25 February 2023: Clinical Research
Comparison of the Fractured and Non-Fractured Orbit Before and After Surgery Using a Titanium Implant or a Resorbable Poly-d,l-lactic Acid (PDLLA) Implant: A Study from a Single Center in Niš, Serbia of 58 Patients with Unilateral Orbital Floor Fracture Using Volumetric Measurement
Predrag Radović1ABDEF*, Sonja Janković2BDF, Milovan Papović3BC, Marša Leone Dimitrijević3BDF, Dragan Krasić1ABDEDOI: 10.12659/MSM.939144
Med Sci Monit 2023; 29:e939144
Abstract
BACKGROUND: A fracture of the orbital floor can lead to complications such as enophthalmos, impaired eye motility, or diplopia, which is why it is necessary to reconstruct the bony walls of the orbit. This study from a single center in Niš, Serbia, included 58 patients with unilateral orbital floor fracture and aimed to use volumetric measurement to compare the fractured and non-fractured orbit before and after surgery using a titanium implant or a resorbable poly-d, l-lactic acid (PDLLA) implant.
MATERIAL AND METHODS: From 2018 to 2022, a total of 58 patients with unilateral orbital floor fractures were treated at the Clinic of Dental Medicine, Niš. Computed tomography examination was used for volumetric measurement of the fractured and non-fractured (contralateral) orbit before and after the surgical procedure. A titanium implant was used in 31 patients, and a PDLLA implant was used in 27 patients.
RESULTS: Orbital volume ratio did not differ statistically significantly in relation to the type of implant (P=0.591). The postoperative volume did not differ statistically significantly from the volume of the contralateral side (titanium, P=0.212; PDLLA, P=0.232). There was a significant correlation between orbital volume and enophthalmos both before and after surgery (P=0.012, P=0.018, respectively).
CONCLUSIONS: Measuring the preoperative volume of the injured orbit is sufficient data for an indication because reconstruction depends primarily on the correlation between the volume and enophthalmos. The findings from this study showed that preoperative orbital volumetry using computed tomography evaluated enophthalmos and provide data to assist orbital floor reconstruction.
Keywords: enophthalmos, Orbital Fractures, Orbital Implants, Humans, Orbit, Titanium, Serbia, Plastic Surgery Procedures
Background
Orbital fractures involve isolated fractures of the inferior or medial wall of the eye socket, without orbital rim(s) fracture or fracture of other facial bones [1–3]. The European Project on Maxillofacial Trauma (EURMAT) states that, of the total number of maxillofacial injuries, about 16% are injuries to the orbit [4]. Unoperated orbital fractures can lead to significant enophthalmos, which is masked during primary evaluation by posttraumatic soft tissue swelling and hematoma [5]. In addition to enophthalmos, a fracture of the orbital floor can lead to complications, such as eye motility impairment or diplopia, which necessitates the reconstruction of the bone walls of the orbit [6].
Entrapment of bulbar muscles, diplopia, orbital wall defects larger than 2 cm2, and bulbar malposition are indications for orbital reconstruction [7–9]. Regarding globe malposition, enophthalmos in particular correlates with increased orbital volume (as has been reported, a volume change of as little as 5% causes clinically significant posttraumatic enophthalmos) [10–12]. Also, radiological findings are important for surgical treatment decisions.
The use of computerized tomography (CT) for the purpose of measuring the orbital volume is a useful tool for surgeons in cases of treating enophthalmos caused by trauma to the bone walls of the orbit, as well as for the purpose of reconstructing the orbital volume. The aim of surgical intervention after a fracture of the orbital floor is to correct the position of the eyeball and restore impaired function. Multiplanar reconstruction using CT is the most precise procedure, providing the most detailed resolution of the bony walls of the orbit, but it takes the longest time for measurement [13–15]. Today, there are several methods for volumetric analysis based on DICOM images. Volume measurements can be performed by manual, semi-automatic, and automatic processes. With the development of new software, semi-automatic and automatic segmentations are increasingly being used. Nonetheless, manual segmentation “slice-by-slice” by an expert is considered the criterion standard; it is more precise but very time-consuming and prone to intra- and interobserver variability [15]. Other studies determined the values of orbital volume change as an indication for surgery. However, measurement methods and fracture types vary. Numerous studies have focused on changes in orbital volume [16,17]. Enophthalmos associated with orbital floor fracture is the result of various factors, among which the increase in orbital volume is considered the most common factor, and there are numerous studies that have attempted to evaluate the success of surgical reconstruction by measuring orbital volume [18]. The analysis and quantification of orbital trauma are complex because orbital volume depends on soft tissue components that change over time (posttraumatic edema, periorbital tissue incarceration, late fibrosis, and atrophy) [19]. On the other hand, the size and localization of bone defects, as well as changes in the volume of the orbit, can be of great importance for the final outcome of the treatment. The reason for the frequent preoperative occurrence of enophthalmos in orbital floor fractures is the increase in pressure and weight of the bulb itself on the lower wall [20].
Defects of the orbital floor could be reconstructed using individual orbital implants from the alloplastic group, such as resorbable poly-d, l-lactic acid (PDLLA) implants [21], as well as titanium mesh [22].
Koenen et al analyzed the etiology, pathophysiology, epidemiology, and treatment of this type of injury and concluded that an interprofessional approach to a blow-out fracture is recommended, with a team consisting of an ophthalmologist, maxillofacial surgeon, and radiologist [23].
Therefore, this study, from a single center in Niš, Serbia, included 58 patients with unilateral orbital floor fracture and aimed to use volumetric measurement to compare the fractured and non-fractured orbit before and after surgery using a titanium implant or a PDLLA implant.
Material and Methods
OPHTHALMOLOGICAL TESTS:
The Hess-Lancaster diplopia test and Hertel exophthalmometry test were performed before surgical treatment. Hypoesthesia of the infraorbital nerve was identified.
The Hess-Lancaster test schematizes the position of the eyes and the function of the extraocular muscles and was applied to all patients 6 months after surgery.
The Hertel exophthalmometry determines the position of the eyeball in the eye socket. In this test, the distance between the apex and the outside of the cornea and the lengths of the orbit edges were simultaneously measured in both eyes. Although the absolute values themselves are not very important, they should be compared and monitored.
A difference of up to ±2 mm from the normal value is negligible, while values greater or less than this indicate the existence of a pathological process in the orbit. In order to assess the presence of secondary enophthalmos, the Hertel test was applied 6 months after surgery.
SURGICAL TECHNIQUE:
Surgical interventions were performed under general endotracheal anesthesia, after adequate preoperative preparation. Access to the fracture site was performed through a transpalpebral approach with a sharp and blunt preparation, the lower orbital edge was accessed, and after the orbital tissue exploration, the existing orbital floor defect was identified. The trapped orbital content was released and returned to the orbit by the periosteal elevator. Defects of the orbital floor were reconstructed using individual orbital implants from the alloplastic group, namely a 0.3 mm-thick titanium mesh (Mikro-Orbita-Mesh, KLS Martin, Tuttlingen, Germany) and resorbable poly-d, and manually modeled l-lactic acid (PDLLA) implant (Resorb X, KLS Martin, Tuttlingen, Germany). The mesh plate was cut and molded to match the anatomy of the fracture site and inserted under the periosteum on the floor of the eye socket. Resorbable PDLLA implant plates were modeled using a bath of a warm physiological solution at a temperature of 55°C to 70°C. The physical properties of the plates can be changed because they become soft and can be modeled without altering their chemical characteristics. They were placed in the region of the orbital floor defect. The implant covered the defect, passing over the peripheral edges of the healthy bone by 2 to 3 mm. The forced withdrawal test was performed to check if there was no limitation in bulbar mobility.
