, we will evaluate if EIT-guided PEEP titration can improve postoperative cardiopulmonary exercise capacity and reduce postoperative complications in elderly patients undergoing open abdominal surgery plus general anesthesia. If the result is in accordance with the hypothesis, it would provide evidence to aid the perioperative management for these patients.
Keywords: Exercise Test, perioperative period, Postoperative Complications, Humans, Aged, Electric Impedance, Prospective Studies, Exercise Tolerance, Positive-Pressure Respiration, Tomography, X-Ray Computed, Randomized Controlled Trials as Topic
Background
Due to the aging of the population, the proportion of elderly patients in surgery is increasing [1]. In addition to the gradual degradation of physiological function in elderly patients, there are often comorbid chronic diseases of the respiratory, cardiovascular, and endocrine systems [2,3]. The requirements of patients for surgical anesthesia have also gradually improved, not only for the assurance of the safety in the perioperative period, but also for economic reasons and a comfortable and rapid recovery, which creates higher requirements for anesthesiologists. Perioperative-related complications, especially cardiopulmonary complications, have always been a concern and have been an important factor for the increase of perioperative mortality, the prolongation of hospital stays, and the risk of transfer to the intensive care unit [4]. Therefore, it is very important to reduce the occurrence of perioperative complications though making reasonable preoperative evaluations, predicting the occurrence of perioperative complications, and using corresponding protective measures during surgery. There are many routine evaluation methods for perioperative patients, mainly based on medical history, signs, and auxiliary examinations. In recent years, the use of the cardiopulmonary exercise test (CPET) to evaluate patients’ cardiopulmonary function has attracted increased attention [5]. CPET is a dynamic and noninvasive evaluation method that evaluates the cardiopulmonary system under rest and stress. It is considered the criterion standard for measuring human dynamic oxygen consumption [6]. Multiple physiological indexes can be observed at the same time, including respiratory parameters, heart rate, and respiratory gas composition. At present, the application of CPET can help guide clinical decision making. Its common uses include assessing the severity of exercise intolerance and dyspnea and identifying damage associated with heart failure and chronic obstructive pulmonary disease [7]. CPET is also used as a preoperative risk assessment tool to predict postoperative mortality, length of hospital stay, and complication rates [8]; however, its accuracy still needs further experiments to verify these results. In this study, CPET will be conducted twice, before and after surgery, to evaluate the effects of EIT-guided PEEP titration on cardiopulmonary exercise capacity of elderly patients undergoing laparotomy.
For patients with mechanical ventilation during general anesthesia, a protective ventilation strategy is often adopted during surgery, and a low level of PEEP is used to reduce alveolar collapse, which can effectively reduce the occurrence of atelectasis [9]. However, the use of PEEP has also led to the occurrence of lung injury in some patients. The research shows that the inhomogeneity of pulmonary ventilation during mechanical ventilation is common, and atelectasis and lung hyperinflation can occur at the same time. The application of a fixed PEEP level may not be the best ventilation strategy, and can even lead to the increase of lung injury [10]. Therefore, the best scheme of intraoperative lung protection ventilation might be an individualized ventilation strategy for patients. This requires that the patients can be monitored in real time. The existing intraoperative pulmonary function monitoring methods have limitations. Conventional imaging monitoring, such as X-ray and computed tomography scanning, which are limited to the conditions of equipment and the operation room, are inconvenient to use; however, there are radiation safety problems. The anesthesia machine can routinely monitor airway pressure, but the changes of gas distribution in the lungs cannot be observed directly. EIT technology is a recently developed technology for real-time imaging of the lung [11]. It has the advantages of being noninvasive and providing real-time imaging. It can observe the gas distribution state of the lung in real time, diagnose atelectasis and hyperinflation, and be applied to monitor the state of the lung during surgery. In this study, we intend to determine the effect of an individuated PEEP setting on postoperative cardiopulmonary exercise capacity and other postoperative complications in elderly patients undergoing laparotomy surgery.
