10 August 2023: Clinical Research
Impact of Maternal Preoperative Hemoglobin Levels on Intraoperative Hemorrhage Risk in Placenta Accrete Spectrum Disorders: A Retrospective Cross-Sectional Study
Baolian Zhang1AE, Hong Liu1AEF, Wenli Wang2BCD, Jia Wang3BCD, Yuping Jiang2CD, Wen Jia2BCD, Haiyan Li2C, He Zhu2C, Xiaona Du2EF, Xianghua Huang2EFG*DOI: 10.12659/MSM.940443
Med Sci Monit 2023; 29:e940443
Abstract
BACKGROUND: Placenta accreta spectrum (PAS) disorders involve abnormal adhesion or invasion of chorionic villi through the myometrium and uterine serosa. Maternal anemia during pregnancy is common and may contribute to complications during delivery, particularly with abnormal placentation. This study examines the association between preoperative maternal hemoglobin levels and the risk of intraoperative massive hemorrhage in pregnant women with PAS disorders.
MATERIAL AND METHODS: A retrospective study included 538 consecutive participants (mean age=31.12±4.68 years) who underwent cesarean sections and met the diagnostic criteria for PAS disorders. Logistic regression analysis was performed to investigate the relationship between maternal preoperative hemoglobin levels and the risk of massive intraoperative hemorrhage (blood loss ≥1500 mL).
RESULTS: The incidence of intraoperative massive hemorrhage among patients with PAS disorders was 38.66%. The mean preoperative maternal hemoglobin level was 10.99±1.39 g/dL, and overall anemia incidence (<11 g/dL) was 48.88% in our study. After adjusting for potential confounders, a non-linear relationship was observed between preoperative maternal hemoglobin levels and the risk of intraoperative massive hemorrhage. When the preoperative hemoglobin level of pregnant women was below 11.5 g/dL (OR=0.52, 95% CI 0.39-0.70), the lower hemoglobin level significantly increased the risk of intraoperative hemorrhage.
CONCLUSIONS: Maternal preoperative hemoglobin levels were inversely associated with the risk of massive intraoperative hemorrhage in PAS disorders. A non-linear relationship was identified, with a turning point at 11.5 g/dL. These findings emphasize the importance of monitoring and managing maternal hemoglobin levels to mitigate the risk of intraoperative hemorrhage in pregnant women with PAS disorders.
Keywords: Hemorrhage, Placenta, hemoglobin, Pregnancy, Female, Humans, Adult, Retrospective Studies, Cross-Sectional Studies, Placenta Accreta, Blood Loss, Surgical, Hemoglobins
Background
Placenta accreta spectrum (PAS) disorders are diseases in which all or part of the placenta tissue invades the maternal uterine myometrium to varying degrees, and the disorders encompass a heterogeneous group of anomalies characterized by an abnormal adhesion or invasion of chorionic villi through the myometrium and uterine serosa [1]. Irving and Hertig first described PAS disorders in 1937 as different degrees of villous tissue invading the muscle layer, and even the surrounding pelvic organs [2]. When placental tissue does not completely or partially spontaneously separate at delivery [3], it often causes life-threatening postpartum hemorrhage, resulting in multisystem organ failure, disseminated intravascular coagulation, need for admission to an intensive care unit, peripartum hysterectomy, and even death [4,5]. PAS disorders has always been a challenge for obstetricians to enhance clinical management, and their prevalence is increasing. Previous studies had suggested that planned delivery [6,7], reasonable time for pregnancy termination [8], delayed hysterectomy [9], and a variety of surgery approaches [10–13] for hemostatic control and minimizing adjacent organ injury reduced maternal mortality and morbidity in patients with PAS disorders.
A previous study demonstrated that women with gestational anemia are at an increased risk of severe postpartum hemorrhage during and after delivery [14]. However, there has been little research investigating the association between preoperative maternal hemoglobin levels and severe postpartum hemorrhage in PAS disorders. Therefore, we conducted a cross-sectional study to explore the relationship between the preoperative maternal hemoglobin level and the risk of intraoperative massive hemorrhage in pregnant women with PAS disorders, exploring potential etiological factors of intraoperative massive hemorrhage in PAS disorders.
Material and Methods
STUDY DESIGN AND PARTICIPANTS:
This cross-sectional retrospective study identified patients with discharge diagnosis of PAS disorders [15] (ICD-o72, ICD-o73), according to the codes of the International Classification of Disease, 10th revision. Data were extracted out of the electronic medical records at the Second Hospital of Hebei Medical University from January 1, 2014, to December 31, 2020. The basic clinical data were collected using a standard clinical audit protocol, and all data were fully anonymous before being submitted for analysis. This study was approved by the Research Ethics Committee of the hospital. The requirement for patient informed consent was waived because of the retrospective nature of this study.
EXCLUSION CRITERIA AND INCLUSION CRITERIA:
The patient exclusion criteria were as follows: (1) participants with internal and surgical diseases, such as diabetes mellitus or gestational diabetes mellitus, hypertensive disorder, thyroid diseases, hepatic diseases, heart disease, and acute pancreatitis; (2) stillbirth, fetal growth restriction, and placental abruption; (3) gestational age <28 weeks or >42 weeks; and (4) missing data on maternal preoperative hemoglobin level and intraoperative blood loss. The inclusion criteria of patients were as follows: (1) met the diagnose of PAS disorders according to FIGO classification for the clinical diagnosis of PAS disorders [15]; (2) gestational age 28 to 42 weeks; and (3) termination of pregnancy by cesarean delivery.
