11 May 2023: Clinical Research
Hippocampal Metabolic Alterations and Cognitive Dysfunction in Varicella Zoster Virus Meningitis: A Single-Center Case Series Study
Huili Liu1ABEF, Jun Wang1BCDE, Yanrong Yuan1BCF, Jing Gu1BCF, Yongxing Yan1ACDEG*DOI: 10.12659/MSM.939670
Med Sci Monit 2023; 29:e939670
Abstract
BACKGROUND: Meningitis has been found to be associated with dementia. Different pathogens of meningitis lead to different cognitive impairments. However, the change of cognitive function and cellular metabolism in the hippocampus in varicella zoster virus (VZV) meningitis has received little attention. We aimed to explore the cognitive function and changes of cellular metabolism in bilateral hippocampal regions in VZV meningitis.
MATERIAL AND METHODS: We used magnetic resonance spectroscopy to check the cellular metabolism in the bilateral hippocampal region in 23 VZV meningitis patients and 19 controls in our hospital from June 2020 to November 2022. Also, cognitive function was assessed using the Montreal Cognitive Assessment (MoCA) scale, and the correlation between MoCA scores and cellular metabolism in the bilateral hippocampal region was analyzed.
RESULTS: Six (26.1%) of the 23 patients with VZV meningitis had cognitive decline. Compared with that of the control group, the MoCA scores of VZV meningitis patients was much impaired (25.88±2.31 vs 27.74±1.94, P<0.05), visual-spatial executive function and delayed recall were significantly decreased (P<0.05), and N-acetylaspartate (NAA)/creatine (Cr) ratios in the bilateral hippocampus of patients with VZV meningitis were significantly lower (P<0.05). NAA/Cr ratios in the left and right hippocampus were positively correlated with MoCA scores (r=0.4158, P=0.0385; r=0.5274, P=0.0010, respectively), and negatively correlated with white blood cell count (P<0.01) and protein content in cerebrospinal fluid (P<0.05).
CONCLUSIONS: Patients with VZV meningitis had cognitive dysfunction and altered cellular metabolism of bilateral hippocampal regions. The main cognitive abnormalities were visual-spatial executive function and delayed recall.
Keywords: cognitive dysfunction, Magnetic Resonance Spectroscopy, Meningitis, Humans, Herpesvirus 3, Human, Hippocampus
Background
Varicella zoster virus (VZV) is a human-specific alfa-herpesvirus. Primary infection of VZV causes varicella. After infection, the virus continues to hide in ganglion cells. When the latent VZV in sensory cranial ganglia and dorsal root ganglia of the spinal cord is reactivated, vesicles and rashes distributed along the nerves can appear, which are called herpes zoster [1] that can cause complications of the peripheral nervous system, such as post-herpetic neuralgia, eye lesions, and cranial neuritis. Some VZV can retrogradely infect the central nervous system (CNS), causing VZV meningitis, encephalitis, myelitis, cerebrovascular disease, and other CNS complications [2–4]. In addition to somatic symptoms, such as neuralgia and cranial nerve paralysis, herpes zoster can be accompanied by non-somatic symptoms, such as depression, anxiety, and even cognitive impairment.
As a noninvasive imaging technique, magnetic resonance spectroscopy (MRS) can detect cellular metabolic changes before the changes of histomorphology and has played a huge role in disease diagnosis, therapeutic efficacy evaluation, and other aspects. At present, MRS has been applied to various fields of cognitive neuroscience. The hippocampus is an important structure that is closely related to cognitive function, and cellular metabolism alterations in the hippocampus are observed in patients with cognitive impairment [5]. Many scholars have found that there is a certain relationship between herpes zoster and dementia. Compared with in healthy people, the risk of dementia is significantly increased after 5 to 10 years in patients with herpes zoster [6–8]. These studies also found that the patients with acute herpes zoster had no obvious clinical dementia symptoms, but their hippocampal cellular metabolism changed. It was speculated that the changes of hippocampal cellular metabolism in patients with acute herpes zoster might be related to the patients’ later cognitive impairment [9]. Previous studies have also found that patients with meningitis can have cognitive impairment and that the impairment differs when caused by different pathogens [10–12].
VZV meningitis is one of the CNS complications caused by VZV reactivation, and the site of injury and clinical manifestations of patients are significantly more severe than that of herpes zoster. Since the lesions of VZV meningitis patients involve meninges, but not brain parenchyma, their clinical manifestations are less than those of patients with encephalitis, with a certain degree of self-limitation, and the prognosis is relatively good [13,14]. However, the changes of cognitive function and cellular metabolism in the bilateral hippocampus of patients with VZV meningitis are not well known. Therefore, in this study, we evaluated the cognitive changes of patients with VZV meningitis and used MRS to detect the cellular metabolism of the bilateral hippocampus to improve the understanding of cognitive function of patients with VZV meningitis and provide a reference for further understanding of its pathological mechanism.
Material and Methods
PATIENTS AND GROUPING:
We selected 23 patients with VZV meningitis hospitalized in our center from June 2020 to November 2022, and selected 19 healthy controls in the same period. The Ethics Committee of Hangzhou Third People’s Hospital approved our study (NO: 2021KA013).
INCLUSION AND EXCLUSION CRITERIA:
The inclusion criteria for patients with VZV meningitis were as follows: 1) aged over 18 years; 2) herpes zoster diagnosis within 4 weeks; 3) signs and/or symptoms of meningitis involvement in patients within 4 weeks of herpes zoster diagnosis; 4) any 2 of the following symptoms: headache, fever, neck stiffness, nausea, vomiting; 5) cerebrospinal fluid (CSF) white blood cell count (WBC) >5×106/L or the content of protein >45 mg/dL, and VZV DNA(+) in the CSF; and 6) negative bacterial, fungus culture from the CSF. The exclusion criteria were as follows: 1) incomplete clinical data records; 2) aged over 95 years; 3) meningitis caused by other causes; 4) HIV-infected patient or women in pregnancy; 5) patients with mental illness, severe dementia, or severe hearing or vision impairment who could not complete the Montreal Cognitive Assessment (MoCA) examination; and 6) patients with other severe brain organic diseases or severe liver and kidney dysfunction.
GENERAL DATA OF PATIENTS:
We systematically collected the age, sex, years of education, comorbidity, herpes location and other data of those included patients with VZV meningitis. At the same time, general data of the control group were collected.
CEREBROSPINAL FLUID TEST:
The patients with VZV meningitis completed lumbar puncture within 48 h after admission. An amount of 5 mL of CSF was collected with sterile tubes, and the WBC count, glucose, protein, chlorine, adenosine deaminase, and lactate dehydrogenase levels of the CSF were measured.
EVALUATION OF COGNITIVE FUNCTION:
The MoCA scale was used to assess the cognitive function of all patients. The MoCA scale includes the assessment of visual-spatial executive function, naming, attention, language fluency, abstraction, delayed recall, and orientation, with a high total score of 30 points. A score of less than 26 points indicates cognitive impairment (adding 1 point adjustment on the test results in patients with years of education less than or equal to 12 years,). The cognitive function assessment of all patients was completed within 48 h after admission and was completed by uniformly trained assessors.
