17 July 2023: Meta-Analysis
Unraveling the Interplay between Nonalcoholic Fatty Liver Disease and Polycystic Ovary Syndrome in Adolescents: Pathogenesis, Prevalence, and Management Strategies
Kinga PółkośnikDOI: 10.12659/MSM.940398
Med Sci Monit 2023; 29:e940398
Abstract
BACKGROUND: With the expanding understanding of conditions contributing to heightened cardiovascular risk, emerging pathologies like nonalcoholic fatty liver disease (NAFLD) and polycystic ovary syndrome (PCOS) are being recognized as hepatic and ovarian manifestations of metabolic syndrome, respectively. This study aims to elucidate the recent advancements in our comprehension of the link between these conditions in the pediatric demographic, focusing on pathogenesis, incidence, diagnostic methods, and effective therapeutic strategies.
MATERIAL AND METHODS: A systematic review was conducted following the PRISMA 2020 guidelines, with a search of the PubMed database for eligible studies published in the ten years leading up to January 2023.
RESULTS: Out of 23 reports based on 16 original studies, we found a significantly higher prevalence of NAFLD in adolescents with PCOS compared to healthy controls. Factors such as increased de novo lipogenesis, alterations in gut microbiota, and a deficiency in growth differentiation factor-15 have been implicated in their pathogenesis. Additionally, novel biomarker S100A4, a clinical prediction score for hepatic steatosis in PCOS, and pharmacotherapy involving low-dose spironolactone, pioglitazone, and metformin have been proposed to enhance the management of these conditions.
CONCLUSIONS: A meticulous approach to the prevention, detection, and treatment of NAFLD in adolescents with PCOS is paramount to mitigate further complications. The study underlines the need for ongoing research to refine our understanding and management of these interconnected metabolic disorders.
Keywords: Adolescent, metabolic syndrome, Non-alcoholic fatty liver disease, Polycystic Ovary Syndrome, Female, Humans, Child, Non-alcoholic fatty liver disease, Prevalence, Insulin Resistance
Background
With the increased understanding of pathogenesis of conditions associated with elevated metabolic risk, subsequent pathologies are being included in the spectrum of conditions associated with metabolic dysfunction. Two such pathologies are nonalcoholic fatty liver disease (NAFLD) and polycystic ovary syndrome (PCOS), which are regarded as hepatic and ovarian manifestations of metabolic syndrome [1,2]. An appropriate approach to these conditions is also important as they are strong risk factors for cardiovascular diseases [3].
PCOS is one of the most common endocrinopathies in adolescent girls [4]. Its prevalence increases with age, and it is estimated to affect about 10% of the pediatric population [4]. A key feature of the disorder is clinical and/or biochemical hyperandrogenism [5]. Clinical hyperandrogenism can manifest as hirsutism and inflammatory acne that is resistant to typical therapy [5]. Another constituent of PCOS is menstrual irregularities persisting over 2 years after menarche, defined by the Endocrine Society (https://www.endocrine.org/) as menstrual cycles shorter than 21 or longer than 35 days, suggesting ovulation disturbances [6]. However, menstrual irregularities should not be used as the only criterion for PCOS in adolescents, as menstrual irregularities may be present for up to 5 years post-menarche without ovarian disturbances/PCOS [5]. Polycystic ovarian morphology (PCOM), defined as the presence of 12 or more follicles 2–9 mm in diameter and/or an increased ovarian volume over 10 mL [6], considered as the third diagnostic criterion in adults, has limited significance in children [5,7,8]. As these criteria were established with the use of transvaginal ultrasound, applying them in children using abdominal ultrasonography may result in overdiagnosis [5]. Even after increasing the ovarian volume limit to 12 ml and dropping the follicle count as a criterion [9], no correlation between hyperandrogenism and PCOM has been observed in adolescents [10]. Therefore, most researchers refer to the 1990 National Institutes of Health (NIH) guidelines, which require both irregular menses and evidence of hyperandrogenism for the diagnosis of PCOS in adolescents [11]. Furthermore, these criteria are also thought to be better at identifying whether a population has an increased metabolic risk, which is associated with hyperandrogenism [6]. As confirmed in various studies, adolescents with PCOS have a high prevalence of metabolic-associated comorbidities, such as obesity, hypertension, dyslipidemia, impaired glucose metabolism, or hepatic steatosis (HS) [5,7,8,12].
NAFLD seems to be the most common cause of chronic liver disease, both among children and adults [13,14]. Its prevalence in the pediatric population is estimated at 8–16% in lean children [15] and up to 29–38% in obese ones, with an increasing trend in recent decades [13]. The term “NAFLD” covers a wide range of liver pathologies, from simple HS, through nonalcoholic steatohepatitis (NASH) with or without fibrosis, to end-stage liver disease [16]. Morphologically, it is defined as an excess fat accumulation involving more than 5% of hepatocytes on histopathological examination, after excluding other causes of steatosis, ie, excessive alcohol intake, drug toxicity, and metabolic, genetic, or systemic disorders [14]. Clinically, NAFLD usually remains a silent condition, until incidental detection of elevated aminotransferase activity or HS in imaging testing [13]. The gold standard diagnostic method is liver biopsy; however, this method is not widely used because of its invasiveness and expense, and the availability of other methods [16].
With the growing understanding of the dominant role of metabolic dysregulation in the pathogenesis of HS, changing the nomenclature from the negative term NAFLD to metabolic- dysfunction-associated fatty liver disease (MAFLD) has been proposed, initially in adults [17], and, in 2021, also in children [18]. The new criteria emphasize that the existence of other disorders leading to liver pathology does not preclude the development of MAFLD, with all its metabolic health implications [18]. They have also led to better understanding of the disorder by patients and physicians, and, consequently, have served to highlight the importance of prevention, diagnosis, and treatment [19]. Due to a recent change in the nomenclature of fatty liver disease in the pediatric population, papers using both terminologies, NAFLD and MAFLD, were included in our review.