DATA COLLECTION AND MEASUREMENT:
EpiData v3.1 Software was used to extract and manage study data. The baseline characteristics of participants included age, occupation, region, body mass index (BMI), gravidity, and parity. Some potential confounders, such as weight changes, uterine anomalies, placental location, type of placenta previa, grade of PAS disorders, hemorrhage during pregnancy, assisted reproduction technology, and multiple pregnancies, were also collected. Intraoperative blood loss was measured according to cell protector and swab measurements from the surgical skin incision to the end of surgery within 2 h after delivery. Intraoperative massive hemorrhage was considered when the volume of postpartum hemorrhage exceeded more than 1500 mL within 2 h after surgery [16]. The level of preoperative maternal hemoglobin was determined from the last tested hemoglobin before delivery by a blood cell analyzer (UniCel DxH 800, Beckman Coulter). In addition, the location of placenta attachment and type of placenta previa were examined using the American GE-E10 color Doppler ultrasound by transabdominal or transabdominal combined with transvaginal scan before surgery [17]. BMI was calculated by the maternal height and the pregnant weight based on hospital inpatient report (kg/m2). The following variables were also collected: gestational age at delivery, as determined by last menstrual period and confirmed by ultrasound in all patients (week); uterine anomalies, including fibroid tumors or previous uterine surgery, such as dilatation and curettage, myomectomy, endometrial resection, or surgical abortion; hemorrhage during pregnancy with at least 1 episode of bleeding from the genital tract during the antenatal period; and grade of PAS disorders was defined according to the standardized terminology and clinical diagnosis on “placenta accrete spectrum disorders” in China [18].
STATISTICAL ANALYSIS:
Continuous variables were expressed as mean±SD (normal distribution) or median (quartile; skewed distribution), and categorical variables were expressed as a percentage. According to the recommendation of the STROBE statement [19], logistic regression was used to quantify unadjusted ORs and adjusted ORs and 95% CIs expressing the association between preoperative maternal hemoglobin level and the risk of intraoperative massive hemorrhage. We selected the potential confounders on the basis of their association with the outcomes of interest or a change in effect estimate of more than 10%. In addition, based on 5 replications and a chained equation approach method in the R MI procedure, the missing data of the covariates were imputed by multiple imputations. All of the analyses were performed with the statistical software package R (http://www.R-project.org, The R Foundation) and Empower Stats (http://www.empowerstats.com, X&Y Solutions, Inc, Boston, MA). P<0.05 was considered statistically significant.
Results
BASELINE CHARACTERISTICS OF PARTICIPANTS:
Among all pregnant women with caesarean delivery from January 1, 2014, to December 31, 2020, at the Second Hospital of Hebei Medical University, there were 1015 patients diagnosed with PAS disorders. Among them, 477 patients were excluded due to internal and surgical diseases (n=414), gestational age <28 weeks or >42 weeks (n=62), and missing data (n=1). Ultimately, a total of 538 consecutive patients met the inclusion criteria (Figure 1). The demographics and clinical characteristics of all participants are shown in Table 1. The average age of the participants was 31.12±4.68 years, and approximately 23.61% of them were older than 35 years. The mean level of preoperative maternal hemoglobin was 10.99±1.39 g/dL, the overall incidence of anemia (hemoglobin <11 g/dL) was 48.88%, and the incidence of intraoperative massive hemorrhage was 38.66%.
UNIVARIATE ANALYSIS:
The univariate analysis showed that age, parity, number of cesarean section, placental location (posterior, lateral, anterior or central), type of placenta previa, grade of PAS, hemorrhage during pregnancy, and antepartum anemia were positively associated with the risk of intraoperative massive hemorrhage. Univariate analysis also showed that the grade of PAS disorders was significantly associated with the risk of intraoperative massive hemorrhage. While, age ≥35 years, occupation, BMI, weight changes, gravidity, uterine anomalies or not, and multiple pregnancy were not associated with the risk of intraoperative massive hemorrhage. In contrast, urban or not (OR=0.60, 95% CI 0.41–0.87), gestational age at delivery (OR=0.82, 95% CI 0.76–0.89), vaginal delivery or not (OR=0.29, 95% CI 0.164.53), assistant reproduction technology (OR=0.45, 95% CI 0.21–0.97), and the level of preoperative maternal hemoglobin (OR=0.64, 95% CI 0.55–0.73) were significantly negatively correlated with the risk of intraoperative massive hemorrhage (Table 2).
UNIVARIATE LINEAR REGRESSION MODEL:
The univariate linear regression model was used to evaluate the association between the level of preoperative maternal hemoglobin and the risk of intraoperative massive hemorrhage. The original model, minimally adjusted model, and fully adjusted model are shown in Table 3. In the non-adjusted model, the level of preoperative maternal hemoglobin had a significant negatively correlation with the risk of intraoperative massive hemorrhage (OR=0.64, 95% CI 0.55–0.73, P<0.0001). In the minimally adjusted model (adjusted for gestational age at delivery, parity, type of placenta previa, and grade of PAS) and the fully adjusted model (adjusted for age, region, gestational age at delivery, gravidity, parity, vaginal delivery, uterine anomalies, assistant reproduction technology, placental location, type of placenta previa, hemorrhage during pregnancy, and grade of PAS), the changes of the effect were not obvious (OR=0.66, 95% CI 0.55–0.79, P<0.0001 and OR=0.65, 95% CI 0.53–0.79, P<0.0001, respectively). For sensitivity analysis, the level of preoperative maternal hemoglobin was a categorical variable; the fully adjusted ORs for patients in tertile 2 and tertile 3, when compared with those in tertile 1, were 0.35 (95% CI 0.19–0.64; P<0.001) and 0.31 (95% CI 0.16–0.58; P<0.001), respectively.