:
A 1.5T magnetic resonance scanner (General Electric Co, USA) was used, and the standard head coil was used as the transmitting and receiving coil. The examination sequence included T1-weighted imaging, T2-weighted imaging, fluid-attenuated inversion recovery, and 1H-MRS. 1H-MRS uses chemical displacement imaging for voxel collection and point-analyzed spectral sequence scanning. The metabolites measured by 1H-MRS mainly include N-acetylaspartate (NAA), choline (Cho), and creatine (Cr). The spectral analysis software was used to automatically complete the baseline calibration and metabolite identification, with Cr as the reference, and the magnetic resonance scanner automatically calculated the ratio of every metabolite (including Cho/Cr, NAA/Cr, and NAA/Cho). Every patient underwent 1H-MRS examination within 48 h after admission.
STATISTICAL ANALYSIS:
Statistical analysis was performed with SPSS version 25.0 for Windows (IBM Corp, Armonk, NY, USA). The Kolmogorov-Smirnov test was used to test the normality of each group of data. Quantitative data conforming to a normal distribution were expressed as mean±standard deviation, and comparison between the 2 groups was conducted by
Results
BASELINE CHARACTERISTICS OF PATIENTS IN DIFFERENT GROUPS:
Among the 23 patients in the VZV meningitis group, 16 were men and 7 women, with a mean age of 56.0±15.3 years (range: 25–79 years). Years of education was 8.57±4.70. In the control group, there were 11 men and 8 women, with a mean age of 56.2±20.3 years (range: 27–93 years). Years of education was 8.68±3.73. There were no marked differences in age, sex, years of education, and concomitant diseases between the groups (P>0.05; Table 1).
CHANGES OF COGNITIVE FUNCTION:
The MoCA scores of VZV meningitis patients were 25.88±2.31, and those of the control group were 27.74±1.94; the MoCA scores of VZV meningitis patients were significantly lower than those of the control group (P<0.05). Six (26.1%) of 23 patients with VZV meningitis had MoCA scores lower than 26 points, compared with only 2 (10.5%) of 19 patients in the control group. Among them, the visual-spatial executive function scores in patients with VZV meningitis were 3.29±1.12, and the delayed recall scores were 4.21±0.66; they were all significantly lower than those of the control group (P<0.05; Table 2).
CHANGES OF CELLULAR METABOLISM IN THE BILATERAL HIPPOCAMPUS:
1H-MRS examination was performed on patients, and clear images and data were obtained, as shown in Figure 1. Compared with the control group, the NAA/Cr ratios of the left and right hippocampus in patients with VZV meningitis were significantly lower (1.01±0.45 vs 1.63±1.19; 1.29±0.45 vs 1.81±0.90, respectively; P<0.05). However, the ratios of Cho/Cr and NAA/Cho in the bilateral hippocampus were not different (P>0.05). Further analysis showed that there were no statistically significant differences in NAA/Cr, Cho/Cr, and NAA/Cho ratios between the bilateral hippocampus in the VZV meningitis group and control group (Table 3).
CORRELATION ANALYSIS:
The MoCA scores of the VZV meningitis patients and the control group were positively correlated with the NAA/Cr ratios in the left and right hippocampus (r=0.4158, P=0.0385; r=0.5274, P=0.0010 and r=0.4555, P=0.0440; r=0.4600, P=0.0435, respectively), as shown in Figures 2–5. However, no significant correlation was found between MoCA scores and the ratios of Cho/Cr and NAA/Cho (P>0.05). The WBC count and protein content in the CSF of patients with VZV meningitis were significantly negatively correlated with NAA/Cr levels in the left and right hippocampus (r=−0.4566, P=0.0285; r=−0.5542, P=0.0061; r=−0.4713, P=0.0232; r=−0.5680, P=0.0047, respectively), as shown in Figures 6–9.
Discussion
Cognition is the process in which the human brain receives external information and transforms it into internal mental activities. It includes memory, calculation, language, attention, understanding and judgment, visual space, and execution. Cognitive impairment refers to impairment of one or more of the above cognitive functions. When 2 or more of these cognitive domains are involved and affect the daily life or social ability of a patient, dementia can be considered. The early diagnosis and treatment of dementia is very important. Timely and accurate recognition of cognitive impairment of patients has important clinical value in preventing the occurrence of dementia. Infection is an important cause of dementia. Meningitis is an inflammatory disease involving the brain and perispinal membrane that can be caused by a variety of pathogens, including bacteria, viruses, Cryptococcus, and parasites. Cognitive impairment is a common symptom of patients with meningitis [15–17]. Many researchers have found that patients with meningitis often have certain cognitive impairments, and the cognitive impairments caused by different pathogens vary. Compared with that of the healthy control group, the attention/executive function, memory, and psychomotor function of patients with pneumococcal meningitis were significantly impaired, and the difference was statistically significant. In another 8 to 10-year study on cognitive function and quality of life of patients with bacterial meningitis, it was also found that there were certain impairments in memory, attention, executive function, and reaction speed [18]. Ganaraja et al [19] studied 60 patients with acute tuberculous meningitis and found that 93.3% of the patients had at least 1 cognitive field impairment (including attention, executive ability, working memory, learning, and memory) and that 33.3% had moderate to severe cognitive impairment. Patients with viral meningoencephalitis can also have mild and moderate cognitive impairment, especially in memory, executive function, and attention [10,15,20].
At present, neuropsychological examination is the most direct way to diagnose and evaluate cognitive dysfunction in clinical practice. Generally, various neuropsychological testing scales are adopted to evaluate cognitive impairment after brain injury. Among them, the MoCA scale is a commonly used screening tool by clinicians to test the cognitive function of patients. Many studies showed that the sensitivity of the MoCA scale to assess cognitive impairment caused by multiple causes was superior to that of the Mini-Mental State Examination [21–23]. The MoCA scale includes multiple subtests, which are comprehensive in the examination of cognitive function. In particular, it assesses language, complex spatial ability, and memory, and at the same time, the scale is good for assessing patient attention and executive ability. A total score of 30 is possible on the MoCA scale, and a score of 26 to 30 is generally considered the normal range. Therefore, the MoCA scale was used in this study to evaluate the cognitive function of patients with VZV meningitis. We found that 6 of 23 patients (26.1%) with VZV meningitis had an MoCA score lower than 26, an early stage of cognitive impairment. Further analysis showed that the cognitive impairment of patients with VZV meningitis was mainly the decline of visual-spatial executive function and delayed recall. Our results reaffirm that viral infection is an important cause of dementia, and that cognitive impairment is one of the common clinical symptoms in patients with VZV meningitis.
MRS is a current imaging technology that can study the metabolism of living tissues, organs, and related biochemical changes under noninvasive conditions. MRS can also conduct quantitative analysis of some compounds. It is a good tool for the examination of brain pathology and physiological changes in cognitive impairment patients. At present, the most commonly used magnetic resonance wave in MRS examination is 1H-MRS, which can detect metabolites, such as NAA, Cho, and Cr, in the brain. NAA mainly exists in axons, dendrites, and neurons, and it is one of the recognized indicators for detecting the integrity, vitality, and density of neurons. A decrease of NAA level is generally caused by nerve loss or dysfunction [24,25]. Cho is involved in the formation of the cell membrane and is closely related to the metabolism of phospholipids. Cr acts as a buffer between adenosine triphosphate and adenosine diphosphate. Research shows that the total amount of Cr is relatively constant in different individuals under different metabolic conditions; therefore, Cr is generally used as a reference for the standardization of body metabolites [26]. The results of our study showed that the cellular metabolism of the bilateral hippocampus in patients with VZV meningitis was abnormal, similar to previous reports, and that MRS is one of the effective tools for assessing cognitive impairment. Patients with VZV meningitis have not only cognitive impairment, but also changes in cellular metabolism in the bilateral hippocampus and neuronal dysfunction.
