16 September 2023: Review Articles
A Review of the Role of Tendon Stem Cells in Tendon-Bone Regeneration
Junjie Chen1EFG, Chuanfeng Jiang1EF, Lu Yin1EF, Yingqi Liu2EF, Yue He3EF, Sen Li4EF, Huarui Shen1FG*DOI: 10.12659/MSM.940805
Med Sci Monit 2023; 29:e940805
Abstract
ABSTRACT: Tendon-bone injuries are a prevalent health concern associated with sports and other physically demanding activities. These injuries have a limited innate healing ability, often leading to the formation of scar tissue rather than the regeneration of healthy tendon tissue. This scar tissue results from excessive fibrosis during the early healing process and often leads to reduced tendon function and an increased risk of reinjury. Traditionally, surgical reconstruction has been the primary treatment for tendon-bone injuries. However, restoring the natural structure and mechanical properties of tendons after surgical reconstruction presents a considerable challenge. Recently, the potential of stem cell therapy has been explored as an alternative treatment approach. In particular, a new type of pluripotent stem cell known as tendon stem cells (TDSCs) has been identified within tendon tissue. These cells exhibit the potential for self-renewal and multidirectional differentiation, meaning they can differentiate into fibroblasts and chondrocytes. These differentiated cells can aid in the repair and regeneration of new tissues by producing collagen and other matrix molecules that provide structural support. TDSCs have become a focal point in research for treating tendon-bone injuries and related conditions. The potential use of these cells provides a basis for both basic research and clinical applications, particularly in understanding the tendon-bone healing process and identifying factors that affect the ability of TDSCs to promote this healing. This review article aims to analyze the role of TDSCs in tendon-bone healing, understanding their therapeutic potential and contributing to the development of effective treatment strategies for tendon-bone injuries.
Keywords: Epidermal Growth Factor, Stem Cell Transplantation, Bone-Patellar Tendon-Bone Grafts, Humans, Cicatrix, Tendons, Tendon Injuries, Pluripotent Stem Cells, Bone Regeneration
Background
Tendon-bone injury is a kind of tendon interface injury represented by cruciate ligament and rotator cuff injuries. Now that society pays increasing attention to physical activities, the incidence of tendon injuries has also increased. Tendon-bone healing is common in both the workplace and sports, with more than 30 million anterior cruciate ligament injuries reported globally each year in the United States, and tendon strains accounting for about 30% to 50% of sports injuries [1,2]. In addition, the incidence of rotator cuff injuries, which gradually increases with age, is approximately 200 000 annually, with $7 billion per year spent on the treatment and rehabilitation of rotator cuff diseases [3,4]. At present, most treatment strategies cannot prevent scarring and change the natural course of the disease. Shoulder arthroscopic rotator cuff repair surgery can achieve good clinical results, but there is still a 13% to 94% re-tear rate after surgery [5]. After tendon insertion injury, tendon-bone healing is mainly due to the proliferation of scar tissue, and the biomechanical strength of tendon insertion is significantly reduced [6]. Tendon-bone healing after tendon insertion reconstruction is mainly indirect healing. That is, the formation of initial fibrous scars between interfaces and then the formation of collagen fibers are gradually organized, thereby forming Sharpey-like collagen fibers, which are related to interface strength; and their number and size have a great influence on the biomechanical effects of grafts [7–9]. A variety of clinical treatment options have been adopted to prevent the healing of tendon-bone injury scars, all of which have a poor therapeutic effect. Therefore, how to better treat tendon-bone injury, alleviate the clinical symptoms of patients, and delay the progression of the disease have become problems in the field of orthopedics [10]. With the development of tissue engineering technology, stem cells for tendon-bone injury treatment methods have achieved ideal results. Current research has shown tendon stem cells (TDSCs) are the most suitable stem cells for the tendon interface, and TDSCs can differentiate into tendon cells, chondrocytes, and bone cells. This produces tissues with similar properties to native fibrocartilage and promotes the repair of tendon injuries. Therefore, the use of tissue engineering technology to promote the repair of tendon-bone injury has become a research hotspot. In this paper, we aim to review the mechanism of TDSCs in tendon-bone healing.