Sutures were removed 7 days after surgery. Patients were monitored once a week for the first month after surgery, and then monthly. In the first and sixth month after surgery, clinical improvement was assessed by clinical signs and diplopia was assessed within the central visual field. “No diplopia” was defined as an absence of diplopia.
RADIOLOGY:
CT examinations were performed using a 64-detector CT scanner (GE Revolution EVO 64; GE Healthcare, Milwaukee, WI, USA). The standard CT protocol for orbit examinations included scanning fields from the hard palate level to the level superior to the frontal sinuses. The patients were examined in the supine position. Scanning parameters included 120 kV and 220 mA, with an X-ray tube rotation of 0.33 s and slice thickness of 0.625 mm. After the software reconstruction of CT scans, all datasets were transferred and processed in 2-dimensional (2D) and 3D projections on a diagnostic workstation (Advantage Workstation 4.7; GE Healthcare, Milwaukee, WI, USA) using AW Volume Share 7 software (General Electric Company, Waukesha, WI, USA). Then, postprocessing techniques of 3D volume rendering and multiplanar reconstruction were used. A multiplanar analysis was conducted in the coronal, sagittal, and axial planes.
All the volumetric measurements were semi-automatically conducted. First, manual segmentation was performed by using a freehand drawing cursor region of interest in the axial plane by an experienced radiologist. Next, the isolated regions were visually inspected and manually adjusted. Segmented orbital contours were reconstructed as 3D images, and orbital volumes were automatically calculated. All measurements were evaluated 3 times by one radiologist, and the arithmetical value was estimated to reduce the measurement error. The anterior orbital border was defined as a line connecting medial and lateral orbital rims, while the posterior orbital border was defined as the orbital apex. A CT examination was used for the volumetric measurement of the fractured and non-fractured (contralateral) orbit before and 6 months after the surgical procedure. In addition, we preoperatively observed the location of orbital floor fractures, incarceration of orbital soft tissue, and extraocular muscles. Orbital implant position and the effect of orbital wall reconstruction were evaluated after surgery.
A CT examination was used for volumetric measurement of the fractured and contralateral (uninjured) orbit before and after the surgical procedure. The calculation formula for orbital volume ratio is: orbital volume ratio (OVR)=(1-(A-B/B))×100% (A: volume of fractured orbit after surgery; B: volume of contralateral [uninjured] orbit).
In the situation when the volume of the fractured orbit after surgery is equal to that of the uninjured orbit, the OVR is 100%. When the volume of the fractured orbit after surgery is 20% greater than that of the uninjured orbit, the OVR is 80%.
STATISTICAL ANALYSIS:
Data are statistically presented in the form of arithmetic mean ± standard deviations, as well as minimum and maximum values. The comparison of numerical variables in relation to implants and time between trauma and surgery was performed by a
Results
DEMOGRAPHICS:
In the period from 2018 to 2022, 58 patients (49 men and 9 women) who were injured were included in the research. The average age of the studied population was 43.60±16.42 years (range 18 to 86 years).
OPERATIVE DETAILS:
A titanium implant was used in 31 patients, and a resorptive PDLLA was used in 27 patients (Figures 1, 2).
The average number of days from injury to surgery was 8.37±8.14. After trauma, diplopia and paresthesia were present in 60.3% and 98.3% of patients, respectively. Postoperative diplopia and paresthesia were present in 6.9% and 43.1% of patients, respectively (Table 1).
Preoperative limited extraocular muscle motility was present in 56.9% of patients, and postoperative limited motility was present in 6.9% of patients. The preoperative volume of the fractured side was statistically significantly higher than that of the contralateral side (
OUTCOMES:
The mean volume of all reconstructed orbits was 25.20±2.75 cm3. The mean postoperative volume difference was 0.81±1.51cm3. The postoperative OVR was 96.50% (Figure 3A–3D).
The comparison of demographic and clinical parameters in relation to the installed implants showed a statistically significant difference in the preoperative volume (P=0.028), postoperative volume (P=0.005), and volume of the contralateral side (P=0.008). The OVR was not statistically significantly different in relation to the type of implant (P=0.591). With both implants, the preoperative volume of the fractured side was statistically significantly higher than that of the contralateral side (titanium, P<0.001; PDLLA, P<0.001), and the postoperative volume of the fractured side was not statistically significantly different than that of the contralateral side (titanium, P=0.212; PDLLA, P=0.232) (Table 2).
Follow-up was statistically significantly longer in patients in whom titanium was used (P<0.001). There was a significant correlation between orbital volume and enophthalmos both before and after surgery (P=0.012, P=0.018, respectively). Similarly, a statistically significant correlation was found between orbital volume difference and enophthalmos both before and after surgery (P<0.001, P=0.003, respectively). Early enophthalmos of at least 1 mm was found in 46 patients (79.3%). Postoperative diplopia was resolved in all but 4 patients (6.9%) who had persisting ocular motility impairment that needed correction with prism glasses (Table 3).
Exophthalmometry at the last follow-up appointment showed a normal globe position in 32 patients (55.1%) and enophthalmos ≤2 mm in 23 patients (39.7%), and 3 of the patients (5.1%) were subjectively disturbed by their globe position.
Discussion
The main goals of surgical intervention in orbital floor fractures are the decarceration of herniated orbital tissues and the reconstruction of bone parts of the orbit, with the restoration of its shape and volume. Also, correction of diplopia, enophthalmos, and limitations of extraocular muscle movement are important tasks [24,25]. Usual indications are the presence of diplopia that lasts longer than 2 weeks, restriction of extraocular muscle movement, radiological finding of extensive fracture, and enophthalmos caused by increased orbital volume [26].
The most important criterion for orbital reconstruction is orbital volume change. It is commonly accepted that the increase in orbital volume is accompanied by the appearance of enophthalmos [11].
Approximately half of the patients (53.4%) who were surgically treated were asymptomatic before surgery, and 32.8% had only mild symptoms such as diplopia or pain in extreme gaze directions.
In this study, the mean age of patients with titanium implants was 46.13 years, and in the group with PDLLA it was 40.70 years; the male: female ratios of these groups were 9.3: 1 and 3.5: 1, respectively. Enophthalmos was persistent in 44.8% (≤2 mm) of patients but clinically relevant in only 5.1% (≥2 mm).
The results of orbital floor reconstructions were obtained by calculating the volume ratio between the injured and the contralateral orbit before and after surgery. In this study, orbital volume was measured in a smaller number of patients (59) and a longer follow-up period of 6 months.
In this study, we performed a manual segmentation with semiautomatic calculation but without mirroring the contralateral orbit. Also, the results showed that the linear correlation between postoperative orbital volume and long-term enophthalmos was statistically significant. The mean volume of the contralateral (uninjured) orbit was 24.38±2.64 cm3, which agrees with the results of Baek et al, Choi et al, and Andrades et al [27–29]. Our research results agree with those of Park et al [30] because the enophthalmos correction in our patients was 0.82 mm per 1 cm3 of orbital volume reduction.
