14 October 2023: Clinical Research
Influence of Age, Gender, Frailty, and Body Mass Index on Serum IL-17A Levels in Mature Type 2 Diabetic Patients
Zvonimir Bosnić1ABEF, František BabičDOI: 10.12659/MSM.940128
Med Sci Monit 2023; 29:e940128
Abstract
BACKGROUND: The cytokine IL-17A is emerging as a marker of chronic inflammation in cardio-metabolic conditions. This study aimed to identify relevant factors that in older primary care patients with type 2 diabetes (T2D) could influence serum IL-17A concentrations. The results have a potential to improve risk stratification and therapy options for these patients.
MATERIAL AND METHODS: The study was conducted during a period of 4 months, in 2020, in the south-eastern region of Croatia. Patients from primary health care, diagnosed with T2D (N=170, M: F 75: 95, ≥50 years old), were recruited at their visits. Those with malignant diseases, on chemotherapy or biological therapy, with amputated legs, or at hemodialysis, were excluded. The multinomial regression models were used to determine independent associations of the groups of variables, indicating sociodemographic and clinical characteristics of these patients, with increasing values (quartiles) of serum IL-17A.
RESULTS: The regression models indicated the frailty index and sex bias are the key modifying factors in associations of other variables with IL-17A serum values.
CONCLUSIONS: Sex bias and the existence of different frailty phenotypes could be the essential determining factors of the serum IL-17A levels in community-dwelling patients with T2D age 50 years and older. The results support the concept of T2D as a complex disorder.
Keywords: adaptive immunity, Aging, Diabetes Mellitus, Type 2, Inflammation, Interleukin-17, Humans, Aged, Middle Aged, frailty, Body Mass Index, Cytokines
Background
Chronic inflammation is considered the main driving force in accelerated aging; that is, aging burdened with cardio-metabolic conditions, such as type 2 diabetes (T2D), atherosclerosis, chronic heart disease (CHD) and chronic kidney disease (CKD), and other age-related chronic conditions [1]. The main sources of inflammation in older individuals are senescent cells and a chronically activated innate immune system. Various stimuli, like molecules that are released from damaged tissues and disturbed gut microbiota, can trigger receptors of the innate immune system, leading to increased production of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), IL-18, and IL-6 [2,3]. Obesity exacerbates age-related inflammation. This is mainly the effect of pro-inflammatory cytokines and other pro-inflammatory mediators produced by dysfunctional adipocytes and monocyte/macrophages that abundantly infiltrate adipose tissue of obese individuals [1,4]. In addition, metabolic intermediates, produced in excess in obesity and obesity-related conditions, including metabolic syndrome (MS) (a cluster of metabolic disorders and hypertension, associated with abdominal-type obesity) and T2D, such as free fatty acids, advanced glycation end-products, and oxidized lipoproteins, have been recognized as strong pro-inflammatory signals [2]. The pro-inflammatory cytokines induce insulin resistance (impaired insulin-mediated glucose utilization in metabolically active tissues), which further exacerbates metabolic disorders and inflammation, leading to the accelerated atherosclerosis and target organ damage [1].
In addition to secretory active macrophages, many other immune cells have been found in obesity-related adipose tissue [4,5]. Thus, the number of pro-inflammatory CD8+ T and CD4+ Th1 lymphocytes is increased, while the number of anti-inflammatory CD4+ Th2 lymphocytes is decreased, compared to adipose tissue of non-obese individuals. Recent evidence suggests that obesity-related pathologies, which are usually characterized by a high level of insulin resistance, including MS, hypertension, T2D, and their related vascular complications and target organ disease, and which play a major role in maintaining inflammation, involve pro-inflammatory CD4+ Th17 lymphocytes [6–10]. The role of Th17 lymphocytes in sustaining chronic inflammation has already been recognized in autoimmune and other inflammation-mediated diseases, such as inflammatory bowel disease, osteoarthritis, and periodontitis [6,11].
In the inflamed tissue microenvironment, the focus is turning from the predomination of anti-inflammatory T regulatory (Treg) lymphocytes toward a predomination of the pro-inflammatory Th1/Th17 pathway, which is associated with increased production of cytokines of the IL-17 family, known as a driver in maintaining tissue inflammation and damage [12,13]. Besides changes in the cytokine profile, changes in metabolic conditions can also shift the balance between Treg and Th17 cell lines. Both of these cell lines have a high level of adaptability to conditions in the microenvironment, which allows functional adaptation of the immune system to variations in physiological situations [14,15]. Th17/Treg polarization is imperfect, which makes inflammation and tissue remodeling/fibrosis stay even, with the balance oscillating between the predomination of either of these processes. For example, besides increased production of anti-inflammatory cytokines, an expansion of Treg is also associated with increased production of Transforming Growth Factor Beta (TGF-β), which is a major fibrotic factor [10].
The cytokine IL-17A is the most thoroughly investigated member of the IL-17 cytokine family, and its role in development of cardiovascular disease (CVD) and target organ damage has been demonstrated in both experimental and clinical conditions [13,16–18]. Some of the proposed mechanisms include increased mobilization of inflammatory and immune cells (eg, monocytes, neutrophils, and T lymphocytes) from circulation to tissues, increased production of pro-inflammatory molecules such as cytokines, chemokines, and adhesion molecules, and induction of extra-cellular matrix degradation and tissue fibrosis [19,20]. As in autoimmune diseases, neutrophils play a significant role in tissue damage caused by inflammation in cardio-metabolic disorders [21]. The therapeutic potential of anti-IL-17A antibodies for curing cardio-metabolic disorders has been demonstrated in animal models [19].
However, before routine implementation of IL-17A for diagnostic and therapeutic purposes in patients with T2D and CVD, it is necessary to understand factors that influence its serum level variations in these patients. This is important because, unlike in autoimmune diseases, where polymorphism of genes involved in immune reaction is the principal determinant of the clinical phenotype, cardio-metabolic disorders are complex, which means that share a common pathophysiology background, and that a variety of sociodemographic, lifestyle, and environmental factors contribute to inter-individual variations in disease expression [22,23]. In addition, evidence from experimental studies indicates involvement of this cytokine in various signaling cascades in both homeostatic (regulatory) and pathological pathways, so its regulation is highly context-dependent [24]. Besides the number of Th17 lymphocytes, local expression of IL-17A also depends on the size of the network of innate immune and residential tissue cells that in damaged tissue acquire the ability to produce IL-17A [25]. Ultimately, serum IL-17A concentrations depend on the net-effect of the synergistic and antagonistic signals from different organs and tissues [24]. For example, previous clinical studies investigating the classical inflammatory markers C-reactive protein (CRP) and neutrophil-to-lymphocyte ratio (NLR) have shown that particular sociodemographic and clinical factors affect their serum concentrations [26–28].
It is especially important to understand how particular factors influence serum IL-17A levels in older patients with T2D. The fact that T2D is mostly an age-related disease contributes to the complexity of these patients, by increasing their potential for development of multiple comorbidities and geriatric conditions such as sarcopenia, malnutrition, and frailty [29]. These conditions can change the body shape and thus also affect metabolic, inflammatory, and hormonal parameters, while increasing the inter-individual heterogeneity [30]. For example, in contrast to the conventional belief that only T2D duration and the quality of hyperglycemia control determine the outcomes in these patients, results of large epidemiologic studies suggest the importance of other factors such as current patient age and age at T2D onset [31]. In addition, frailty is emerging as being a major modulator of outcomes in diabetic patients [32]. Frailty is a progressive disorder (classified as pre-frailty and frailty), considered to be a result of exhaustion of homeostatic mechanisms, with nonspecific symptoms and signs such as sarcopenia (muscle loss), slow walking, weakness, and low activity levels [33]. The prevalence of frailty increases with age and the level of comorbidity and is higher in women than in men [34,35]. T2D and its related comorbidites – MS, CVD, and CKD – are strongly associated with frailty [36–38]. The pathogenesis of frailty is not well understood, and chronic inflammation is considered one of the several mechanisms involved [39]. There are no data showing how frailty relates to serum IL-17A levels in older diabetic patients.
Therefore, the aim of this study was to explore the relevance of sociodemographic and clinical factors in determining serum levels of IL-17A in patients with T2D who are age 50 years and older. The results are expected to inform future clinical studies and to accelerate implementation of the cytokine IL-17A in prediction of outcomes and treatment schemes in these patients. Of particular importance for clinical practice would be understanding the relationships between frailty and serum IL-17A levels in the context of other factors, especially when considering recent evidence on the existence of at least 2 well-defined metabolic frailty phenotypes – one associated with obesity and the other associated with weight loss [40].
Material and Methods
ETHICS STATEMENT:
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Expert and Ethics Council of the Health Centre Slavonski Brod, Croatia (ID: 1433-1/020).
STUDY DESIGN AND PARTICIPANTS:
The study was conducted during a 4-month period in 2020 at the Health Centre of Slavonski Brod, a town in the south-eastern part of Croatia, with a population of about 50 000. We included only patients diagnosed with T2D and on therapy with antiglycemic agents, age 50 years and older, and of both sexes. They were recruited from 4 general practice offices out of a total of 20 that exist in this health center, as their general practitioners (GPs) agreed to participate in the study. This selection process did not, however, hamper the representativeness of the sample, because GPs in Croatia have a gatekeeping role, and almost all adults are registered with a GP. These 4 selected GP offices covered a total number of about 7000 adults. In addition, older people living in the area have similar living conditions and are generally of lower socioeconomic status.