NAFLD and PCOS are 2 disturbances associated with insulin resistance [20,21]. However, the exact link between them is not fully understood [20]. Likely factors contributing to the higher incidence of NAFLD in PCOS patients, in addition to the aforementioned insulin resistance, are hyperandrogenism, visceral obesity, altered lipid metabolism, and inflammation [20,21]. Moreover, both diseases are known as risk factors for the development of further metabolic dysfunction, leading to increased cardiovascular risk [20,21].
Several systematic reviews and meta-analyses evaluating the association between PCOS and NAFLD/MAFLD have been published so far [20]; however, there are still many considerations in this field that require further research, especially with regard to the pediatric population. Reaching consensus on appropriate diagnostic criteria for PCOS, finding effective biomarkers and simple screening methods for HS, and developing successful therapeutic approaches could reduce the risk of complications in this group of patients, which is especially important given the many future years they have to live with the disease. Thus, our aim was to undertake a systematic review of studies published between 2013 and 2022 on NAFLD and PCOS in adolescents, and to summarize the data reported in these studies, to make these findings more accessible and more widely disseminated.
Material and Methods
ETHICAL STATEMENT:
This study was approved by the Ethics Committee of the Medical University of Białystok– resolution: APK.002.498.2021.
SEARCH STRATEGY:
The PubMed database was systematically searched in July 2022, then the search was repeated in January 2023 to update the content to include recent publications. The keywords consisted of combinations of different terms for 3 components concerning the syndrome: liver pathology, ovarian pathology, and the adolescent population. Specifically, the keywords used within these 3 areas were: liver pathology (“nonalcoholic fatty liver disease”, “non-alcoholic fatty liver disease”, “NAFLD”, “metabolic dysfunction associated fatty liver disease”, “metabolic-dysfunction associated fatty liver disease”, “metabolic associated fatty liver disease”, “metabolic- associated fatty liver disease”, “MAFLD”, “nonalcoholic steatohepatitis”, “non-alcoholic steatohepatitis”, “NASH”, “hepatic steatosis”, “hepatic fat”, “hepatic fat fraction”, “liver steatosis”, “liver fat”, “fatty liver”); ovarian pathology (“polycystic ovary syndrome”, “PCOS”); and population (“children”, “adolescents”). Searches were restricted to studies published in English in the last 10 years (2013–2022).
INCLUSION AND EXCLUSION CRITERIA:
The inclusion criteria were: 1) original studies, either observational or interventional; 2) conducted on adolescent patients (<21 years old); 3) diagnosed with PCOS and/or NAFLD; 4) published in English; 5) published in the last 10 years (2013–2022). Reviews, meta-analyses, letters to the editor, author corrections, responses to the article, studies with animal research models, and studies based only on an adult population (exclusively >18 years old) were excluded.
Regarding appropriate diagnosis of NAFLD, the findings of liver steatosis in imaging studies and/or elevated liver enzymes in laboratory tests were recognized. Accepted PCOS criteria included NIH guidelines (hyperandrogenism and menstrual irregularities; and exclusion of other etiologies) or the Rotterdam 2003 Guidelines (2 out of 3: oligo- and/or anovulation, hyperandrogenism, PCOM; and exclusion of other etiologies).
STUDY SELECTION:
All retrieved studies, after removing duplicates, were included in the initial screening of titles and abstracts, conducted by 2 independent researchers. Unrelated publications were removed and the second review of the full text of the remaining documents was then conducted, assessing them with regard to the adopted eligibility criteria. In the case of disagreement, studies were discussed between all the authors to reach a consensus on inclusion or exclusion.
Results
CHARACTERISTICS OF THE INCLUDED STUDIES:
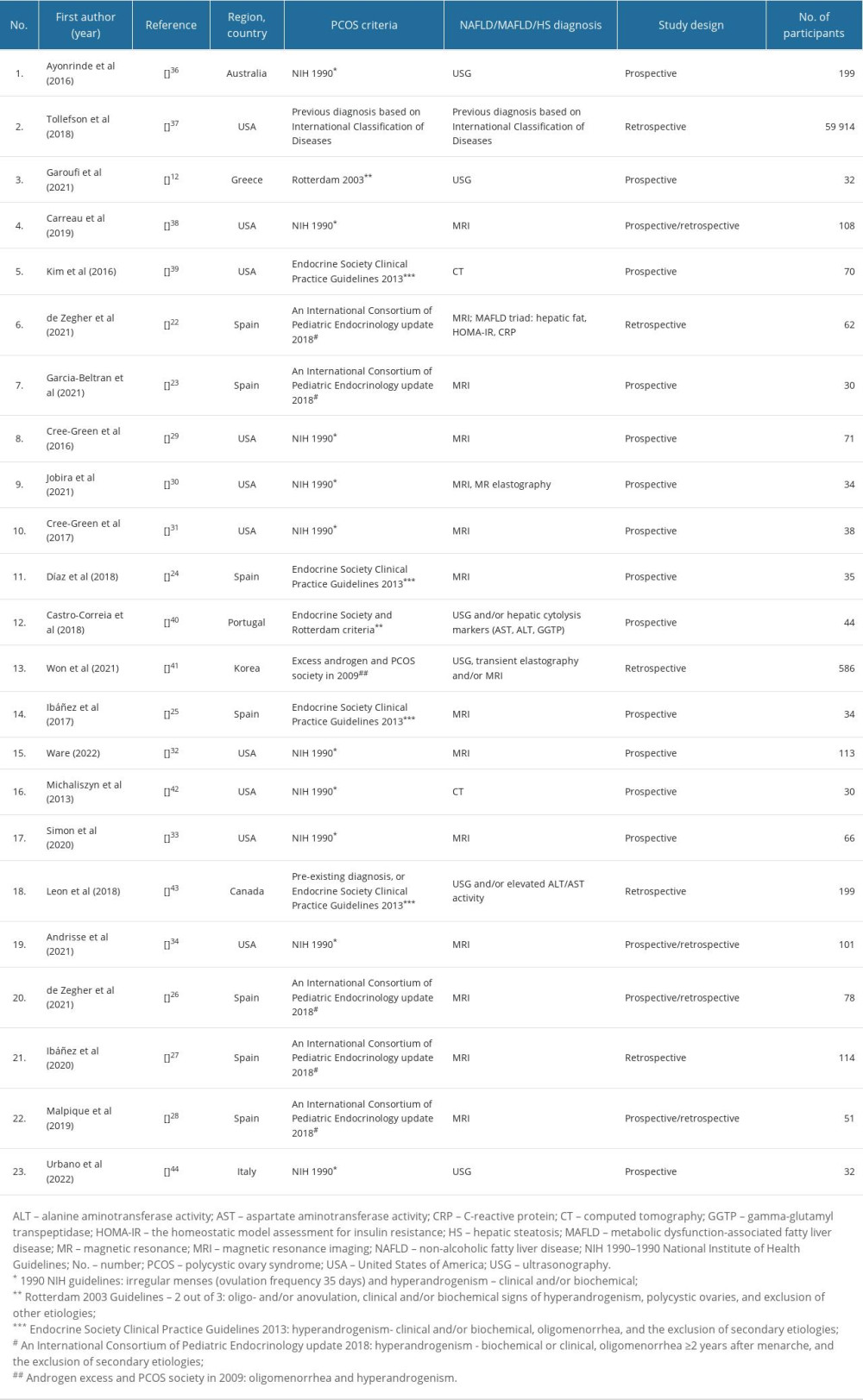
Initially, a total of 1384 records were identified. After removing duplicates, 98 records were included for further screening of titles and abstracts. According to the adopted eligibility criteria, 74 articles were rejected. Full texts of the remaining 24 articles were subjected to an in-depth analysis. That resulted in the final inclusion of 23 papers, which are based on 16 unique studies. Among these, 7 articles were based on 2 studies conducted in Catalan on a group of normal-weight girls with PCOS [22–28], while 6 others were based on the results of 4 studies conducted in the United States of America [29–34]. One of the reports evaluated for eligibility was excluded [35], because the criteria for the diagnosis of PCOS (PCOM and hirsutism) were inadequate relative to the other accepted papers, making comparison of the reports impossible. Moreover, the study participants consisted of both girls and boys, making it impossible to compare the PCOS vs non-PCOS groups in terms of, for example, occurrence of HS or insulin resistance.
The characteristics of the final, included publications are summarized in Table 1.
PREVALENCE OF NAFLD IN PCOS:
According to the analyzed data, the prevalence of PCOS in the pediatric population has increased, especially when obesity is present. PCOS was reported in 12% of obese girls [43]. Only a single study determined the prevalence of PCOS in the general adolescent population [43]; in the others, a diagnosis of PCOS was an inclusion criterion. Moreover, 2 studies revealed that psoriasis and diabetes mellitus type 1 seem to play a role in PCOS development, even in normal-weight patients, with prevalence levels of 49% and 17–21%, respectively [37,40].
The incidence of NAFLD in a population of PCOS girls was estimated to be 6.7–60%, depending on the study [12,29,32,36,41,42], and was significantly higher than in a non-PCOS control group, differing from it by 2.5–4.3 times [29,32,36].
RISK FACTORS FOR HEPATIC STEATOSIS:
The higher percentage of liver fat in PCOS adolescents seems to be associated with: obesity [12,36,41,42], a higher concentration of serum androgens [38,41], high-sensitivity C-reactive protein (CRP) [36], aminotransferase activity [30,38,41], hemoglobin A1c (HbA1c) [30,38], insulin resistance as assessed by homeostatic model assessment for insulin resistance (HOMA-IR) [30,36,38,41,42], dyslipidemia [38,41,42], alteration of gut microbiota [23,30], sleep disturbances [33], higher pancreatic fat fraction [32], and lower sex-hormone binding globulin (SHBG) concentration [36,38,41]. Racial differences appeared to be very subtle; prevalence in racial subgroups was similar to the diverse, general population [34]. Moreover, similarly to PCOS, psoriasis seems to be a risk factor for NAFLD development, increasing its risk by 76%, regardless of obesity [37].
COMORBIDITIES ASSOCIATED WITH PCOS AND NAFLD:
Several studies highlighted the more frequent co-occurrence of PCOS and NAFLD with other conditions associated with underlying insulin resistance [39,41]. This frequent co-occurrence, compared with occurrence in metabolically healthy patients, justified calling the syndromes, respectively, ovarian and hepatic manifestations of metabolism impairment. This terminology emphasizes the heightened risk for cardiovascular disorders and earlier development of subclinical atherosclerosis [12], even in normal-weight girls [31].