Material and Methods
TRIAL DESIGN:
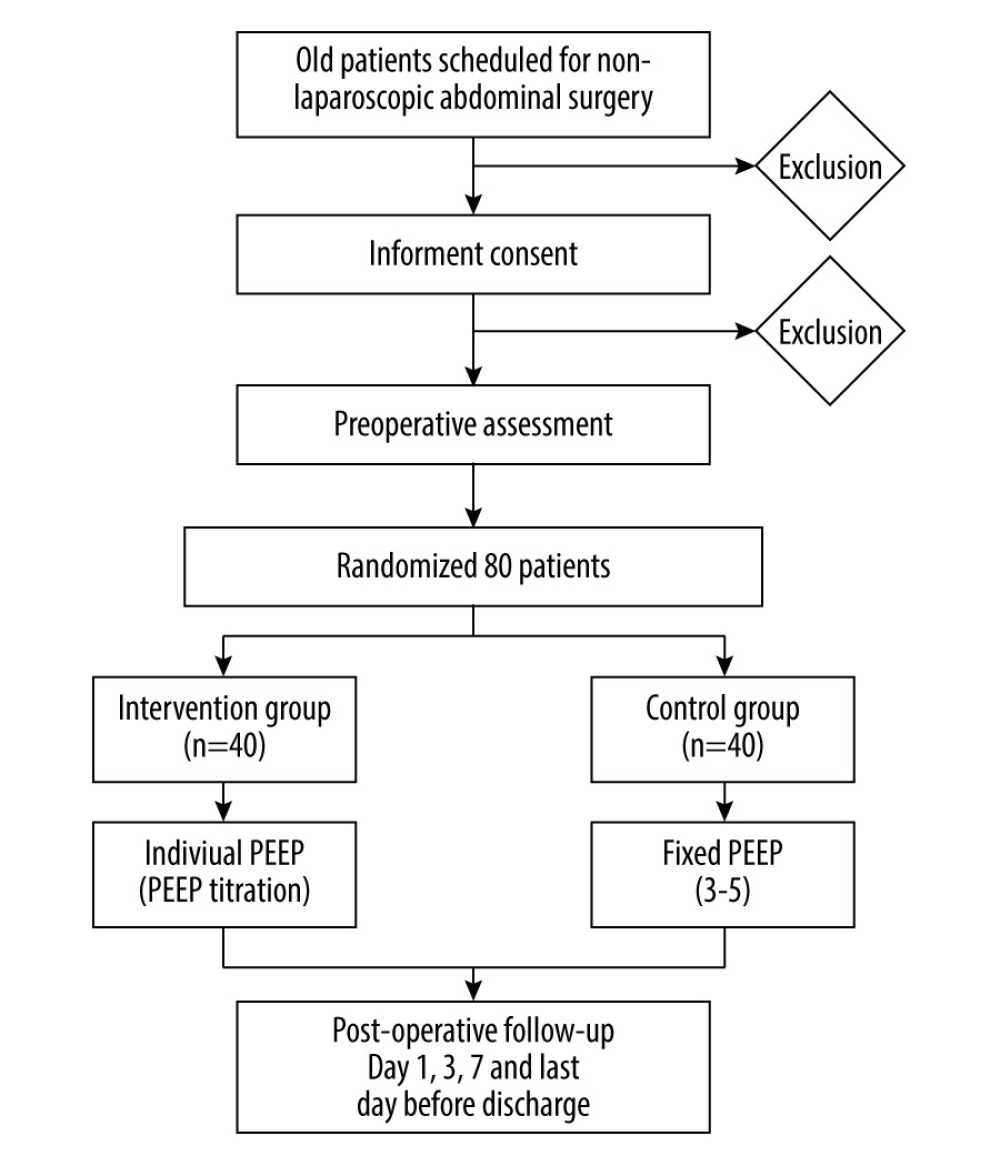
This is a single center, prospective, randomized controlled trial, which is planned to include 80 patients. The research will be conducted in Beijing Shijitan Hospital, Capital Medical University. This research complies with the Declaration of Helsinki and the guidelines for human biomedical research. It was reviewed by the hospital Ethics Committee (approval no. sjtky11-lx-2022[17]). The trial is registered at www.chictr.com.org.cn (registration no. ChiCTR2200058293) on April 4, 2022. The study complies with the Consolidated Standards of Reporting Trials (CONSORT) diagram [12] (Figure 1) and conforms to the Recommendations for Interventional Trials (SPIRIT 2013) reporting guidelines [13–15].
STUDY POPULATION:
We will randomly select 80 patients aged 65 years or older, regardless of sex, with elective laparotomy under general anesthesia and an estimated operation time ≥2 h. The patients will be divided into 2 groups: an intervention group and a control group. At least 2 researchers will participate in the study: one for intraoperative mechanical ventilation management and the other for blind enrollment and follow-up. The Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) score will be used to screen patients at intermediate and high risk before surgery, with a score ≥26 [16,17]. The exclusion criteria are laparoscopic surgery, estimated operation time of <2 h, body mass index (BMI) >35kg/m2, younger than 65 years old, American Society of Anesthesiologists Physical Status of IV or higher, existing orthopedic or neuromuscular disorders, preoperative bronchiectasis/respiratory infection, contraindications to the use of EIT (eg, pacemaker, automatic implantable defibrillator, implantable pump), preoperative hemodynamic instability, suspected PEEP titration intolerance, involvement in another clinical study, or refusal to participate. All patients signed informed consent.