The fact that participating GPs were all specialists, with at least of 7 years of working experience, secured the high level of accuracy of the collected data. The fact that the GPs all worked in the same health center means that they used similar professional vocabulary and content meaning of encoded terminology, which is important for data consistency. For diagnosis and treatment criteria for T2D, GPs in Croatia follow the guidelines of the European Society of Cardiology (ESC) and the European Association of the Study of Diabetes (EASD) [41]. Patient management is supported by the ICT system and electronic health records (eHRs). Although the general health check-ups for people aged 45–65 years include assessment of major cardiovascular (CV) risk factors, performed as a “fee-for-service reimbursement program” that has been in place for many years, screening for T2D is not fully successful, and the rate of undiagnosed diabetic patients in Croatia reaches 40% [42].
We used the age threshold of 50 years as a criterion for patient selection because the evidence suggests that the incidence rate of T2D is highest in people age 45–64 years [43]. Only patients who were able to come independently or with assistance to their GPs were included in the study. Exclusion criteria were acute health conditions, malignant diseases, autoimmune system diseases, and the use of corticosteroids or being on biological treatments. We also excluded patients with visible cognitive dysfunctions, leg amputation, had a transplanted kidney, and those who were on continuous renal replacement therapy.
The power analysis was performed to determine the study size. With the significance level of 0.05 and power of 0.8, and the moderate effect size of f2=0.15, the estimated sample size was 180 participants (G*Power, 3.1.9.4.). Patients were recruited during their regular GP visits. GPs explained the purpose of the study and the study protocol to all eligible patients, and asked for signed informed consent. The study lasted until the required number of subjects was reached. Some patients did not go to the laboratory to provide blood for testing, and we made a slight adjustment to keep participant numbers within the capacity of 1 cytokine kit, so that the final number of participants included in data analysis was 170 (M: F, 75: 95).
DATASET:
Participants were thoroughly described by using 62 variables (Table 1A, 1B). Variables were selected to indicate: patient age and sex, smoking habits, anthropometric measures, MS, the number of comorbidities, diagnoses of CVD, the main groups of other chronic diseases, some common geriatric conditions such as malnutrition and frailty, the total number of prescribed drugs, the prescription rates of the main groups of antidiabetic and antihypertensive drugs, the hypolipidemic drug statins, and non-steroidal anti-inflammatory drugs (NSAIDs), as these are the most widely prescribed anti-inflammatory drugs [44].
Regarding selection of prescribed drugs, special focus was put on newly recommended cardio- and renal-protective antidiabetic drugs, glucagon-like peptide-1 receptor agonist (GLP1ra), and sodium-glucose cotransporter-2 inhibitors (SGLT2inh), for their potential anti-inflammatory effects [41,45]. An old-fashioned first-line antidiabetic drug, metformin, and statins are widely prescribed to diabetic patients, and for both drugs, besides their metabolic effects, direct involvement in IL-17A signaling has been identified [46,47]. Antidiabetic drugs of special interest due to their demonstrated effect in regulating the Th17/Treg balance also include dipeptidyl peptidase-4 inhibitors (DPP4inhs) [48,49]. Among antihypertensive drugs, the effect on serum IL-17A concentrations has been proposed for angiotensin-converting enzyme inhibitors (ACE-INHs) and angiotensin receptor blockers (ARBs) due to their effects on ameliorating tissue inflammation, which is mediated by cooperation of tissue-type angiotensin II and IL-17A [13].
Anthropometric measures included body mass index (BMI) (kg/m2), which is a measure of general obesity, waist circumference (wc), which is a measure of visceral obesity, and a marker of MS, and mid-arm circumference (mac), which is a measure of sarcopenia (muscle loss) [50]. Clinically relevant sarcopenia was defined as mac ≤22 cm [51].
To assess the nutritional status, we used the Mini Nutritional Assessment – Short Form (MNA-SF) test, a version with 18 questions [52]. This test examines patients’ eating habits, types of food consumed, number of long-term medications, and self-reported health conditions. The maximum number of points that can be scored is 30. A score ranging from 24 to 30 indicates good nutrition, 17 to 23 indicates a risk of malnutrition, and less than 17 points indicate malnutrition. The frailty status was assessed by using the Fried’s phenotype model [53], which considers 5 criteria – weight loss, feeling of exhaustion, low-level activity, slow walking, and hand grip strength measured using a hand dynamometer. Three to 5 positive criteria indicate frailty, 1 to 2 positive criteria indicate pre-frailty, and all negative criteria mean robustness. We also assessed participants on MS, following evidence indicating that diabetic patients with MS, compared to those without, have a higher predisposition for complications [54]. For this purpose, we used the modified definition of the National Cholesterol Education Program, Adult Treatment Panel III (NCP ATP III) [55]. The NCP ATP III definition of MS is at least 2 of the following: wc ≥102 cm (88 cm for F), triglycerides (TG) ≥1.7, HDL (high-density lipoprotein) cholesterol <1.0 (1.2 for F), and diagnosis of hypertension.
Some standard blood laboratory tests were performed to better characterize patient metabolic status, nutrition, and inflammation, and to determine the level of renal function decline. These tests included: erythrocyte number, leukocyte number, lymphocyte number and percentage, neutrophil number and percentage, hemoglobin (Hb), CRP, fasting glucose, glycosylated hemoglobin (HbA1c), total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, and serum creatinine, needed for estimation of glomerular filtration rate (eGFR), a measure of renal function decline [56]. HbA1c, a measure of average blood glucose in the last 2 or 3 months, indicates the quality of hyperglycemia control [57]. According to the EAS/EASD guidelines, the target HbA1c in diabetic patients should be, in general, <7%, but for older patients with comorbidities, less-stringent HbA1c goals, <8% or even ≤9%, may be adequate (26) [41]. The US National Kidney Foundation Guidelines recognize 4 stages of renal function decline (not counting for the terminal stage), where stage 2 (eGFR <90 >60 ml/min) indicates mild renal impairment, and stages 3 and 4 (eGFR of ≤60 ml/min) indicate moderately and severely decreased renal function [56]. In the laboratory panel test, we also included Thyroid-Stimulating Hormone (TSH), to detect latent hypothyroidism, a common disorder in older diabetic patients, and associated with metabolic disorders and increased risk of diabetic complications [58]. Latent hypothyroidism is indicated if TSH is ≥4 mU/L [59].
We used classical markers of inflammation, CRP, Hb, and neutrophil-lymphocyte ratio (NLR), to compare their levels of variation with that of IL-17A and their associations with IL-17A (Table 2) [60,61]. For variable Hb, evidence indicates that it can be considered as a marker of chronic inflammation associated with the presence of chronic health conditions, and as a predictor of frailty [62,63].
THE DATA COLLECTION PROCEDURE:
Data on age, sex, comorbidities, and treatment options were obtained from eHRs. In Croatia, the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10) is used for disease classification. Having ≥3 diagnoses of chronic diseases was considered as multimorbidity [35]. As CVD, we considered diagnoses of coronary artery disease (CAD), CHD, cerebrovascular disease, and periphery artery disease. Diabetic retinopathy was used as a microvascular complication, but it was not considered as CVD [57]. These diagnoses were recorded in eHRs if confirmed by the specialist examination. Information on grades of CHD is missing, because the heart ultrasound examination, necessary for grading, has not been systematicaly performed and recorded in eHRs [64]. From eHRs, we also used information on T2D and hypertension duration, as 2 frequently overlapping disorders, and checked it by talking with patients [65]. From T2D duration and patient age, we were able to conclude about the age at diagnosis of T2D, and this all information is prognostically important [31].
If not older than 6 months, data for anthropometric measurements was used from eHRs; otherwise, these measures were taken from participants during their visits. In addition, participants were asked about smoking habits and were evaluated for the presence of frailty, malnutrition, and MS. A detailed description of the screening procedure on frailty is provided in a previous publication [66].
LABORATORY TESTING:
Participants were referred to the county hospital’s central laboratory for laboratory testing. For this purpose, 10 ml of venous blood (2 Vacutainer tubes) was obtained from participants by cubital venipuncture. The clot was removed by centrifugation, and serum was stored at −20°C until analyzed. A part of serum was separated and stored at −70°C for the purpose of measuring IL-17A. These specimens were transported in the transporter refrigerator to the Laboratory for Clinical Immunology and Allergology Diagnostics of the University Hospital Centre of Osijek, the capital of eastern Croatia.
Erythrocyte and leukocyte numbers and hemoglobin were assesssed by a routine analysis using a Sysmex XN 1000 (SYSMEX UK LTD, Wymbush, Milton Keynes, GB) hematology analyzer. For counting leukocyte subpopulations, including the number of lymphocytes and neutrophils, the Coulter impedance method was used. The NLR was estimated from the complete blood count with differential [61].
Biochemical analyses were performed in the automated process analyzer DxC 700AU (Beckman Coulter, Fullerton, SAD). Lipid parameters, including total cholesterol, LDL and HDL cholesterol, and triglycerides, were determined by enzymatic staining. The enzymatic method was also used to determine serum creatinine. For assessing fasting blood glucose, the enzymatic UV test (with hexokinase) was used, while HbA1c was analyzed by the turbidimetric method. CRP was analyzed by the immunoturbidimetric method. To determine TSH, the chemiluminescent microparticle immunoassay was performed in an Alinity, Architect analyzer (Abbot Laboratories, Abbot Park, IL 60064, SAD). From information on serum creatinine, sex, and age, we calculated eGFR according to the Modification of Diet in Renal Disease (MDRD) formula, using the online calculation system of the US National Kidney Fundation [67].
DETERMINING SERUM IL-17A:
To analyze IL-17A, we used a high-sensitive IL-17A human ELISA kit (Invitrogen, ThermoFisher Scientific, SAD). Enzyme-linked immunosorbent assay (ELISA) was performed according to the manufacturer’s protocol. The company has stated the test’s sensitivity to be 4 pg/mL. Drawing from previous experiences in measuring IL-17A in human serum, we decided to add an additional calibration point, performed only with the puffer system and reagents, to allow determination of IL-17A at very low concentrations, starting from 0 pg/mL.