Tendon Stem Cells
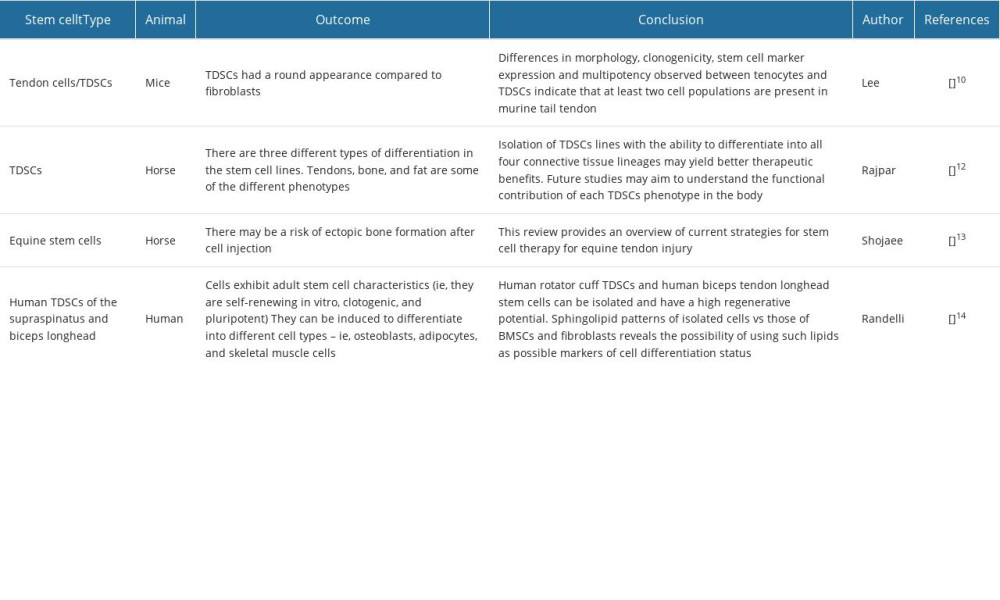
Lee et al [10] isolated tendon cells and TDSCs from rat tail tendon, and TDSCs exhibited a round morphology compared with the fibroblast morphology of tendon cells. In addition, the morphology, colony formation, and proliferation of TDSCs and tendon cells showed cells differentiated to form tendon- and bone-like tissues in vivo [11]. Rajpar et al [12] found that tendon stem cells of the superficial flexor tendon of horses differed in differentiation and proliferation potential, even from the same individual and the same tendon.
According to Shojaee et al [13], the extracellular matrix of tendons is primarily composed of type I collagen fibers, which are transcribed by tendon cells responsible for the synthesis of collagen and extracellular matrix components. Terminally differentiated tendon cells are elongated cells that have an expanded nucleus. Between the bundles of tendon cells are round stem/progenitor cells (TSPCs) with periocular nuclei. In general, little is known about where the different types of TSPCs are located. In addition, TDSCs have the ability to proliferate and differentiate. Randelli et al [14] isolated stem cell populations from the long heads of the supraspinatus muscle and biceps tendon, and the cells exhibited adult stem cell characteristics, which could be induced to differentiate into different cell types-osteoblasts, adipocytes, and skeletal muscle cells. Simultaneously, exogenous growth factors can regulate the proliferation and differentiation of TDSCs. Rui et al investigated the effect of BMP-2 on the differentiation of TDSCs and proteoglycan production in vitro culture. Studies have shown that BMP-2 promotes osteogenesis, lipogenesis, and chondrogenic differentiation of TDSCs, but inhibits the expression of tendon-forming markers. Yin et al [15] showed that nanostructures have a differentiation-promoting effect on human tendon stem/progenitor cells (hTSPCs). Many studies have shown that some active ingredients of traditional Chinese medicine have a promoting effect on stem cells to chondrocytes. However, there is no research on inducing TDSCs differentiation into tendon cells; therefore, further studies are needed (Table 1).
Physiological Structure of Tendon Bone Tissue
Macroscopically, 3 types of tendon-bone junction exist, namely muscle-tendon junctions (MTJs), osteotendinous junctions (OTJs), and central muscular abdomens (CAMs), each with its own characteristics and blood supply. Tendon collagen fibers form the MTJ, which is most vulnerable to tearing. The tendon is attached to the bone at the OTJ, which consists of 4 different areas: zone I-dense connective tissue, zone II-uncalcified fibrocartilage, zone III-calcified fibrocartilage, and zone IV-bone (Figure 1) [16].