VZV meningitis is one of the common CNS complications caused by VZV reactivation. The pathophysiological mechanism of VZV meningitis can be related to direct virus infection, persistent inflammation, hematogenous invasion of the virus, and virus-induced hypercoagulability. [2,4,27,28]. At present, most scholars believe that its main mechanism is vasculitis. The present study showed that the WBC count and protein content of the CSF in patients with VZV meningitis were negatively correlated with the NAA/Cr ratio. The increased WBC count and protein content in the CSF of patients with VZV meningitis was mainly caused by the damage of the blood-brain barrier due to an inflammatory reaction and increased permeability. Our results indicate that after the reactivation of VZV, the inflammatory reaction caused the damage of hippocampal neurons, further leading to cognitive impairment. An inflammatory reaction may have an important role in the mechanism of CNS injury caused by VZV reactivation.
Our study also had some limitations. First, our research was conducted in a single center study and the sample size was small. Second, to assess the cognitive impairment of patients, we used the MoCA scale, which is mainly a screening tool lacking comprehensive details. Third, the detection time points of MRS and MoCA were single and not dynamic, and it is necessary to detect their changes dynamically in prospective case-cohort studies to make their direct relationship clear.
Conclusions
The patients with VZV meningitis in the acute phase had changes in cellular metabolism in the bilateral hippocampus and cognitive impairment mainly involving visual-spatial executive function and delayed recall. Cognitive impairment in patients with VZV meningitis may be due to changes in the hippocampal cellular metabolism, caused by an inflammatory reaction, which may lead to cognitive impairment in VZV meningitis patients. Further prospective studies are needed to confirm our results.
Figures
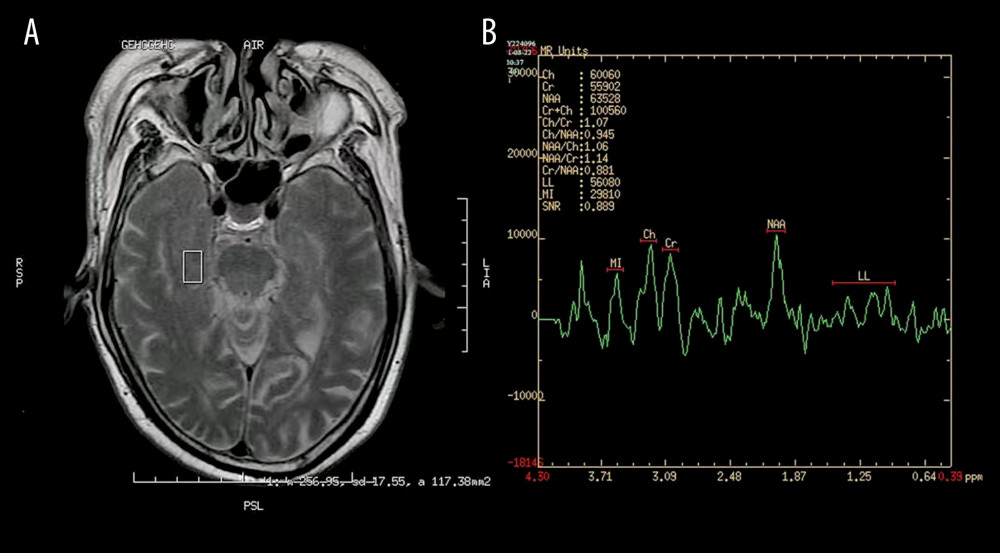 Figure 1. Images of magnetic resonance spectroscopy. (A) Region of interest (ROC); (B) right hippocampus 1H-MRS.
Figure 1. Images of magnetic resonance spectroscopy. (A) Region of interest (ROC); (B) right hippocampus 1H-MRS. 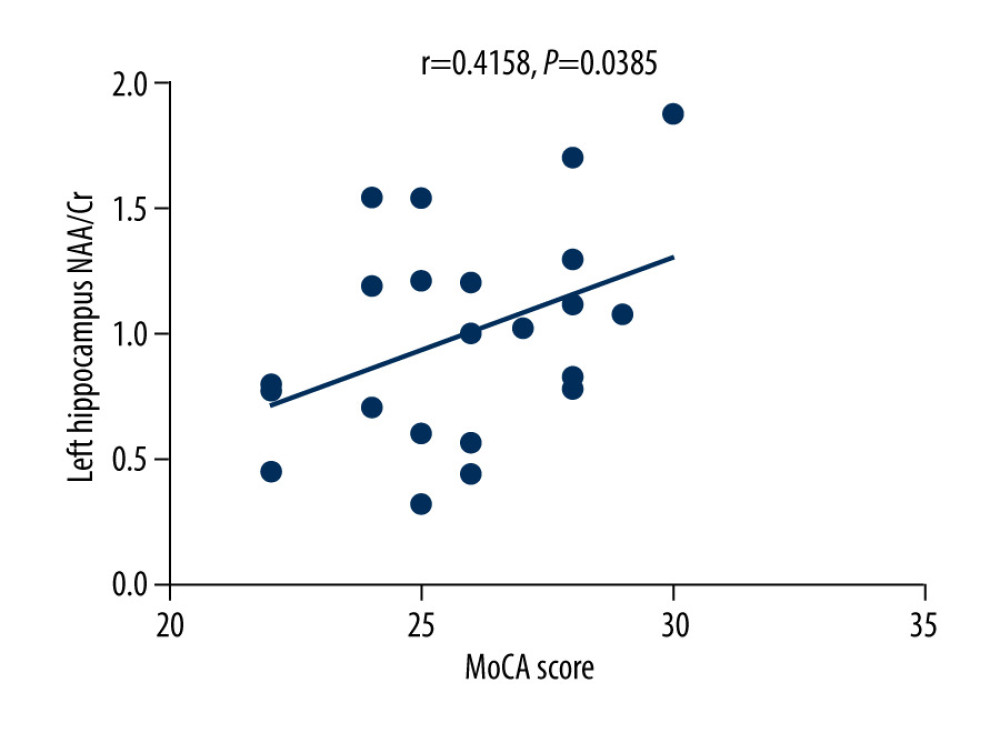 Figure 2. There was a positive correlation between Montreal Cognitive Assessment score and NAA/Cr ratio in the left hippocampus in patients with varicella zoster virus meningitis (r=0.4158, P=0.0385).