RANDOMIZATION AND INTERVENTION:
The patients will be randomly divided into the 2 groups, intervention and control, with equal numbers. A computer generated randomization table will be used, with 20 blocks of 4 patients per block. Distributions will be stored in numbered, sealed, and opaque envelopes. Patients will be included and assigned in numerical order. Before anesthesia, the anesthesiologist will open the envelope to determine the grouping of patients. Intraoperative EIT will be used to monitor the optimal PEEP for titration 5 min after intubation in the intervention group. The control group used a fixed PEEP of 3 to 5 cmH2O. The rest of the management procedure will be consistent between the 2 groups.
STANDARD PROCESS:
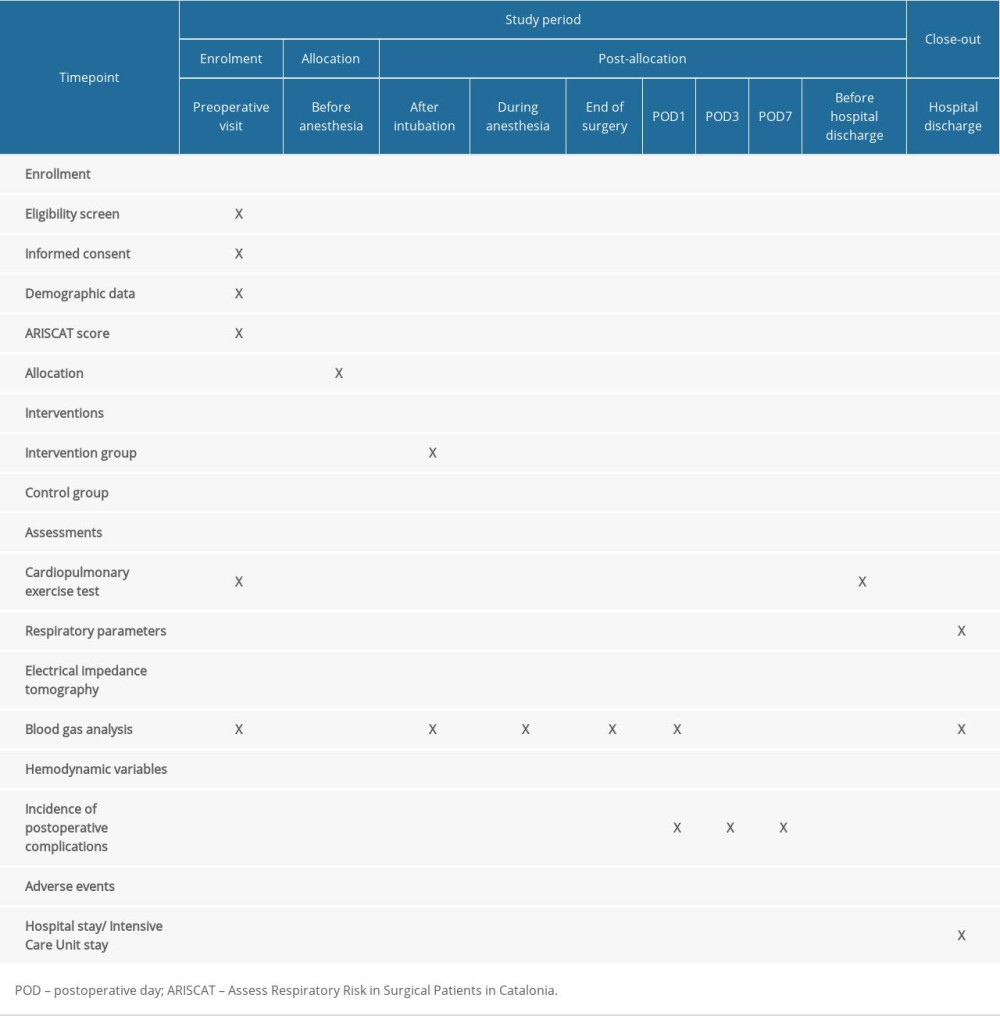
Eligible patients will be enrolled during the preoperative evaluation. All patients in the 2 groups will receive the same perioperative management process (Table 1). CPET will be completed at least 1 day before surgery. Prevention and treatments of nausea and vomiting and postoperative analgesia will be performed after surgery. The anesthetic procedure will be undertaken by the anesthetists in the same subspecialty anesthesia team for both the intervention and control groups. The anesthesia scheme of the 2 groups will be intravenous rapid induction of endotracheal intubation, mechanical ventilation, and intraoperative intravenous inhalation combined with maintenance anesthesia. Standard intraoperative monitoring will be used, including ECG, blood pressure, invasive radial artery pressure, bispectral index, pulse oximetry, continuous monitoring, and urine volume. Ventilation parameters monitored by anesthesia machine will be the fraction of inspired oxygen (FiO2), tidal volume (Vt), the respiratory rate (RR), PEEP, plateau pressure (Pplat), peak airway pressure, and respiratory system compliance. Postoperative monitoring will include at least ECG, pulse oximetry and noninvasive blood pressure measurements. Preoxygenation will be with FiO2 1.0 for 5 min before induction of intraoperative respiratory management. The volume-controlled mode will be used after intubation: Vt 8mL/(predicted body weight) and Pplat of 25 cmH2O. If the Pplat ≥25 cmH2O, the Vt will decrease at the level of 1 mL/kg to achieve the target of Pplat <25 cmH2O. The RR will be maintained with an inspiratory to expiratory ratio of 1: 2 at the end expiratory carbon dioxide partial pressure (ETCO2) between 35 and 45 mmHg. In the whole process, FiO2 will be set to 0.5. If SpO2 <95%, FiO2 will be raised by 0.1 until SpO2 is higher than 95%.
ALVEOLAR RECRUITMENT MANEUVERS:
The alveolar recruitment maneuvers will be performed at 5 min, 1 h, and 2 h after intubation and at the end of the operation. The ventilator mode will be changed from volume-controlled ventilation to pressure-controlled ventilation, with driving pressure of 20 cmH2O, respiratory rate of 15 times/min, inspiratory to expiratory ratio of 1: 1, PEEP 5 cmH2O, and FiO2 remaining unchanged. The PEEP level will be increased by 5 cmH2O every 10 respiratory cycles to 20 cmH2O, and the maximum airway pressure will be set to 40 cmH2O and maintained for 15 respiratory cycles [18]. If hemodynamic instability occurs in the rehabilitation period (cardiac index or mean arterial pressure decreases by 50%), norepinephrine or ephedrine will be given. When the hemodynamics are stable, other alveolar recruitment maneuvers will be performed. Then the individualized PEEP group will titrate the best PEEP through the decreasing PEEP test.
PEEP TITRATION:
At the end of the alveolar recruitment maneuvers, volume-controlled ventilation mode will be set, with Vt 8 mL/kg, RR of 15 respirations per min, and expiratory ratio of 1: 2. After that, PEEP will be decreased by 2 cmH2O every 1 min from 20 to 0 cmH2O, monitoring the changes in the slope of the EIT tracing. A decrease in slope indicates derecruitment, whereas an increase in slope indicates recruitment. When we get a horizontal tracing, it corresponds to a stable end-expiratory lung volume and indicates that PEEP is optimum [19,20]. This individual PEEP will be maintained throughout mechanical ventilation.
CPET:
From study recruitment to the day before surgery, patients will receive a doctor-supervised symptom-limited incremental CPET on a computer-controlled electromagnetic braking cycle dynamometer [21,22]. Before CPET, each patient will undergo a lung activity measurement of forced inspiratory and expiratory flow volume circulation. The subsequent incremental exercise test will take 8 to 12 min to complete. During this period, patients will sit on a bicycle dynamometer and have cardiovascular and respiratory measurements taken, followed by 3 min unloaded cycling as a warm-up. At the test sites where the circulating power meter cannot be set to unloaded cycling, the no-load cycle stage will be set to the lowest possible working load on the local circulating power meter. Then, the pedal resistance will be gradually increased every minute, and the ramp scheme will be adopted. During this process, patients will pedal at a speed of 60 rotations per min. Typically, untrained individuals increase their productivity by 10 watts per min, while participants who regularly participate in sports activities increase their productivity by 20 to 30 watts per min [23,24]. The main recorded indicators are, peak oxygen consumption (VO2peak), and anaerobic threshold.
The same test will be performed again the day before discharge from hospital, and VO2peak and anaerobic threshold will be recorded.
PRIMARY OUTCOMES:
The primary outcomes of the study will be change of postoperative cardiopulmonary exercise capacity (VO2peak and anaerobic threshold).
SECONDARY OUTCOMES:
The secondary outcomes will be incidence of pulmonary complications and other postoperative complications in 1, 3, and 7 days after surgery, length of hospital stay, and intensive care unit stay.