In general, the demonstrated orbital volume reconstruction showed satisfactory correction. We found mild enophthalmos (≤2 mm) in patients with an under-correction of the orbital volume. These findings underline that bone orbital volume plays an important role in the development of enophthalmos and diplopia lately. During the postoperative follow-up period, no infection caused by the orbital implant, dislocation or exposure of the implant, or loss of vision due to the presence of the orbital implant was observed. The non-significant difference between contralateral orbital volumes before and after surgery indicates the reliability of the contralateral (healthy) orbit measurements.
There was no significant difference in OVR groups dependent on the type of orbital implant during postoperative follow-up. Regarding postoperative complications, we did not find any except for 4 patients with persistent diplopia that needed correction with prismatic glasses. No patients required reoperation. Most cases of enophthalmos were well corrected after orbital floor reconstruction.
The results of this study showed that both reconstructive materials had similar effects on the outcome of treatment. Although we expected significantly higher persistent enophthalmos in patients with PDLLA implants, both implant types showed good results without significant differences in the follow-up period. We can safely use both types of implants. We found a statistically significant correlation between orbital volume difference and enophthalmos both before and after surgery. Both implant types showed no significant differences regarding extraocular muscle motility and diplopia. These results suggest that factors such as a surgeon’s preference, patients’ requests, or cost-effectiveness, could be appropriate for implant selection. Namely, both implant types seem to be equally effective and safe for orbital floor reconstruction. In cases of inferior orbital wall fractures, these implants are particularly effective for improving enophthalmos. The preoperative volume calculation of the fractured orbital forms sufficient data for an orbital reconstruction if there is a correlation between the increased volume and enophthalmos.
Alloplast materials, both resorptive and permanent (non-resorptive), are commonly used in the reconstruction of orbital floor defects. The choice of materials for orbital floor repair remains controversial, as there is no perfect implant available for all types of fractures and each one has advantages and disadvantages [26]. A resorptive implant should have a tensile strength greater than that of the orbital walls and gradually transfer the load from the disintegrating implant, allowing time for adequate bone and/or fibrous tissue formation in the bone defect. It should be completely resorbed to minimize the risk of foreign body reaction, infection, and the need for removal [3145]. Permanent implants such as titanium mesh have the benefits of good tensile strength and support of the orbital tissue. Multiple studies that compared the clinical outcomes of orbital fracture reconstruction using resorptive implants and permanent implants (including diplopia, enophthalmos, and ocular motility restriction) demonstrated comparable results, and their authors concluded that resorptive implants are suitable for isolated orbital floor reconstructions [31,36–39]. Some surgeons used resorptive mesh plates owing to their ease of use and complete resorption [40]. Some authors showed that the connective tissues around the resorptive mesh plate could not completely replace the bone structures after plate resorption, which can result in late enophthalmos [41].
Additionally, postoperative fibrosis cannot support the orbital structure, and it could persistently lead to enophthalmos [42]. Titanium mesh is known to be biocompatible and has been widely used for various craniofacial fractures. It can be easily adapted to bone structures and cut to the desired shape [43]. As foreign bodies, titanium plates could also lead to different late complications, such as infection, extrusion, implant migration, and permanent diplopia [44].
Resorb X, used in this study, is a resorptive system that is solely derived from 50: 50 poly (D, L-lactide) lactide. PDLLA is a purely amorphous compound that degrades in vitro without releasing crystalline by-products that might be involved in causing foreign-body reactions. An in vivo light microscopic examination showed that pure PDLLA disappears from the extracellular space within 72 weeks of implantation. Moreover, PDLLA maintains 90% of its bending strength at 6 weeks after implantation and 60% at 12 weeks [45]. Orbital volume measurements have been used for direct comparisons, OVR calculation, or the measurement of herniated tissue volume. Most authors calculate the OV using manual segmentation. Several studies have shown a correlation between an increased OV and enophthalmos. OVR is a parameter that can standardize this inter-individual variability. It has been described as a better predictor of enophthalmos onset [11,18,46–51].
Measuring orbital volume using slice-by-slice manual segmentation is currently considered the criterion standard and has been used as a reference for assessing different automatic and semiautomatic segmentation methods [52–54]. However, this process is relatively time-consuming, and its results can be somewhat observer-dependent. In this study, we used a semiautomatic method for achieving more precise results.
Several studies investigated the relation between the orbital volume change caused by an orbital fracture and, consequently, diplopia or enophthalmos [12,47,55,56]. The literature data has shown that increased orbital volume of 1 cm3 could lead to enlargement of enophthalmos for 1 mm [10,11,46,48].
Also, it has been demonstrated that intra-individual volume differences are quite small, and mirroring technique of uninjured orbit is a suitable for fracture volume estimation [57,58]. In a healthy population, there are also examples in which intra-individual volume differences might be greater than 1.5 cm3 [57–59]. Thus, a simple measurement of volume difference by mirroring the contralateral orbit might not always correctly predict postoperative ocular symptoms and the need for surgical treatment.
A recent study by Schönegg et al revealed no significant correlation between preoperative orbital volume difference and late enophthalmos when the mirroring technique was used [60]. This might be explained by the fact that other factors, such as anatomical location, and other characteristics also affect the development of symptoms [56,60].
The present study had some limitations in terms of the small number of patients with postoperative diplopia. One possible solution would be to compare the shape of fractured orbits with a statistical shape model derived from a large sample of uninjured orbits. Also, a statistical shape model could be used for the analysis of bilateral orbital fractures [61]. Patients with muscle entrapment often have severe symptoms despite a relatively small change in orbital volume, which explains why some patients with a small volume change require surgical intervention. Early malposition of the eyeball itself, as well as surgical assessment of the extent of the fracture that can cause enophthalmos, are indications for surgical treatment [56]. Young et al showed a mean persistent enophthalmos of 2.1 mm as clinically irrelevant. Most non-surgically treated patients at the 12-month follow-up had no aesthetically unattractive enophthalmos [58,59]. Nevertheless, enophthalmos could be persistent even after successful surgery [61].
Previously, Shin et al and Oh et al reported a 4: 1 male: female ratio with mean ages of 31.5 and 27.2 years, respectively, in surgically treated orbital fracture patients [27,61]. Underlying diseases and anesthesia eligibility can certainly influence the surgical decision; however, older age should not be automatically considered to be an exclusionary factor for surgery.
Fan et al reported that 0.89 mm of enophthalmos was improved by the reconstruction of fractured orbits by 1 cm3 in 16 patients [6]. Park et al reported an improvement in enophthalmos of 0.67 mm, with an orbital volume reduction of 1 cm3 in 14 examinees using a copolymer mesh orbital implant [30]. Ploder et al showed that enophthalmos of 1.2 mm was improved by a 1 cm3 reduction in refracted orbital volume in 38 patients [62]. Raskin et al concluded that enophthalmos of 0.47 mm was improved by reducing the volume of fractured orbits by 1 cm3 in 30 patients [48]. Ye et al found that 0.66 mm of enophthalmos was enhanced by reducing 1 cm3 of fractured orbital volume in 16 patients with blow-out fractures using a porous polyethylene orbital implant [63]. This finding is in accordance with the results of other studies reporting a linear correlation between increased orbital volume and enophthalmos [55].