Figure 2. There was a positive correlation between Montreal Cognitive Assessment score and NAA/Cr ratio in the left hippocampus in patients with varicella zoster virus meningitis (r=0.4158, P=0.0385). 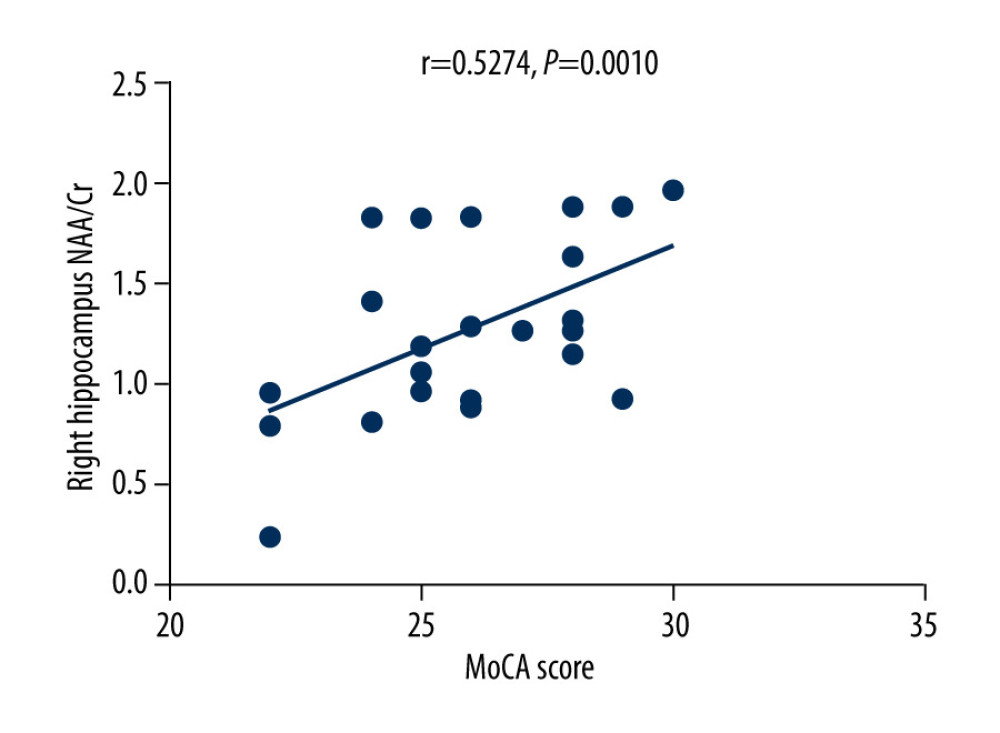 Figure 3. There was a positive correlation between Montreal Cognitive Assessment score and NAA/Cr ratio in the right hippocampus in patients with varicella zoster virus meningitis (r=0.5274, P=0.0010).
Figure 3. There was a positive correlation between Montreal Cognitive Assessment score and NAA/Cr ratio in the right hippocampus in patients with varicella zoster virus meningitis (r=0.5274, P=0.0010). 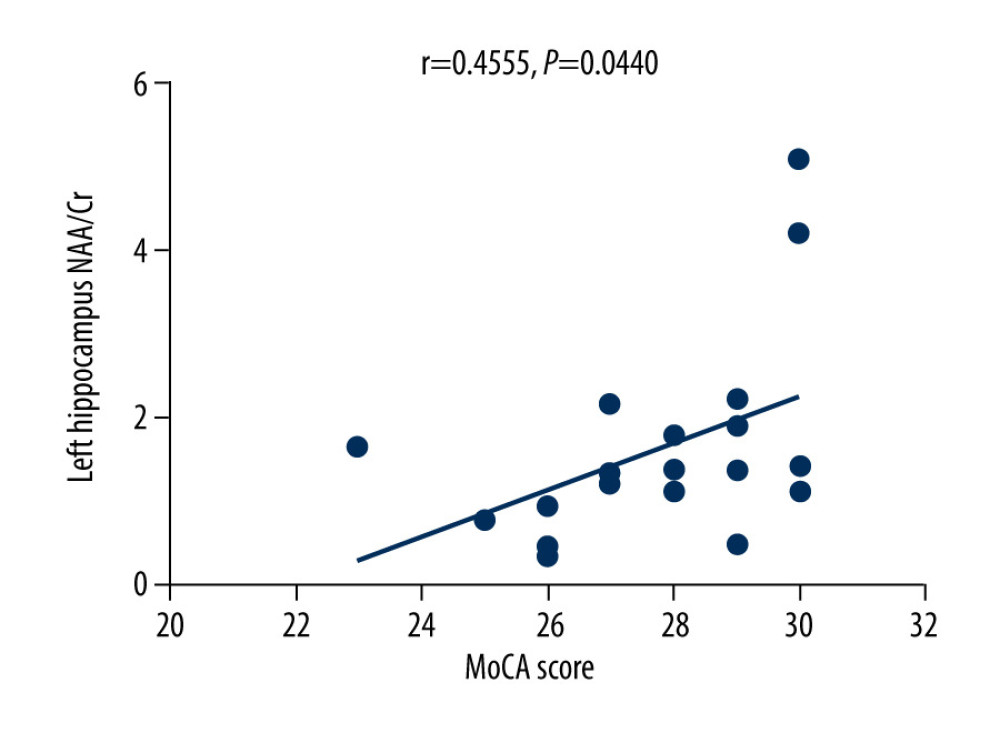 Figure 4. There was a positive correlation between Montreal Cognitive Assessment score and NAA/Cr ratio in the left hippocampus in the control group (r=0.4555, P=0.0440).
Figure 4. There was a positive correlation between Montreal Cognitive Assessment score and NAA/Cr ratio in the left hippocampus in the control group (r=0.4555, P=0.0440). 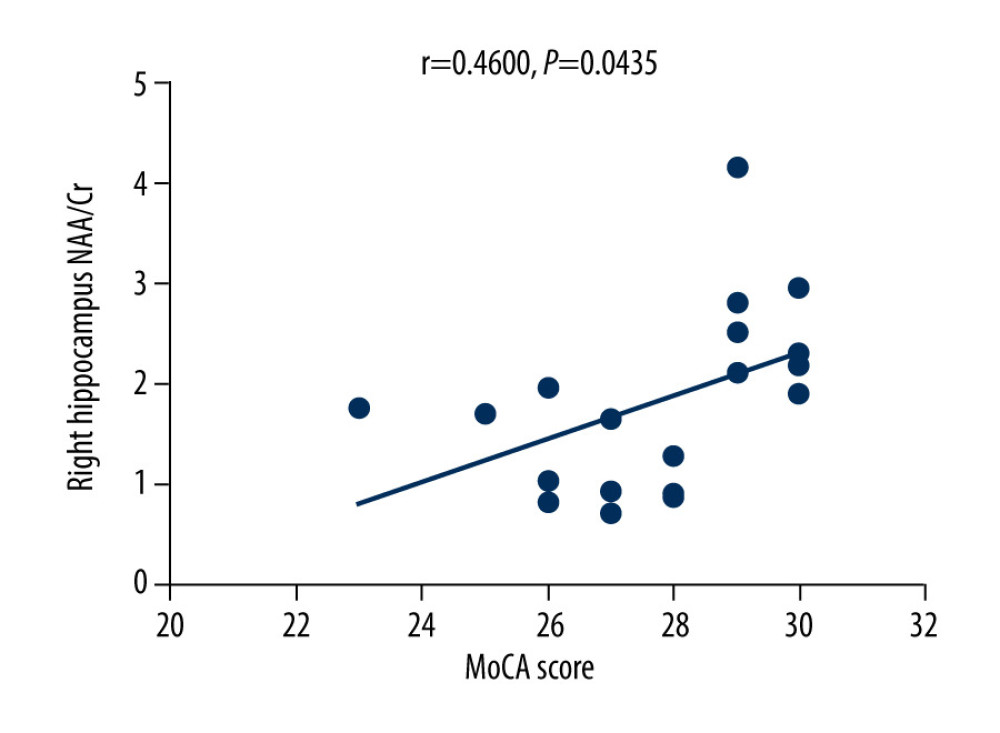 Figure 5. There was a positive correlation between Montreal Cognitive Assessment score and NAA/Cr ratio in the right hippocampus in the control group (r=0.4600, P=0.0435).