NON-LINEAR ANALYSIS AND 2-PIECEWISE LINEAR REGRESSION MODEL:
The relationship between the level of preoperative maternal hemoglobin and the risk of intraoperative massive hemorrhage was non-linear after adjusting for age, region, gestational age at delivery, gravidity, parity, vaginal delivery, uterine anomalies (fibroid tumors or previous uterine surgery or surgery abortion), assisted reproduction technology, placental location, type of placenta previa, hemorrhage during pregnancy, and grade of PAS (Figure 2). Moreover, a 2-piecewise linear regression model was used to calculate the inflection point (11.5 g/dL; Table 4). When the level of preoperative maternal hemoglobin was <11.5 g/dL, the preoperative maternal hemoglobin level increased by 1 g/dL, and the adjusted risk of intraoperative hemorrhage was reduced by 0.48 (OR=0.52; 95% CI 0.39–0.70; P<0.0001). However, there was no significant association between the level of preoperative maternal hemoglobin and the risk of intraoperative massive hemorrhage (OR=1.06, 95% CI 0.64–1.75 and P=0.7602) when the level of preoperative maternal hemoglobin was ≥11.5 g/dL.
Discussion
Our study showed that the median intraoperative blood lost was 1100 mL (range, 200–9850 mL) and the incidence of the intraoperative massive hemorrhage was 38.66%, which was slightly lower than that of reported studies [20]; the reason may be the current multidisciplinary approach and ongoing process improvement in the management of this disease. This study also provided some new insights. Lower preoperative maternal hemoglobin concentration, as a changeable factor, was found to be a predictor for intraoperative massive hemorrhage in PAS disorders. In this retrospective, cross-sectional study, we found that the correlation was non-linear between the level of preoperative maternal hemoglobin and the risk of intraoperative massive hemorrhage after adjusting for age, region, gestational age at delivery, gravidity, parity, vaginal delivery, uterine anomalies, assisted reproduction technology, placental location, type of placenta previa, hemorrhage during pregnancy, and grade of PAS disorders. Moreover, when the preoperative hemoglobin level of pregnant women was lower than 11.5 g/dL, there was a significant negative correlation between the preoperative hemoglobin level and risk of intraoperative massive hemorrhage in PAS disorders. However, when the preoperative maternal hemoglobin level was more than 11.5 g/dL, there was no significant correlation.
The presence of PAS disorders is correlated with the incidence of maternal diseases, such as life-threatening massive hemorrhage, which leads to secondary complications, including multisystem organ failure and hysterectomy [5,21–23]. Consequently, it is particularly important to optimize the preoperative hemoglobin levels in PAS disorders in order to improve the tolerance of intraoperative bleeding. According to evidence-based guidelines for the management of abnormally invasive placenta recommendations by the International Society for Abnormally Invasive Placenta, if the level of hemoglobin is lower than 11 g/dL before 28 weeks of gestation or lower than 10.5 g/dL after 28 weeks of gestation, iron supplementation should be given to optimize the patient’s hemoglobin level before surgery [24]. Retrospective cohort studies have reported that patients with anemia had a higher risk of postpartum hemorrhage than those without prenatal anemia [20,25]. Also, a meta-analysis suggested that severe prenatal anemia increased postpartum hemorrhage risk [26]. In the present study, we found similar results, namely that anemia was associated with a higher rate of intraoperative massive hemorrhage, and the correlation between the preoperative maternal hemoglobin level and the risk of intraoperative massive hemorrhage was a non-linear relationship [14]. However, a prospective cohort study by Parks et al [27] noted that only women with severe anemia had a significantly higher rate of postpartum hemorrhage, not found in mild and moderate anemia. The possible reason may be that our patients had lower tolerance for postpartum hemorrhage than those in the previous study. A slightly lower hemoglobin level has been found to be negatively associated with severe postpartum hemorrhage even without severe anemia.
The mechanisms underlying the association are not yet very clear. Soltan et al reported that women with anemia are prone to have postpartum hemorrhage, possibly due to uterine systolic fatigue caused by the raised nitric oxide levels [28]. A previous study also found a potential role that an impaired coagulation profile in anemic pregnant women could lead to postpartum hemorrhage [29]. Another possibility was the presence of low-grade prenatal fibrinolysis in severe anemic pregnant women, which could increase postpartum hemorrhage during delivery [30]. Further, more basic experimental studies are required to investigated if this connection is causal and to identify the underlying mechanism.
There were some limitations in this study. First, the study was a retrospective, cross-sectional observational study conducted in patients with PAS disorders at a single center; therefore, it reflected only the clinical characteristics and pregnancy outcomes of PAS disorders in this study population. Second, we did not review the use of medicines such as oxytocin, misoprostol, prostaglandin, and tranexamic acid in the operation and the surgical methods. It is noteworthy that the potential exposure factors resulting from such errors would bias the results toward a reduced occurrence of postpartum hemorrhage, and thus the results are an underestimation of the association between maternal preoperative hemoglobin concentration and massive intraoperative hemorrhage. Further, we should conduct multi-center clinical studies or prospective cohort studies to reduce selection bias in the future.
Conclusions
In conclusion, there was a non-linear relationship between preoperative maternal hemoglobin level and intraoperative massive hemorrhage; namely, the maternal preoperative hemoglobin level was significant negatively related to the risk of massive intraoperative hemorrhage when the hemoglobin was less than 11.5 g/dL. Given the high prevalence of PAS disorders, the result of the study might be of significance for clinicians to enhance antenatal care, and could help obstetricians in their evaluations and planning treatment strategies for patients with PAS disorders to reduce adverse pregnant outcomes.
