08 December 2023: Clinical Research
Efficacy Analysis of Neoadjuvant versus Adjuvant Cisplatin-Paclitaxel Regimens for Initial Treatment of FIGO Stages IB3 and IIA2 Cervical Cancer
Wei TaoDOI: 10.12659/MSM.940545
Med Sci Monit 2023; 29:e940545
Abstract
BACKGROUND: Large cancer lesions are often challenging to treat with surgical intervention alone. Neoadjuvant chemotherapy is frequently used for FIGO stage IB3 and IIA2 cervical cancers to optimize the outcomes of radical surgeries. This study aimed to compare the effectiveness of neoadjuvant chemotherapy, followed by adjuvant chemotherapy and radiotherapy, if necessary, with the traditional approach of adjuvant chemotherapy and radiotherapy after radical hysterectomy in treatment-naïve patients with cervical cancer of specified stages.
MATERIAL AND METHODS: A total of 245 female patients were administered either 70 to 85 mg/m² cisplatin and 165 to 175 mg/m² paclitaxel every 21 days (2 cycles) prior to radical hysterectomy, followed by adjuvant chemotherapy and radiotherapy if needed (neoadjuvant therapy, NT cohort, n=105), or received adjuvant chemotherapy and radiotherapy after radical hysterectomy adjuvant therapy, AT cohort, n=140).
RESULTS: In the NT cohort, 76% of patients responded to neoadjuvant chemotherapy, while 24% did not. Adverse operative, intraoperative, and postoperative outcomes were significantly more common among the non-responders (P<0.05). After 5 years, 91% of responders and 72% of non-responders survived without recurrence (P=0.0372), and 3% of responders and 28% of non-responders had died (P=0.0005).
CONCLUSIONS: The resistance to neoadjuvant chemotherapy is a poor prognostic factor. Neoadjuvant chemotherapy followed by radical hysterectomy and adjuvant chemotherapy/radiotherapy appears to be advantageous for cervical cancer patients who respond well to neoadjuvant chemotherapy.
Keywords: Carcinoma, Gynecology, Hysterectomy, Neoadjuvant Therapy, Uterus
Background
Cervical cancer is a major issue for women globally [1]. Cervical cancer is the second most common malignancy in Chinese women [2]. In the last 20 years, the incidence rate of cervical cancer in younger Chinese women has increased [3]. For non-metastatic cervical cancer, excluding International Federation of Gynecology and Obstetrics (FIGO) stages IA, IB1, IB2, and IIA1 cancers, concurrent platinum-based chemoradiation is recommended by the National Comprehensive Cancer Network (NCCN) guidelines [4]. However, clinical applications of these guidelines are limited because of the unavailability of radiotherapy facilities in China [5]. Concurrent platinum-based chemoradiation has significant adverse effects on reproductive organs during clinical management of cervical cancer [6], for example, vaginal injuries, ovarian failure, damage to endocrine function, pelvic tissue degeneration, and impaired sexual lifestyle [7]. Women who received concurrent platinum-based chemoradiation therapies are mostly unable to receive surgical resections because of severe pelvic tissue injuries. Therefore, gynecologists and oncologists want to improve current surgical practices for cervical cancers [5].
In women with cervical cancer stage IB3 (larger than 4 cm) and IIA2 (spread from the cervix to the upper two-thirds of the vagina), radical surgery is preferred. However, huge cancerous lesions are difficult to handle with only surgery [5]. In many parts of the world, especially in China, neoadjuvant concurrent platinum-based chemotherapy is a more accepted approach for stage IB3 and IIA2 cervical cancers to overcome the errors of radical surgeries [5]. It is also recommended by the National Health Commission of the People’s Republic of China [8]. Neoadjuvant chemotherapy has fewer vaginal injuries caused by radical surgeries, better outcomes for quality of life, and better ovarian functions for premenopausal women [9–11]. Trials on Chinese women with stage IB3 and IIA2 cervical cancer [5,12] reported better efficacy and safety of neoadjuvant chemotherapy. However, neoadjuvant chemotherapy delays the surgical resection process and the concurrent platinum-based chemoradiation therapies that would worsen the clinical conditions of patients, especially if they were irresponsive to neoadjuvant chemotherapy [5,13,14].
The objectives of this retrospective study were to evaluate the efficacy of neoadjuvant chemotherapy following radical hysterectomy and adjuvant chemotherapy and radiotherapy, if required, against adjuvant chemotherapy and radiotherapy, if required, after radical hysterectomy in treatment-naïve patients with cervical cancer stages IB3 and IIA2.
Material and Methods
ETHICS APPROVAL AND CONSENT TO PARTICIPATE:
The designed protocol of the established study (approval number: 15XCH17 dated March 21, 2018) was approved by the Xuzhou Central Hospital Review Board and the Chinese Society of Clinical Oncology. The study follows the 2008 Declarations of Helsinki and the law of China. Because this was a retrospective study, informed consent was waived by the Xuzhou Central Hospital Review Board.
INCLUSION CRITERIA:
Female patients who were 18 to 60 years of age and had pathologically and radiologically confirmed stage IB3 and IIA2 cervical cancer before the start of any chemotherapies and/or radiotherapies were included in the analysis.
EXCLUSION CRITERIA:
Patients who received any kind of chemotherapies and/or radiotherapies for other kinds of cancer were excluded from the study. Patients with respiratory, cardiac, kidney, and/or liver diseases were excluded from the study.
SAMPLE SIZE CALCULATION:
The sample size was 100 based on the assumption that the 5-year survival would be 60% or more in patients who would receive neoadjuvant chemotherapy following radical hysterectomy plus adjuvant chemoradiotherapy or who would receive radical hysterectomy plus adjuvant chemoradiotherapy [15], with α=0.05 and β=0.2.
COHORTS:
A total of 105 patients received 70 to 85 mg/m2 cisplatin plus 165 to 175 mg/m2 paclitaxel every 21 days (2 cycles) before radical hysterectomy and adjuvant chemotherapy and radiotherapy (if required) after radical hysterectomy (NT cohort). A radical hysterectomy was performed 3 weeks after the last cycle of neoadjuvant chemotherapy. A total of 140 patients received adjuvant chemotherapy and radiotherapy (if required) after radical hysterectomy (AT cohort). The treatment was suspended due to hematological toxicity (anemia, neutropenia) and/or allergic reactions (vomiting, skin peeling, unconsciousness).
RADICAL HYSTERECTOMY:
Robot-assisted or laparoscopic radical hysterectomy or open surgery (ie, abdominal radical hysterectomy) was performed for radical surgeries.
ADJUVANT CHEMOTHERAPY AND RADIOTHERAPY FOR NT COHORT:
After surgery, pathological analysis was performed for the resected part. Risk factors, for example, lymph node metastasis, two-thirds or more depth of interstitial infiltration, parametrial infiltration, and lymph vascular space invasion, were evaluated. If patients had any of the risk factors, they received 4 cycles of 70 to 85 mg/m2 cisplatin plus paclitaxel 165 to 175 mg/m2 every 21 days. If patients had none of the risk factors, they received 2 cycles of 70 to 85 mg/m2 cisplatin plus paclitaxel 165 to 175 mg/m2 every 21 days. If patients had positive pelvic lymph nodes, they received 6 cycles of 70 to 85 mg/m2 cisplatin plus paclitaxel 165 to 175 mg/m2 every 21 days. In addition, patients with positive vaginal margins received additional radiation therapy [5].