Figure 5. There was a positive correlation between Montreal Cognitive Assessment score and NAA/Cr ratio in the right hippocampus in the control group (r=0.4600, P=0.0435). 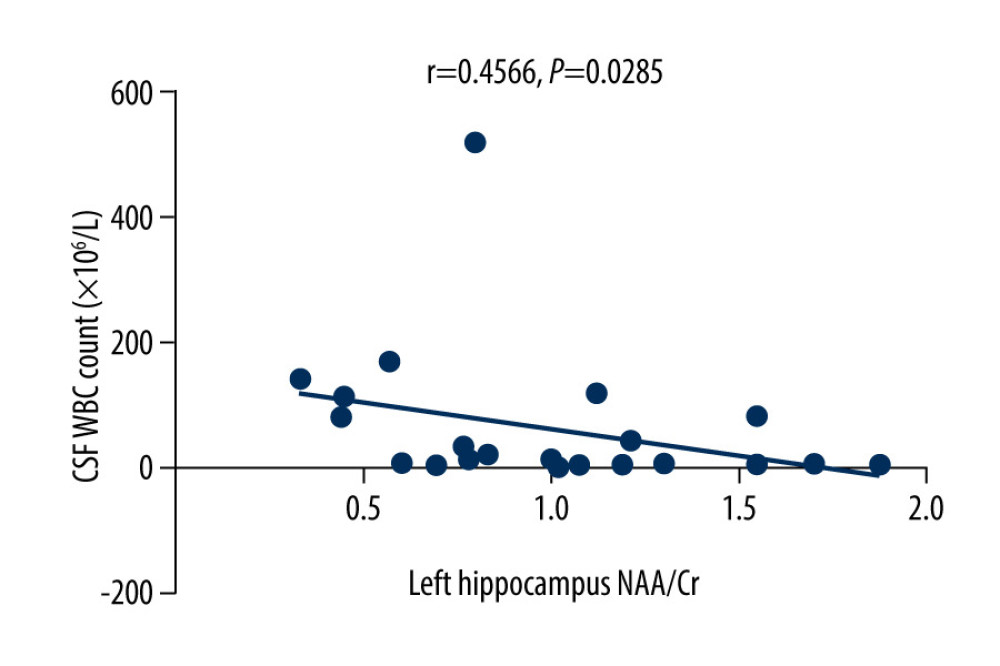 Figure 6. There was a negative correlation between the NAA/Cr ratio in the left hippocampus and the WBC count in the cerebrospinal fluid cerebrospinal fluid of patients with varicella zoster virus meningitis (r=−0.4566, P=0.0285).
Figure 6. There was a negative correlation between the NAA/Cr ratio in the left hippocampus and the WBC count in the cerebrospinal fluid cerebrospinal fluid of patients with varicella zoster virus meningitis (r=−0.4566, P=0.0285). 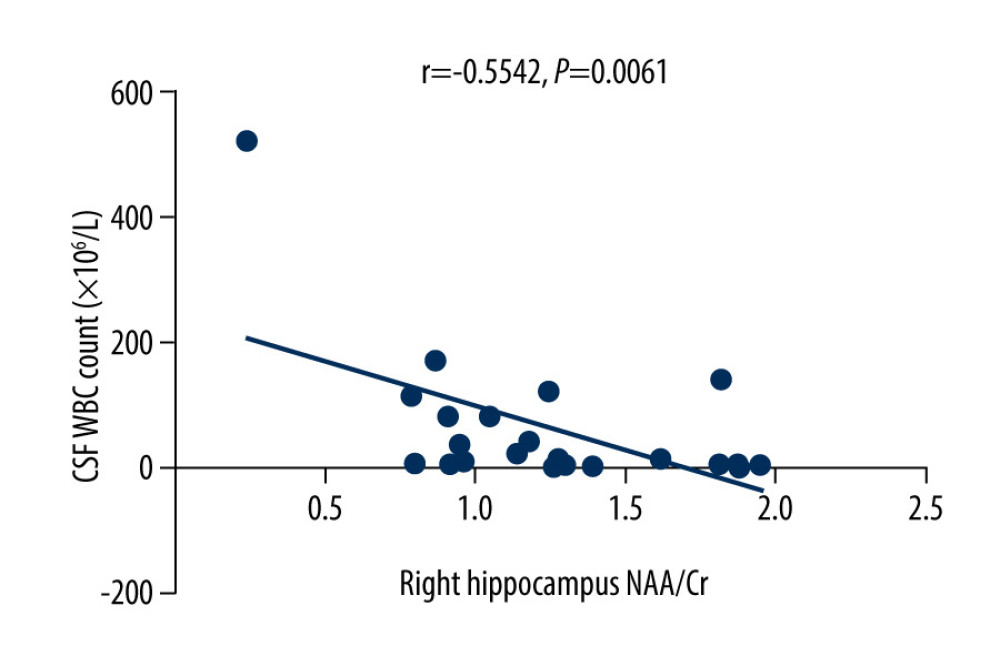 Figure 7. There was a negative correlation between NAA/Cr ratio in the right hippocampus and the WBC count in the cerebrospinal fluid of patients with varicella zoster virus meningitis (r=−0.5542, P=0.0061).
Figure 7. There was a negative correlation between NAA/Cr ratio in the right hippocampus and the WBC count in the cerebrospinal fluid of patients with varicella zoster virus meningitis (r=−0.5542, P=0.0061). 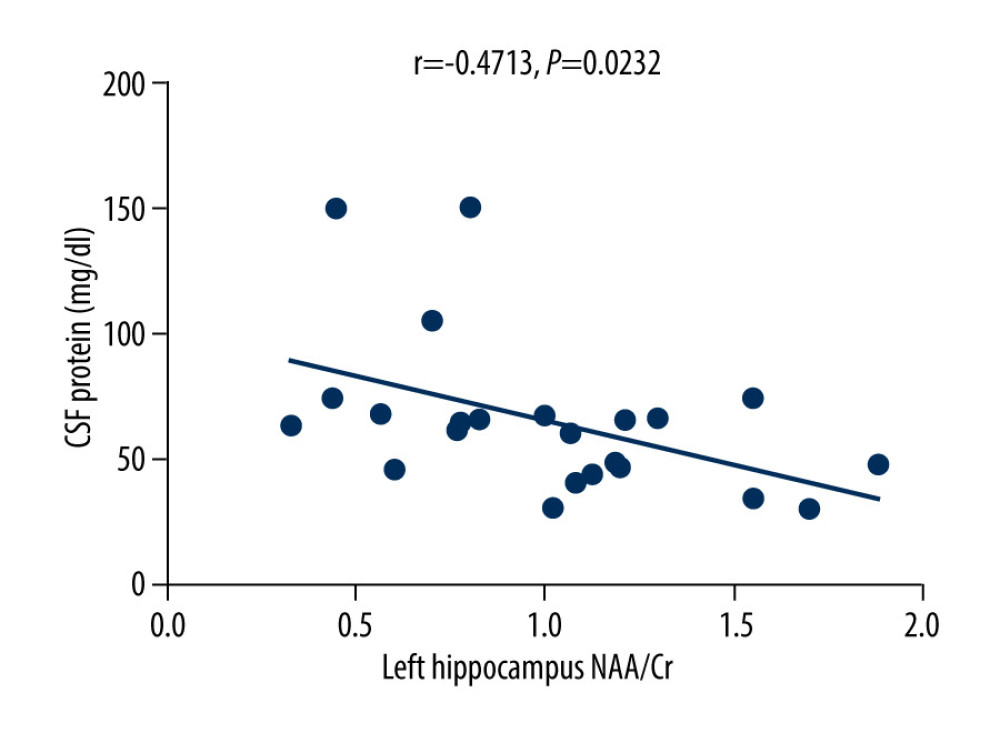 Figure 8. There was a negative correlation between NAA/Cr ratio in the left hippocampus and the protein content in the cerebrospinal fluid of patients with varicella zoster virus meningitis (r=−0.4713, P=0.0232).