Figures
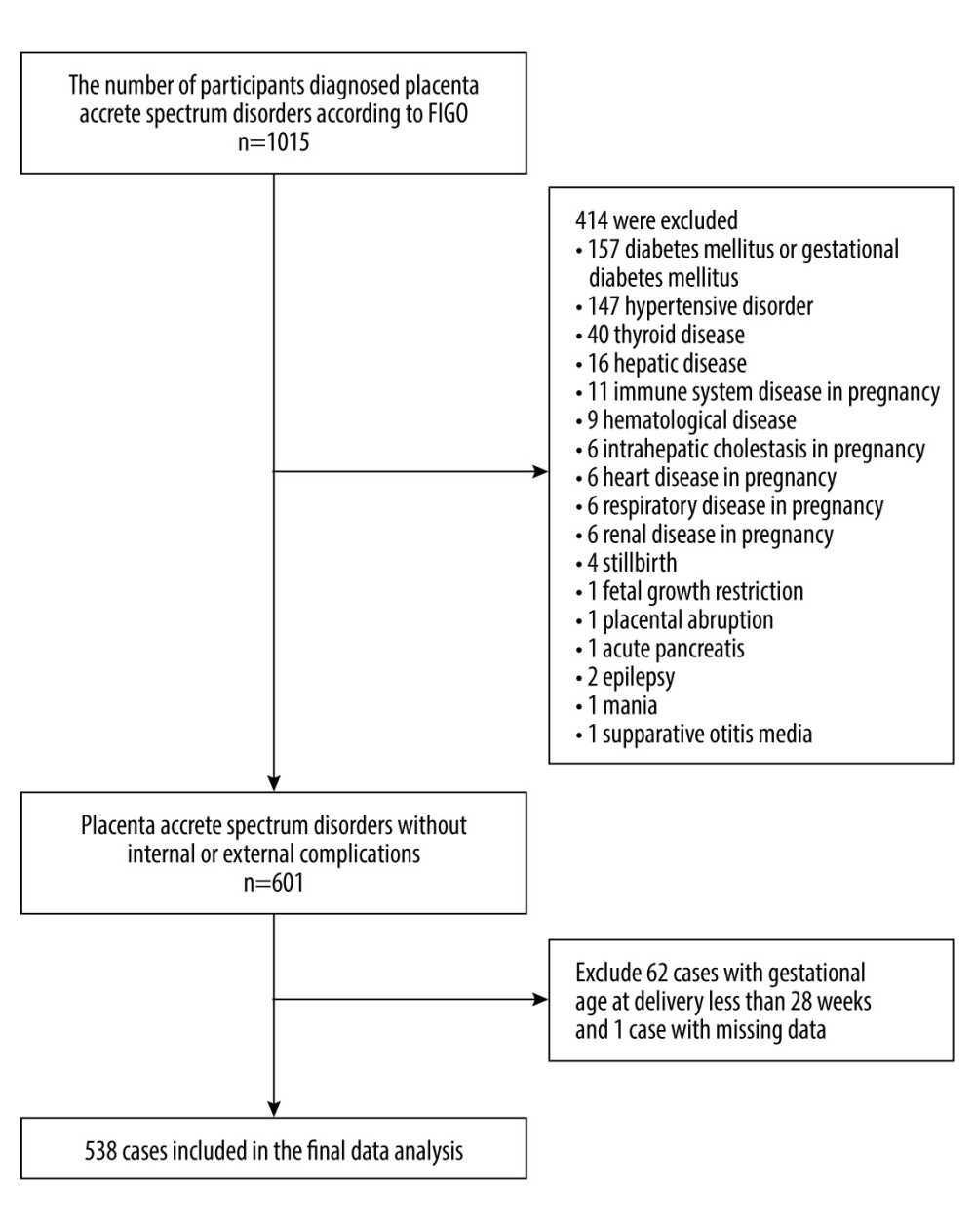 Figure 1. Flow chart of patient selection.
Figure 1. Flow chart of patient selection. ![The relationship between level of maternal preoperative hemoglobin and risk of massive intraoperative hemorrhage. (The statistical software package R [http://www.R-project.org, The R Foundation] and Empower Stats [http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA]). The horizontal coordinate presents the level of maternal preoperative hemoglobin, and the ordinate presents the risk of massive intraoperative hemorrhage (0, referred to non-massive intraoperative hemorrhage; 1, referred to massive intraoperative hemorrhage). A non-linear relationship between the level of maternal preoperative hemoglobin and risk of massive intraoperative hemorrhage was detected after adjusting confounders.](https://jours.isi-science.com/imageXml.php?i=medscimonit-29-e940443-g002.jpg&idArt=940443&w=1000) Figure 2. The relationship between level of maternal preoperative hemoglobin and risk of massive intraoperative hemorrhage. (The statistical software package R [http://www.R-project.org, The R Foundation] and Empower Stats [http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA]). The horizontal coordinate presents the level of maternal preoperative hemoglobin, and the ordinate presents the risk of massive intraoperative hemorrhage (0, referred to non-massive intraoperative hemorrhage; 1, referred to massive intraoperative hemorrhage). A non-linear relationship between the level of maternal preoperative hemoglobin and risk of massive intraoperative hemorrhage was detected after adjusting confounders.