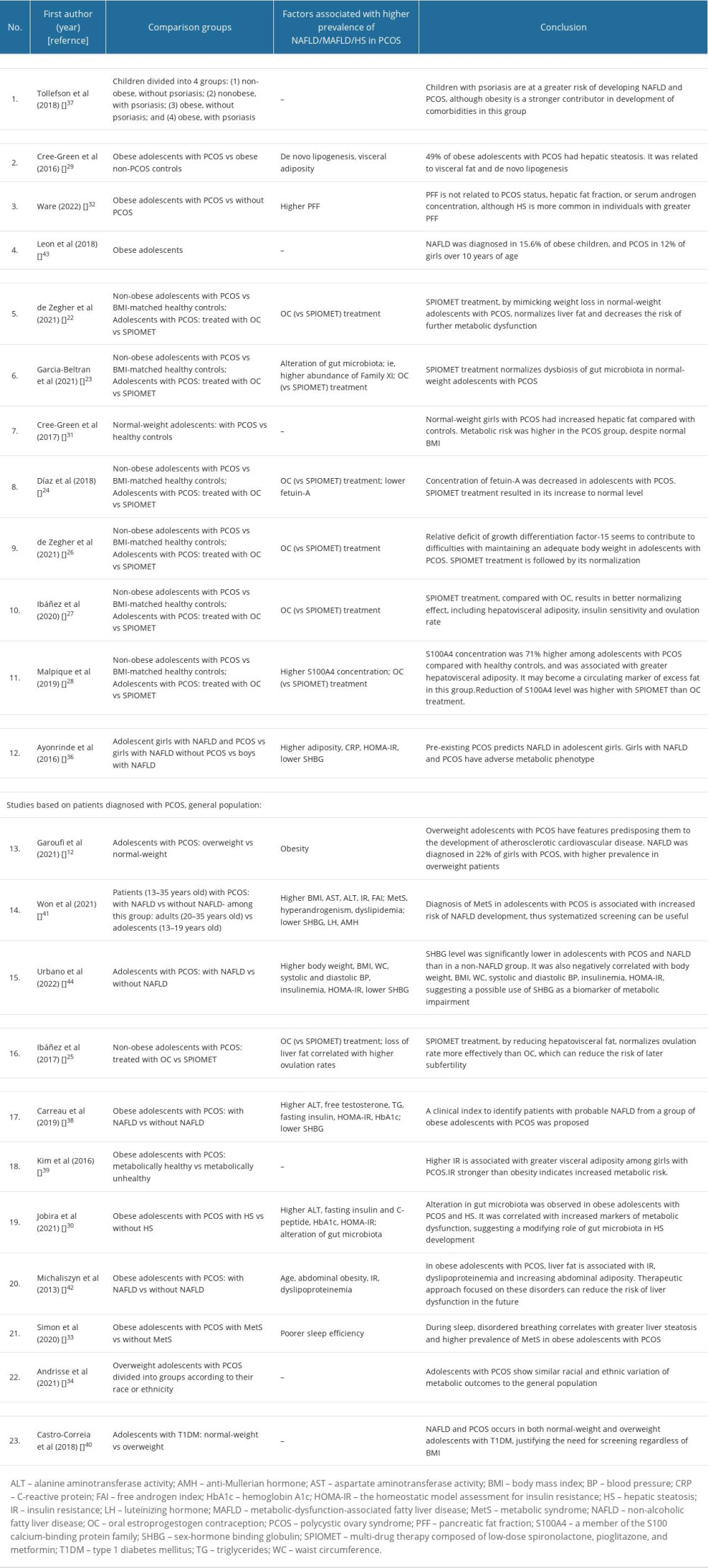
Comparison of the characteristics of the research and control groups, and the main outcomes of each study included in this systematic review, are shown in Table 2.
Discussion
The papers included in the present review appear to shed new light on PCOS and NAFLD in adolescents. Increased de novo lipogenesis (DNL) [29], changes in gut microbiota [30], or a deficit of growth differentiation factor-15 (GDF15) [26] have been identified as possible pathomechanisms for PCOS and NAFLD development. Moreover, the biomarker S100A4, a member of the S100 calcium-binding protein family [28], and a clinical prediction score of HS in PCOS have been reported to improve the diagnosis [38]. To better manage these disorders, pharmacotherapy based on low doses of spironolactone, pioglitazone, and metformin has been proposed [22–28].
While searching the database, we found no systematic review describing the relationship between NAFLD and PCOS specifically, in the pediatric population. In a review based on adults, a strong association between these conditions was reported, showing an increased risk of NAFLD among women with PCOS [20]. In the aforementioned meta-analysis, hyperandrogenism was indicated as a strong risk factor for the development of HS in patients with PCOS. In the pediatric population, such a relationship was also demonstrated [41].
It has already been reported that HS is related to insulin resistance in girls with PCOS, although the initial mechanism remains unclear. Cree-Green et al conducted a study aiming to assess potential causes of this phenomenon [29]. They observed a markedly increased prevalence of HS and higher insulin resistance in the PCOS group, compared with equally obese girls without the syndrome. Surprisingly, the percentage of liver fat, as assessed by magnetic resonance imaging, correlated with biomarkers of DNL, not with androgens or peripheral insulin resistance. That may indicate that DNL is a mechanism underlying HS in obese girls with PCOS. Previous studies of the NAFLD population, regardless of PCOS status, have also suggested a significant contribution of DNL to HS, but attributed the cause to reduced adipose tissue and muscle insulin sensitivity [45,46]. Regardless of the discrepancies, insulin resistance and DNL seem to play an important role in the development of HS in NAFLD and PCOS [47].
Another possible mechanism considers the development of PCOS and HS as an adaptive process, initiated by multifactorial mismatch between lesser prenatal and greater postnatal weight gain [48]. Disproportionately abundant nutrition after birth leads to excess fat in subcutaneous adipose tissue and, as a consequence, excess hepatovisceral fat [5,48]. To reduce central obesity, body growth and maturation accelerates. Probable mediators of this process include higher concentrations of insulin and insulin-like growth factor 1, known as strong anabolic factors responsible for insulin resistance development. This adaptive feedback mode is lost about 2 years after menarche, when girls reach their adult height; however, other accelerators persist, leading to hepatovisceral fat accumulation and PCOS. Other factors, such as lifestyle, genetic polymorphisms, or epigenetic changes are considered as modulators of the process.
Another factor with involvement in the pathogenesis, as well as potential use in the diagnosis of NAFLD, is the hepatokine fetuin-A. It is an organokine secreted mainly by hepatocytes, and is known to have an effect on lipid and glucose metabolism. Its secretion is impaired in people with NAFLD [49]. So far, fetuin-A level has been shown to be elevated in children with NAFLD [50], although its role in the development of HS in PCOS patients is not well assessed.
Adolescents with PCOS are characterized by an increased risk of obesity. A potential determinant of this phenomenon is GDF15, a stress- and inflammation-induced cytokine that is expressed mainly in liver, lung, and kidney. An elevated level of GDF15 leads to significant weight loss [51], by reducing appetite through action on specific receptors in the brainstem [52]. GDF15 has also been suggested as a potential biomarker of NAFLD, as its plasma concentration seems to correlate with changes in intrahepatic fat content [53]. Insulin resistance, obesity, diabetes, high CRP level, aging, and metformin intake lead to higher concentrations of GDF15 and, consequently, weight loss, and this could be a protective mechanism against the development of further metabolic disorders [54,55].
De Zegher et al observed a relative deficit of GDF15 in adolescents with PCOS, taking into consideration the ratio between concentrations of GDF15 and its secretagogues, insulin and CRP [26]. This indicates the presence of impairment of the protective mechanism of appetite attenuation, and a deficiency of GDF15, despite high availability of compounds that usually result in increased secretion. GDF15 deficiency could therefore explain the excess weight gain in these patients and their difficulty in achieving weight loss.