Figure 8. There was a negative correlation between NAA/Cr ratio in the left hippocampus and the protein content in the cerebrospinal fluid of patients with varicella zoster virus meningitis (r=−0.4713, P=0.0232). 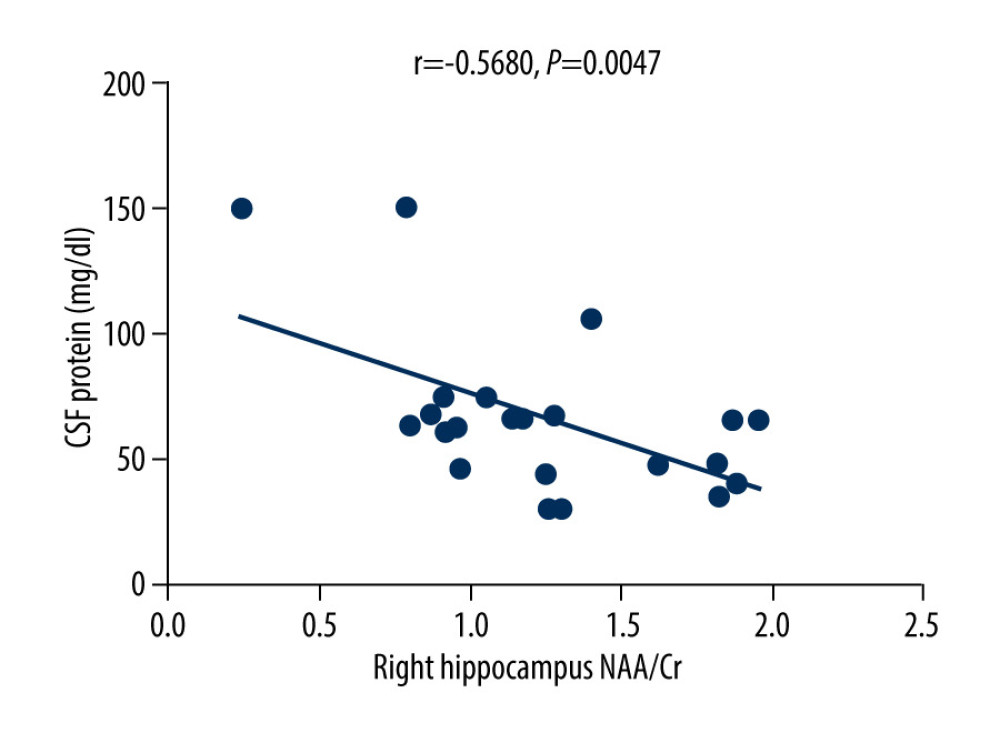 Figure 9. There was a negative correlation between NAA/Cr ratio in the right hippocampus and the protein content in the cerebrospinal fluid of patients with varicella zoster virus meningitis (r=−0.5680, P=0.0047).
Figure 9. There was a negative correlation between NAA/Cr ratio in the right hippocampus and the protein content in the cerebrospinal fluid of patients with varicella zoster virus meningitis (r=−0.5680, P=0.0047). References
1. Schmader K, Herpes zoster: Clin Geriatr Med, 2016; 32; 539-53
2. Weitzman D, Shavit O, Stein M, A population based study of the epidemiology of herpes zoster and its complications: J Infect, 2013; 67; 463-69
3. John AR, Canaday DH, Herpes zoster in the older adult: Infect Dis Clin North Am, 2017; 31(4); 811-26
4. van Oorschot D, Vroling H, Bunge E, Diaz-Decaro J, A systematic literature review of herpes zoster incidence worldwide: Hum Vaccin Immunother, 2021; 17(6); 1714-32
5. Greicius MD, Krasnow B, Boyett-Anderson JM, Regional analysis of hippocampal activation during memory encoding and retrieval: fMRI study: Hippocampus, 2003; 13; 164-74
6. Grahn A, Nilsson S, Nordlund A, Cognitive impairment 3 years after neurological Varicella-zoster virus infection: A long-term case control study: J Neurol, 2013; 260(11); 2761-69
7. Lopatko Lindman K, Hemmingsson ES, Weidung B, Herpesvirus infections, antiviral treatment, and the risk of dementia – a registry-based cohort study in Sweden: Alzheimers Dement (N Y), 2021; 7; e12119
8. Tzeng NS, Chung CH, Lin FH, Anti-herpetic medications and reduced risk of dementia in patients with herpes simplex virus infections-a nationwide, population-based cohort study in Taiwan: Neurotherapeutics, 2018; 15; 417-29
9. Wang J, Yuan YR, Liu HL, Cellular metabolism changes in bilateral hippocampus in patients with herpes zoster: Am J Transl Res, 2022; 14; 3980-87
10. McGill F, Griffiths MJ, Bonnett LJ, Incidence, aetiology, and sequelae of viral meningitis in UK adults: A multicentre prospective observational cohort study: Lancet Infect Dis, 2018; 18; 992-1003
11. Schiess N, Groce NE, Dua T, The impact and burden of neurological sequelae following bacterial meningitis: A narrative review: Microorganisms, 2021; 9; 900
12. Ene L, Marcotte TD, Umlauf A, Latent toxoplasmosis is associated with neurocognitive impairment in young adults with and without chronic HIV infection: J Neuroimmunol, 2016; 299; 1-7
13. Gabutti G, Valente N, Kuhdari P, Prevention of herpes zoster and its complications: From the clinic to the real-life experience with the vaccine: J Med Microbiol, 2016; 65(12); 1363-69
14. Shikova E, Kumanova A, Tournev I, Varicella zoster virus infection in neurological patients in Bulgaria: J Neurovirol, 2021; 27(2); 272-78
15. Schmidt H, Heimann B, Djukic M, Neuropsychological sequelae of bacterial and viral meningitis: Brain, 2006; 129; 333-45
16. Damsgaard J, Hjerrild S, Andersen H, Leutscher PDC, Long-term neuropsychiatric consequences of aseptic meningitis in adult patients: Infect Dis (Lond), 2015; 47; 357-63
17. Ungureanu A, van der Meer J, Bicvic A, Meningitis, meningoencephalitis and encephalitis in Bern: An observational study of 258 patients: BMC Neurol, 2021; 21; 474
18. Schmand B, de Bruin E, de Gans J, van de Beek D, Cognitive functioning and quality of life nine years after bacterial meningitis: J Infect, 2010; 61; 330-34
19. Ganaraja VH, Jamuna R, Nagarathna C, Long-term cognitive outcomes in tuberculous meningitis: Neurol Clin Pract, 2021; 11; e222-e31
20. Sittinger H, Müller M, Schweizer I, Merkelbach S, Mild cognitive impairment after viral meningitis in adults: J Neurol, 2002; 249; 554-60
21. Nasreddine ZS, Phillips NA, Bédirian V, The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment: J Am Geriatr Soc, 2005; 53(4); 695-99
22. Smith T, Gildeh N, Holmes C, The Montreal Cognitive Assessment: Validity and utility in a memory clinic setting: Can J Psychiatry, 2007; 52(5); 329-32
23. Pendlebury ST, Mariz J, Bull L, MoCA, ACE-R, and MMSE versus the National Institute of Neurological Disorders and Stroke-Canadian Stroke Network Vascular Cognitive Impairment Harmonization Standards Neuropsychological Battery after TIA and stroke: Stroke, 2012; 43(2); 464-69