STATISTICAL ANALYSIS:
Numerical data are presented as the mean±standard deviation (SD) or as the median and interquartile range (IQR), depending on the type of distribution (standard or not). Categorical data are presented as absolute and relative frequencies. To examine distributions of different variables according to the increasing values of IL-17A, we divided a range value of IL-17A into quartiles.
The collinearity and multicollinearity were investigated for numerical attributes using correlation analysis (Spearman’s correlation coefficient) and variance inflation factor (VIF). A value of VIF between 1 and 5 indicates a moderate correlation between the given predictor variable and other predictor variables in the model, but this is often not severe enough to require attention. A value greater than 5 indicates a potentially strong correlation between the given predictor variable and other predictor variables in the model. In this case, the coefficient estimates and
Results
Participants were patients in primary health care, diagnosed with T2D, age 50 years and older, who were able to walk independently, and without severe conditions. They were selected by a consecutive sampling method. The sample was a good representation of older, community-dwelling, diabetic patients from the study area, since residents had good access to GPs, and GPs employ similar methods in screening and managing diabetic patients.
Most participants were age 50–75 years (median 66), with slightly more women than men. Almost equal proportions of participants had T2D of a short duration (0–5 years) and longer duration (>5 years). Most participants had hypertension (89.4%), mostly for a longer period (>5 years). Most participants were overweight/obese and most had abdominal (visceral) type of obesity. There was a high proportion of those with MS, especially among women. Many patients had multimorbidity. They were mostly in a good nutritional state, without signs of marked sarcopenia (none had mac ≤22 cm), and none were malnourished. About 43% of participants were pre-frail or frail, while fully frail individuals counted for about 20% of participants in the sample, with more women than men (Table 1A, 1B).
In most patients, HbA1c was <8.5% (median 6.9, ICR 1.7), indicating well-controlled hyperglycemia. Good metabolic control was confirmed also by LDL cholesterol, maintained in most patients within the recommended values of less than 5.0 mmol/L (mean 3.21, SD 1.04). Concerning CV complications, about one-third of patients were diagnosed with CAD, and almost a half of patients were diagnosed with CHD, although information on degree severity of CHD was missing. About two-thirds of participants had decreased renal function, but in most cases it was mild (eGFR 90–60 mL/min) or moderate (eGFR <60–45 mL/min). Of non-CV comorbidities, the most prevalent were musculoskeletal diseases and anxiety disorders (Table 1A, 1B).
Many patients had been prescribed ACE-INH/ARB antihypertensive drugs, the traditional antidiabetic drug metformin, and statins. Few participants were prescribed newer antidiabetic drugs such as GLP1ra and SGLT2inh. NSAID was prescribed to about two-thirds of participants (Table 1B).
Participants in the sample showed a narrow range of cytokine IL-17A (median 1.71, ICR 1.50 pg/mL), corresponding to its low variability. This was also a characteristic of the classical markers of inflammation, NLR and CRP (Table 2). The quartiles were calculated by the internal R function according to the standard rule: 1st quartile (25th percentile of the data under the produced value), 2nd quartile (50th percentile of the data under the produced value), and 3rd quartile (75th percentile of the data under the produced value). As shown in Figure 1, interquartile cut-offs of IL-17A were 1.42 pg/ml (between 1st and 2nd quartiles), 1.71 pg/ml (between 2nd and 3rd quartiles), and 2.92 pg/ml (between 3rd and 4th quartiles), indicating that the 2 middle quartiles (2nd and 3rd) were very close to each other, and that IL-17A was slightly higher in the upper quartile.
Correlation analyses (Table 3) showed that several variables were strongly correlated with each other. It is necessary to consider this information during the regression models specification (1 of the paired variables should not be used in prediction models). By VIF, we detected strong multicollinearity (>5) within the following variables: LDL (8.55), total cholesterol (9.36), neutrophils% (11.32), neutrophils (71.6), lymphocytes (63.84), lymphocytes% (10.42), and NLR (8.08). These variables were excluded from the respective models.
Variables showing significant differences among quartiles of IL-17A were those indicating metabolic disorders (LDL cholesterol and HbA1c), chronic inflammation associated with the burden of comorbidities (hemoglobin), CVD (CAD and CHD), and some non-CV chronic conditions (eg, gastrointestinal disorders, low back pain, urogenital disorders, incontinent urinary, and anxiety disorders). In addition, some drugs, such as newer oral antidiabetics (including DPP4inh, GLP-1ra, and SGLT-2inh) and beta-blockers (Tables 4, 5) differed among quartiles of IL-17A.
The values of these variables did not increase linearly, in parallel to the increasing quartile ranks of IL-17A. For example, participants in the lower and upper quartiles of IL-17A were more often diagnosed with CAD and CHD than those in the middle quartiles. There were more patients prescribed newer oral antidiabetics in lower quartiles of IL-17A than in the upper quartiles. Unexpectedly, some variables, including markers of inflammation, sex, age, BMI, the frailty index, and eGFR, as well as some of the drugs, did not show significant differences among quartiles of IL-17A. This could be also a result of collinearity or multicollinearity (Table 3), which in the context of patient complexity (great inter-individual variations and the existence of multiple patient subgroups) can differently, non-linearly, and unexpectedly affect serum IL-17A levels in particular patient subgroups. For this reason, we prepared several regression models to identify variables independently associated with increasing values (quartiles) of serum IL-17A levels.
As indicated by the regression models (Table 6), variables indicating comorbidity- and frailty-related inflammation (hemoglobin), glucose-related metabolic disorders (HbA1c), and some frailty-associated chronic health conditions such as osteoporosis, osteoarthritis, and urogenital diseases were independently associated with quartiles of IL-17A. Among geriatric syndromes, indicated by the model “Comorbidity level and functional disorders,” only frailty status was selected. Prescribed drugs selected by the regression models were antidiabetic drugs DPP4inh and pioglitazone. In models indicating comorbidities, the frailty index was the modifying factor.
Since regression models emphasized frailty as the key factor that modifies associations of other variables with variations in serum IL-17A levels, we performed several graphical presentations (Figures 2–5) to reveal associations of some factors, for which evidence indicates their associations with frailty, with the frailty status of patients in the sample. In addition, Figure 6A, 6B show how several factors considered together influence variations in serum IL-17A levels.
Figure 2 shows that women predominate over men among frail patients. The proportion test across the frailty status categories showed a significant difference for men (
In men (Figure 3A), frailty status did not change according to changes in BMI; the proportion test (men) across the BMI categories showed no significant difference for “nonfrail” (
Figure 4 shows that there are no significant differences in age among patient subgroups defined by differences in the frailty status: nonfrail vs pre-frail (
In Figure 5, the relationships between status CVD and the frailty index categories are detailed.
Figure 6 shows sex-dependent variations in serum IL-17A levels among patient subgroups defined by frailty status and BMI categories, depending on a difference in age. Figure 6A shows that in diabetic patients who are under 65 years of age, if they are not obese (BMI ≤30 kg/m2), frailty affects serum IL-17A levels, but this concerns only women, as they are tending to achieve frailty younger than men (in ages before 65 years) (
Discussion
LIMITATIONS OF THE STUDY:
Limitations of this study include the relatively small number of participants for the number of variables used for analysis, which could have caused overestimation of the effect size in differences and regression analyses, and non-randomized participant selection, which could have impacted the variable selection in regression models. In addition, multivariable interactions and the presence of confounding factors could have masked or falsely demonstrated the actual associations. The mitigating circumstances are that residents in the study area have good access to GPs and that GPs employ similar methods in screening and managing diabetic patients, and that the applied regression models do not require many of the principles of linear regression models, and randomization does not justify the assumptions behind the model.
The recognized effect of sex bias and frailty phenotypes on IL-17A serum values should be addressed in future studies to separately assess several patient subgroups, defined at least by variables such as age, sex, frailty status, and BMI. The new methods for the complex data analysis from machine learning could provide benefits by separating diabetic patients into discrete subgroups, defined by a set of variables that tend to cluster together, or by phenotypes.
Other limitations are that individuals with subclinical atherosclerotic disease have not been identified, which could have influenced serum IL-17A levels in cases without a diagnosis of CVD, and there was no information on the grade severity of CHD.
Conclusions
This study is the first attempt to identify sociodemographic and clinical determinants of IL-17A serum levels in T2D patients age 50 years and older. The results have revealed the complexity of these patients, by means of the existence of multiple patient subgroups that can differentially impact IL-17A serum levels. Future studies should address the modifying role on IL-17A serum levels of sex, age, and frailty phenotypes.
Figures
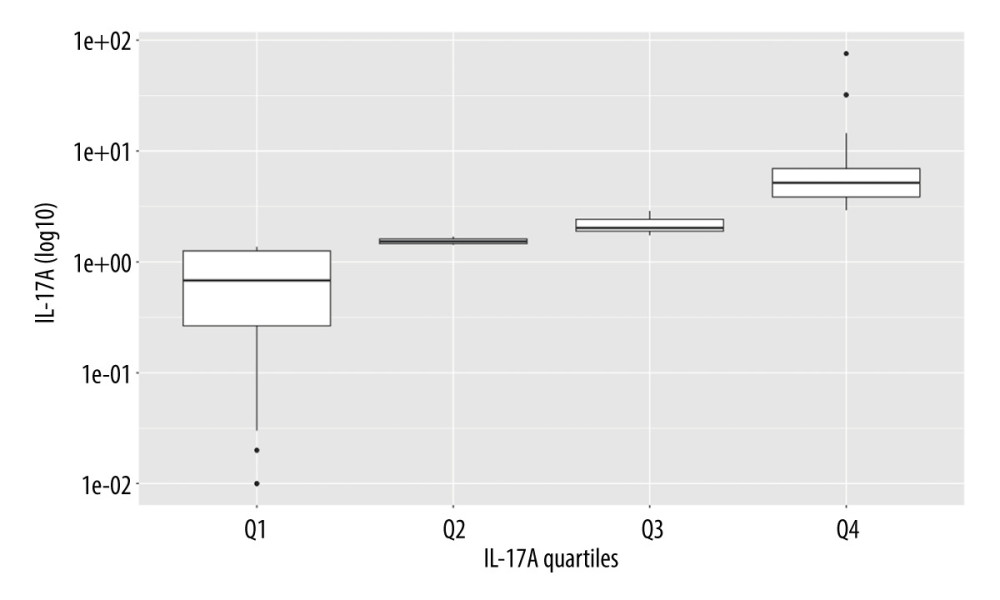 Figure 1. The quartiles of IL-17A. R, 4.3.1, R Development Core Team.