The gut microbiome, sometimes called a second genome of the human body, also appears to have a modifying effect on the pathogenesis of both PCOS and NAFLD/MAFLD. Gut microbiota are known to play important roles in host organism homeostasis, acting in immune response modulation, metabolism regulation, fat redistribution, and provision of barrier protection in the gut [56]. The significant alteration of gut microbiota composition and the contribution of this altered gut flora to disease development has already been shown in both PCOS and NAFLD patients [23,57–60]. Dysbiosis of the gut microbiome is associated with, among other factors, poor eating habits and obesity. This dysbiosis seems to increase the permeability of the intestinal mucosa, which results in immune system activation and insulin resistance through interference with insulin receptor function [60]. Moreover, toxins entering the “leaky” gut affect the liver, causing hepatic damage due to the existence of the gut-hepatic axis [59].
Jobira et al, after demonstrating a unique gut microbiome profile in adolescents with PCOS [56], examined whether the microbiome profile differs depending on the presence or absence of HS [30]. They observed altered composition of the gut microbiome, depending on HS status. Moreover, microbiome composition was significantly correlated with central obesity, higher alanine aminotransferase (ALT) activity, hepatic fat fraction, and insulin resistance indices, suggesting a relationship between HS and gut microbiota in obese girls with PCOS, without any correlation with androgen concentration.
SHBG is a hepatic glycoprotein that regulates circulating androgens by taking part in their transport. It is known that hyperinsulinemia reduces SHBG level, which is confirmed to be associated with increased risk of obesity and NAFLD [44]. Urbano et al conducted a study based on an adolescent PCOS sample that was divided according to the presence or absence of NAFLD [61]. The researchers found that decreased levels of SHBG seem to correlate with a higher risk of developing liver pathology. Moreover, independently of NAFLD status, SHBG was negatively correlated with other indicators of metabolic alterations, such as elevated blood pressure, insulin resistance, or increased body weight, which were also significantly higher in individuals with NAFLD. This suggests a role for SHBG deficiency in the development of liver pathology and its possible use as a biomarker for this condition.
Scientific societies creating recommendations for the management of PCOS in adolescents emphasize an increased risk of developing NAFLD. Thus, there is an emerging need for greater vigilance in detecting HS, which would allow for earlier implementation of appropriate management, preventing the development of complications in the future. Unfortunately, due to the lack of adequately sensitive and specific screening tests, regular screening is not recommended for all patients to date. Carreau et al tried to create a simple method to identify individuals who would benefit from further evaluation for NAFLD, to avoid unnecessary large group testing [38]. A proposed score, called by the authors the PCOS-HS index, requires only ALT and SHBG concentrations, which are standard laboratory tests commonly obtained during initial diagnosis for PCOS, and 2 measurements taken during physical examination: waist circumference and body mass index (BMI) percentile. The proposed model to assess the probability of NAFLD is based on the following formula: 1/(1+(exp(−(25.19+ (−0.3411* BMI percentile)+ (0.06149* waist circumference (cm))+ (0.09374* ALT (U/L))+ (−0.07954* SHBG (nmol/L)).
It was created using backwards stepdown elimination, based on the data obtained from 87 overweight/obese girls with PCOS, aged between 14 and 17. NAFLD was diagnosed in 52% of the attendees. When using a threshold of 0.43 to predict high risk of NAFLD, specificity of the test was 69%, sensitivity 82%, positive predictive value 74%, and negative predictive value 78%. The researchers also performed validation, based on 21 participants, obtaining similar results in test reliability (specificity 70%, sensitivity 91%). They specified different cut-off points to assess low, intermediate, and high risk of NAFLD.
Certainly, implementation of such an index in daily clinical practice would improve HS risk assessment, allowing earlier diagnosis and initiation of appropriate treatment. Moreover, the requirement of only basic parameters makes it widely available, even in the primary care setting.
As PCOS and NAFLD become more prevalent, there is a growing need to search for markers providing a simple, low-cost way for screening for metabolic disorders in apparently healthy individuals. Malpique et al hypothesized that a member of the S100 calcium-binding protein family, S100A4, could reflect the level of hepatovisceral fat [28]. They based it on the previously known role of this factor as an adipokine secreted by subcutaneous white adipose tissue in response to its dysfunction, leading to increased insulin resistance or inflammation, independently of BMI [62]. They found that the mean concentration of circulating S100A4 was 71% higher in the PCOS group, and that it correlated with hepatovisceral adiposity. This provides hope for the establishment of a new marker for easier identification of HS without performing complex diagnostics on the entire risk group.
Although PCOS is a common disorder in the adolescent population, no pharmacological treatment has yet been approved by the Food and Drug Administration or the European Medicines Agency. Lifestyle modification, primarily aimed at reducing excess body weight and increasing physical activity, remains an essential first-line therapy, and these measures have been shown to normalize androgen level and ovulation rate and to reduce cardiovascular risk. Unfortunately, in most cases, such interventions are not sufficient and additional therapeutic options are necessary. The most commonly used drugs are: metformin, which lowers insulin resistance by increasing tissue insulin sensitivity; oral estroprogestogen contraception (OC), which regulates menstrual cycles, inhibits excessive endometrial proliferation, and decreases the free androgen index; and anti-androgenic drugs, which reduce hyperandrogenism and its consequences [4,5].
In choosing the appropriate pharmacotherapy, it is essential to consider its predictable impact on metabolic health and lifetime reduction of cardiovascular risk, which is particularly high in the adolescent population, considering the long span of future life for these patients. So far, the most commonly used treatment in this group is OC, which, by silencing the gonadotropic axis, leads to anovulation and normalization of androgen levels, but has no effect on the reduction of excessive fat accumulated in hepatovisceral stores. Moreover, it has been shown that OC significantly affects metabolic alterations by exacerbating insulin resistance and lipid disorders, as well as by influencing weight gain [5,6]. These observations have prompted researchers to begin searching for combination therapy approaches, to achieve a better effect.