ADJUVANT CHEMOTHERAPY AND RADIOTHERAPY FOR AT COHORT:
After surgery, pathological analysis was performed for the resected part. Risk factors, for example, lymph node metastasis, two-thirds or more depth of interstitial infiltration, parametrial infiltration, and lymph vascular space invasion, were evaluated. If patients had any of the risk factors, they received 6 cycles of 70 to 85 mg/m2 cisplatin plus paclitaxel 165 to 175 mg/m2 every 21 days. If patients had none of the risk factors, they received 4 cycles of 70 to 85 mg/m2 cisplatin plus paclitaxel 165 to 175 mg/m2 every 21 days. If patients had positive pelvic lymph nodes, they received 8 cycles of 70 to 85 mg/m2 cisplatin plus paclitaxel 165 to 175 mg/m2 every 21 days. In addition, patients with positive vaginal margins received additional radiation therapy [5].
FOLLOW-UP PERIOD:
Patients were followed up every 3 months for the first 2 years, every 6 months from 3 to 5 years, and once a year after 5 years.
RESPONSE TO NEOADJUVANT CHEMOTHERAPY:
In the NT cohort, after neoadjuvant chemotherapy and before radical hysterectomy, a biopsy was performed from the cervix. The absence of tumor cells and lymph nodes in the pathology results was defined as a complete response, and a partial response was defined as a 30% or greater decrease in the sum of the longest diameter of the tumor in size. A total of 20% or greater increase in the sum of longest diameters with an absolute increase of 5 mm or more, or detection of new lesions in the pathology report, was defined as progressive disease. Neither partial response nor progressive disease was defined as stable disease. The Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1) was used to evaluate response in pathological examinations [16]. Complete response and partial response were defined as sensitivity to neoadjuvant chemotherapy, and progressive disease and stable disease were defined as insensitivity to neoadjuvant chemotherapy.
PROGRESSION-FREE SURVIVAL:
Progression-free survival was defined as patient survival without recurrence.
OVERALL SURVIVAL:
Overall survival was defined as the survival of patients from detection of disease to death by any cause.
During routine follow-up, the biopsy was performed from the cervix. A total of 20 % or more increase in the sum of longest diameters with an absolute increase of 5 mm or more, or detection of new lesions in pathology was defined as disease.
STATISTICAL ANALYSIS:
InStat 3.01 GraphPad Software (San Diego, CA, USA) was used for statistical analysis. Categorial parameters are depicted as frequency (percentages), and continuous parameters are depicted as mean±standard error of the mean or median (Q3-Q1). The Mann-Whitney test was performed for nonlinear continuous parameters. The linearity of continuous parameters was evaluated using the Kolmogorov-Smirnov method. An unpaired
Results
STUDY POPULATION:
From January 15, 2017, to March 20, 2018, in total, 264 female patients aged 18 to 60 years with pathologically and radiologically confirmed stage IB3 and IIA2 cervical cancer were reported at the Xuzhou Central Hospital, China, and referring hospitals. The data of 19 patients were excluded from the study: 15 patients had received chemotherapy and/or radiotherapy for cancer, 1 patient had respiratory disease, 1 patient had cardiac disease, 1 patient had kidney disease, and 1 patient had liver disease. Data from a total of 245 patients in response to neoadjuvant chemotherapy of the NT cohort, response of adjuvant chemoradiotherapy of both cohorts, and follow-up time were included in the analysis (medical records were accessed to obtain patients information). The flowchart of the study is presented in Figure 1.
DEMOGRAPHICAL AND CLINICAL CHARACTERISTICS:
Before chemoradiotherapy, the age, hemoglobin content, platelet count, body mass index, tumor size, and FIGO stages of patients were comparable between cohorts (P>0.05 for all; Table 1).
RESPONSE TO NEOADJUVANT CHEMOTHERAPY:
A total of 80 (76%) patients in the NT cohort were sensitive to neoadjuvant chemotherapy, and 25 (24%) patients in the NT cohort were insensitive to neoadjuvant chemotherapy. The details of response to neoadjuvant chemotherapy are reported in Table 2.
DEMOGRAPHICAL AND CLINICAL CHARACTERISTICS OF THE NT COHORT:
Patients who were insensitive to neoadjuvant chemotherapy were older and had lower hemoglobin content, higher platelet count, lower body mass index, higher tumor size, and higher IIA2 stage than those who were sensitive to neoadjuvant chemotherapy before chemoradiotherapies (P<0.05 for all; Table 3).
OPERATIVE PARAMETERS:
Operative parameters of patients were comparable between cohorts (P>0.05 for all). In the NT cohort, more patients underwent robot-assisted radical hysterectomy who were sensitive to neoadjuvant chemotherapy, and more patients underwent abdominal open surgeries who were insensitive to neoadjuvant chemotherapy. Intraoperative hemorrhage, parametrium excision, gastrointestinal tract injuries, infection, catheter time, and postoperative drainage were higher in patients who were insensitive to neoadjuvant chemotherapy than in those who were sensitive to neoadjuvant chemotherapy (P<0.05 for all). The details of the operative parameters of patients of the NT cohort are shown in Table 4.
PROGRESSION-FREE SURVIVAL: After 5 years, 90 (86%) patients from the NT cohort and 111 (79%) patients from the AT cohort survived without recurrence (P=0.1745, Fisher exact test). Progression-free survival in 5 years between the NT cohort and the AT cohort was similar (P=0.9999, χ2 test, degree of freedom (df): 14; Figure 2). After 5 years 73 (91%) patients who were sensitive to neoadjuvant chemotherapy and 18 (72%) patients who were insensitive to neoadjuvant chemotherapy survived without recurrence (P=0.0372, Fisher exact test). Progression-free survival in 5 years was similar for patients who were sensitive to neoadjuvant chemotherapy and for those who were insensitive to neoadjuvant chemotherapy (P=0.9999, χ2 test, df: 14; Figure 3).
OVERALL SURVIVAL: After 5 years, 9 (9%) patients from the NT cohort and 20 (14%) patients from the AT cohort died (P=0.2307, Fisher exact test). Overall survival of patients in 5 years was the same between the NT cohort and the AT cohort (P=0.9999, χ2 test, df: 14, Figure 4). After 5 years, 2 (3%) patients sensitive to neoadjuvant chemotherapy and 7 (28%) patients insensitive to neoadjuvant chemotherapy died (P=0.0005, Fisher exact test). Overall survival in 5 years was similar for patients who were sensitive to neoadjuvant chemotherapy and for those who were insensitive to neoadjuvant chemotherapy (P=0.9996, χ2 test, df: 14; Figure 5).
INDEPENDENT PARAMETER:
Age higher than 40 years, hemoglobin level of 9.5 g/dL or lower, platelet count higher than 300×109/L, body mass index of 23.5 kg/m2 or lower, tumor size of 5 cm or higher, and stage IIA2 were independent parameters for an insensitive response to neoadjuvant chemotherapies. The details of the independent parameters of patients with insensitive response to neoadjuvant chemotherapies are shown in Table 5.