Figure 1. The quartiles of IL-17A. R, 4.3.1, R Development Core Team. 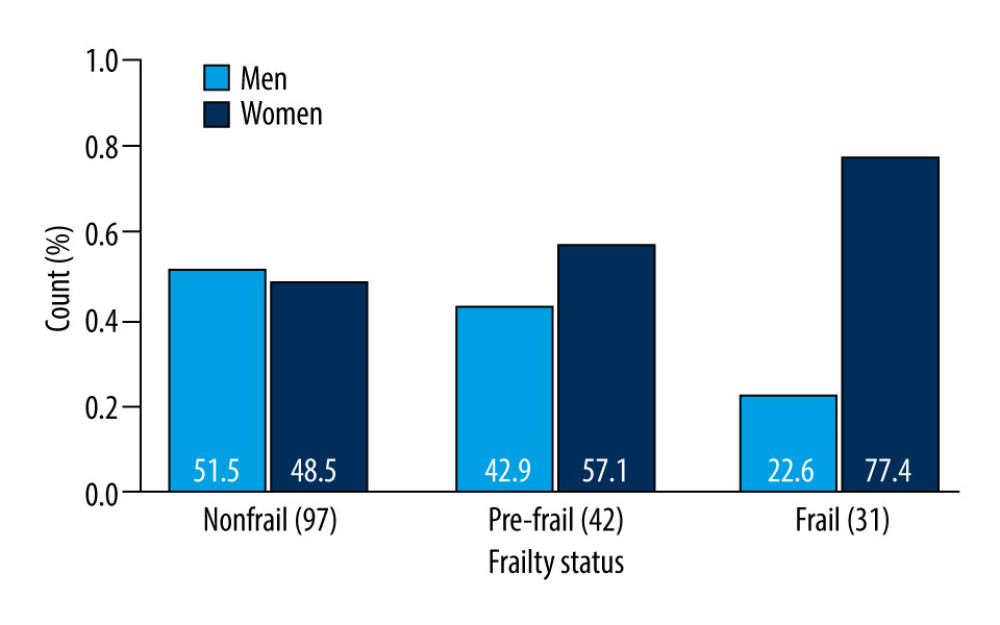 Figure 2. Sex-dependent differences in frailty status: nonfrail, pre-frail and frail. R, 4.3.1, R Development Core Team.
Figure 2. Sex-dependent differences in frailty status: nonfrail, pre-frail and frail. R, 4.3.1, R Development Core Team. 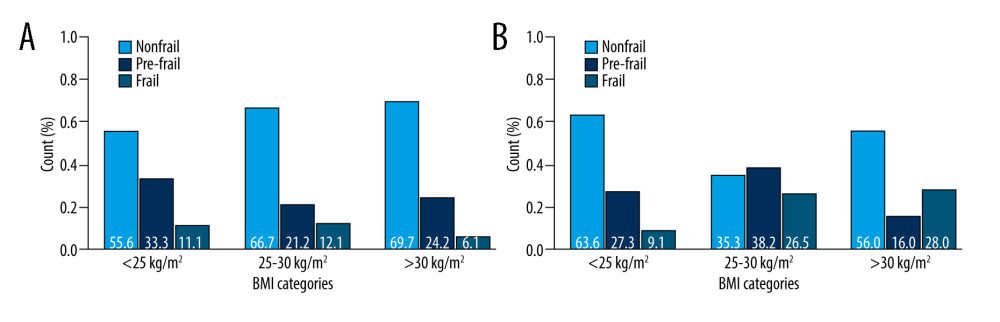 Figure 3. (A) Sex (men)-dependent distribution of older diabetic patients according to frailty status and BMI categories. (B) Sex (women)-dependent distribution of older diabetic patients according to frailty status and BMI categories. R, 4.3.1, R Development Core Team.
Figure 3. (A) Sex (men)-dependent distribution of older diabetic patients according to frailty status and BMI categories. (B) Sex (women)-dependent distribution of older diabetic patients according to frailty status and BMI categories. R, 4.3.1, R Development Core Team. 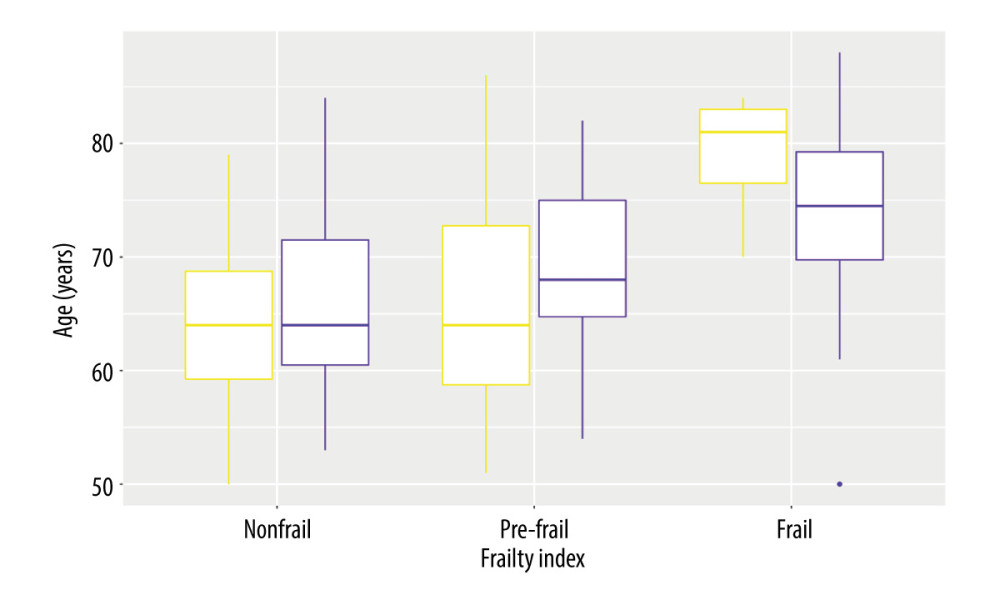 Figure 4. Sex-dependent differences of diabetic patients according to the frailty status (yellow – men, violet – women). R, 4.3.1, R Development Core Team.
Figure 4. Sex-dependent differences of diabetic patients according to the frailty status (yellow – men, violet – women). R, 4.3.1, R Development Core Team.  Figure 5. Distribution of diabetic patients with/without CVD according to the frailty status. R, 4.3.1, R Development Core Team.
Figure 5. Distribution of diabetic patients with/without CVD according to the frailty status. R, 4.3.1, R Development Core Team. 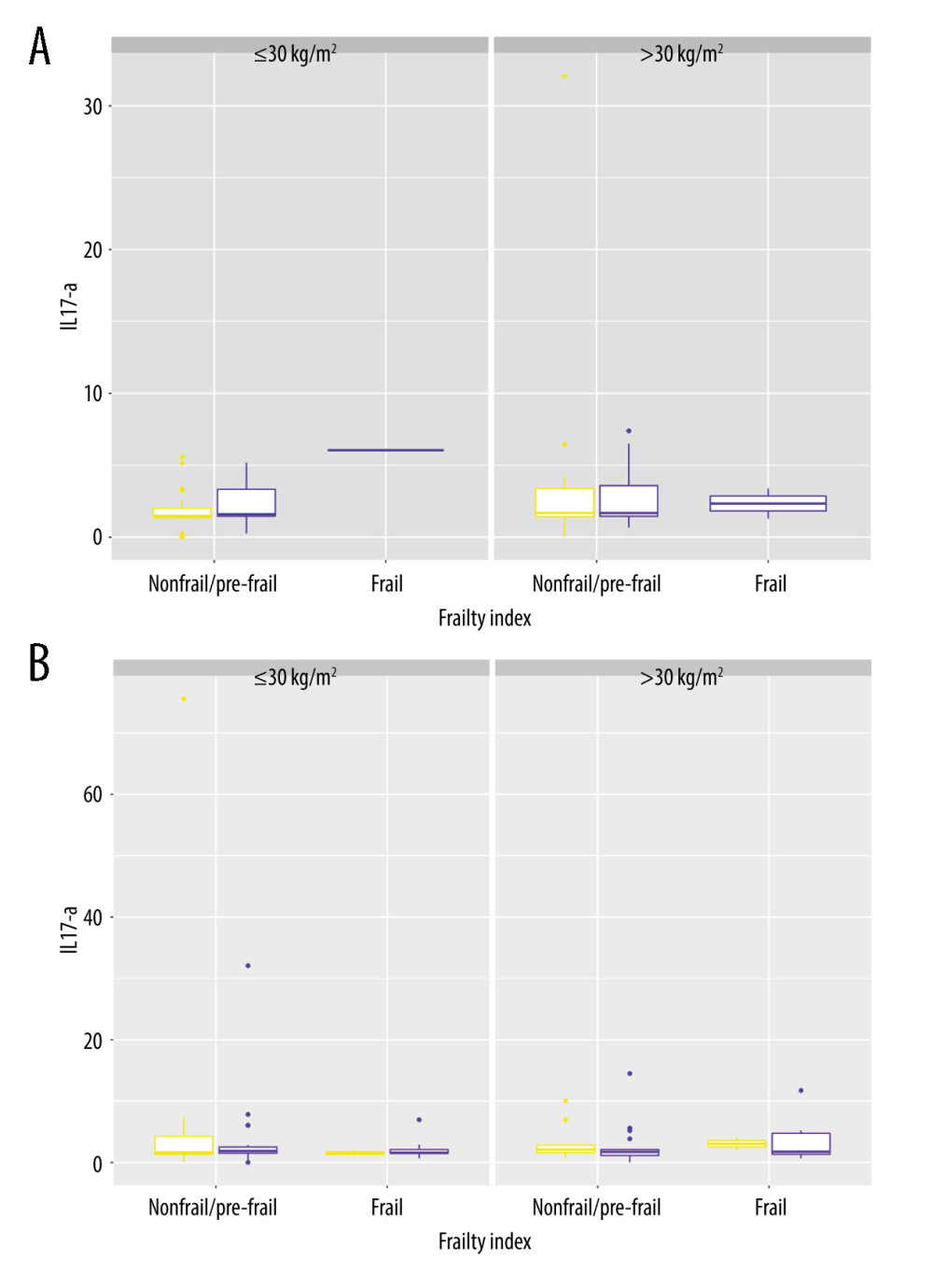 Figure 6. (A) IL-17A in men and women <65 years old, distributed according to differences in the frailty status and BMI categories. (B) IL-17A in men and women ≥65 years old, distributed according to differences in the frailty status and BMI categories. R, 4.3.1, R Development Core Team.