24. Mandal PK, In vivo proton magnetic resonance spectroscopic signal processing for the absolute quantitation of brain metabolites: Eur J Radiol, 2012; 81; e653-64
25. Rosso LM, Crowley DJ, Silveri MM, Hippocampus glutamate and N-acetyl aspartate markers of excitotoxic neuronal compromise in posttraumatic stress disorder: Neuropsychopharmacology, 2017; 42; 1698-705
26. Walecki J, Barcikowska M, Ćwikła JB, Gabryelewicz T, N-acetylaspartate, choline, myoinositol, glutamine and glutamate (glx) concentration changes in proton MR spectroscopy (1H MRS) in patients with mild cognitive impairment (MCI): Med Sci Monit, 2011; 17; MT105-11
27. Gilden D, White T, Khmeleva N, Prevalence and distribution of VZV in temporal arteries of patients with giant cell arteritis: Neurology, 2015; 84; 1948-55
28. Koskiniemi M, Piiparinen H, Rantalaiho T, Acute central nervous system complications in varicella zoster virus infections: J Clin Virol; 25; 293-301
Figures
 Figure 1. Images of magnetic resonance spectroscopy. (A) Region of interest (ROC); (B) right hippocampus 1H-MRS.
Figure 1. Images of magnetic resonance spectroscopy. (A) Region of interest (ROC); (B) right hippocampus 1H-MRS. Figure 2. There was a positive correlation between Montreal Cognitive Assessment score and NAA/Cr ratio in the left hippocampus in patients with varicella zoster virus meningitis (r=0.4158, P=0.0385).
Figure 2. There was a positive correlation between Montreal Cognitive Assessment score and NAA/Cr ratio in the left hippocampus in patients with varicella zoster virus meningitis (r=0.4158, P=0.0385). Figure 3. There was a positive correlation between Montreal Cognitive Assessment score and NAA/Cr ratio in the right hippocampus in patients with varicella zoster virus meningitis (r=0.5274, P=0.0010).
Figure 3. There was a positive correlation between Montreal Cognitive Assessment score and NAA/Cr ratio in the right hippocampus in patients with varicella zoster virus meningitis (r=0.5274, P=0.0010). Figure 4. There was a positive correlation between Montreal Cognitive Assessment score and NAA/Cr ratio in the left hippocampus in the control group (r=0.4555, P=0.0440).
Figure 4. There was a positive correlation between Montreal Cognitive Assessment score and NAA/Cr ratio in the left hippocampus in the control group (r=0.4555, P=0.0440). Figure 5. There was a positive correlation between Montreal Cognitive Assessment score and NAA/Cr ratio in the right hippocampus in the control group (r=0.4600, P=0.0435).
Figure 5. There was a positive correlation between Montreal Cognitive Assessment score and NAA/Cr ratio in the right hippocampus in the control group (r=0.4600, P=0.0435). Figure 6. There was a negative correlation between the NAA/Cr ratio in the left hippocampus and the WBC count in the cerebrospinal fluid cerebrospinal fluid of patients with varicella zoster virus meningitis (r=−0.4566, P=0.0285).
Figure 6. There was a negative correlation between the NAA/Cr ratio in the left hippocampus and the WBC count in the cerebrospinal fluid cerebrospinal fluid of patients with varicella zoster virus meningitis (r=−0.4566, P=0.0285). Figure 7. There was a negative correlation between NAA/Cr ratio in the right hippocampus and the WBC count in the cerebrospinal fluid of patients with varicella zoster virus meningitis (r=−0.5542, P=0.0061).
Figure 7. There was a negative correlation between NAA/Cr ratio in the right hippocampus and the WBC count in the cerebrospinal fluid of patients with varicella zoster virus meningitis (r=−0.5542, P=0.0061). Figure 8. There was a negative correlation between NAA/Cr ratio in the left hippocampus and the protein content in the cerebrospinal fluid of patients with varicella zoster virus meningitis (r=−0.4713, P=0.0232).
Figure 8. There was a negative correlation between NAA/Cr ratio in the left hippocampus and the protein content in the cerebrospinal fluid of patients with varicella zoster virus meningitis (r=−0.4713, P=0.0232). Figure 9. There was a negative correlation between NAA/Cr ratio in the right hippocampus and the protein content in the cerebrospinal fluid of patients with varicella zoster virus meningitis (r=−0.5680, P=0.0047).