Figure 2. The relationship between level of maternal preoperative hemoglobin and risk of massive intraoperative hemorrhage. (The statistical software package R [http://www.R-project.org, The R Foundation] and Empower Stats [http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA]). The horizontal coordinate presents the level of maternal preoperative hemoglobin, and the ordinate presents the risk of massive intraoperative hemorrhage (0, referred to non-massive intraoperative hemorrhage; 1, referred to massive intraoperative hemorrhage). A non-linear relationship between the level of maternal preoperative hemoglobin and risk of massive intraoperative hemorrhage was detected after adjusting confounders. Tables
Table 1. The demographic clinical characteristics of participants in this study.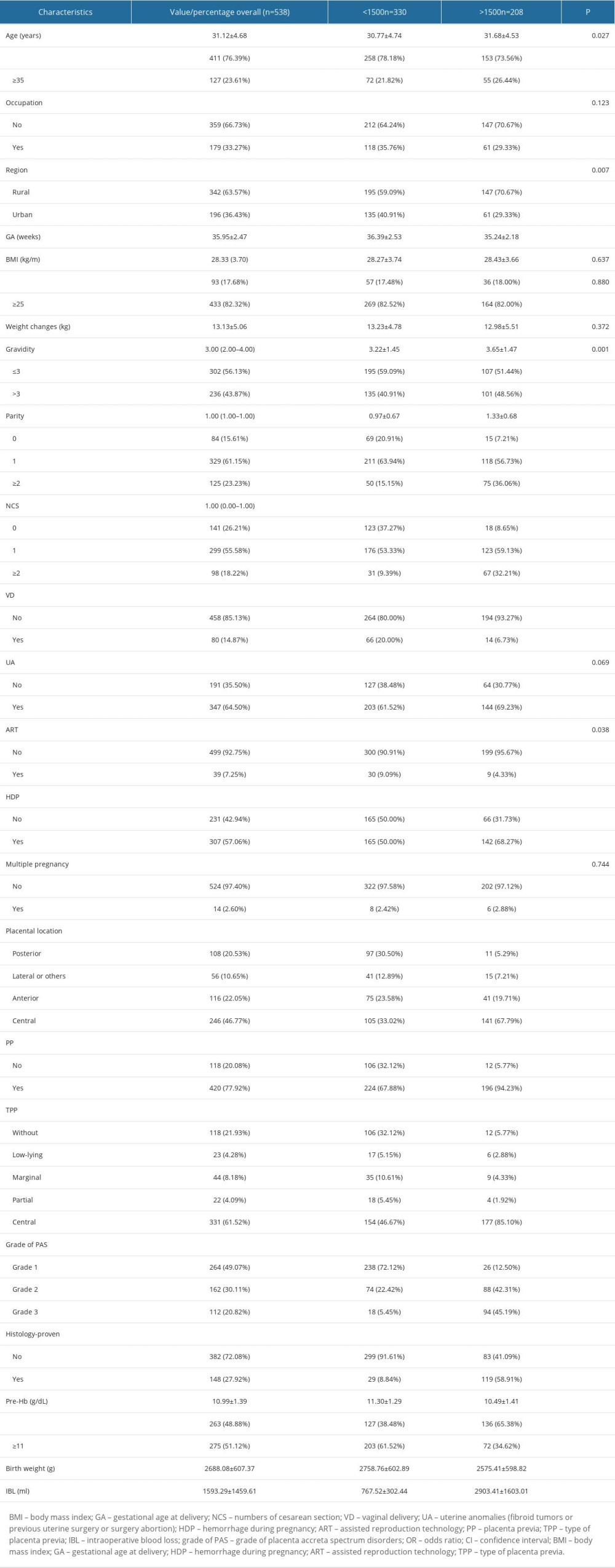 Table 2. The results of univariate analysis related to level of maternal preoperative hemoglobin and risk of massive intraoperative hemorrhage.
Table 2. The results of univariate analysis related to level of maternal preoperative hemoglobin and risk of massive intraoperative hemorrhage.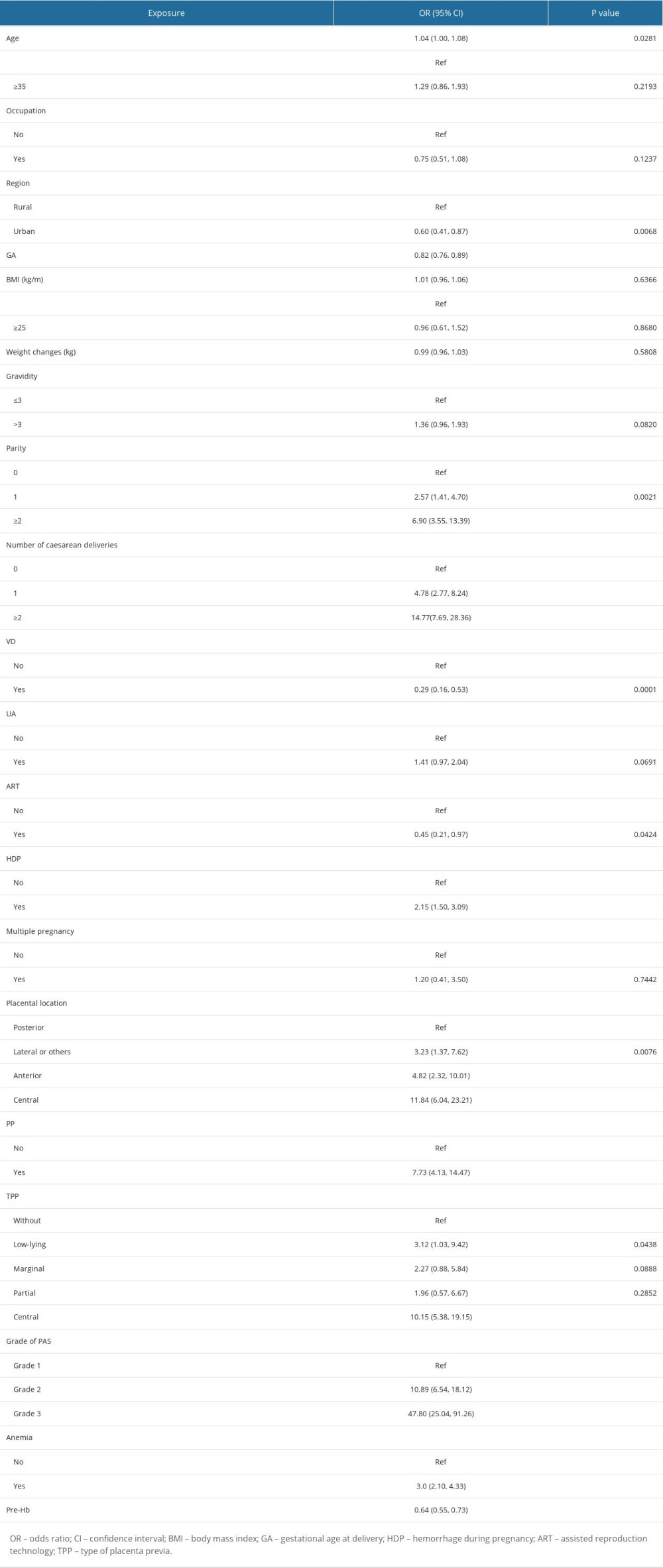 Table 3. Association between level of maternal preoperative hemoglobin and risk of massive intraoperative hemorrhage.