Figure 6. (A) IL-17A in men and women <65 years old, distributed according to differences in the frailty status and BMI categories. (B) IL-17A in men and women ≥65 years old, distributed according to differences in the frailty status and BMI categories. R, 4.3.1, R Development Core Team. Tables
Table 1A. Participant characteristics (numerical variables). Table 1B. Participant characteristics (categorical variables).
Table 1B. Participant characteristics (categorical variables).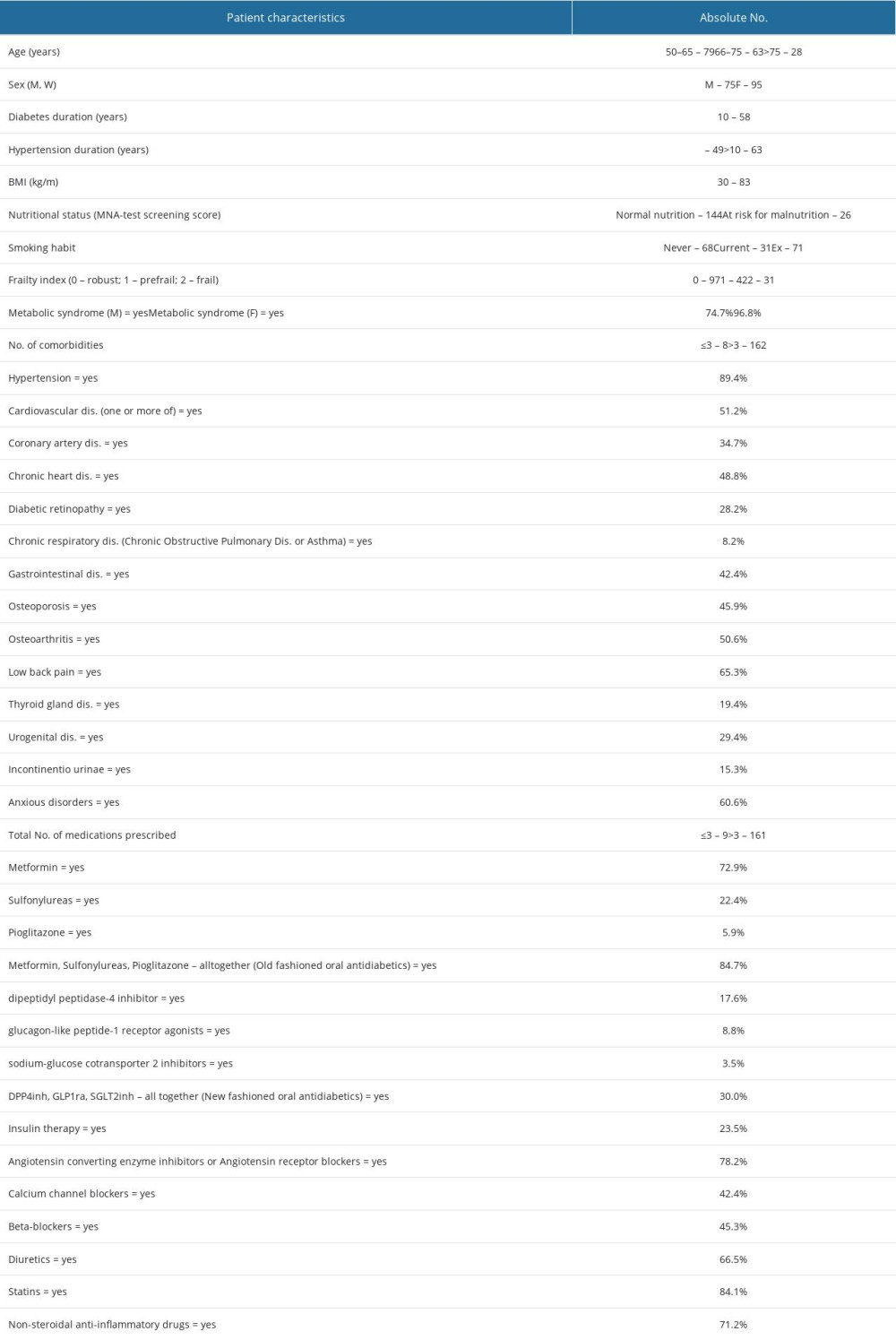 Table 2. Markers of inflammation.
Table 2. Markers of inflammation.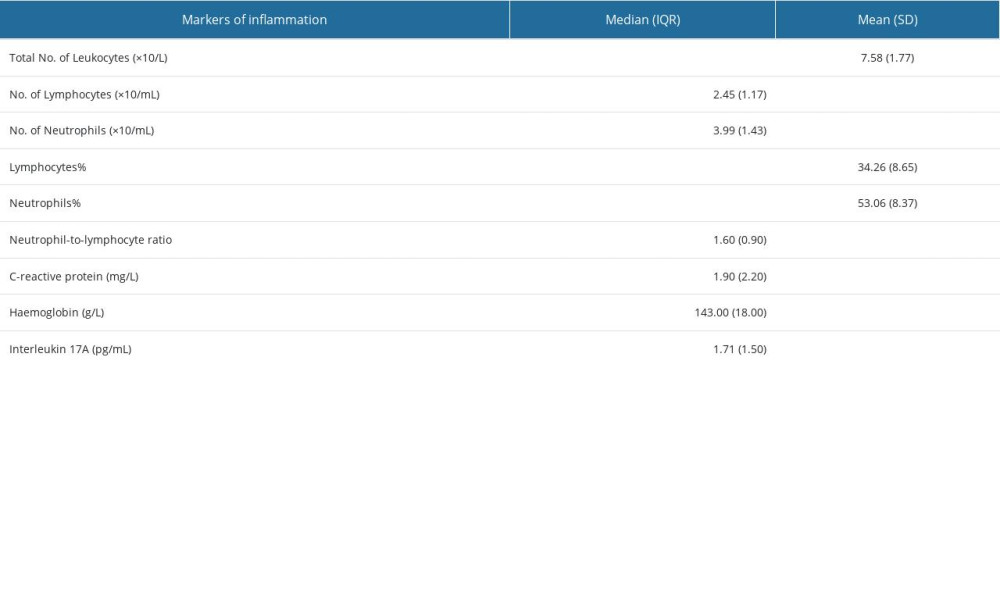 Table 3. The result of correlation analysis.
Table 3. The result of correlation analysis. Table 4. Differences in distributions of examined variables among quartiles of IL-17A. Numerical variables.