In recent years, major achievements in this field have been made by de Zegher et al, who proposed a new multi-drug therapy composed of low-dose spironolactone, pioglitazone, and metformin (SPIOMET). Considering excessive steatosis as an initiating factor in the development of metabolic disorders, the multidirectional action of this drug combination, by reducing ectopic lipid accumulation, serves to prevent the development of further metabolic disturbances. This is possible due to the synergistic effect of low doses of spironolactone (50 mg/d), an anti-androgenic and anti-mineralocorticoid drug that activates brown adipose tissue; pioglitazone (7.5 mg/d), an insulin sensitizer that leads to a doubling of circulating high-molecular-weight adiponectin, playing an important role in maintaining glucose homeostasis; and metformin (850 mg/d), which reduces insulin resistance and increases (approximately triples) the concentration of appetite-attenuating GDF15 [26].
The researchers conducted 2 randomized clinical trials based on a group of non-obese adolescent girls with PCOS [26]. They compared the efficacy of 1 year of SPIOMET vs OC therapy, both of which included followup for 1 year after treatment. They found that SPIOMET leads to redistribution of excess fat from ectopic to subcutaneous stores, mimicking the metabolic changes that occur during weight normalization, without affecting actual weight. This is important in consideration of an increased risk of developing NAFLD and cardiovascular risk in girls with PCOS, regardless of obesity [31,63]. As a consequence of this action, SPIOMET resulted in normalization of ovulation frequency and androgen level, increased insulin sensitivity and GDF15 level, reduced organ steatosis, and regulation of the previously altered composition of the gut microbiome [22,23,25–27,48,64]. Moreover, together with lowering adiposity, a trend toward normalization of serum S100A4 level was observed [28], justifying its use as a marker for excessive hepatovisceral adiposity.
SPIOMET treatment also resulted in faster normalization of fetuin-A level [24]. However, this level was lower at the baseline in the group of adolescent girls with PCOS compared with healthy controls, showing an inverse relationship to that observed in previously published works. The researchers explained this as being related to the high activity of proinflammatory cytokines, which inhibit hepatic production of fetuin-A.
Finally, SPIOMET treatment seems to have better long-term effectiveness, and has the ability to decrease the risk of future MAFLD development [22], defined as the presence of the “MAFLD triad” - increased HOMA-IR, HS, and circulating CRP. This analysis revealed that OC increased the prevalence of MAFLD from 13% to 35%, while SPIOMET decreased it from 13% to 0%. OC also caused a statistically significant increase in patients’ BMI. Therefore, the common use of OC as a treatment for PCOS seems to neglect the problem of metabolic dysregulation underlying the development of the described disorders. In contrast, SPIOMET shows a more pathophysiologically beneficial approach.
Taking into account all demonstrated results, SPIOMET seems to be a particularly encouraging therapeutic option for PCOS, especially in sexually inactive adolescents, in whom contraception is not required, because of its positive effect not only on the ovaries, but on overall metabolic health and, consequently, long-term prognosis. Furthermore, OC should be prescribed with more caution, taking into account the need to detect and correct emerging metabolic dysregulation.
We are aware that our study has some limitations. Searching of the database was done manually, without using automatic tools, and the numbers of searched terms and obtained results were high, causing a risk of error in this phase of the work. Moreover, due to the diversity of the groups, including age, body weight, criteria used for PCOS diagnosis, and assessed factors, it was difficult to reliably compare the groups and perform statistical calculations. Therefore, we were also not able to perform a meta-analysis, which would certainly have increased the value of the review.
Conclusions
In recent years, PCOS and NAFLD/MAFLD have been affecting an increasing percentage of the pediatric population, and are therefore of growing interest for scientists. Researchers are trying to better understand the processes underlying the development of these disorders, find points of convergence in pathogenesis, and, consequently, better methods of prevention, diagnosis, and treatment. It is very important, since children, with their potentially long futures of living with the disease, are at significantly higher risk of developing severe comorbidities and complications during their lifetime. The achievements so far are very promising. According to recent scientific reports, there is a strong link between NAFLD and PCOS, as well as with other conditions resulting from insulin resistance. This justifies designating NAFLD and PCOS, respectively, as hepatic and ovarian manifestations of metabolic syndrome. Published reports in the last 10 years have suggested several possible pathomechanisms linking PCOS and NAFLD, such as DNL, relative GDF15 deficiency, and gut microbiota disorders. New biomarkers, such as fetuin-A, SHBG, S100A4, and the PCOS-HS index, have been proposed in the diagnosis of HS among adolescent girls with PCOS. Among therapeutic options, the use of the combination treatment SPIOMET, with its beneficial effect on overall metabolic health, has been proposed as promising. Nevertheless, further studies in larger populations to confirm these findings, and studies of subsequent management possibilities for HS in PCOS, are still warranted.