PULMONARY COMPLICATIONS:
Pulmonary complications in the study are as follows: (1) atelectasis: a chest X-ray shows pulmonary opacity, mediastinum, hilum, or diaphragm offset to the affected areas and compensatory hyperinflation of adjacent non-atelectasis lungs; (2) hypoxemia: SpO2 less than 92% with FiO2 0.21 or SpO2 less than 95% with FiO2 0.5; (3) acute respiratory distress syndrome: defined by Berlin definition [25]; (4) pneumonia: new pulmonary infiltration or progression of previous infiltration confirmed by chest X-ray, with at least 2 of the 3 criteria: (i) blood leukocytosis ≥12×109/L or <4×109/L, (ii) temperature >38.5°C or <36°C, (iii) increased secretion with purulent sputum; (5) pneumothorax: displacement of air and mediastinum in pleural cavity (chest X-ray examination for suspected hoarseness); (6) pleural effusion: the costophrenic angle becomes blunt, the adjacent anatomical structures are displaced on the chest radiographs; (7) aspiration pneumonia: respiratory failure after inhalation of reflux; (8) bronchospasm: use bronchodilators to treat expiratory wheezing; and (9) early extubation failure or need for re-intubation.
Other postoperative complications will have follow-up with the Postoperative Morbidity Survey instrument [26].
Baseline variables, including age, sex, height, weight, BMI, American Association of Anesthesiologists grade physical condition, ARISCAT risk score, medical history, and type of intervention, will be recorded. The parameters recorded at 4 points during surgery (5 min, 60 min, and 120 min after induction and the end of the operation) are FiO2, arterial blood gas, SpO2, respiratory parameter, and hemodynamics parameters. Additional data will be recorded, such as urine volume and pharyngeal temperature, the type of narcotic drugs, the type and amount of fluid, blood loss and transfusion, the demand for vasoactive drugs, operation time, mechanical ventilation time, the times of alveolar recruitment maneuvers, and the need for rescue treatments.
DATA AND STATISTICAL ANALYSIS:
According to previous studies [27,28], it is estimated that the incidence of postoperative cardiopulmonary exercise capacity declines in elderly patients, with intermediate or high risk of 30% to 45%. Based on these reports and the results of a preliminary trial, we assume that titration of iPEEP will reduce the decline of postoperative cardiopulmonary exercise capacity by 50% of fixed PEEP. The sample size calculation is based on α=0.05 allowing type 1 errors, β=0.2 allowing type 2 errors, and power=80%. Both groups require 36 cases, according to the mean value of the 2 groups in the literature, using the PASS 21.0 software. Considering patients who could be lost to follow-up, 40 patients in each group will be enrolled.
First, the baseline variables of these patients will be described, and the homogeneity of the groups will be assessed using appropriate statistical tests due to the types of variables analyzed (proportional mean difference, chi-square, analysis of variance, confidence interval of 95%). Second, calculating the bivariate variables association between patient characteristics, primary endpoints, and secondary endpoints, and in the case of quantitative results, analysis of variance (ANOVA) tests will be used.
Then, the relevance of the intervention to the primary and secondary results will be analyzed, the corresponding odds ratio will be calculated, and the ANOVA of quantitative results will be used. In all cases, the respective means or proportions will be estimated with their respective 95% confidence intervals. The primary outcomes, pulmonary complication, non-pulmonary complications, and mortality measurements analysis will be repeated using a multivariable logistic regression model and will be adjusted for any patient characteristics with clinical relevance shown in previous bivariate analysis.
DATA MANAGEMENT:
This study consists of a chief researcher and other researchers who participate in the design of this research scheme, the experimental process, and recording of the experimental data. The data collection will be conducted by an external independent researcher who will not participate in the quality control of this study. Monitors will assess the progress of the study and verify the accuracy and completeness of data records. All research data will include consent, and case report forms will be retained for 10 years after the trial ends at Beijing Shijitan Hospital. Finally, the original data and results will be released at
Discussion
This study is designed to verify that individualized PEEP is superior to the traditional lung protective ventilation strategy (low tidal volume + fixed low PEEP) in cardiopulmonary exercise capacity and postoperative pulmonary complications in elderly patients undergoing mechanical ventilation during surgery. Previous studies [18,19,35,36] have shown that individualized PEEP has certain advantages for patients with mechanical ventilation under general anesthesia. But there is little research of elderly patients undergoing major laparotomy surgery under general anesthesia, and there are no reports on the changes of cardiopulmonary exercise capacity after surgery. In this study, cardiopulmonary exercise capacity will be tested before and after surgery to test whether the protective effect can be observed.