Table 4. Differences in distributions of examined variables among quartiles of IL-17A. Numerical variables.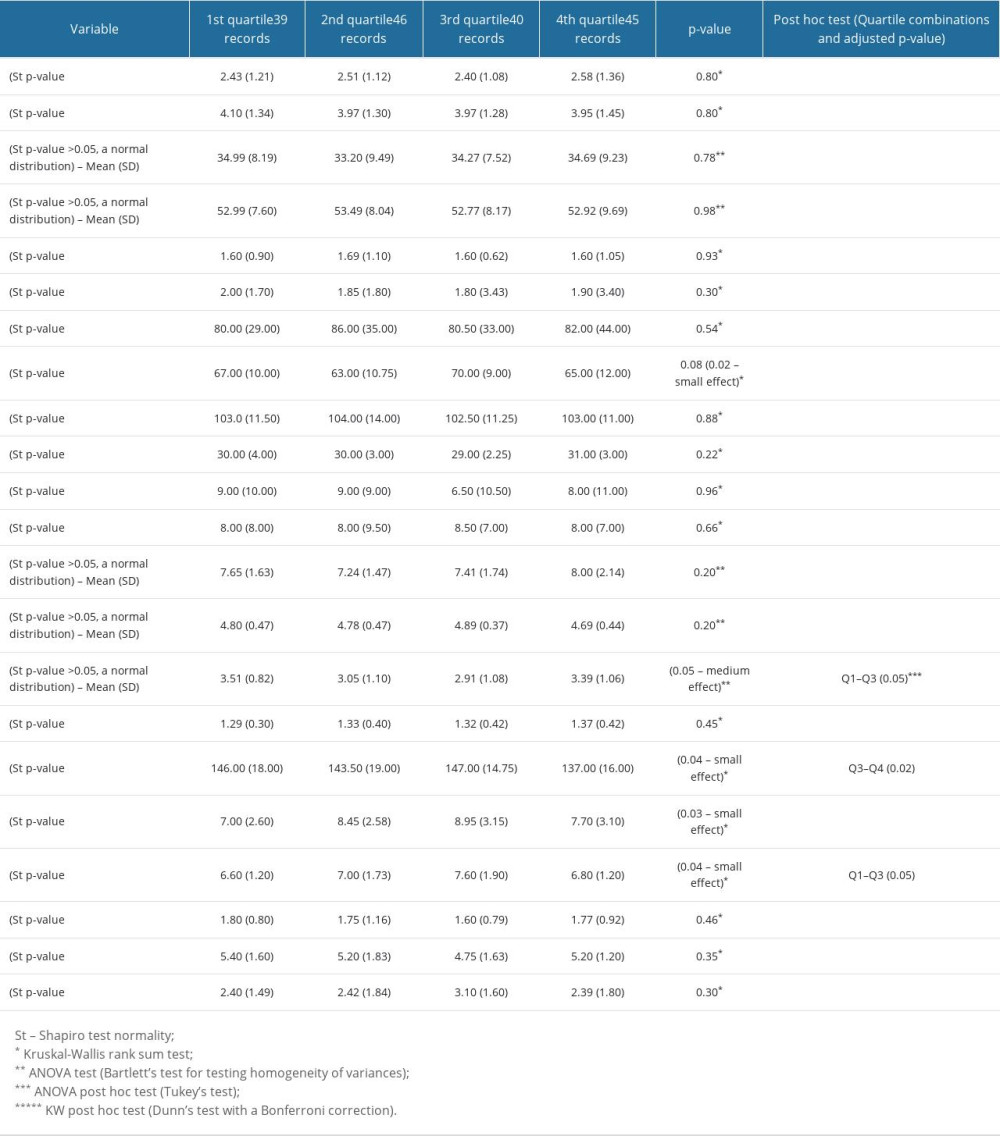 Table 5. Differences in examined variables among quartiles of IL-17A. Categorical variables.
Table 5. Differences in examined variables among quartiles of IL-17A. Categorical variables. Table 6. Multinomial logistic regression models for quartiles of IL-17A. All models were adjusted for age, sex, T2D duration, and frailty index.
Table 6. Multinomial logistic regression models for quartiles of IL-17A. All models were adjusted for age, sex, T2D duration, and frailty index.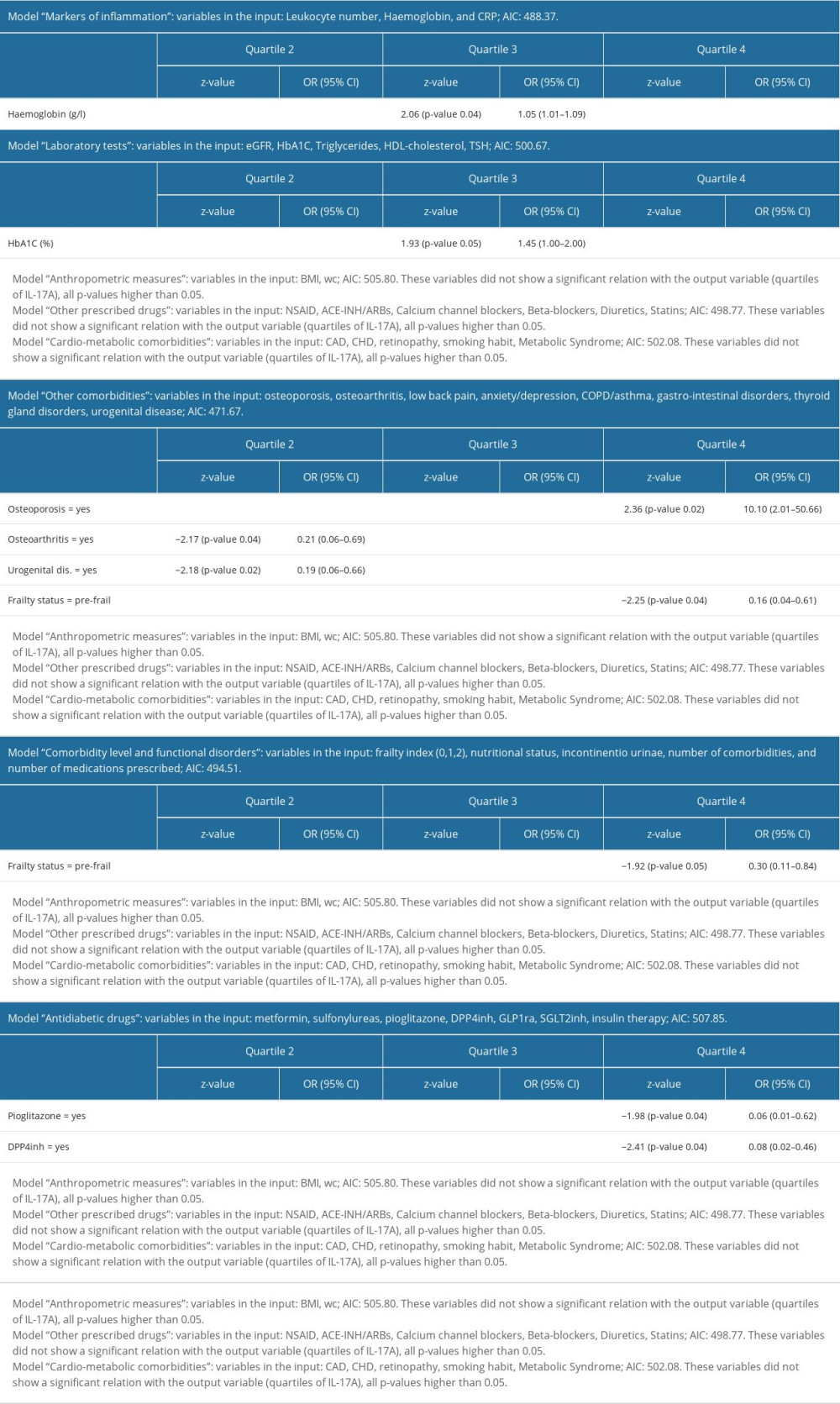
References
1. Franceschi C, Garagnani P, Parini P, Inflammaging: A new immune-metabolic viewpoint for age-related diseases: Nat Rev Endocrinol, 2018; 14(10); 576-90
2. Sepehri Z, Kiani Z, Afshari M, Inflammasomes and type 2 diabetes: An updated systematic review: Immunol Lett, 2017; 192; 97-103
3. Lazar V, Ditu LM, Pircalabioru GG, Gut microbiota, host organism, and diet trialogue in diabetes and obesity: Front Nutr, 2019; 6; 21
4. Kawai T, Autieri MV, Scalia R, Adipose tissue inflammation and metabolic dysfunction in obesity: Am J Physiol Cell Physiol, 2021; 320(3); C375-C91
5. Zatterale F, Longo M, Naderi J, Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes: Front Physiol, 2020; 10; 1607
6. Chehimi M, Vidal H, Eljaafari A, Pathogenic role of IL-17-producing immune cells in obesity, and related inflammatory diseases: J Clin Med, 2017; 6(7); 68
7. Chang YC, Hee SW, Chuang LM, T helper 17 cells: A new actor on the stage of type 2 diabetes and aging?: J Diabetes Investig, 2021; 12(6); 909-13
8. Ip B, Cilfone NA, Belkina AC, Th17 cytokines differentiate obesity from obesity-associated type 2 diabetes and promote TNFα production: Obesity (Silver Spring), 2016; 24(1); 102-12
9. Wang Q, Wang Y, Xu D, Research progress on Th17 and T regulatory cells and their cytokines in regulating atherosclerosis: Front Cardiovasc Med, 2022; 9; 929078
10. Kong L, Andrikopoulos S, MacIsaac RJ, Role of the adaptive immune system in diabetic kidney disease: J Diabetes Investig, 2022; 13(2); 213-26
11. Kleinewietfeld M, Hafler DA, The plasticity of human Treg and Th17 cells and its role in autoimmunity: Semin Immunol, 2013; 25(4); 305-12
12. Zhang S, Gang X, Yang S, The alterations in and the role of the Th17/Treg balance in metabolic diseases: Front Immunol, 2021; 12; 678355
13. Rodrigues-Diez RR, Tejera-Muñoz A, Orejudo M, Interleukin-17A: Possible mediator and therapeutic target in hypertension: Nefrologia (Engl Ed), 2021; 41(3); 244-57
14. Brown CY, Sadlon T, Hope CM, Molecular insights into regulatory T-cell adaptation to self, environment, and host tissues: Plasticity or loss of function in autoimmune disease: Front Immunol, 2020; 11; 1269
15. Shen H, Shi LZ, Metabolic regulation of TH17 cells: Mol Immunol, 2019; 109; 81-87
16. Zi C, He L, Yao H, Changes of Th17 cells, regulatory T cells, Treg/Th17, IL-17 and IL-10 in patients with type 2 diabetes mellitus: A systematic review and meta-analysis: Endocrine, 2022; 76(2); 263-72
17. Yuan S, Zhang S, Zhuang Y, Interleukin-17 stimulates STAT3-mediated endothelial cell activation for neutrophil recruitment: Cell Physiol Biochem, 2015; 36(6); 2340-56
18. Schüler R, Efentakis P, Wild J, T cell-derived IL-17A induces vascular dysfunction via perivascular fibrosis formation and dysregulation of •NO/cGMP signaling: Oxid Med Cell Longev, 2019; 2019; 6721531
19. Ma J, Li YJ, Chen X, Interleukin 17A promotes diabetic kidney injury: Sci Rep, 2019; 9(1); 2264
20. Cortvrindt C, Speeckaert R, Moerman A, The role of interleukin-17A in the pathogenesis of kidney diseases: Pathology, 2017; 49(3); 247-58
21. Silvestre-Roig C, Braster Q, Ortega-Gomez A, Soehnlein O, Neutrophils as regulators of cardiovascular inflammation: Nat Rev Cardiol, 2020; 17(6); 327-40
22. McCarthy MI, Painting a new picture of personalised medicine for diabetes [published correction appears in Diabetologia. 2017;60(5):940]: Diabetologia, 2017; 60(5); 793-99
23. Udler MS, McCarthy MI, Florez JC, Mahajan A, Genetic risk scores for diabetes diagnosis and precision medicine: Endocr Rev, 2019; 40(6); 1500-20
24. Bechara R, McGeachy MJ, Gaffen SL, The metabolism-modulating activity of IL-17 signaling in health and disease: J Exp Med, 2021; 218(5); e20202191
25. Higaki A, Caillon A, Paradis P, Schiffrin EL, Innate and innate-like immune system in hypertension and vascular injury: Curr Hypertens Rep, 2019; 21(1); 4
26. Pfützner A, Forst T, High-sensitivity C-reactive protein as cardiovascular risk marker in patients with diabetes mellitus: Diabetes Technol Ther, 2006; 8(1); 28-36
27. Howard R, Scheiner A, Kanetsky PA, Egan KM, Sociodemographic and lifestyle factors associated with the neutrophil-to-lymphocyte ratio: Ann Epidemiol, 2019; 38; 11-21e6
28. Trtica Majnarić L, Guljaš S, Bosnić Z, Neutrophil-to-lymphocyte ratio as a cardiovascular risk marker may be less efficient in women than in men: Biomolecules, 2021; 11(4); 528
29. Bellary S, Kyrou I, Brown JE, Bailey CJ, Type 2 diabetes mellitus in older adults: Clinical considerations and management: Nat Rev Endocrinol, 2021; 17(9); 534-48
30. Saedi AA, Feehan J, Phu S, Duque G, Current and emerging biomarkers of frailty in the elderly: Clin Interv Aging, 2019; 14; 389-98
31. Zoungas S, Woodward M, Li Q, Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes: Diabetologia, 2014; 57(12); 2465-74
32. Strain WD, Down S, Brown P, Diabetes and frailty: An expert consensus statement on the management of older adults with type 2 diabetes: Diabetes Ther, 2021; 12(5); 1227-47
33. Hoogendijk EO, Afilalo J, Ensrud KE, Frailty: Implications for clinical practice and public health: Lancet, 2019; 394; 1365-75
34. Vetrano DL, Palmer K, Marengoni A, Frailty and multimorbidity: A systematic review and meta-analysis: J Gerontol A Biol Sci Med Sci, 2019; 74(5); 659-66
35. Barnett K, Mercer SW, Norbury M, Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study: Lancet, 2012; 380(9836); 37-43
36. Sinclair AJ, Rodriguez-Mañas L, Diabetes and frailty: Two converging conditions?: Can J Diabetes, 2016; 40(1); 77-83
37. Chowdhury R, Peel NM, Krosch M, Hubbard RE, Frailty and chronic kidney disease: A systematic review: Arch Gerontol Geriatr, 2017; 68; 135-42
38. Wleklik M, Denfeld Q, Lisiak M, Frailty syndrome in older adults with cardiovascular diseases-what do we know and what requires further research?: Int J Environ Res Public Health, 2022; 19(4); 2234
39. Heinze-Milne SD, Banga S, Howlett SE, Frailty and cytokines in preclinical models: Comparisons with humans: Mech Ageing Dev, 2022; 206; 111706
40. Abdelhafiz AH, Emmerton D, Sinclair AJ, Impact of frailty metabolic phenotypes on the management of older people with type 2 diabetes mellitus: Geriatr Gerontol Int, 2021; 21(8); 614-22
41. Cosentino F, Grant PJ, Aboyans V, 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD [published correction appears in Eur Heart J. 2020;41(45):4317]: Eur Heart J, 2020; 41(2); 255-323