Discussion
After 5 years, a statistically equivalent but higher number of patients with progression-free survival and overall survival was found in the NT cohort than in the AT cohort. The results of progression-free survival and overall survival of the present study agree with those of the NCT03308591 trial [5] in China, the phase III trial in Japan [15], and a meta-analysis [17]. In addition, gynecologic oncologists in China prefer adjuvant chemotherapy and radiotherapy after radical hysterectomy for patients with intermediate or high risk factors (IB3 and IIA2) [5]. Therefore, of neoadjuvant chemotherapy following adjuvant chemotherapy and radiotherapy is a better recommendation in treatment-naïve patients with stage IB3 and IIA2 cervical cancer.
In the present study, operative, intraoperative, and postoperative complications were worse and progression-free survival and overall survival were lower in patients who were insensitive to neoadjuvant chemotherapy than in those who were sensitive to neoadjuvant chemotherapy. Response to neoadjuvant chemotherapy predicts favorable prognosis in cervical cancer patients [18]. The results of operative complications, progression-free survival, and overall survival for patients who received neoadjuvant chemotherapy in the present study are not consistent with those of the NCT03308591 trial [5] on Chinese patients, but are consistent with results from a meta-analysis [19]. The NCT03308591 trial [5] is not completely balanced at baseline, which affects the efficacy of neoadjuvant chemotherapy. In the present study, neoadjuvant chemotherapy following adjuvant chemotherapy and radiotherapy was beneficial for treatment-naïve patients with stage IB3 and IIA2 cervical cancer who were sensitive to neoadjuvant chemotherapy.
The clinical parameters of patients were independent factors for response to neoadjuvant chemotherapy. The association of clinical parameters of patients with the response to neoadjuvant chemotherapy in the present study agrees with that of the NCT03308591 trial [5] on Chinese patients and a meta-analysis [20]. Prognostic factors of treatment-naïve patients with stage IB3 and IIA2 cervical cancer may have favorable clinical outcomes after neoadjuvant chemotherapy.
In the present study, the cisplatin-based regimen was preferred for neoadjuvant chemotherapy. In the literature, both cisplatin- and carboplatin-based treatments were used in neoadjuvant cervical cancer studies. It is known that objective responses are higher with cisplatin-based treatments [21]. Therefore, in the present study, cisplatin was specifically preferred for its high response rates.
The present study was built on the concept that it did not follow the international guidelines because of limitations in the radiotherapy machines; however, we mentioned that radiotherapy was used when needed. The possible justification for this is that the proper treatment of the selected cohort should have been definitive chemoradiotherapy.
Although the clinical parameters of patients were comparable between cohorts before chemotherapies were administered, there are some limitations of the study. For example, this was a retrospective, single-institution, small-sample study. The authors have not shown any differences regarding overall survival and progression free survival in the 2 studied cohorts, neoadjuvant chemotherapies vs adjuvant chemoradiotherapies. The only new information, namely significant differences in overall survival and progression-free survival, within the neoadjuvant cohort referred to the response to neoadjuvant chemotherapy; however, the sensitive subpopulation was not compared with the adjuvant chemotherapies cohort, which is the standard of care. No information of the type of irradiation (brachytherapy or external beam radiotherapy) or the total radiative doses were given. The discussion is based mainly on local Chinese recommendations and studies. The sample size calculation, that was based in a very low outcome rate (60% 5-year survival) for this group of patients, that was expected to be above 75%; the number of patients submitted to radiotherapy was not specified; and patients with worse response to the NT cohort were at the same time patients already known to have worse prognosis, among others limitations. The safety of neoadjuvant chemotherapy was not evaluated and reported for the patients, and could be part of a future study.
Conclusions
Neoadjuvant chemotherapy and adjuvant chemoradiotherapy are better recommendations for treatment-naïve patients with stage IB3 and IIA2 cervical cancer and are beneficial for this group of patients who are more sensitive to neoadjuvant chemotherapy than adjuvant chemotherapy. The prognostic factors of treatment-naïve patients with stage IB3 and IIA2 cervical cancer may have favorable clinical outcomes after neoadjuvant chemotherapy. Prognostic factors of treatment-naïve patients with cervical cancer stages IB3 and IIA2 may have favorable clinical outcomes after neoadjuvant chemotherapy. The findings would be helpful for places where radiotherapy is not available. The data contained in this manuscript are interesting and helpful for the design of future clinical trials. We do not suggest that preoperative chemotherapy results may be a biomarker for prognosis, ie, chemotherapy results in ovarian cancer.
Figures
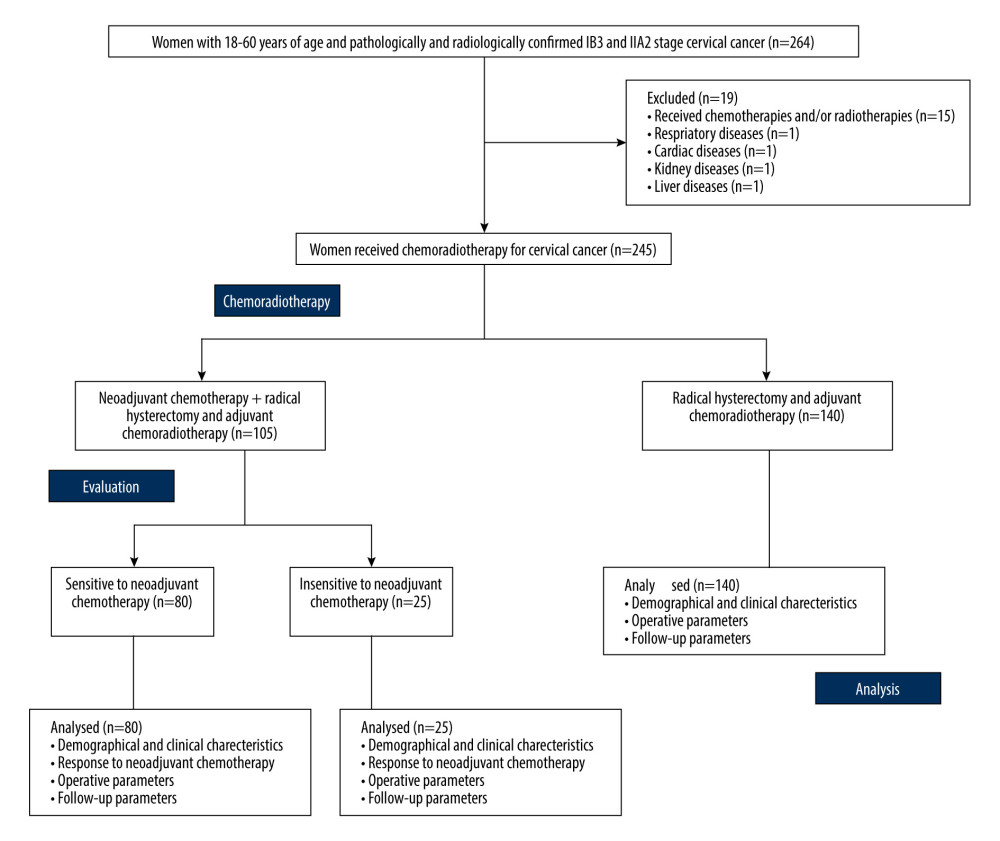 Figure 1. The flowchart of the study.