References
1. Weihe P, Weihrauch-Blüher S, Metabolic syndrome in children and adolescents: Diagnostic criteria, therapeutic options and perspectives: Curr Obes Rep, 2019; 8(4); 472-79
2. Baranova A, Tran TP, Birerdinc A, Younossi ZM, Systematic review: Association of polycystic ovary syndrome with metabolic syndrome and non-alcoholic fatty liver disease: Aliment Pharmacol Ther, 2011; 33(7); 801-14
3. Chociej AB, Wasilewska N, Flisiak-Jackiewicz M, Lebensztejn D, Cardiovascular risk in children with nonalcoholic fatty liver disease (NAFLD): Curr Pediatr Rev, 2020; 16(4); 294-97
4. Ibáñez L, de Zegher F, Polycystic ovary syndrome in adolescent girls: Pediatr Obes, 2020; 15(2); e12586
5. Ibáñez L, Oberfield SE, Witchel S, An International Consortium update: Pathophysiology, diagnosis, and treatment of polycystic ovarian syndrome in adolescence: Horm Res Paediatr, 2017; 88(6); 371-95
6. Legro RS, Arslanian SA, Ehrmann DA, Diagnosis and treatment of polycystic ovary syndrome: An Endocrine Society clinical practice guideline: J Clin Endocrinol Metab, 2013; 98(12); 4565-92
7. Rosenfield RL, The diagnosis of polycystic ovary syndrome in adolescents: Pediatrics, 2015; 136(6); 1154-65
8. Al Wattar BH, Fisher M, Bevington L, Clinical practice guidelines on the diagnosis and management of polycystic ovary syndrome: A systematic review and quality assessment study: J Clin Endocrinol Metab, 2021; 106(8); 2436-46
9. Witchel SF, Oberfield S, Rosenfield RL, The diagnosis of polycystic ovary syndrome during adolescence: Horm Res Paediatr, 2015 Online ahead of print
10. Merino PM, Villarroel C, Jesam C, New diagnostic criteria of polycystic ovarian morphology for adolescents: impact on prevalence and hormonal profile: Horm Res Paediatr, 2017; 88(6); 401-7
11. Zawadski J, Dunaif A, Diagnostic criteria for polycystic ovary syndrome; Towards a rational approach: Polycystic ovary syndrome, 1992; 377-84, Blackwell Scientific
12. Garoufi A, Pagoni A, Papadaki M, Cardiovascular risk factors and subclinical atherosclerosis in Greek adolescents with polycystic ovary syndrome: Its relationship with body mass index: Children (Basel), 2021; 9(1); 4
13. Vos MB, Abrams SH, Barlow SE, NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN): J Pediatr Gastroenterol Nutr, 2017; 64(2); 319-34
14. European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO), EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease: J Hepatol, 2016; 64(6); 1388-402
15. Conjeevaram Selvakumar PK, Kabbany MN, Lopez R, Prevalence of suspected nonalcoholic fatty liver disease in lean adolescents in the United States: J Pediatr Gastroenterol Nutr, 2018; 67(1); 75-79
16. Vajro P, Lenta S, Socha P, Diagnosis of nonalcoholic fatty liver disease in children and adolescents: Position paper of the ESPGHAN Hepatology Committee: J Pediatr Gastroenterol Nutr, 2012; 54(5); 700-13
17. Eslam M, Newsome PN, Sarin SK, A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement: J Hepatol, 2020; 73(1); 202-9
18. Eslam M, Alkhouri N, Vajro P, Defining paediatric metabolic (dysfunction)-associated fatty liver disease: An international expert consensus statement: Lancet Gastroenterol Hepatol, 2021; 6(10); 864-73
19. Flisiak-Jackiewicz M, Bobrus-Chociej A, Wasilewska N, Lebensztejn DM, From Nonalcoholic Fatty Liver Disease (NAFLD) to Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) – new terminology in pediatric patients as a step in good scientific direction?: J Clin Med, 2021; 10(5); 924
20. Wu J, Yao XY, Shi RX, A potential link between polycystic ovary syndrome and non-alcoholic fatty liver disease: An update meta-analysis: Reprod Health, 2018; 15(1); 77
21. Macut D, Božić-Antić I, Bjekić-Macut J, Tziomalos K, Management of endocrine disease: Polycystic ovary syndrome and nonalcoholic fatty liver disease: Eur J Endocrinol, 2017; 177(3); R145-R58
22. de Zegher F, Diaz M, Ibañez L, From adolescent PCOS to adult MAFLD: Opposing effects of randomised interventions: BMJ Open Gastroenterol, 2021; 8(1); e000574
23. Garcia-Beltran C, Malpique R, Carbonetto B, Gut microbiota in adolescent girls with polycystic ovary syndrome: Effects of randomized treatments: Pediatr Obes, 2021; 16(4); e12734
24. Díaz M, Gallego-Escuredo JM, López-Bermejo A, Low-dose spironolactone-pioglitazone-metformin normalizes circulating fetuin-A concentrations in adolescent girls with polycystic ovary syndrome: Int J Endocrinol, 2018; 2018; 4192940
25. Ibáñez L, Del Río L, Díaz M, Normalizing ovulation rate by preferential reduction of hepato-visceral fat in adolescent girls with polycystic ovary syndrome: J Adolesc Health, 2017; 61(4); 446-53
26. de Zegher F, Díaz M, Villarroya J, The relative deficit of GDF15 in adolescent girls with PCOS can be changed into an abundance that reduces liver fat: Sci Rep, 2021; 11(1); 7018
27. Ibáñez L, Díaz M, García-Beltrán C, Toward a treatment normalizing ovulation rate in adolescent girls with polycystic ovary syndrome: J Endocr Soc, 2020; 4(5); bvaa032
28. Malpique R, Sánchez-Infantes D, Garcia-Beltran C, Towards a circulating marker of hepato-visceral fat excess: S100A4 in adolescent girls with polycystic ovary syndrome – evidence from randomized clinical trials: Pediatr Obes, 2019; 14(5); e12500
29. Cree-Green M, Bergman BC, Coe GV, Hepatic steatosis is common in adolescents with obesity and PCOS and relates to de novo lipogenesis but not insulin resistance: Obesity (Silver Spring), 2016; 24(11); 2399-406
30. Jobira B, Frank DN, Silveira LJ, Hepatic steatosis relates to gastrointestinal microbiota changes in obese girls with polycystic ovary syndrome: PLoS One, 2021; 16(1); e0245219
31. Cree-Green M, Rahat H, Newcomer BR, Insulin resistance, hyperinsulinemia, and mitochondria dysfunction in nonobese girls with polycystic ovarian syndrome: J Endocr Soc, 2017; 1(7); 931-44
32. Ware MA, Kaar JL, Diniz Behn C, Pancreatic fat relates to fasting insulin and postprandial lipids but not polycystic ovary syndrome in adolescents with obesity: Obesity (Silver Spring), 2022; 30(1); 191-200