42. Nelken-Bestvina D, Kurc-Bionda A, Vojvodić Z, Trends in preventive activities for the adult population in family medicine in Croatia: 1995–2012: Coll Antropol, 2014; 38(Suppl 2); 61-66
43. Poljičanin T, Šekerija M, Metelko ŽCroDiab web and improvement of diabetes care at the primary health care level: Acta Medica Croatica, 2010; 64(5); 349-54 [in Croatian]
44. Majnarić LT, Wittlinger T, Stolnik D, Prescribing analgesics to older people: A challenge for GPs: Int J Environ Res Public Health, 2020; 17(11); 4017
45. Mascolo A, Di Napoli R, Balzano N, Safety profile of sodium glucose co-transporter 2 (SGLT2) inhibitors: A brief summary: Front Cardiovasc Med, 2022; 9; 1010693
46. Moon J, Lee SY, Choi JW, Metformin ameliorates scleroderma via inhibiting Th17 cells and reducing mTOR-STAT3 signaling in skin fibroblasts [published correction appears in J Transl Med. 2021;19(1):266]: J Transl Med, 2021; 19(1); 192
47. Zhang X, Jin J, Peng X, Ramgolam VS, Markovic-Plese S, Simvastatin inhibits IL-17 secretion by targeting multiple IL-17-regulatory cytokines and by inhibiting the expression of IL-17 transcription factor RORC in CD4+ lymphocytes: J Immunol, 2008; 180(10); 6988-96
48. Aso Y, Fukushima M, Sagara M, Sitagliptin, a DPP-4 inhibitor, alters the subsets of circulating CD4+ T cells in patients with type 2 diabetes: Diabetes Res Clin Pract, 2015; 110(3); 250-56
49. Tian Y, Chen T, Wu Y, Pioglitazone stabilizes atherosclerotic plaque by regulating the Th17/Treg balance in AMPK-dependent mechanisms: Cardiovasc Diabetol, 2017; 16(1); 140
50. Başıbüyük GÖ, Ayremlou P, Saeidlou SN, A comparison of the different anthropometric indices for assessing malnutrition among older people in Turkey: A large population-based screening: J Health Popul Nutr, 2021; 40(1); 13
51. Gingrich A, Volkert D, Kiesswetter E, Prevalence and overlap of sarcopenia, frailty, cachexia and malnutrition in older medical inpatients: BMC Geriatr, 2019; 19(1); 120
52. Kalan U, Arik F, Isik AT, Soysal P, Nutritional profiles of older adults according the Mini-Nutritional Assessment: Aging Clin Exp Res, 2020; 32(4); 673-80
53. Fried LP, Tangen CM, Walston J, Frailty in older adults: Evidence for a phenotype: J Gerontol A Biol Sci Med Sci, 2001; 56(3); M146-56
54. Zadhoush F, Sadeghi M, Pourfarzam M, Biochemical changes in blood of type 2 diabetes with and without metabolic syndrome and their association with metabolic syndrome components: J Res Med Sci, 2015; 20(8); 763-70
55. Huang PL, A comprehensive definition for metabolic syndrome: Dis Model Mech, 2009; 2(5–6); 231-37
56. Ikizler TA, Cuppari L, The 2020 updated KDOQI clinical practice guidelines for nutrition in chronic kidney disease: Blood Purif, 2021; 50(4–5); 667-71
57. Davies MJ, Aroda VR, Collins BS, 2019 Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD): Diabetologia, 2022; 65; 1925-66
58. Han C, He X, Xia X, Subclinical hypothyroidism and type 2 diabetes: A systematic review and meta-analysis: PLoS One, 2015; 10(8); e0135233
59. Pearce SH, Brabant G, Duntas LH, 2013 ETA guideline: Management of subclinical hypothyroidism: Eur Thyroid J, 2013; 2(4); 215-28
60. Pfützner A, Forst T, High-sensitivity C-reactive protein as cardiovascular risk marker in patients with diabetes mellitus: Diabetes Technol Ther, 2006; 8(1); 28-36
61. Bhat T, Teli S, Rijal J, Neutrophil to lymphocyte ratio and cardiovascular diseases: A review: Expert Rev Cardiovasc Ther, 2013; 11(1); 55-59
62. Weiss G, Ganz T, Goodnough LT, Anemia of inflammation: Blood, 2019; 133(1); 40-50
63. Steinmeyer Z, Delpierre C, Soriano G, Hemoglobin concentration; A pathway to frailty: BMC Geriatr, 2020; 20(1); 202
64. McDonagh TA, Metra M, Adamo M, 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure [published correction appears in Eur Heart J. 2021;42(48):4901]: Eur Heart J, 2021; 42(36); 3599-726
65. Tsimihodimos V, Gonzalez-Villalpando C, Hypertension and diabetes mellitus: Coprediction and time trajectories: Hypertension, 2018; 71(3); 422-28
66. Wittlinger T, Bekić S, Guljaš S, Patterns of the physical, cognitive, and mental health status of older individuals in a real-life primary care setting and differences in coping styles: Front Med (Lausanne), 2022; 9; 989814
67. National Kidney Fundation: eGFR Calculator Available from: https://www.kidney.org/professionals/kdoqi/gfr_calculator
68. Kritharides L, Inflammatory markers and outcomes in cardiovascular disease: PLoS Med, 2009; 6(9); e1000147
69. Mancusi C, Izzo R, di Gioia G, Insulin resistance the hinge between hypertension and type 2 diabetes: High Blood Press Cardiovasc Prev, 2020; 27(6); 515-26
70. Marwan AL, Increased serum cytokines levels in type 2 diabetes mellitus associated with arterial hypertension: A link to cardio-metabolic risk factors: Turkish Journal of Endocrinology and Metabolism, 2016; 20; 127-31
71. Alberro A, Iribarren-Lopez A, Sáenz-Cuesta M, Inflammaging markers characteristic of advanced age show similar levels with frailty and dependency: Sci Rep, 2021; 11(1); 4358
72. Welstead M, Muniz-Terrera G, Russ TC, Inflammation as a risk factor for the development of frailty in the Lothian Birth Cohort 1936: Exp Gerontol, 2020; 139; 111055
73. Venturini C, Sampaio RF, de Souza Moreira B, A multidimensional approach to frailty compared with physical phenotype in older Brazilian adults: Data from the FIBRA-BR study: BMC Geriatr, 2021; 21(1); 246
74. Gale CR, Baylis D, Cooper C, Sayer AA, Inflammatory markers and incident frailty in men and women: The English Longitudinal Study of Ageing: Age (Dordr), 2013; 35(6); 2493-501
75. Samson LD, Buisman AM, Ferreira JA, Inflammatory marker trajectories associated with frailty and ageing in a 20-year longitudinal study: Clin Transl Immunology, 2022; 11(2); e1374
76. Gordon EH, Peel NM, Samanta M, Sex differences in frailty: A systematic review and meta-analysis: Exp Gerontol, 2017; 89; 30-40
77. Cohen AA, Legault V, Li Q, Men sustain higher dysregulation levels than women without becoming frail: J Gerontol A Biol Sci Med Sci, 2018; 73(2); 175-84
78. Halter JB, Musi N, McFarland Horne F, Diabetes and cardiovascular disease in older adults: Current status and future directions: Diabetes, 2014; 63(8); 2578-89
79. Wang Y, O’Neil A, Jiao Y, Sex differences in the association between diabetes and risk of cardiovascular disease, cancer, and all-cause and cause-specific mortality: A systematic review and meta-analysis of 5,162,654 participants: BMC Med, 2019; 17(1); 136
80. Denfeld QE, Habecker BA, Camacho SA, Characterizing sex differences in physical frailty phenotypes in heart failure: Circ Heart Fail, 2021; 14(9); e008076
81. AlBadri A, Lai K, Wei J, Inflammatory biomarkers as predictors of heart failure in women without obstructive coronary artery disease: A report from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE): PLoS One, 2017; 12(5); e0177684
82. Huebschmann AG, Huxley RR, Kohrt WM, Sex differences in the burden of type 2 diabetes and cardiovascular risk across the life course: Diabetologia, 2019; 62(10); 1761-72
83. Wu JD, Liang DL, Xie Y, Prediabetes and risk of heart failure: The link grows stronger: Cardiovasc Diabetol, 2021; 20(1); 112