Figure 1. The flowchart of the study. 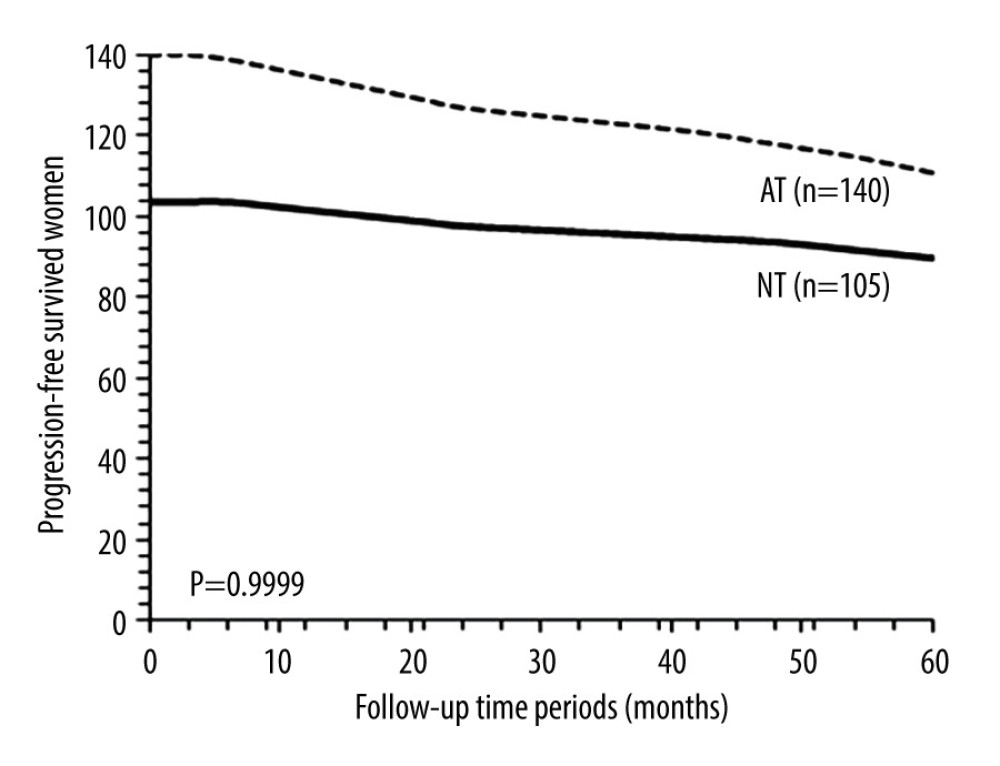 Figure 2. Progression-free survival between the NT and the AT cohorts. Progression-free survival: survival of patients after adjuvant chemotherapy without recurrence or detection of disease. NT cohort: patients received 70–85 mg/m2 cisplatin plus 165–175 mg/m2 paclitaxel at every 21 days before radical hysterectomy and adjuvant chemotherapy and radiotherapy (if required) after radical hysterectomy; AT cohort: patients received adjuvant chemotherapy and radiotherapy (if required) after radical hysterectomy. χ2 test was used for statistical analysis.
Figure 2. Progression-free survival between the NT and the AT cohorts. Progression-free survival: survival of patients after adjuvant chemotherapy without recurrence or detection of disease. NT cohort: patients received 70–85 mg/m2 cisplatin plus 165–175 mg/m2 paclitaxel at every 21 days before radical hysterectomy and adjuvant chemotherapy and radiotherapy (if required) after radical hysterectomy; AT cohort: patients received adjuvant chemotherapy and radiotherapy (if required) after radical hysterectomy. χ2 test was used for statistical analysis. 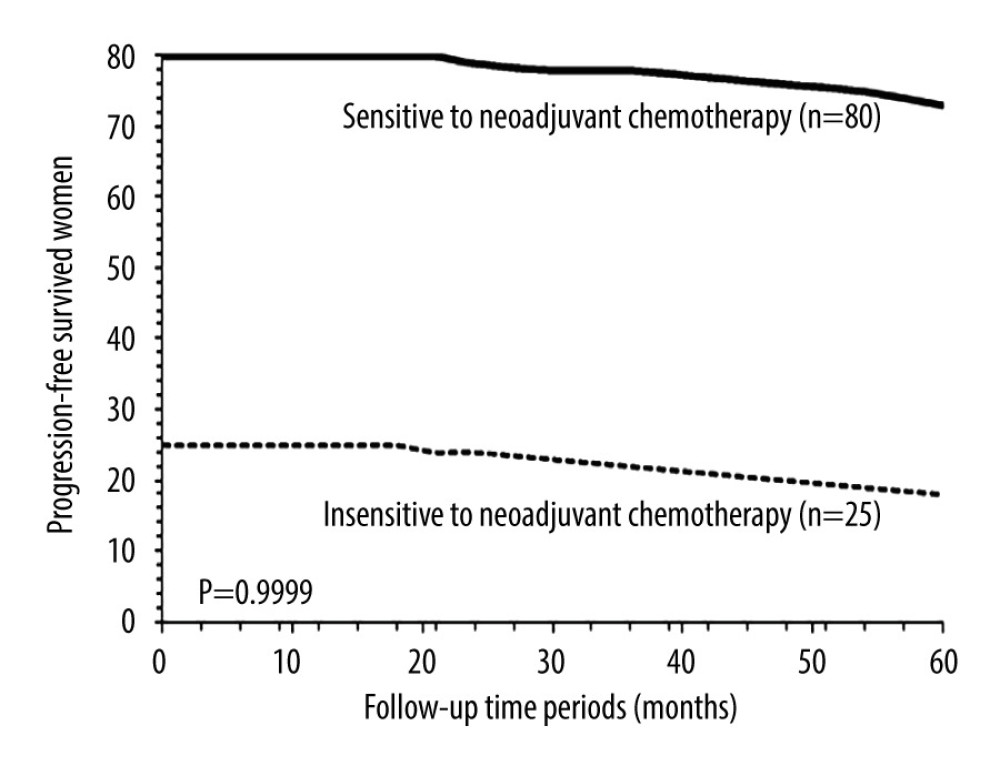 Figure 3. Progression-free survival between patients sensitive to neoadjuvant chemotherapy and patients insensitive to neoadjuvant chemotherapy. Progression-free survival: survival of patients after adjuvant chemotherapy without recurrence or detection of disease. χ2 test was used for statistical analysis.