Figure 9. There was a negative correlation between NAA/Cr ratio in the right hippocampus and the protein content in the cerebrospinal fluid of patients with varicella zoster virus meningitis (r=−0.5680, P=0.0047). Tables
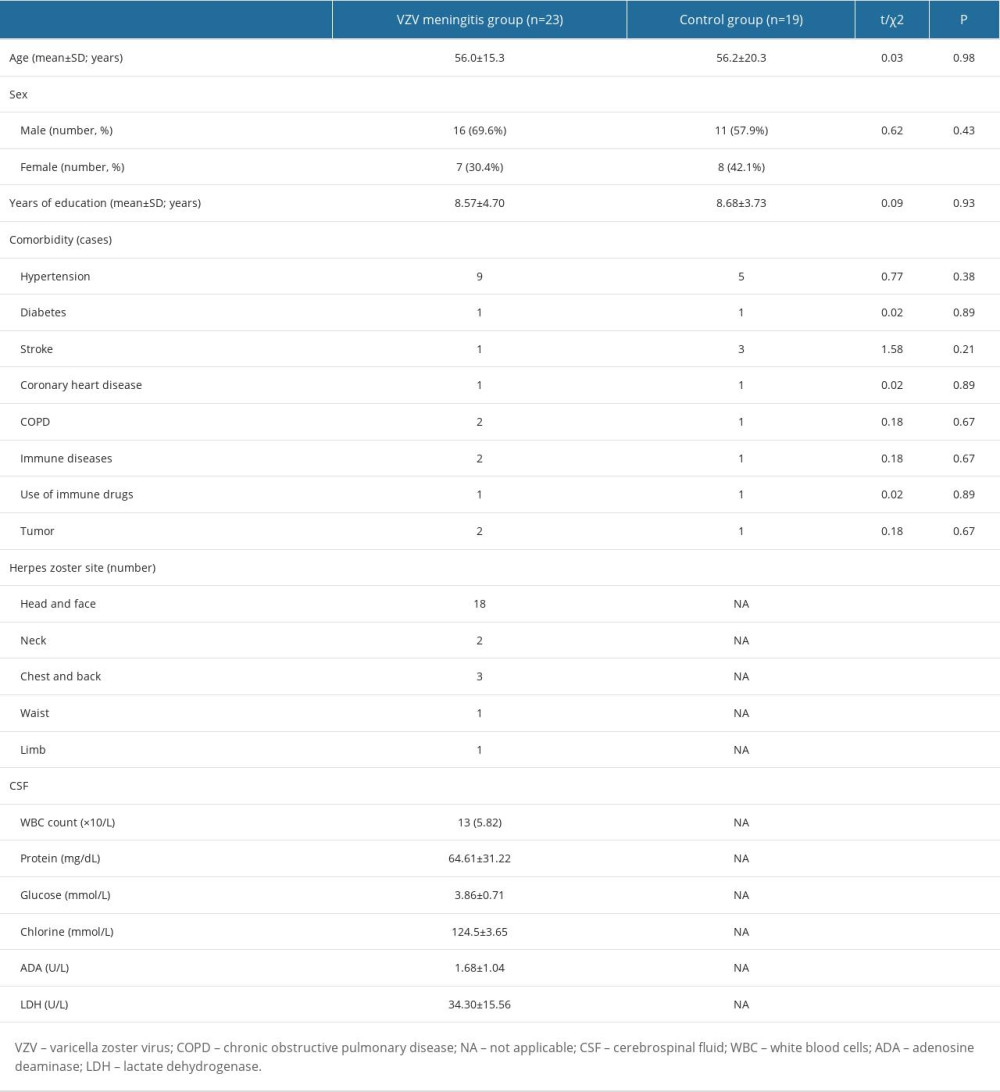 Table 1. Baseline characteristics of patients in the 2 groups on admission.
Table 1. Baseline characteristics of patients in the 2 groups on admission.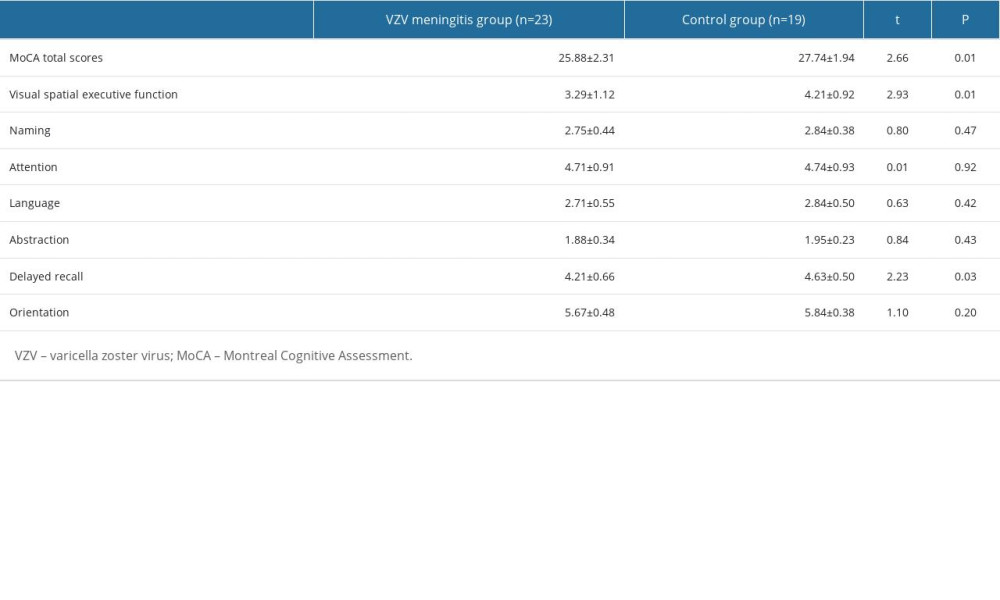 Table 2. Comparison of cognitive function scores between the 2 groups (mean±SD).
Table 2. Comparison of cognitive function scores between the 2 groups (mean±SD).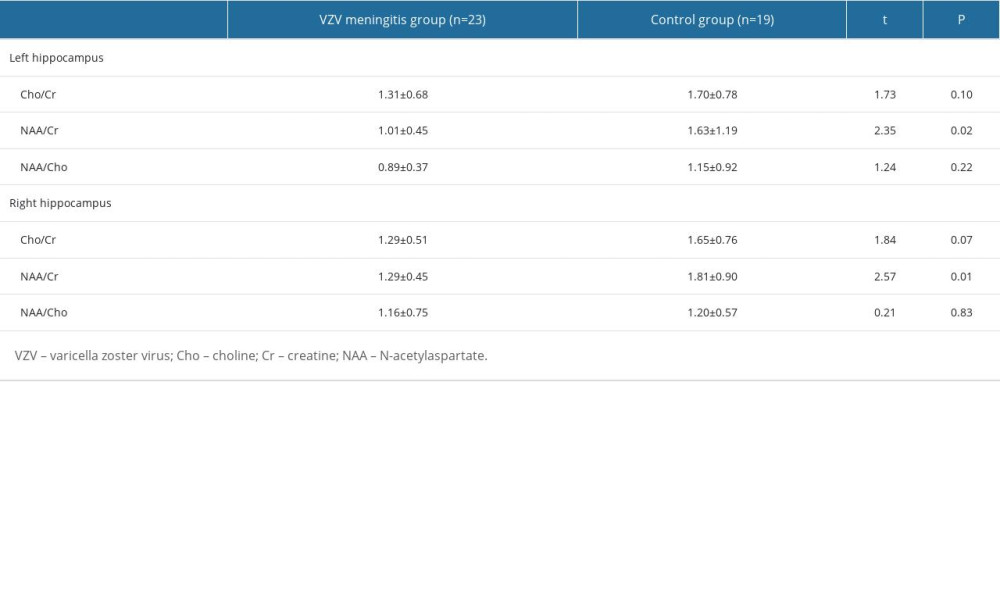 Table 3. Comparison of hippocampus magnetic resonance spectroscopy between the 2 groups (mean±SD).
Table 3. Comparison of hippocampus magnetic resonance spectroscopy between the 2 groups (mean±SD). Table 1. Baseline characteristics of patients in the 2 groups on admission.
Table 1. Baseline characteristics of patients in the 2 groups on admission. Table 2. Comparison of cognitive function scores between the 2 groups (mean±SD).
Table 2. Comparison of cognitive function scores between the 2 groups (mean±SD). Table 3. Comparison of hippocampus magnetic resonance spectroscopy between the 2 groups (mean±SD).
Table 3. Comparison of hippocampus magnetic resonance spectroscopy between the 2 groups (mean±SD). In Press
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