84. Iglay K, Hannachi H, Engel SS, Comorbidities in type 2 diabetes patients with and without atherosclerotic cardiovascular disease: A retrospective database analysis: Curr Med Res Opin, 2021; 37(5); 743-51
85. Cicek M, Buckley J, Pearson-Stuttard J, Gregg EW, Characterizing multimorbidity from type 2 diabetes: Insights from clustering approaches: Endocrinol Metab Clin North Am, 2021; 50(3); 531-58
86. Sözen T, Başaran NÇ, Tınazlı M, Özışık L, Musculoskeletal problems in diabetes mellitus: Eur J Rheumatol, 2018; 5(4); 258-65
87. Tembo MC, Mohebbi M, Holloway-Kew KL, The contribution of musculoskeletal factors to physical frailty: A cross-sectional study: BMC Musculoskelet Disord, 2021; 22(1); 921
88. Zhang J, Fu Q, Ren Z, Changes of serum cytokines-related Th1/Th2/Th17 concentration in patients with postmenopausal osteoporosis: Gynecol Endocrinol, 2015; 31(3); 183-90
89. Suskind AM, Frailty and lower urinary tract symptoms: Curr Urol Rep, 2017; 18(9); 67
90. Afonso C, Sousa-Santos AR, Santos A, Frailty status is related to general and abdominal obesity in older adults: Nutr Res, 2021; 85; 21-30
91. de Hollander EL, Bemelmans WJ, Boshuizen HC, The association between waist circumference and risk of mortality considering body mass index in 65- to 74-year-olds: A meta-analysis of 29 cohorts involving more than 58 000 elderly persons: Int J Epidemiol, 2012; 41(3); 805-17
92. Yuan L, Chang M, Wang J, Abdominal obesity, body mass index and the risk of frailty in community-dwelling older adults: A systematic review and meta-analysis: Age Ageing, 2021; 50(4); 1118-28
93. Ciardullo S, Ballabeni C, Trevisan R, Perseghin G, Metabolic syndrome, and not obesity, is associated with chronic kidney disease: Am J Nephrol, 2021; 52(8); 666-72
94. Wu PY, Chao CT, Chan DC, Contributors, risk associates, and complications of frailty in patients with chronic kidney disease: A scoping review: Ther Adv Chronic Dis, 2019; 10; 2040622319880382
Figures
 Figure 1. The quartiles of IL-17A. R, 4.3.1, R Development Core Team.
Figure 1. The quartiles of IL-17A. R, 4.3.1, R Development Core Team. Figure 2. Sex-dependent differences in frailty status: nonfrail, pre-frail and frail. R, 4.3.1, R Development Core Team.
Figure 2. Sex-dependent differences in frailty status: nonfrail, pre-frail and frail. R, 4.3.1, R Development Core Team. Figure 3. (A) Sex (men)-dependent distribution of older diabetic patients according to frailty status and BMI categories. (B) Sex (women)-dependent distribution of older diabetic patients according to frailty status and BMI categories. R, 4.3.1, R Development Core Team.
Figure 3. (A) Sex (men)-dependent distribution of older diabetic patients according to frailty status and BMI categories. (B) Sex (women)-dependent distribution of older diabetic patients according to frailty status and BMI categories. R, 4.3.1, R Development Core Team. Figure 4. Sex-dependent differences of diabetic patients according to the frailty status (yellow – men, violet – women). R, 4.3.1, R Development Core Team.
Figure 4. Sex-dependent differences of diabetic patients according to the frailty status (yellow – men, violet – women). R, 4.3.1, R Development Core Team. Figure 5. Distribution of diabetic patients with/without CVD according to the frailty status. R, 4.3.1, R Development Core Team.
Figure 5. Distribution of diabetic patients with/without CVD according to the frailty status. R, 4.3.1, R Development Core Team. Figure 6. (A) IL-17A in men and women <65 years old, distributed according to differences in the frailty status and BMI categories. (B) IL-17A in men and women ≥65 years old, distributed according to differences in the frailty status and BMI categories. R, 4.3.1, R Development Core Team.
Figure 6. (A) IL-17A in men and women <65 years old, distributed according to differences in the frailty status and BMI categories. (B) IL-17A in men and women ≥65 years old, distributed according to differences in the frailty status and BMI categories. R, 4.3.1, R Development Core Team. Tables
 Table 1A. Participant characteristics (numerical variables).
Table 1A. Participant characteristics (numerical variables). Table 1B. Participant characteristics (categorical variables).
Table 1B. Participant characteristics (categorical variables). Table 2. Markers of inflammation.
Table 2. Markers of inflammation. Table 3. The result of correlation analysis.
Table 3. The result of correlation analysis. Table 4. Differences in distributions of examined variables among quartiles of IL-17A. Numerical variables.
Table 4. Differences in distributions of examined variables among quartiles of IL-17A. Numerical variables. Table 5. Differences in examined variables among quartiles of IL-17A. Categorical variables.
Table 5. Differences in examined variables among quartiles of IL-17A. Categorical variables. Table 6. Multinomial logistic regression models for quartiles of IL-17A. All models were adjusted for age, sex, T2D duration, and frailty index.
Table 6. Multinomial logistic regression models for quartiles of IL-17A. All models were adjusted for age, sex, T2D duration, and frailty index. Table 1A. Participant characteristics (numerical variables).
Table 1A. Participant characteristics (numerical variables). Table 1B. Participant characteristics (categorical variables).
Table 1B. Participant characteristics (categorical variables). Table 2. Markers of inflammation.
Table 2. Markers of inflammation. Table 3. The result of correlation analysis.
Table 3. The result of correlation analysis. Table 4. Differences in distributions of examined variables among quartiles of IL-17A. Numerical variables.
Table 4. Differences in distributions of examined variables among quartiles of IL-17A. Numerical variables. Table 5. Differences in examined variables among quartiles of IL-17A. Categorical variables.
Table 5. Differences in examined variables among quartiles of IL-17A. Categorical variables. Table 6. Multinomial logistic regression models for quartiles of IL-17A. All models were adjusted for age, sex, T2D duration, and frailty index.
Table 6. Multinomial logistic regression models for quartiles of IL-17A. All models were adjusted for age, sex, T2D duration, and frailty index. In Press
12 Mar 2024 : Clinical Research
Comparing Neuromuscular Blockade Measurement Between Upper Arm (TOF Cuff®) and Eyelid (TOF Scan®) Using Miv...Med Sci Monit In Press; DOI: 10.12659/MSM.943630
11 Mar 2024 : Clinical Research
Enhancement of Frozen-Thawed Human Sperm Quality with Zinc as a Cryoprotective AdditiveMed Sci Monit In Press; DOI: 10.12659/MSM.942946
12 Mar 2024 : Database Analysis
Risk Factors of Age-Related Macular Degeneration in a Population-Based Study: Results from SHIP-TREND-1 (St...Med Sci Monit In Press; DOI: 10.12659/MSM.943140
12 Mar 2024 : Clinical Research
Preoperative Blood Transfusion Requirements for Hemorrhoidal Severe Anemia: A Retrospective Study of 128 Pa...Med Sci Monit In Press; DOI: 10.12659/MSM.943126
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