Figure 3. Progression-free survival between patients sensitive to neoadjuvant chemotherapy and patients insensitive to neoadjuvant chemotherapy. Progression-free survival: survival of patients after adjuvant chemotherapy without recurrence or detection of disease. χ2 test was used for statistical analysis. 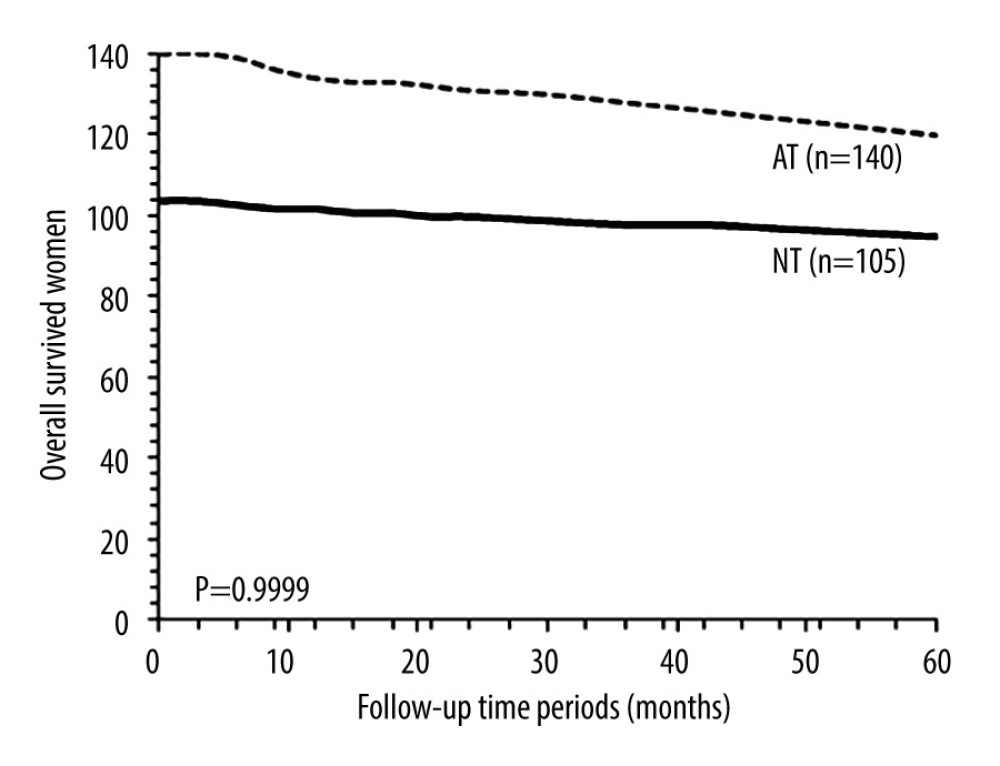 Figure 4. Overall survival between the NT and the AT cohorts. Overall survival: survival of patients from detection of disease to death after adjuvant chemotherapy by any cause. NT cohort: patients received 70–85 mg/m2 cisplatin plus 165–175 mg/m2 paclitaxel at every 21 days before radical hysterectomy and adjuvant chemotherapy and radiotherapy (if required) after radical hysterectomy; AT cohort: patients received adjuvant chemotherapy and radiotherapy (if required) after radical hysterectomy. χ2 test was used for statistical analysis.
Figure 4. Overall survival between the NT and the AT cohorts. Overall survival: survival of patients from detection of disease to death after adjuvant chemotherapy by any cause. NT cohort: patients received 70–85 mg/m2 cisplatin plus 165–175 mg/m2 paclitaxel at every 21 days before radical hysterectomy and adjuvant chemotherapy and radiotherapy (if required) after radical hysterectomy; AT cohort: patients received adjuvant chemotherapy and radiotherapy (if required) after radical hysterectomy. χ2 test was used for statistical analysis. 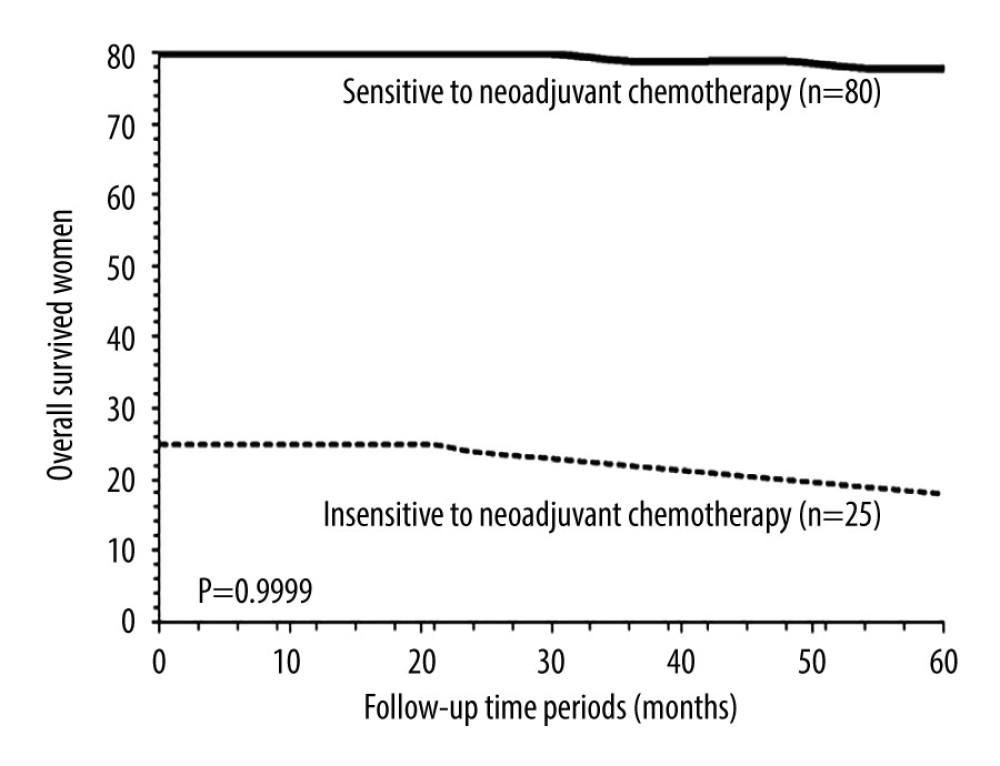 Figure 5. Overall survival between patients sensitive to neoadjuvant chemotherapy and patients insensitive to neoadjuvant chemotherapy. Overall survival: survival of patients from detection of disease to death after adjuvant chemotherapy by any cause. χ2 test was used for statistical analysis.
Figure 5. Overall survival between patients sensitive to neoadjuvant chemotherapy and patients insensitive to neoadjuvant chemotherapy. Overall survival: survival of patients from detection of disease to death after adjuvant chemotherapy by any cause. χ2 test was used for statistical analysis. Tables
Table 1. Demographic and clinical characteristics of patients before chemoradiotherapies.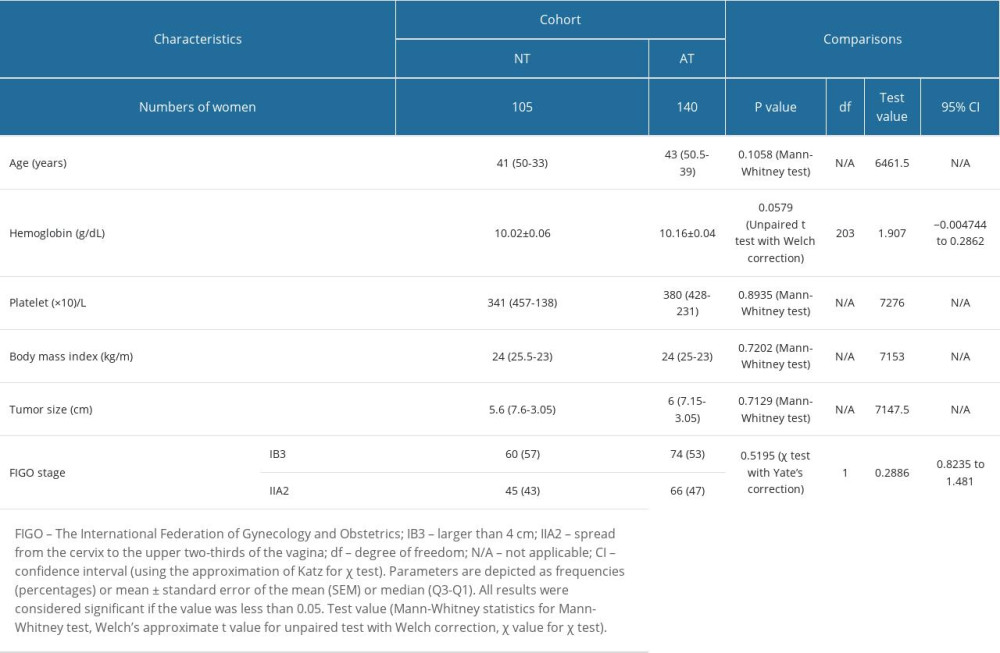 Table 2. Response to neoadjuvant chemotherapy for patients in the NT cohort.