33. Simon S, Rahat H, Carreau AM, Poor sleep is related to metabolic syndrome severity in adolescents with PCOS and Obesity: J Clin Endocrinol Metab, 2020; 105(4); e1827-e34
34. Andrisse S, Garcia-Reyes Y, Pyle L, Racial and ethnic differences in metabolic disease in adolescents with obesity and polycystic ovary syndrome: J Endocr Soc, 2021; 5(4); bvab008
35. Gawlik A, Shmoish M, Hartmann MF, Steroid metabolomic disease signature of nonsyndromic childhood obesity: J Clin Endocrinol Metab, 2016; 101(11); 4329-37
36. Ayonrinde OT, Adams LA, Doherty DA, Adverse metabolic phenotype of adolescent girls with non-alcoholic fatty liver disease plus polycystic ovary syndrome compared with other girls and boys: J Gastroenterol Hepatol, 2016; 31(5); 980-87
37. Tollefson MM, Van Houten HK, Asante D, Association of psoriasis with comorbidity development in children with psoriasis: JAMA Dermatol, 2018; 154(3); 286-92
38. Carreau AM, Pyle L, Garcia-Reyes Y, Clinical prediction score of nonalcoholic fatty liver disease in adolescent girls with polycystic ovary syndrome (PCOS-HS index): Clin Endocrinol (Oxf), 2019; 91(4); 544-52
39. Kim JY, Tfayli H, Michaliszyn SF, Distinguishing characteristics of metabolically healthy versus metabolically unhealthy obese adolescent girls with polycystic ovary syndrome: Fertil Steril, 2016; 105(6); 1603-11
40. Castro-Correia C, Santos-Silva R, Pinheiro M, Metabolic risk factors in adolescent girls with type 1 diabetes: J Pediatr Endocrinol Metab, 2018; 31(6); 631-35
41. Won YB, Seo SK, Yun BH, Non-alcoholic fatty liver disease in polycystic ovary syndrome women: Sci Rep, 2021; 11(1); 7085
42. Michaliszyn SF, Lee S, Tfayli H, Arslanian S, Polycystic ovary syndrome and nonalcoholic fatty liver in obese adolescents: Association with metabolic risk profile: Fertil Steril, 2013; 100(6); 1745-51
43. Leon G, de Klerk E, Ho J, Prevalence of comorbid conditions pre-existing and diagnosed at a tertiary care pediatric weight management clinic: J Pediatr Endocrinol Metab, 2018; 31(4); 385-90
44. Goldštajn M, Toljan K, Grgić F, Sex hormone binding globulin (SHBG) as a marker of clinical disorders: Coll Antropol, 2016; 40(3); 211-18
45. Jacome-Sosa MM, Parks EJ, Fatty acid sources and their fluxes as they contribute to plasma triglyceride concentrations and fatty liver in humans: Curr Opin Lipidol, 2014; 25(3); 213-20
46. Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ, Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease: Gastroenterology, 2014; 146(3); 726-35
47. Wasilewska N, Lebensztejn DM, Non-alcoholic fatty liver disease and lipotoxicity: Clin Exp Hepatol, 2021; 7(1); 1-6
48. de Zegher F, López-Bermejo A, Ibáñez L, Central obesity, faster maturation, and ‘PCOS’ in girls: Trends Endocrinol Metab, 2018; 29(12); 815-18
49. Lebensztejn DM, Flisiak-Jackiewicz M, Białokoz-Kalinowska I, Hepatokines and non-alcoholic fatty liver disease: Acta Biochim Pol, 2016; 63(3); 459-67
50. Lebensztejn DM, Białokoz-Kalinowska I, Kłusek-Oksiuta M, Serum fetuin A concentration is elevated in children with non-alcoholic fatty liver disease: Adv Med Sci, 2014; 59(1); 81-84
51. Patel S, Alvarez-Guaita A, Melvin A, GDF15 provides an endocrine signal of nutritional stress in mice and humans: Cell Metab, 2019; 29(3); 707-18e8
52. Mullican SE, Lin-Schmidt X, Chin CN, GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates: Nat Med, 2017; 23(10); 1150-57
53. Galuppo B, Agazzi C, Pierpont B, Growth differentiation factor 15 (GDF15) is associated with non-alcoholic fatty liver disease (NAFLD) in youth with overweight or obesity: Nutr Diabetes, 2022; 12(1); 9
54. Schernthaner-Reiter MH, Kasses D, Tugendsam C, Growth differentiation factor 15 increases following oral glucose ingestion: effect of meal composition and obesity: Eur J Endocrinol, 2016; 175(6); 623-31
55. Coll AP, Chen M, Taskar P, GDF15 mediates the effects of metformin on body weight and energy balance: Nature, 2020; 578(7795); 444-48
56. Jobira B, Frank DN, Pyle L, Obese adolescents with PCOS have altered biodiversity and relative abundance in gastrointestinal microbiota: J Clin Endocrinol Metab, 2020; 105(6); e2134-e44
57. Zhao X, Jiang Y, Xi H, Exploration of the relationship between gut microbiota and polycystic ovary syndrome (PCOS): A review: Geburtshilfe Frauenheilkd, 2020; 80(2); 161-71
58. Del Chierico F, Nobili V, Vernocchi P, Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach: Hepatology, 2017; 65(2); 451-64
59. Tokuhara D, Role of the gut microbiota in regulating non-alcoholic fatty liver disease in children and adolescents: Front Nutr, 2021; 8; 700058
60. Tremellen K, Pearce K, Dysbiosis of gut microbiota (DOGMA) – a novel theory for the development of polycystic ovarian syndrome: Med Hypotheses, 2012; 79(1); 104-12
61. Urbano F, Chiarito M, Lattanzio C, Sex hormone-binding globulin (SHBG) reduction: The alarm bell for the risk of non-alcoholic fatty liver disease in adolescents with polycystic ovary syndrome: Children (Basel), 2022; 9(11); 1748
62. Arner P, Petrus P, Esteve D, Screening of potential adipokines identifies S100A4 as a marker of pernicious adipose tissue and insulin resistance: Int J Obes (Lond), 2018; 42(12); 2047-56
63. Zdanowicz K, Białokoz-Kalinowska I, Lebensztejn DM, Non-alcoholic fatty liver disease in non-obese children: Hong Kong Med J, 2020; 26(5); 459-62
64. de Zegher F, Ibáňez L, Letter to the editor: Tackling NAFLD in adolescent polycystic ovary syndrome: Reducing liver fat to mimic weight loss: Hepatology, 2021; 73(4); 1623-24
In Press
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
12 Mar 2024 : Clinical Research
Comparing Neuromuscular Blockade Measurement Between Upper Arm (TOF Cuff®) and Eyelid (TOF Scan®) Using Miv...Med Sci Monit In Press; DOI: 10.12659/MSM.943630
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952