Table 3. Association between level of maternal preoperative hemoglobin and risk of massive intraoperative hemorrhage.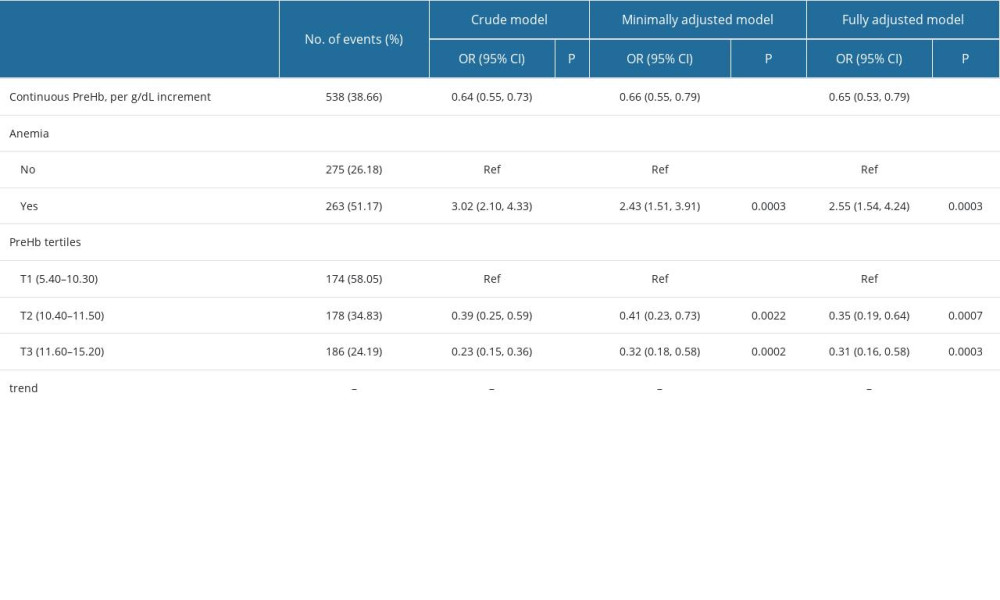 Table 4. The results of 2-piecewise linear regression model of association between level of maternal preoperative hemoglobin and risk of massive intraoperative hemorrhage.
Table 4. The results of 2-piecewise linear regression model of association between level of maternal preoperative hemoglobin and risk of massive intraoperative hemorrhage.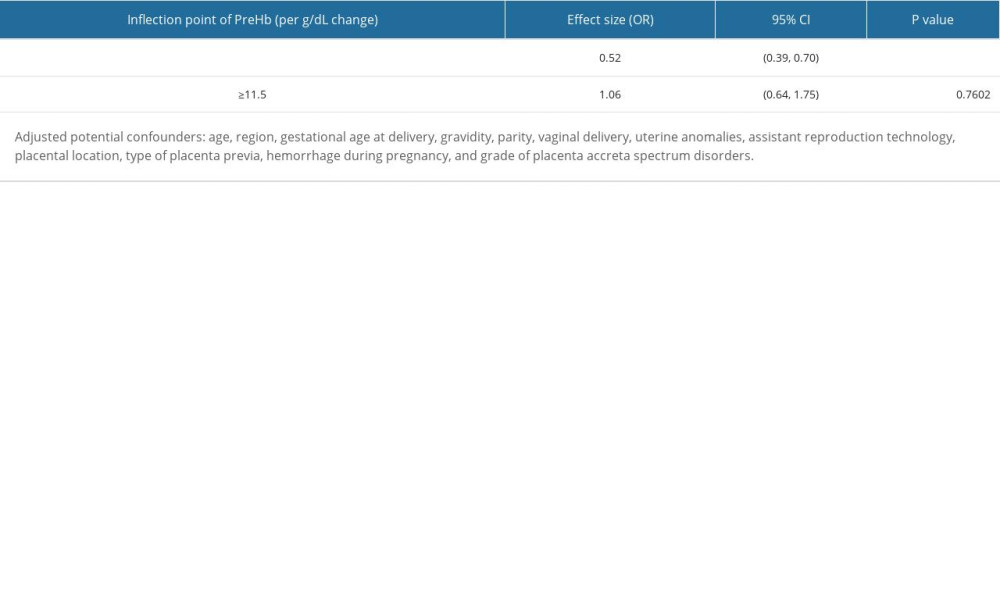
References
1. Belfort MA, Placenta accreta: Am J Obstet Gynecol, 2010; 203(5); 430-39
2. Jauniaux E, Collins S, Burton GJ, Placenta accreta spectrum: Pathophysiology and evidence-based anatomy for prenatal ultrasound imaging: Am J Obstet Gynecol, 2018; 218(1); 75-87
3. Thurn L, Lindqvist PG, Jakobsson M, Abnormally invasive placenta-prevalence, risk factors and antenatal suspicion: Results from a large population-based pregnancy cohort study in the Nordic countries: BJOG, 2016; 123(8); 1348-55
4. Jauniaux E, Bunce C, Grønbeck L, Langhoff-Roos J, Prevalence and main outcomes of placenta accreta spectrum: A systematic review and meta-analysis: Am J Obstet Gynecol, 2019; 221(3); 208-18
5. Marcellin L, Delorme P, Bonnet MP, Placenta percreta is associated with more frequent severe maternal morbidity than placenta accreta: Am J Obstet Gynecol, 2018; 219(2); 193e1-e9
6. Shamshirsaz AA, Fox KA, Erfani H, Outcomes of planned compared with urgent deliveries using a multidisciplinary team approach for morbidly adherent placenta: Obstet Gynecol, 2018; 131(2); 234-41
7. Durukan H, Durukan ÖB, Yazıcı FG, Planned versus urgent deliveries in placenta previa: Maternal, surgical and neonatal results: Arch Gynecol Obstet, 2019; 300(6); 1541-49
8. Wang Y, Zeng L, Niu Z, An observation study of the emergency intervention in placenta accreta spectrum: Arch Gynecol Obstet, 2019; 299(6); 1579-86
9. Zuckerwise LC, Craig AM, Newton JM, Outcomes following a clinical algorithm allowing for delayed hysterectomy in the management of severe placenta accreta spectrum: Am J Obstet Gynecol, 2020; 222(2); 179e1-e9
10. Sanad AS, Mahran AE, Aboulfotouh ME, The effect of uterine artery ligation in patients with central placenta pevia: A randomized controlled trial: BMC Pregnancy Childbirth, 2018; 18(1); 351
11. Shih JC, Liu KL, Kang J, ‘Nausicaa’ compression suture: A simple and effective alternative to hysterectomy in placenta accreta spectrum and other causes of severe postpartum haemorrhage: BJOG, 2019; 126(3); 412-17
12. Sichitiu J, El-Tani Z, Mathevet P, Desseauve D, Conservative surgical management of placenta accreta spectrum: A pragmatic approach: J Invest Surg, 2021; 34(2); 172-80
13. Kingdom JC, Hobson SR, Murji A, Minimizing surgical blood loss at cesarean hysterectomy for placenta previa with evidence of placenta increta or placenta percreta: The state of play in 2020: Am J Obstet Gynecol, 2020; 223(3); 322-29
14. Shields LE, Smalarz K, Reffigee L, Comprehensive maternal hemorrhage protocols improve patient safety and reduce utilization of blood products: Am J Obstet Gynecol, 2011; 205(4); 368.e1-8
15. Jauniaux E, Ayres-de-Campos D, Langhoff-Roos J, FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders: Int J Gynaecol Obstet, 2019; 146(1); 20-24