Table 2. Response to neoadjuvant chemotherapy for patients in the NT cohort.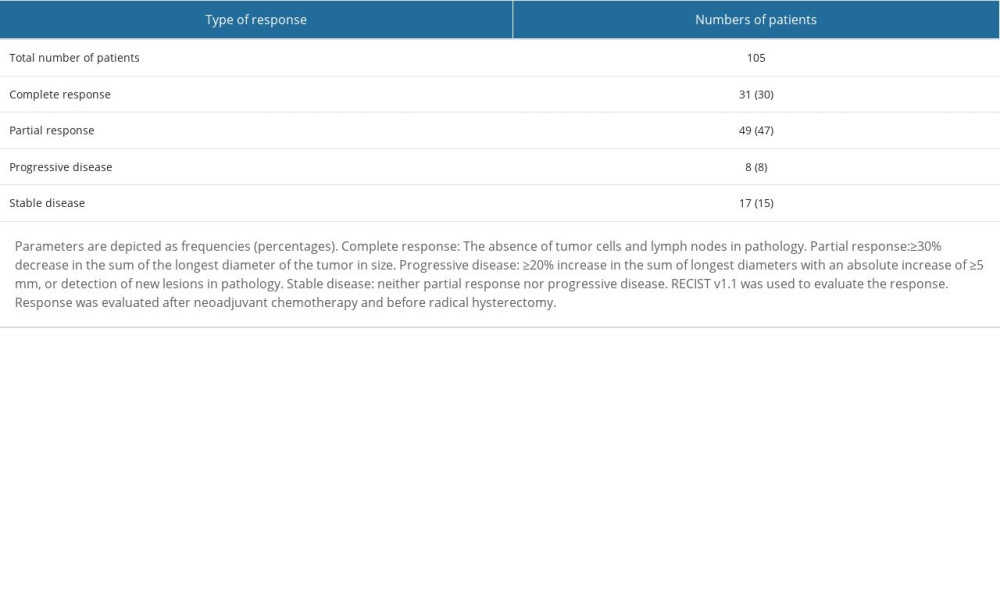 Table 3. Demographic and clinical characteristics of patients before chemoradiotherapies in the NT cohort.
Table 3. Demographic and clinical characteristics of patients before chemoradiotherapies in the NT cohort.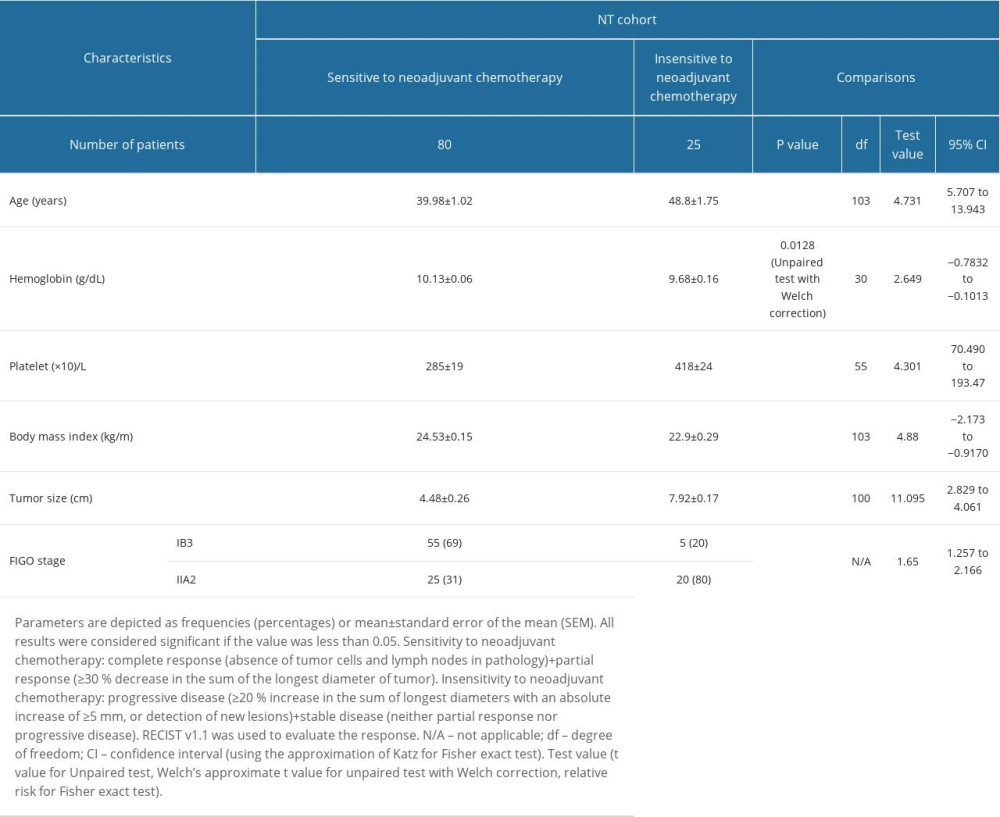 Table 4. Operative parameters of patients in the NT cohort.