Limitations of this study were its retrospective nature and the relatively small number of patients in the groups. As we continue to collect more medical data, we will have stronger statistical power to identify more significant differences.
Conclusions
The measurement of orbital volume is a good tool for predicting enophthalmos and planning orbital surgery. It is necessary to develop an easy-to-use technique that allows us to quickly and precisely perform measurements and calculations and that can be widely applied.
We must keep in mind that after orbital trauma or after orbital surgery, periorbital tissue retraction can lead to enophthalmos, even if the volume of the bony orbit is completely restored. Planimetry remains the most commonly used technique. Modern software packages for image processing will become more precise, and, therefore, important over time.
Measuring the preoperative volume of the injured orbit is sufficient data for an indication because reconstruction depends primarily on the correlation between the volume and enophthalmos. The CT technique used for volumetric measurement in this study was useful for analyzing the results of orbital floor reconstruction, regardless of which type of reconstructive materials were used. The findings from this study showed that preoperative orbital volumetry using CT was shown to evaluate enophthalmos and provide data to assist orbital floor reconstruction.
Figures
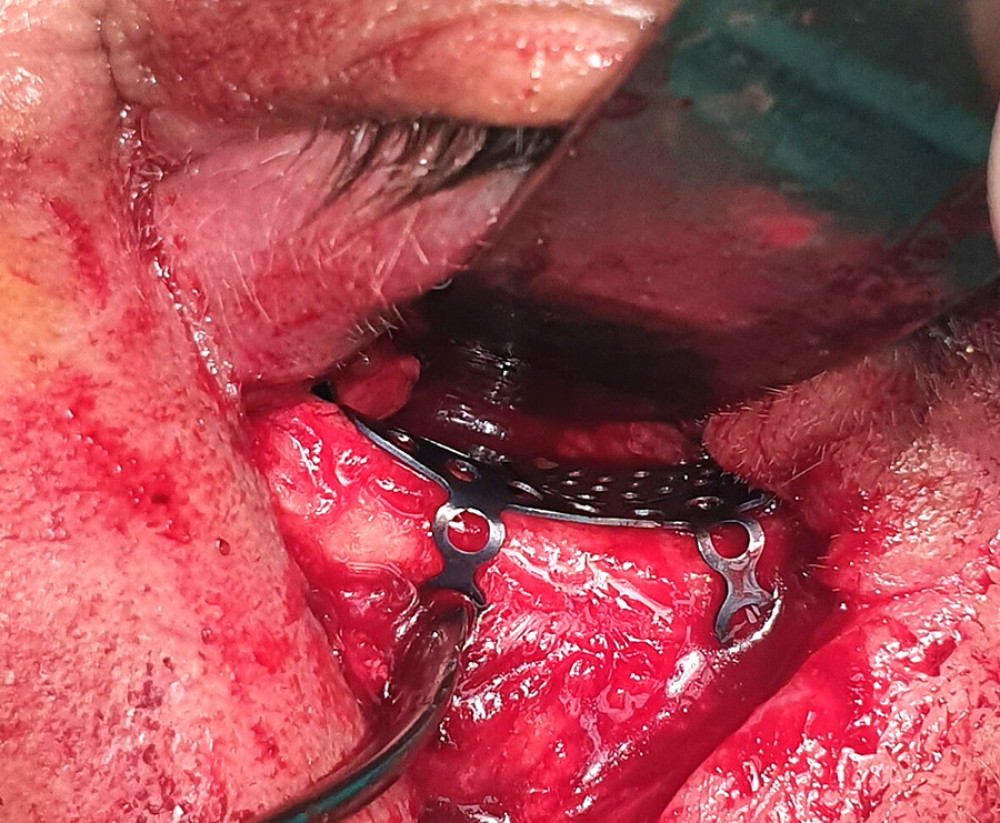 Figure 1. Orbital floor reconstruction with titanium mesh. Windows photo editor 10.0.1001116384 © 2020 Microsoft Corp.
Figure 1. Orbital floor reconstruction with titanium mesh. Windows photo editor 10.0.1001116384 © 2020 Microsoft Corp. 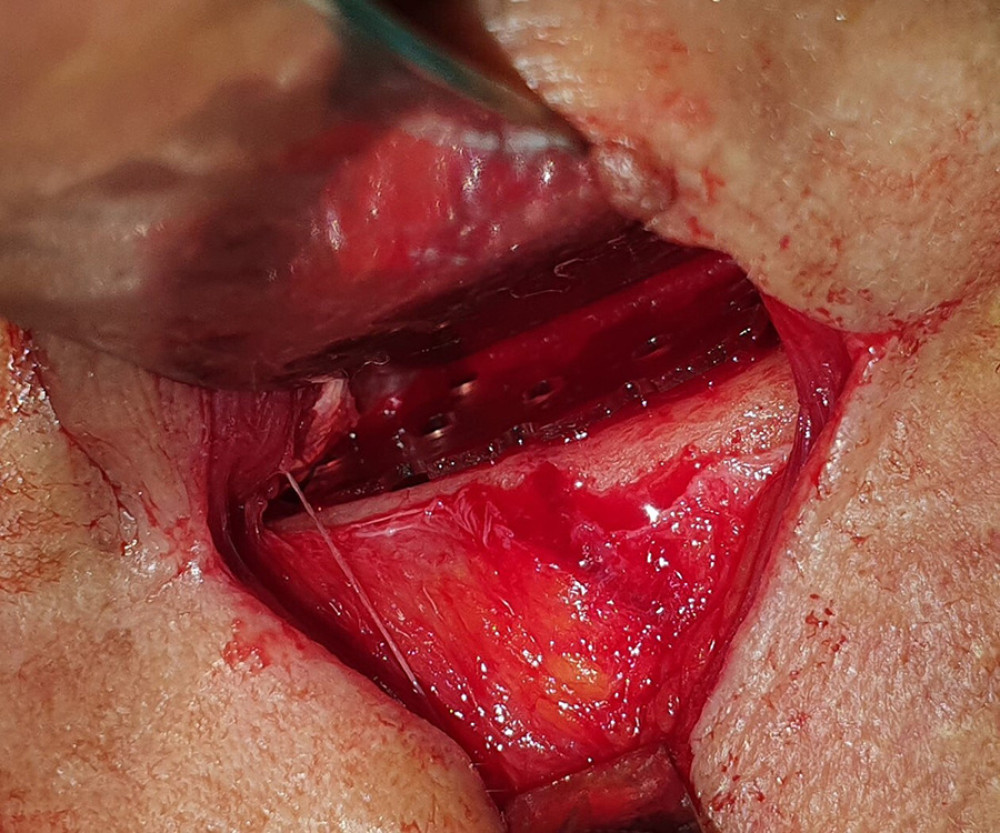 Figure 2. Orbital floor reconstruction with resorbable poly-d, l-lactic acid (PDLLA) implant. Windows photo editor 10.0.1001116384 © 2020 Microsoft Corp.