Postoperative pulmonary complications and other postoperative complications are common problems in elderly patients. Mechanical ventilation can cause lung injury [16,29,30]. Patients can have atelectasis, pneumonia, acute respiratory distress syndrome, and other pulmonary complications after surgery. During ventilation, the inflated part of the lung receives the high tidal volume, potentially causing alveolar expansion, excessive tension, and pressure of the alveolar. The alveolar in a noninflated atelectasis area repeatedly collapse and re-expand, resulting in alveolar shear stress and diffused lung injury [17,31]. This can trigger the inflammation response, which leads to ventilator-induced lung injury. There are evidences that protective mechanical ventilation strategies can reduce lung injury and systemic inflammation response, decreasing the morbidity of these complications [32]. Low Vt as a lung protective mechanical ventilation strategy can reduce over expansion and lung injury not only in patients with acute respiratory distress syndrome, but also in intraoperative patients with non-injured lungs before surgery [33,34]. The use of low Vt can cause atelectasis, so the use of PEEP becomes another key point of the lung-protective ventilation strategy [35]. However, the optimal PEEP level is still unclear because of the high degree of heterogeneity in previous trials on different PEEP levels and the method of PEEP settings. Alveolar recruitment maneuvers are beneficial to reopen the collapsed alveoli. After the collapsed alveoli recover, it is necessary to select an appropriate PEEP level to maintain and prevent the alveoli from collapsing again. Inappropriate PEEP levels can cause alveoli to dilate or collapse, causing lung injury or atelectasis. Hence, it is essential to explore the optimal PEEP level in clinical practice.
The volume of tissue and gas can be calculated by measuring CT scan values. However, CT scans during surgery are difficult, and there are radiation hazards. EIT can monitor the changes of intrathoracic impedance caused by ventilation to see the gas distribution and mechanical characteristics [36,37]. EIT has the benefits of being noninvasive, real-time, and bedside and has no radiation hazards, compared with CT scans. EIT can intuitively evaluate the collapse and re-extension of lung tissue, so as to determine the correlation between lung volume and respiratory mechanics [38].
In previous studies [18–20,39–44], several measurements for PEEP titration by EIT have been reported, but none of them are currently accepted. In this study, the incremental PEEP method will be used for ventilator-driven alveolar lung supplement. Considering the feasibility and relative accuracy of surgery, we chose this method for PEEP titration. Optimal PEEP for patients can be set by titration [19,20]. Previous studies have shown that individualized PEEP titration has certain advantages for postoperative complications in elderly patients, which is usually reflected in respiratory physiological parameters and pulmonary complications, while it usually does not improve the length of hospital stay, mortality, and extrapulmonary complications. In previous perioperative studies, CPET was mainly used for preoperative evaluation and prediction of postoperative complications and it has been proven to predict postoperative mortality and postoperative pulmonary complications [45,46]. CPET is also commonly used to evaluate the recovery of cardiopulmonary capacity after cardiac surgery [47], but there are few studies on the comparison of cardiopulmonary exercise capacity in patients undergoing non-cardiac surgery. This study will use cardiopulmonary exercise capacity as the primary outcome to explore the relationship between perioperative cardiopulmonary exercise capacity and pulmonary complications to further explore the methods to improve prognosis.
Conclusions
In conclusion, we hypothesis that EIT monitoring for titration of individualized PEEP in elderly patients during open abdominal surgery could improve cardiopulmonary exercise capacity and reduce postoperative pulmonary complications, combined with other lung protection strategies. If this trial data is in accordance with the hypothesis, our study would provide evidence for perioperative management in elderly patients.