Table 4. Operative parameters of patients in the NT cohort.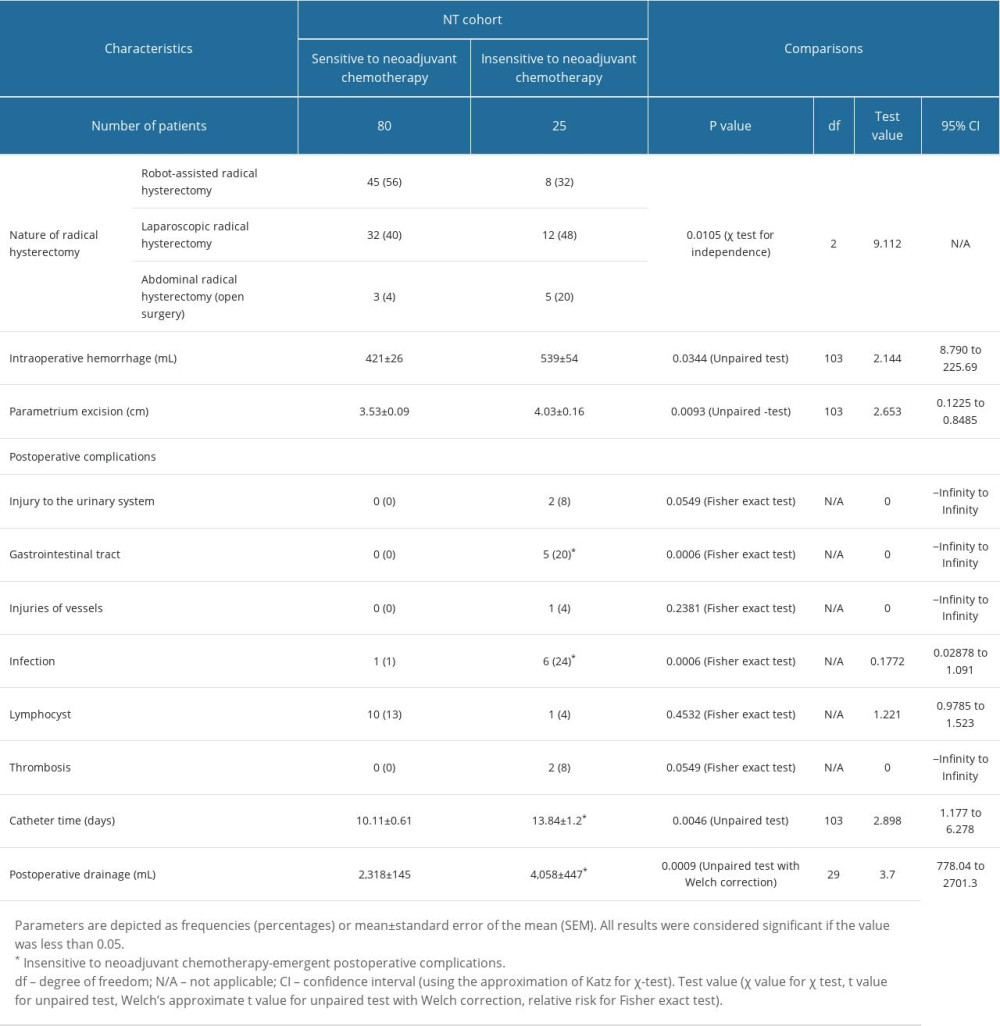 Table 5. Parameters of patients responsible for the insensitive response of neoadjuvant chemotherapies.
Table 5. Parameters of patients responsible for the insensitive response of neoadjuvant chemotherapies.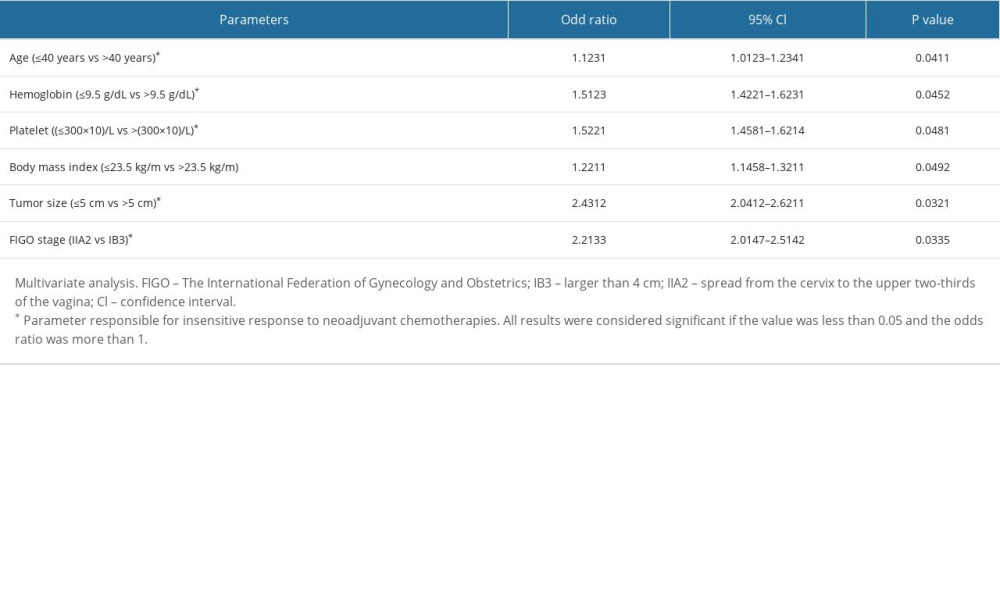
References
1. Sung H, Ferlay J, Siegel RL, Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries: Cancer J Clin, 2021; 71(3); 209-49
2. Xu Q, Wang J, Sun Y, Efficacy and safety of sintilimab plus anlotinib for PD-L1-positive recurrent or metastatic cervical cancer: A multicenter, single-arm, prospective phase II trial: J Clin Oncol, 2022; 40(16); 1795-805
3. Shen X, Cheng Y, Ren F, Shi Z, The burden of cervical cancer in China: Front Oncol, 2022; 12; 979809
4. National Comprehensive Cancer Network, NCCN Clinical Practice Guidelines in Oncology: Cervical Cancer Available from: www.nccn.org/professionals/physician_gls/pdf/cervical.pdf
5. Hu Y, Han Y, Shen Y, Neoadjuvant chemotherapy for patients with international federation of gynecology and obstetrics stages IB3 and IIA2 cervical cancer: A multicenter prospective trial: BMC Cancer, 2022; 22(1); 1270
6. Cosgrove CM, Salani R, Ovarian effects of radiation and cytotoxic chemotherapy damage: Best Pract Res Clin Obstet Gynaecol, 2019; 55; 37-48
7. Suvannasarn R, Muangmool T, Wongpakaran N, Charoenkwan K, Health-related quality of life for early-stage cervical cancer survivors after primary radical surgery followed by radiotherapy versus radical surgery alone: J Obstet Gynaecol, 2022; 42(5); 1217-24
8. National Health Commission of the People’s Republic of China, National guidelines for diagnosis and treatment of cervical cancer 2022 in China (English version): Chin J Cancer Res, 2022; 34(3); 256-69
9. Rob L, Skapa P, Robova H, Fertility-sparing surgery in patients with cervical cancer: Lancet Oncol, 2011; 12(12); 192-200
10. Bentivegna E, Gouy S, Maulard A, Oncological outcomes after fertility-sparing surgery for cervical cancer: A systematic review: Lancet Oncol, 2016; 17(6); e240-e53
11. Dolmans MM, Manavella DD, Recent advances in fertility preservation: J Obstet Gynaecol Res, 2019; 45(2); 266-79
12. Li J, Li Y, Wang H, Neoadjuvant chemotherapy with weekly cisplatin and paclitaxel followed by chemoradiation for locally advanced cervical cancer: BMC Cancer, 2023; 23(1); 51
13. Gupta S, Maheshwari A, Parab P, Neoadjuvant chemotherapy followed by radical surgery versus concomitant chemotherapy and radiotherapy in patients with stage IB2, IIA, or IIB squamous cervical cancer: A randomized controlled trial: J Clin Oncol, 2018; 36(16); 1548-55
14. Hu T, Li S, Chen Y, Matched-case comparison of neoadjuvant chemotherapy in patients with FIGO stage IB1-IIB cervical cancer to establish selection criteria: Eur J Cancer, 2012; 48(15); 2353-60
15. Katsumata N, Yoshikawa H, Kobayashi H, Phase III randomised controlled trial of neoadjuvant chemotherapy plus radical surgery vs radical surgery alone for stages IB2, IIA2, and IIB cervical cancer: A Japan Clinical Oncology Group trial (JCOG 0102): Br J Cancer, 2013; 108(10); 1957-63
16. Ko CC, Yeh LR, Kuo YT, Chen JH, Imaging biomarkers for evaluating tumor response: RECIST and beyond: Biomark Res, 2021; 9(1); 52
17. Kim HS, Sardi JE, Katsumata N, Efficacy of neoadjuvant chemotherapy in patients with FIGO stage IB1 to IIA cervical cancer: An international collaborative meta-analysis: Eur J Surg Oncol, 2013; 39(2); 115-24
18. Ye Q, Yuan HX, Chen HL, Responsiveness of neoadjuvant chemotherapy before surgery predicts favorable prognosis for cervical cancer patients: A meta-analysis: J Cancer Res Clin Oncol, 2013; 139(11); 1887-98
19. Hou Q, Shen J, Zhou S, Early response to neoadjuvant chemotherapy helps decrease recurrence rate of cervical cancer: A systematic review and meta-analysis: Ann Palliat Med, 2021; 10(6); 6092-103
20. Liu B, Gao S, Li S, A comprehensive comparison of CT, MRI, positron emission tomography or positron emission tomography/CT, and diffusion weighted imaging-MRI for detecting the lymph nodes metastases in patients with cervical cancer: A meta-analysis based on 67 studies: Gynecol Obstet Invest, 2017; 82(3); 209-22
21. Ilhan Y, Tatli AM, Teker F, Cisplatin plus paclitaxel and bevacizumab versus carboplatin plus paclitaxel and bevacizumab for the first-line treatment of metastatic or recurrent cervical cancer: Int J Gynecol Cancer, 2022; 32(4); 502-7
Figures
 Figure 1. The flowchart of the study.