Figure 2. Orbital floor reconstruction with resorbable poly-d, l-lactic acid (PDLLA) implant. Windows photo editor 10.0.1001116384 © 2020 Microsoft Corp. 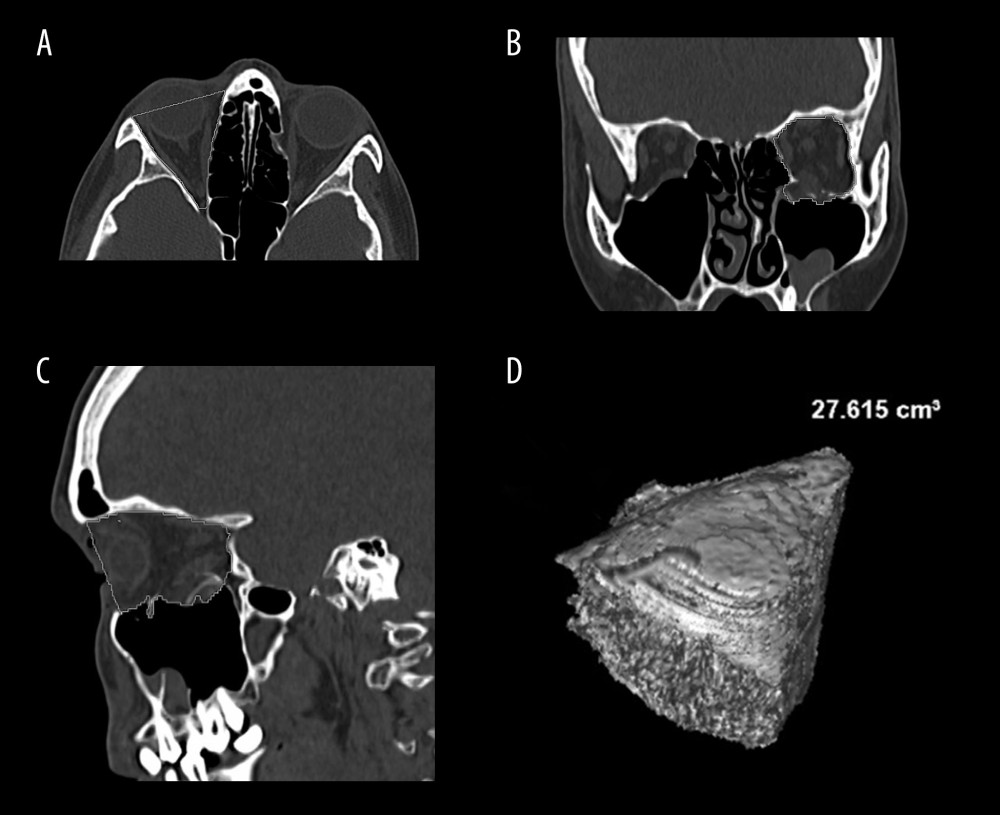 Figure 3. (A) Example of orbital volume measurement in the bone window using multiplanar reconstruction (MPR) in the axial plane. (B) Example of orbital volume measurement in the bone window using MPR in the coronal plane. (C) Example of orbital volume measurement in the bone window using MPR in the sagittal plane. (D) Volume rendering reconstruction of the orbit with calculated volume. Images created with AW Volume Share 7 software (General Electric Company, Waukesha, WI, USA) at Advantage Workstation 4.7 (GE Healthcare, Milwaukee, WI, USA).
Figure 3. (A) Example of orbital volume measurement in the bone window using multiplanar reconstruction (MPR) in the axial plane. (B) Example of orbital volume measurement in the bone window using MPR in the coronal plane. (C) Example of orbital volume measurement in the bone window using MPR in the sagittal plane. (D) Volume rendering reconstruction of the orbit with calculated volume. Images created with AW Volume Share 7 software (General Electric Company, Waukesha, WI, USA) at Advantage Workstation 4.7 (GE Healthcare, Milwaukee, WI, USA). References
1. Jones DEP, Evans JNG, “Blow-out” fractures of the orbit: An investigation into their anatomical basis: J Laryngol Otol”, 1967; 81(10); 1109-20
2. Burm JS, Chung CH, Oh SJ, Pure orbital blowout fracture: New concepts and importance of medial orbital blowout fracture: Plast Reconstr Surg, 1999; 103(7); 1839-49
3. Ellis E, Orbital trauma: Oral Maxillofac Surg Clin North Am, 2012; 24(4); 629-48
4. Boffano P, Roccia F, Zavattero E, European Maxillofacial Trauma (EURMAT) project: A multicentre and prospective study: J Craniomaxillofac Surg, 2015; 43(1); 62-70
5. Catone GA, Morrissette MP, Carlson ER, A retrospective study of untreated orbital blow-out fractures: J Oral Maxillofac Surg, 1988; 46(12); 1033-37
6. Fan X, Li J, Zhu J, Li H, Zhang D, Computer-assisted orbital volume measurement in the surgical correction of late enophthalmos caused by blowout fractures: Ophthal Plast Reconstr Surg, 2003; 19; 207-11
7. Converse JM, Smith B, On the treatment of blow-out fractures of the orbit: Plast Reconstr Surg, 1978; 62(1); 100-4
8. Leatherbarrow B: Oculoplastic surgery, 2010; 547-68, London, CRC Press
9. Manson PN, Mathes SJ: Plastic surgery secrets, 2006; 264-92, Philadelphia, Mosby Elsevier
10. Schuknecht B, Carls F, Valavanis A, Sailer HF, CT assessment of orbital volume in late post-traumatic enophthalmos: Neuroradiology, 1996; 38(5); 470-75
11. Whitehouse RW, Batterbury M, Jackson A, Noble JL, Prediction of enophthalmos by computed tomography after ‘blow out’ orbital fracture: Br J Ophthalmol, 1994; 78(8); 618-20
12. Choi SH, Kang DH, Prediction of late enophthalmos using preoperative orbital volume and fracture area measurements in blowout fracture: J Craniofac Surg, 2017; 28(7); 1717-20
13. Sentucq C, Schlund M, Bouet B, Overview of tools for the measurement of the orbital volume and their applications to orbital surgery: J Plast Reconstr Aesthet Surg, 2021; 74(3); 581-91
14. Bontzos G, Mazonakis M, Papadaki E, Ex vivo orbital volumetry using stereology and CT imaging: A comparison with manual planimetry: Eur Radiol, 2018; 29; 1065-374
15. Forbes G, Gehring DG, Gorman CA, Volume measurements of normal orbital structures by computed tomographic analysis: Am J Roentgenol, 1985; 145(1); 149-54
16. Schouman T, Courvoisier DS, Van Issum C, Can systematic computed tomographic scan assessment predict treatment decision in pure orbital floor blowout fractures?: J Oral Maxillofac Surg, 2012; 70(7); 1627-32
17. Scolozzi P, Bachelet JT, Courvoisier DS, Are inferior rectus muscle displacement and the fracture’s size associated with surgical repair decisions and clinical outcomes in patients with pure blowout orbital fracture?: J Oral Maxillofac Surg, 2020; 78(12); 2280.e1-e10
18. Manson PN, Grivas A, Rosenbaum A, Studies on enophthalmos: II. The measurement of orbital injuries and their treatment by quantitative computed tomography: Plast Reconstr Surg, 1986; 77; 203-14
19. Dubois L, Steenen SA, Gooris PJJ, Controversies in orbital reconstruction – I. Defect-driven orbital reconstruction: A systematic review: Int J Oral Maxillofac Surg, 2015; 44(3); 308-15
20. Haug RH, Nuveen E, Bredbenner T, An evaluation of the support provided by common internal orbital reconstruction materials: J Oral Maxillofac Surg, 1999; 57; 564-70
21. Annunziata M, Nastri L, Cecoro G, Guida L, The use of poly-d,l-lactic acid (PDLLA) devices for bone augmentation techniques: A systematic review: Molecules, 2017; 22(12); 2214