References
1. Hanna K, Ditillo M, Joseph B, The role of frailty and prehabilitation in surgery: Curr Opin Crit Care, 2019; 25(6); 717-22
2. Ilinca S, Calciolari S, The patterns of health care utilization by elderly Europeans: Frailty and its implications for health systems: Health Serv Res, 2015; 50(1); 305-20
3. Kim S, Brooks AK, Groban L, Preoperative assessment of the older surgical patient: Honing in on geriatric syndromes: Clin Interv Aging, 2015; 10; 13-27
4. Boehm O, Baumgarten G, Hoeft A, Epidemiology of the high-risk population: Perioperative risk and mortality after surgery: Curr Opin Crit Care, 2015; 21(4); 322-27
5. Herdy AH, Ritt LE, Stein R, Cardiopulmonary exercise test: Background, applicability and interpretation: Arq Bras Cardiol, 2016; 107(5); 467-81
6. Moran J, Wilson F, Guinan E, Mccormick P, Role of cardiopulmonary exercise testing as a risk-assessment method in patients undergoing intra-abdominal surgery: A systematic review: Br J Anaesth, 2016; 116(2); 177-91
7. Stringer WW, Cardiopulmonary exercise testing: Current applications: Expert Rev Respir Med, 2010; 4(2); 179-88
8. Kallianos A, Rapti A, Tsimpoukis S, Cardiopulmonary exercise testing (CPET) as preoperative test before lung resection: In Vivo, 2014; 28(6); 1013-20
9. Serpa NA, Schultz MJ, Slutsky AS, Current concepts of protective ventilation during general anaesthesia: Swiss Med Wkly, 2015; 145; w14211
10. Guldner A, Kiss T, Serpa NA, Intraoperative protective mechanical ventilation for prevention of postoperative pulmonary complications: A comprehensive review of the role of tidal volume, positive end-expiratory pressure, and lung recruitment maneuvers: Anesthesiology, 2015; 123(3); 692-713
11. Kobylianskii J, Murray A, Brace D, Electrical impedance tomography in adult patients undergoing mechanical ventilation: A systematic review: J Crit Care, 2016; 35; 33-50
12. Moher D, Hopewell S, Schulz KF, CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials: BMJ, 2010; 340; c869
13. Chan AW, Tetzlaff JM, Altman DG, SPIRIT 2013: New guidance for content of clinical trial protocols: Lancet, 2013; 381(9861); 91-92
14. Chan AW, Tetzlaff JM, Gotzsche PC, SPIRIT 2013 explanation and elaboration: Guidance for protocols of clinical trials: BMJ, 2013; 346; e7586
15. Chan AW, Tetzlaff JM, Altman DG, SPIRIT 2013 statement: Defining standard protocol items for clinical trials: Ann Intern Med, 2013; 158(3); 200-7
16. Canet J, Gallart L, Gomar C, Prediction of postoperative pulmonary complications in a population-based surgical cohort: Anesthesiology, 2010; 113(6); 1338-50
17. Nijbroek SG, Schultz MJ, Hemmes S, Prediction of postoperative pulmonary complications: Curr Opin Anaesthesiol, 2019; 32(3); 443-51
18. Ferrando C, Soro M, Unzueta C, Individualised perioperative open-lung approach versus standard protective ventilation in abdominal surgery (iPROVE): A randomised controlled trial: Lancet Respir Med, 2018; 6(3); 193-203
19. Erlandsson K, Odenstedt H, Lundin S, Stenqvist O, Positive end-expiratory pressure optimization using electric impedance tomography in morbidly obese patients during laparoscopic gastric bypass surgery: Acta Anaesth Scand, 2006; 50(7); 833-39
20. Eronia N, Mauri T, Maffezzini E, Bedside selection of positive end-expiratory pressure by electrical impedance tomography in hypoxemic patients: A feasibility study: Ann Intensive Care, 2017; 7(1); 76
21. , ATS/ACCP Statement on cardiopulmonary exercise testing: Am J Respir Crit Care Med, 2003; 167(2); 211-77
22. Balady GJ, Arena R, Sietsema K, Clinician’s Guide to cardiopulmonary exercise testing in adults: A scientific statement from the American Heart Association: Circulation, 2010; 122(2); 191-225
23. Wijeysundera DN, Pearse RM, Shulman MA, Measurement of Exercise Tolerance before Surgery (METS) study: A protocol for an international multicentre prospective cohort study of cardiopulmonary exercise testing prior to major non-cardiac surgery: BMJ Open, 2016; 6(3); e010359
24. Bartoszko J, Thorpe KE, Laupacis A, Wijeysundera DN, Association of preoperative anaemia with cardiopulmonary exercise capacity and postoperative outcomes in noncardiac surgery: A substudy of the Measurement of Exercise Tolerance before Surgery (METS) Study: Br J Anaesth, 2019; 123(2); 161-69
25. Ranieri VM, Rubenfeld GD, Thompson BT, Acute respiratory distress syndrome: the Berlin Definition: JAMA, 2012; 307(23); 2526-33