Figure 1. The flowchart of the study. Figure 2. Progression-free survival between the NT and the AT cohorts. Progression-free survival: survival of patients after adjuvant chemotherapy without recurrence or detection of disease. NT cohort: patients received 70–85 mg/m2 cisplatin plus 165–175 mg/m2 paclitaxel at every 21 days before radical hysterectomy and adjuvant chemotherapy and radiotherapy (if required) after radical hysterectomy; AT cohort: patients received adjuvant chemotherapy and radiotherapy (if required) after radical hysterectomy. χ2 test was used for statistical analysis.
Figure 2. Progression-free survival between the NT and the AT cohorts. Progression-free survival: survival of patients after adjuvant chemotherapy without recurrence or detection of disease. NT cohort: patients received 70–85 mg/m2 cisplatin plus 165–175 mg/m2 paclitaxel at every 21 days before radical hysterectomy and adjuvant chemotherapy and radiotherapy (if required) after radical hysterectomy; AT cohort: patients received adjuvant chemotherapy and radiotherapy (if required) after radical hysterectomy. χ2 test was used for statistical analysis. Figure 3. Progression-free survival between patients sensitive to neoadjuvant chemotherapy and patients insensitive to neoadjuvant chemotherapy. Progression-free survival: survival of patients after adjuvant chemotherapy without recurrence or detection of disease. χ2 test was used for statistical analysis.
Figure 3. Progression-free survival between patients sensitive to neoadjuvant chemotherapy and patients insensitive to neoadjuvant chemotherapy. Progression-free survival: survival of patients after adjuvant chemotherapy without recurrence or detection of disease. χ2 test was used for statistical analysis. Figure 4. Overall survival between the NT and the AT cohorts. Overall survival: survival of patients from detection of disease to death after adjuvant chemotherapy by any cause. NT cohort: patients received 70–85 mg/m2 cisplatin plus 165–175 mg/m2 paclitaxel at every 21 days before radical hysterectomy and adjuvant chemotherapy and radiotherapy (if required) after radical hysterectomy; AT cohort: patients received adjuvant chemotherapy and radiotherapy (if required) after radical hysterectomy. χ2 test was used for statistical analysis.
Figure 4. Overall survival between the NT and the AT cohorts. Overall survival: survival of patients from detection of disease to death after adjuvant chemotherapy by any cause. NT cohort: patients received 70–85 mg/m2 cisplatin plus 165–175 mg/m2 paclitaxel at every 21 days before radical hysterectomy and adjuvant chemotherapy and radiotherapy (if required) after radical hysterectomy; AT cohort: patients received adjuvant chemotherapy and radiotherapy (if required) after radical hysterectomy. χ2 test was used for statistical analysis. Figure 5. Overall survival between patients sensitive to neoadjuvant chemotherapy and patients insensitive to neoadjuvant chemotherapy. Overall survival: survival of patients from detection of disease to death after adjuvant chemotherapy by any cause. χ2 test was used for statistical analysis.
Figure 5. Overall survival between patients sensitive to neoadjuvant chemotherapy and patients insensitive to neoadjuvant chemotherapy. Overall survival: survival of patients from detection of disease to death after adjuvant chemotherapy by any cause. χ2 test was used for statistical analysis. Tables
 Table 1. Demographic and clinical characteristics of patients before chemoradiotherapies.
Table 1. Demographic and clinical characteristics of patients before chemoradiotherapies. Table 2. Response to neoadjuvant chemotherapy for patients in the NT cohort.
Table 2. Response to neoadjuvant chemotherapy for patients in the NT cohort. Table 3. Demographic and clinical characteristics of patients before chemoradiotherapies in the NT cohort.
Table 3. Demographic and clinical characteristics of patients before chemoradiotherapies in the NT cohort. Table 4. Operative parameters of patients in the NT cohort.
Table 4. Operative parameters of patients in the NT cohort. Table 5. Parameters of patients responsible for the insensitive response of neoadjuvant chemotherapies.
Table 5. Parameters of patients responsible for the insensitive response of neoadjuvant chemotherapies. Table 1. Demographic and clinical characteristics of patients before chemoradiotherapies.
Table 1. Demographic and clinical characteristics of patients before chemoradiotherapies. Table 2. Response to neoadjuvant chemotherapy for patients in the NT cohort.
Table 2. Response to neoadjuvant chemotherapy for patients in the NT cohort. Table 3. Demographic and clinical characteristics of patients before chemoradiotherapies in the NT cohort.
Table 3. Demographic and clinical characteristics of patients before chemoradiotherapies in the NT cohort. Table 4. Operative parameters of patients in the NT cohort.
Table 4. Operative parameters of patients in the NT cohort. Table 5. Parameters of patients responsible for the insensitive response of neoadjuvant chemotherapies.
Table 5. Parameters of patients responsible for the insensitive response of neoadjuvant chemotherapies. In Press
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
12 Mar 2024 : Clinical Research
Comparing Neuromuscular Blockade Measurement Between Upper Arm (TOF Cuff®) and Eyelid (TOF Scan®) Using Miv...Med Sci Monit In Press; DOI: 10.12659/MSM.943630
11 Mar 2024 : Clinical Research
Enhancement of Frozen-Thawed Human Sperm Quality with Zinc as a Cryoprotective AdditiveMed Sci Monit In Press; DOI: 10.12659/MSM.942946
12 Mar 2024 : Database Analysis
Risk Factors of Age-Related Macular Degeneration in a Population-Based Study: Results from SHIP-TREND-1 (St...Med Sci Monit In Press; DOI: 10.12659/MSM.943140
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