22. Gear AJ, Lokeh A, Aldridge JH, Safety of titanium mesh for orbital reconstruction: Ann Plast Surg, 2002; 48(1); 1-7 discussion 7–9
23. Koenen L, Waseem M, Orbital floor fracture [Updated 2022 Aug 7]: StatPearls [Internet] Jan, 2022, Treasure Island (FL), StatPearls Publishing Available at:http://www.ncbi.nlm.nih.gov/books/NBK534825/
24. Essig H, Dressel L, Rana M, Precision of posttraumatic primary orbital reconstruction using individually bent titanium mesh with and without navigation: A retrospective study: Head Face Med, 2013; 9(1); 18
25. Jeon SY, Kim C, Ma Y, Hwang E, Microsurgical intranasal reconstruction of blowout fractures of the medial orbital wall: Laryngoscope, 1996; 106; 910-13
26. Mathog RH, Management of orbital blow-out fractures: Otolaryngol Clin North Am, 1991; 24; 79-91
27. Baek SH, Park MS, Choi JH, Lee TS, Bony orbital volume measurement in normal adults using CT scan: J Korean Ophthalmol Soc, 2002; 43; 634-49
28. Choi JH, Park IK, Choi SJ, Shin JH, Measurement of orbital volume from facial CT scans using a semi-automatic computer program: J Korean Ophthalmol Soc, 2015; 56; 168-73
29. Andrades P, Cuevas P, Hernández R, Characterization of the orbital volume in normal population: J Craniomaxillofac Surg, 2018; 46(4); 594-99
30. Park YJ, Chung IY, Seo SW, An analysis of orbital reconstruction with bioresorbable plate through orbital volume assessment: J Korean Ophthalmol Soc, 2008; 49; 1046-53
31. Polacco MA, Kahng PW, Sudoko CK, Gosselin BJ, Orbital floor reconstruction: A comparison of outcomes between absorbable and permanent implant systems: Craniomaxillofac Trauma Reconstr, 2019; 12; 193-98
32. Seen S, Young SM, Teo SJ, Permanent versus bioresorbable implants in orbital floor blowout fractures: Ophthalmic Plast Reconstr Surg, 2018; 34; 536-43
33. Ramesh S, Hubschman S, Goldberg R, Resorbable implants for orbital fractures: A systematic review: Ann Plast Surg, 2018; 81; 372-79
34. Mok D, Lessard L, Cordoba C, A review of materials currently used in orbital floor reconstruction: Can J Plast Surg, 2004; 12; 134-40
35. Athanasiou KA, Agrawal CM, Barber FA, Burkhart SS, Orthopedic applications for PLA-PGA biodegradable polymers: Arthroscopy, 1998; 14; 726-37
36. Young SM, Sundar G, Lim TC, Use of bioresorbable implants for orbital fracture reconstruction: Br J Ophthalmol, 2017; 101; 1080-85
37. Baek WI, Kim HK, Kim WS, Bae TH, Comparison of absorbable mesh plate versus titanium-dynamic mesh plate in reconstruction of blow-out fracture: An analysis of longterm outcomes: Arch Plast Surg, 2014; 41; 355-61
38. Wi J, Sung K, Chi M, Orbital volume restoration rate after orbital fracture a CT-based orbital volume measurement for evaluation of orbital wall reconstructive effect: Eye, 2017; 31; 713-19
39. Magaña FG, Arzac RM, De Hilario Avilés L, Combined use of titanium mesh and resorbable PLLA-PGA implant in the treatment of large orbital floor fractures: J Craniofac Surg, 2011; 22(6); 1991-95
40. Al-Sukhun J, Lindqvist C, A comparative study of 2 implants used to repair inferior orbital wall bony defects: Autogenous bone graft versus bioresorbable poly-L/DL-lactide [P (L/DL) LA 70/30] plate: J Oral Maxillofac Surg, 2006; 64; 1038-48
41. Merten HA, Luhr HGResorbable synthetics (PDS foils) for bridging extensive orbital wall defects in an animal experiment comparison: Fortschr Kiefer Gesichtschir, 1994; 39; 186-90 [in German]
42. Tuncer S, Yavuzer R, Kandal S, Reconstruction of traumatic orbital floor fractures with resorbable mesh plate: J Craniofac Surg, 2007; 18(3); 598-605
43. Dietz A, Ziegler CM, Dacho A, Effectiveness of a new perforated 0.15 mm poly-p-dioxanon-foil versus titanium dynamic mesh in reconstruction of the orbital floor: J Craniomaxillofac Surg, 2001; 29; 82-88
44. Hwang K, Kim DH, Comparison of the supporting strength of a poly-L-lactic acid sheet and porous polyethylene (Medpor) for the reconstruction of orbital floor fractures: J Craniofac Surg, 2010; 21; 847-53
45. Heidemann W, Gerlach KL, Imaging of biodegradable osteosynthesis materials by ultrasound: Dentomaxillofac Radiol, 2002; 31; 155-58
46. Bite U, Jackson IT, Forbes GS, Gehring DG, Orbital volume measurements in enophthalmos using three-dimensional CT imaging: Plast Reconstr Surg, 1985; 75; 502-8
47. Ahn HB, Ryu WY, Yoo KW, Prediction of enophthalmos by computer-based volume measurement of orbital fractures in a Korean population: Ophthal Plast Reconstr Surg, 2008; 24; 36-39
48. Raskin EM, Millman AL, Lubkin V, Prediction of late enophthalmos by volumetric analysis of orbital fractures: Ophthal Plast Reconstr Surg, 1998; 14; 19-26