26. Grocott MP, Browne JP, Van der Meulen J, The Postoperative Morbidity Survey was validated and used to describe morbidity after major surgery: J Clin Epidemiol, 2007; 60(9); 919-28
27. Ovroutski S, Ewert P, Miera O, Long-term cardiopulmonary exercise capacity after modified Fontan operation: Eur J Cardiothorac Surg, 2010; 37(1); 204-9
28. Bartoszko J, Thorpe KE, Laupacis A, Association of preoperative anaemia with cardiopulmonary exercise capacity and postoperative outcomes in noncardiac surgery: A substudy of the Measurement of Exercise Tolerance before Surgery (METS) Study: Brit J Anaesth, 2019; 123(2); 161-69
29. Mazo V, Sabate S, Canet J, Prospective external validation of a predictive score for postoperative pulmonary complications: Anesthesiology, 2014; 121(2); 219-31
30. Serpa NA, Hemmes SN, Barbas CS, Incidence of mortality and morbidity related to postoperative lung injury in patients who have undergone abdominal or thoracic surgery: A systematic review and meta-analysis: Lancet Respir Med, 2014; 2(12); 1007-15
31. Dreyfuss D, Saumon G, Ventilator-induced lung injury: Lessons from experimental studies: Am J Respir Crit Care Med, 1998; 157(1); 294-323
32. Futier E, Constantin JM, Paugam-Burtz C, A trial of intraoperative low-tidal-volume ventilation in abdominal surgery: N Engl J Med, 2013; 369(5); 428-37
33. Treschan TA, Schaefer MS, Subasi L, Evolution of ventilator settings during general anaesthesia for neurosurgery: An observational study in a German centre over 15 years: Eur J Anaesthesiol, 2015; 32(12); 894-96
34. Wanderer JP, Ehrenfeld JM, Epstein RH, Temporal trends and current practice patterns for intraoperative ventilation at U.S. academic medical centers: A retrospective study: BMC Anesthesiol, 2015; 15; 40
35. Chaney MA, Nikolov MP, Blakeman BP, Bakhos M, Protective ventilation attenuates postoperative pulmonary dysfunction in patients undergoing cardiopulmonary bypass: J Cardiothorac Vasc Anesth, 2000; 14(5); 514-18
36. Hsu CF, Cheng JS, Lin WC, Electrical impedance tomography monitoring in acute respiratory distress syndrome patients with mechanical ventilation during prolonged positive end-expiratory pressure adjustments: J Formos Med Assoc, 2016; 115(3); 195-202
37. Long Y, Liu DW, He HW, Zhao ZQ, Positive end-expiratory pressure titration after alveolar recruitment directed by electrical impedance tomography: Chin Med J (Engl), 2015; 128(11); 1421-27
38. He X, Jiang J, Liu Y, Electrical impedance tomography-guided PEEP titration in patients undergoing laparoscopic abdominal surgery: Medicine (Baltimore), 2016; 95(14); e3306
39. Bikker IG, Leonhardt S, Bakker J, Gommers D, Lung volume calculated from electrical impedance tomography in ICU patients at different PEEP levels: Intensive Care Med, 2009; 35; 1362-67
40. Pereira SM, Tucci MR, Morais C, Individual positive end-expiratory pressure settings optimize intraoperative mechanical ventilation and reduce postoperative atelectasis: Anesthesiology, 2018; 129(6); 1070-81
41. Lowhagen K, Lindgren S, Odenstedt H, A new non-radiological method to assess potential lung recruitability: A pilot study in ALI patients: Acta Anaesthesiol Scand, 2011; 55(2); 165-74
42. Karsten J, Luepschen H, Grossherr M, Effect of PEEP on regional ventilation during laparoscopic surgery monitored by electrical impedance tomography: Acta Anaesthesiol Scand, 2011; 55(7); 878-86
43. Zhao Z, Lee LC, Chang MY, The incidence and interpretation of large differences in EIT-based measures for PEEP titration in ARDS patients: J Clin Monit Comput, 2020; 34(5); 1005-13
44. Haase J, Buchloh DC, Hammermuller S, Mechanical ventilation strategies targeting different magnitudes of collapse and tidal recruitment in porcine acid aspiration-induced lung injury: J Clin Med, 2019; 8(8); 1250
45. Stubbs DJ, Grimes LA, Ercole A, Performance of cardiopulmonary exercise testing for the prediction of post-operative complications in non cardiopulmonary surgery: A systematic review: PLoS One, 2020; 15(2); e0226480
46. Hennis PJ, Meale PM, Grocott MP, Cardiopulmonary exercise testing for the evaluation of perioperative risk in non-cardiopulmonary surgery: Postgrad Med J, 2011; 87(1030); 550-57
47. Zanini M, Nery RM, de Lima JB, Effects of different rehabilitation protocols in inpatient cardiac rehabilitation after coronary artery bypass graft surgery: A randomized clinical trial: J Cardiopulm Rehabil Prev, 2019; 39(6); E19-25
In Press
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952