16. Shields LE, Smalarz K, Reffigee L, Comprehensive maternal hemorrhage protocols improve patient safety and reduce utilization of blood products: Am J Obstet Gynecol, 2011; 205(4); 368.e1-8
17. Reddy UM, Abuhamad AZ, Levine D, Saade GRFetal Imaging Workshop Invited Participants, Fetal imaging: Executive summary of a Joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, American Institute of Ultrasound in Medicine, American College of Obstetricians and Gynecologists, American College of Radiology, Society for Pediatric Radiology, and Society of Radiologists in Ultrasound Fetal Imaging Workshop: Am J Obstet Gynecol, 2014; 210(5); 387-97
18. Yang HX, Yan J, Liu XX, Chen DJ, Zhao YYThe standardized terminology and clinical diagnosis on “placenta accrete spectrum disorders” in China: Zhonghua Fu Chan Ke Za Zhi, 2021; 56(6); 377-79 [in Chinese]
19. Vandenbroucke JP, von Elm E, Altman DG, Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration: PLoS Med, 2007; 4(10); e297
20. Schwickert A, van Beekhuizen HJ, Bertholdt CInternational Society for Placenta Accreta Spectrum (IS-PAS), Association of peripartum management and high maternal blood loss at cesarean delivery for placenta accreta spectrum (PAS): A multinational database study: Acta Obstet Gynecol Scand, 2021; 100(Suppl 1); 129-40
21. Seoud MA, Nasr R, Berjawi GA, Placenta accreta: Elective versus emergent delivery as a major predictor of blood loss: J Neonatal Perinatal Med, 2017; 10(1); 9-15
22. Erfani H, Fox KA, Clark SL, Maternal outcomes in unexpected placenta accreta spectrum disorders: Single-center experience with a multidisciplinary team: Am J Obstet Gynecol, 2019; 221(4); 337.e1-e5
23. Zeng C, Yang M, Ding Y, Placenta accreta spectrum disorder trends in the context of the universal two-child policy in China and the risk of hysterectomy: Int J Gynaecol Obstet, 2018; 140(3); 312-18
24. Collins SL, Alemdar B, van Beekhuizen HJ, Evidence-based guidelines for the management of abnormally invasive placenta: Recommendations from the International Society for Abnormally Invasive Placenta: Am J Obstet Gynecol, 2019; 220(6); 511-26
25. Rukuni R, Bhattacharya S, Murphy MF, Maternal and neonatal outcomes of antenatal anemia in a Scottish population: a retrospective cohort study: Acta Obstet Gynecol Scand, 2016; 95(5); 555-64
26. Omotayo MO, Abioye AI, Kuyebi M, Eke AC, Prenatal anemia and postpartum hemorrhage risk: A systematic review and meta-analysis: J Obstet Gynaecol Res, 2021; 47(8); 2565-76
27. Parks S, Hoffman MK, Goudar SS, Maternal anaemia and maternal, fetal, and neonatal outcomes in a prospective cohort study in India and Pakistan: BJOG, 2019; 126(6); 737-43
28. Soltan MH, Ibrahim EM, Tawfek M, Raised nitric oxide levels may cause atonic postpartum hemorrhage in women with anemia during pregnancy: Int J Gynaecol Obstet, 2012; 116(2); 143-47
29. Nair M, Chhabra S, Choudhury SS, Relationship between anaemia, coagulation parameters during pregnancy and postpartum haemorrhage at childbirth: A prospective cohort study: BMJ Open, 2021; 11(10); e050815
30. Bolliger D, Szlam F, Levy JH, Haemodilution-induced profibrinolytic state is mitigated by fresh-frozen plasma: Implications for early haemostatic intervention in massive haemorrhage: Br J Anaesth, 2010; 104(3); 318-25
Figures
 Figure 1. Flow chart of patient selection.