49. Sugiura K, Yamada H, Okumoto T, Quantitative assessment of orbital fractures in Asian patients: CT measurement of orbital volume: J Craniomaxillofac Surg, 2017; 45(12); 1944-47
50. Mohajerani H, Jafari SM, Manoochehri N, Tabrizi R, Does orbital volume change using the mirror technique have a correlation with posttraumatic enophthalmos?: J Craniofac Surg, 2019; 30(4); 369-72
51. Ebrahimi A, KalantarMotamedi MH, Rasouli HR, Naghdi N, Enophthalmos and orbital volume changes in zygomaticomaxillary complex fractures: Is there a correlation between them?: J Oral Maxillofac Surg, 2019; 77; 134.e1-e9
52. Jansen J, Schreurs R, Dubois L, Orbital volume analysis: Validation of a semi-automatic software segmentation method: Int J Comput Assist Radiol Surg, 2016; 11(1); 11-18
53. Chepurnyi Y, Chernohorskyi D, Prykhodko D, Reliability of orbital volume measurements based on computed tomography segmentation: Validation of different algorithms in orbital trauma patients: J Craniomaxillofac Surg, 2020; 48(6); 574-81
54. Wagner ME, Gellrich NC, Friese KI, Model-based segmentation in orbital volume measurement with cone beam computed tomography and evaluation against current concepts: Int J Comput Assist Radiol Surg, 2016; 11(1); 1-9
55. Oh SA, Aum JH, Kang DH, Gu JH, Change of the orbital volume ratio in pure blow-out fractures depending on fracture location: J Craniofac Surg, 2013; 24(4); 1083-87
56. Yang JH, Hwang SB, Shin JY, 3-Dimensional volumetric analysis of relationship between the orbital volume ratio and enophthalmos in unoperated blowout fractures: J Oral Maxillofac Surg, 2019; 77(9); 1847-54
57. Jansen J, Dubois L, Schreurs R, Should virtual mirroring be used in the preoperative planning of an orbital reconstruction?: J Oral Maxillofac Surg, 2018; 76(2); 380-87
58. Lieger O, Schaub M, Taghizadeh E, Büchler P, How symmetrical are bony orbits in humans?: J Oral Maxillofac Surg, 2019; 77(1); 118-25
59. Tandon R, Aljadeff L, Ji S, Finn RA, Anatomic variability of the human orbit: J Oral Maxillofac Surg, 2020; 78(5); 782-96
60. Schönegg D, Wagner M, Schumann P, Correlation between increased orbital volume and enophthalmos and diplopia in patients with fractures of the orbital floor or the medial orbital wall: J Craniomaxillofac Surg, 2018; 46(9); 1544-49
61. Doerfler HM, Huempfner-Hierl H, Kruber D, Template-based orbital wall fracture treatment using statistical shape analysis: J Oral Maxillofac Surg, 2017; 75(7); 1475.e1-e8
62. Ploder O, Klug C, Voracek M, Evaluation of computer-based area and volume measurement from coronal computed tomography scans in isolated blowout fractures of the orbital floor: J Oral Maxillofac Surg, 2002; 60; 1267-72
63. Ye J, Kook KH, Lee SY, Evaluation of computer-based volume measurement and porous polyethylene channel implants in reconstruction of large orbital wall fractures: Invest Ophthalmol Vis Sci, 2006; 47; 509-13
Figures
 Figure 1. Orbital floor reconstruction with titanium mesh. Windows photo editor 10.0.1001116384 © 2020 Microsoft Corp.
Figure 1. Orbital floor reconstruction with titanium mesh. Windows photo editor 10.0.1001116384 © 2020 Microsoft Corp. Figure 2. Orbital floor reconstruction with resorbable poly-d, l-lactic acid (PDLLA) implant. Windows photo editor 10.0.1001116384 © 2020 Microsoft Corp.
Figure 2. Orbital floor reconstruction with resorbable poly-d, l-lactic acid (PDLLA) implant. Windows photo editor 10.0.1001116384 © 2020 Microsoft Corp. Figure 3. (A) Example of orbital volume measurement in the bone window using multiplanar reconstruction (MPR) in the axial plane. (B) Example of orbital volume measurement in the bone window using MPR in the coronal plane. (C) Example of orbital volume measurement in the bone window using MPR in the sagittal plane. (D) Volume rendering reconstruction of the orbit with calculated volume. Images created with AW Volume Share 7 software (General Electric Company, Waukesha, WI, USA) at Advantage Workstation 4.7 (GE Healthcare, Milwaukee, WI, USA).
Figure 3. (A) Example of orbital volume measurement in the bone window using multiplanar reconstruction (MPR) in the axial plane. (B) Example of orbital volume measurement in the bone window using MPR in the coronal plane. (C) Example of orbital volume measurement in the bone window using MPR in the sagittal plane. (D) Volume rendering reconstruction of the orbit with calculated volume. Images created with AW Volume Share 7 software (General Electric Company, Waukesha, WI, USA) at Advantage Workstation 4.7 (GE Healthcare, Milwaukee, WI, USA). Tables
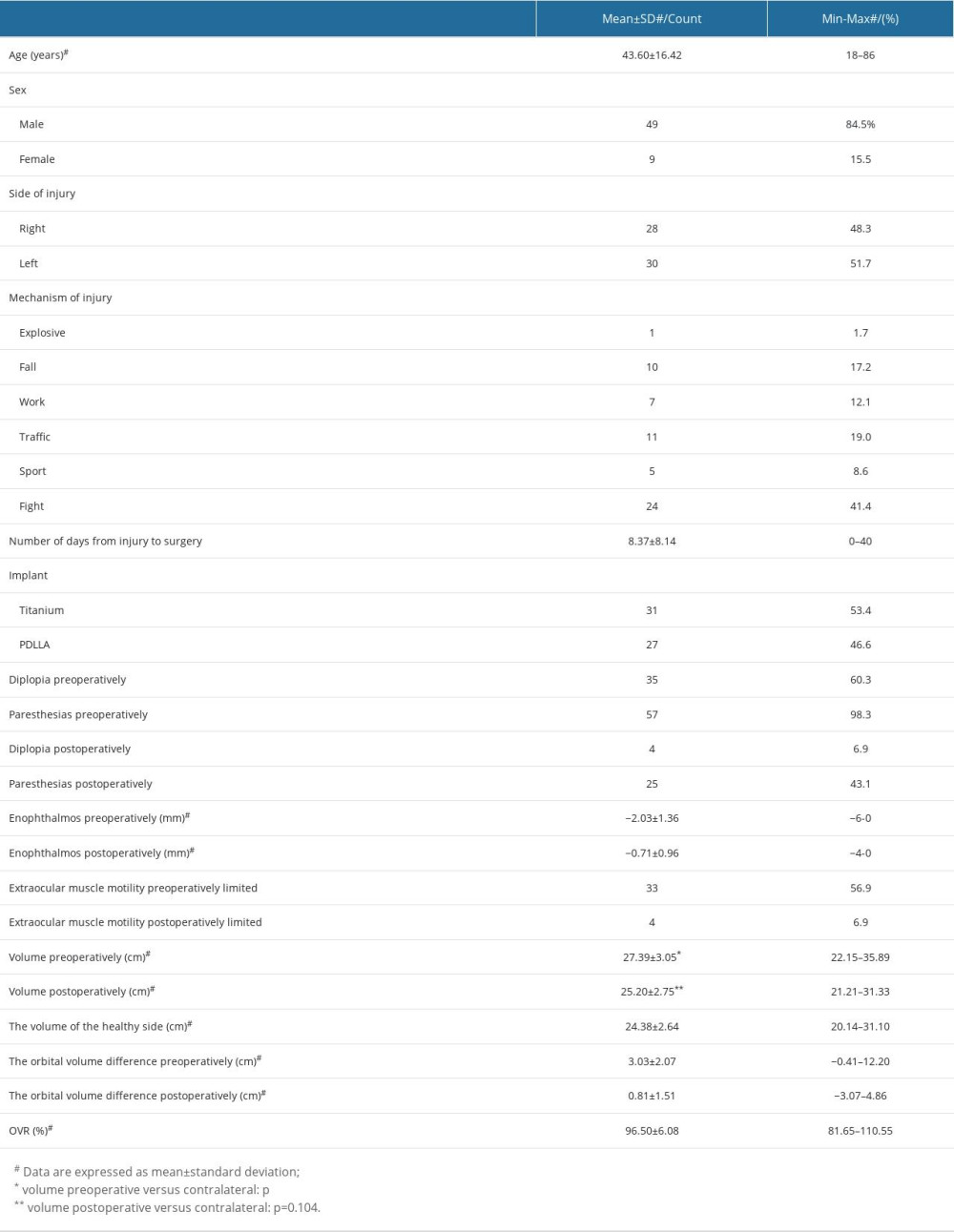 Table 1. Demographic and clinical characteristics of the studied population.