Figure 1. Flow chart of patient selection.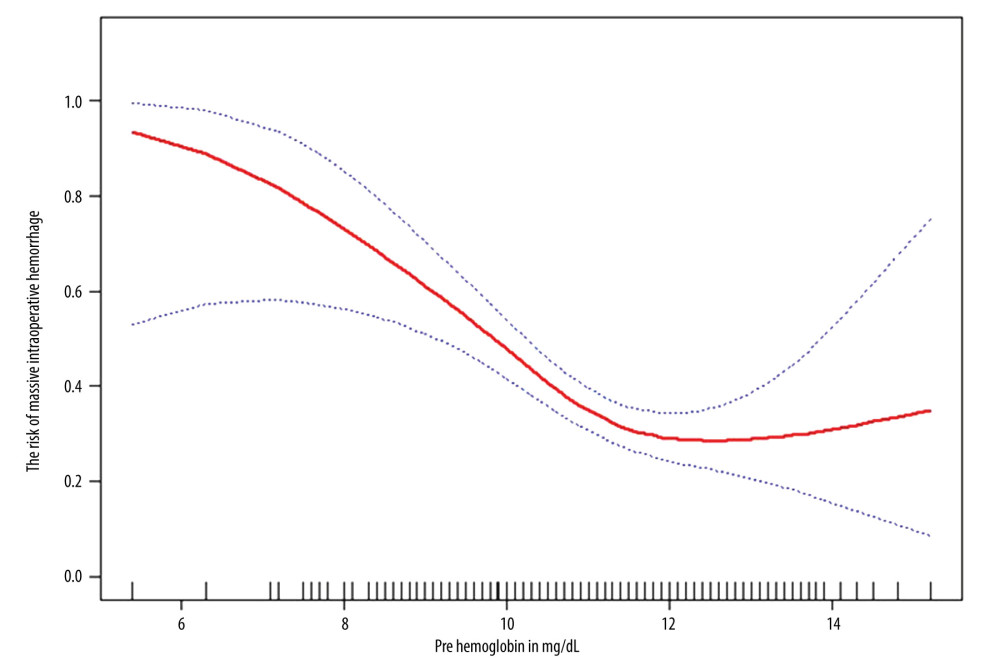 Figure 2. The relationship between level of maternal preoperative hemoglobin and risk of massive intraoperative hemorrhage. (The statistical software package R [http://www.R-project.org, The R Foundation] and Empower Stats [http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA]). The horizontal coordinate presents the level of maternal preoperative hemoglobin, and the ordinate presents the risk of massive intraoperative hemorrhage (0, referred to non-massive intraoperative hemorrhage; 1, referred to massive intraoperative hemorrhage). A non-linear relationship between the level of maternal preoperative hemoglobin and risk of massive intraoperative hemorrhage was detected after adjusting confounders.
Figure 2. The relationship between level of maternal preoperative hemoglobin and risk of massive intraoperative hemorrhage. (The statistical software package R [http://www.R-project.org, The R Foundation] and Empower Stats [http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA]). The horizontal coordinate presents the level of maternal preoperative hemoglobin, and the ordinate presents the risk of massive intraoperative hemorrhage (0, referred to non-massive intraoperative hemorrhage; 1, referred to massive intraoperative hemorrhage). A non-linear relationship between the level of maternal preoperative hemoglobin and risk of massive intraoperative hemorrhage was detected after adjusting confounders. Tables
 Table 1. The demographic clinical characteristics of participants in this study.
Table 1. The demographic clinical characteristics of participants in this study. Table 2. The results of univariate analysis related to level of maternal preoperative hemoglobin and risk of massive intraoperative hemorrhage.
Table 2. The results of univariate analysis related to level of maternal preoperative hemoglobin and risk of massive intraoperative hemorrhage. Table 3. Association between level of maternal preoperative hemoglobin and risk of massive intraoperative hemorrhage.
Table 3. Association between level of maternal preoperative hemoglobin and risk of massive intraoperative hemorrhage. Table 4. The results of 2-piecewise linear regression model of association between level of maternal preoperative hemoglobin and risk of massive intraoperative hemorrhage.
Table 4. The results of 2-piecewise linear regression model of association between level of maternal preoperative hemoglobin and risk of massive intraoperative hemorrhage. Table 1. The demographic clinical characteristics of participants in this study.
Table 1. The demographic clinical characteristics of participants in this study. Table 2. The results of univariate analysis related to level of maternal preoperative hemoglobin and risk of massive intraoperative hemorrhage.
Table 2. The results of univariate analysis related to level of maternal preoperative hemoglobin and risk of massive intraoperative hemorrhage. Table 3. Association between level of maternal preoperative hemoglobin and risk of massive intraoperative hemorrhage.
Table 3. Association between level of maternal preoperative hemoglobin and risk of massive intraoperative hemorrhage. Table 4. The results of 2-piecewise linear regression model of association between level of maternal preoperative hemoglobin and risk of massive intraoperative hemorrhage.
Table 4. The results of 2-piecewise linear regression model of association between level of maternal preoperative hemoglobin and risk of massive intraoperative hemorrhage. In Press
12 Mar 2024 : Clinical Research
Metabolomic Alterations in Methotrexate Treatment of Moderate-to-Severe PsoriasisMed Sci Monit In Press; DOI: 10.12659/MSM.943360
14 Mar 2024 : Clinical Research
Renal Dysfunction Increases Risk of Adverse Cardiovascular Events in 5-Year Follow-Up Study of Intermediate...Med Sci Monit In Press; DOI: 10.12659/MSM.943956
15 Mar 2024 : Clinical Research
Impact of One-Lung Ventilation on Oxygenation and Ventilation Time in Thoracoscopic Heart Surgery: A Compar...Med Sci Monit In Press; DOI: 10.12659/MSM.943089
14 Mar 2024 : Clinical Research
Differential DHA and EPA Levels in Women with Preterm and Term Births: A Tertiary Hospital Study in IndonesiaMed Sci Monit In Press; DOI: 10.12659/MSM.943895
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