Table 1. Demographic and clinical characteristics of the studied population.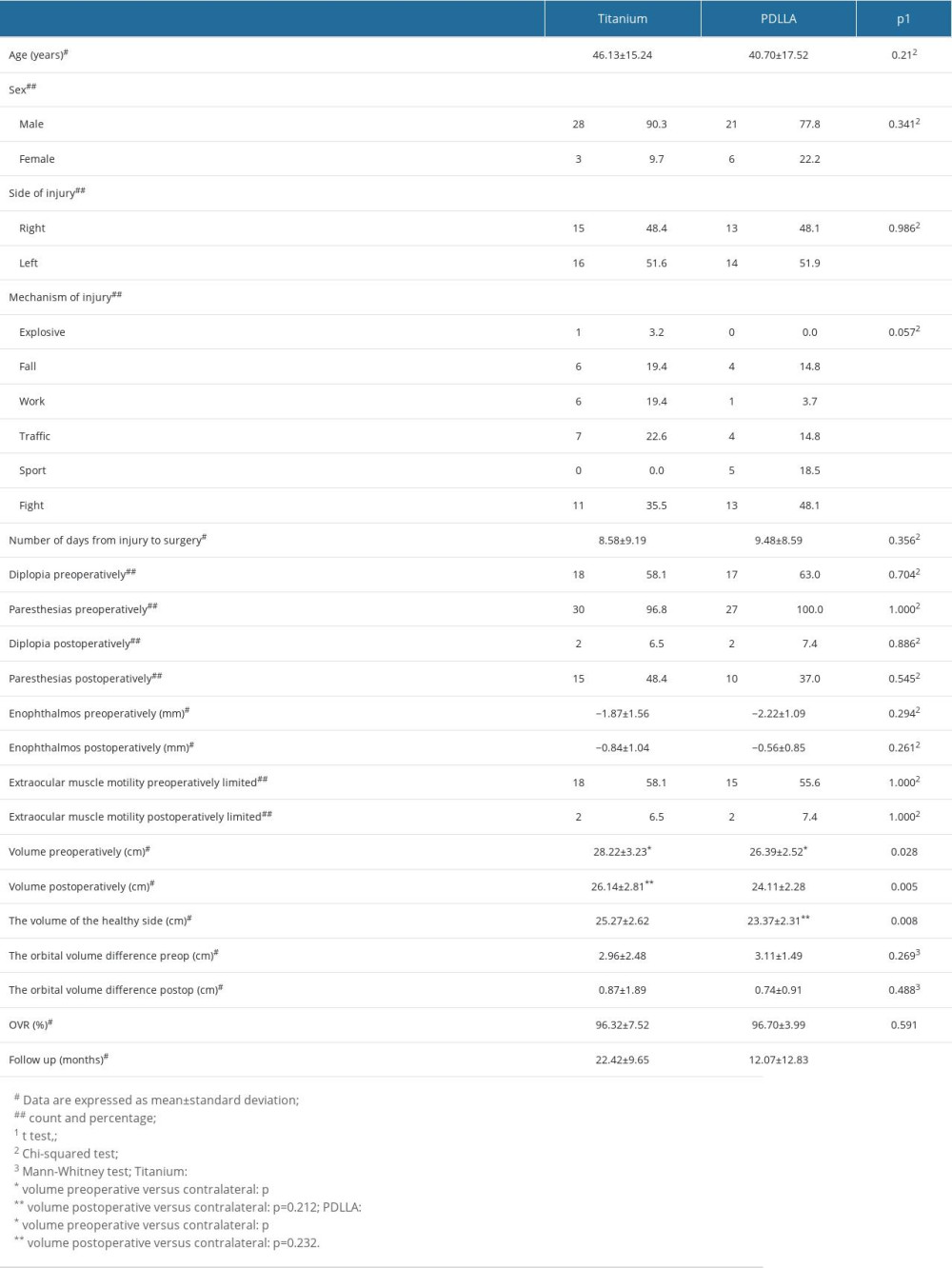 Table 2. Demographic and clinical characteristics of the studied population in relation to the implant.
Table 2. Demographic and clinical characteristics of the studied population in relation to the implant.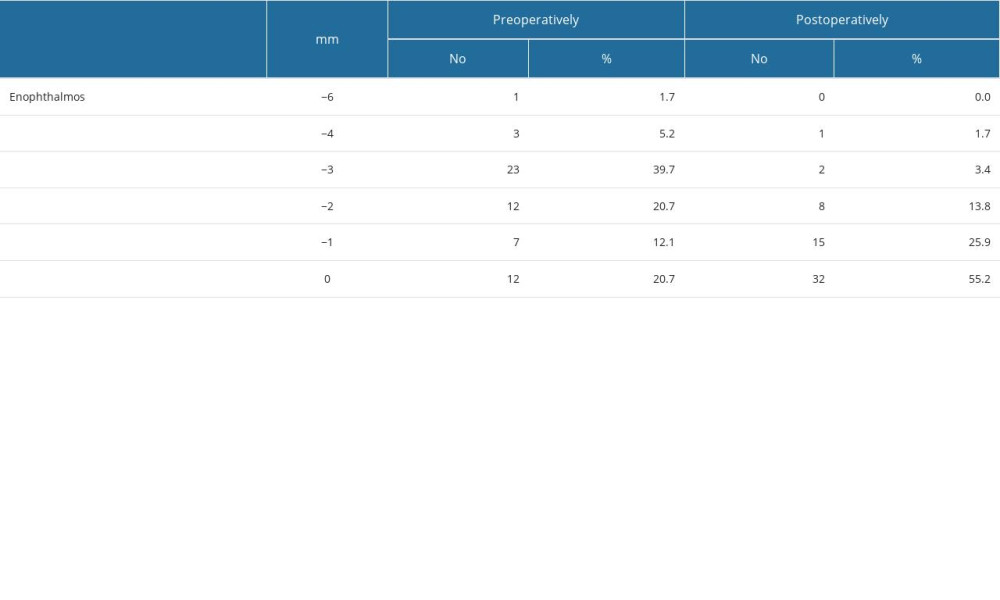 Table 3. Globe position in the study population.
Table 3. Globe position in the study population. Table 1. Demographic and clinical characteristics of the studied population.
Table 1. Demographic and clinical characteristics of the studied population. Table 2. Demographic and clinical characteristics of the studied population in relation to the implant.
Table 2. Demographic and clinical characteristics of the studied population in relation to the implant. Table 3. Globe position in the study population.
Table 3. Globe position in the study population. In Press
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