Regarding changes after tendon-bone injury, at present, there are relatively few clinicopathological studies on tendon healing, and the understanding of the pathological changes of tendon insertion healing after tendon-bone injury repair is mainly based on animal experimental studies. There are 3 main stages in the recovery process after a tendon-bone injury: inflammatory exudation, fibroproliferation, and transformation. The inflammatory exudative period, also known as early healing, usually refers to the necrosis of the graft within 4 weeks after implantation, when its mechanical strength decreases significantly. The fibroproliferative phase refers to 4 to 12 weeks after surgery, when the graft is remodeled and re-vascularized, and the cell activity becomes stronger. During the plastic modification period, graft remodeling can take 6 months or even several years after hyperplasia. Tabuchi et al [17] showed that during the implantation of a rabbit bone tunnel tendon, a period of 12 weeks is required for Sharpey-like fibers made of type III collagen to gradually be replaced by type I collagen, and there was some continuity between Sharpey-like fibers and intratendon fibers at 26 weeks. Chen et al [18] showed that periosteosteal tendon grafts grow progressively to the interfacial fibrous layer at 8 weeks during tendon bone repair in rabbits. At 12 weeks, collagen fibers anchor to the bone, and fibrocartilage was formed in the tissue between the tendons and the bones. Testing showed higher maximum extraction intensity in the periosteal capsule group at all time points, with statistically significant differences at 8 and 12 weeks (Figure 1).
Research Progress of TDSCs on Tendon-Bone Healing
Many experimental studies have proven that mesenchymal stem cells (MSCs) have the ability to differentiate in multiple directions. Stem cells can be directed into a variety of tissues, including tendons, cartilage, and bone, under the stimulation of endogenous factors. At present, stem cell technology is widely used in the field of orthopedic disease research, with existing studies showing that stem cells have a positive role in promoting tendon-bone healing. MSCs come from a variety of sources, including fat, tendons, cord blood, tooth tissue, and epithelium [19–24], and stem cells from different sources differ in physiological characteristics. Rhodes et al investigated the effect of bone marrow mesenchymal stem cells (BM-MSCs) in patients with patellar tendinopathy, and the results showed that the tendon structure improvement in 2-dimensional ultrasound and ultrasonic tissue characterization was statistically significant in the BM-MSC group at 2 months. Therefore, BM-MSC therapy combined with chronic patellar tendinosis rehabilitation was effective in reducing pain and increasing activity levels in active participants [25]. Choi et al [26] found that in a rat model of chronic rotator cuff tear, fat-derived stem cells were designed into sheets and transplanted during surgical repair. Repaired tendon-bone junctions and histologies showed increased fibrocartilage, clear mineralized fibrocartilage boundaries, and abundant type III collagen; the conclusion shows that stem cell tablets enhance the regeneration of tendon-bone junctions in the chronic rotator cuff repair.
TDSCs are a type of stem cell most suitable for tendon interface repair, and several researchers have used stem cell technology to enhance cartilage regeneration at the tendon-bone interface following rotator cuff surgery, so that it can form a structure similar to a normal tendon-bone connection. These cells not only have universal stem cell properties similar to MSCs, but also have strong characteristics of tendon-linked genes and proteins, including collagen I, tenascin C, Scx, and Tnmd [27]. In addition, compared with other adult stem cells, TDSCs are currently recognized as one of the tissue engineering seed cells, owing to their low trauma and strong in vitro proliferation ability. TDSCs have advantages over BMSCs in promoting tendon healing: TDSCs are derived from tendons, are more adapted to the environment of the tendon junction interface, and proliferate faster than BMSC sand. Rat TDSCs have more chondrogenic and osteogenic markers than BMSCs [28]. Chen et al [29] collected TDSC-conditioned culture medium and found that TDSCs can promote tendon-bone healing in the rat rotator cuff. Voss et al [30] used a humeral head puncture technique in arthroscopy to obtain TDSCs, and through the tendon insertion point, the rotator cuff tissue to be repaired was inserted to promote tendon-bone healing. Although the use of TDSCs has shown good results, its use will be limited by donor morbidity, the need for long-term culture of cells, and phenotypic bias during in vitro expansion, and there is still limited research on the use of TSPCs for tendon-bone junction injury. Further studies are needed to determine whether they are effective in large animal models.
Factors Affecting TDSCs Promotion of Tendon Healing
With the rapid progress of tissue engineering technology, the rational application of new therapeutic strategies is necessary to promote tendon-bone healing. Growth factors activate the cascade of tendon repair, repair of the stent mimics the structure, function, and biomechanical properties of rotator cuff tendons, and cell therapy directly places stem cells at the site of injury to promote the reconstruction of tendon interface. Each treatment modality has its own advantages, and a combination of strategies may be the most effective: biological scaffolds provide a substrate for seed cell growth, while acting as drug carriers for slow-release growth factors, which regulate cell proliferation and differentiation and induce different cellular activities. Tendon-bone healing is a complex process that depends on various types of cells, growth factors, cytokines, and extracellular matrix elements.
Growth Factors
Growth factors are expressed in the nucleus and are capable of secreting various active substances, interacting with cytokines, and producing many cell signals. Growth factors affect cell development and growth by regulating the periodic division, differentiation, and proliferation of cells. In general, growth factors regulate the activity of stem cells, such as epidermal growth factor (EGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), and transforming growth factor (TGF).
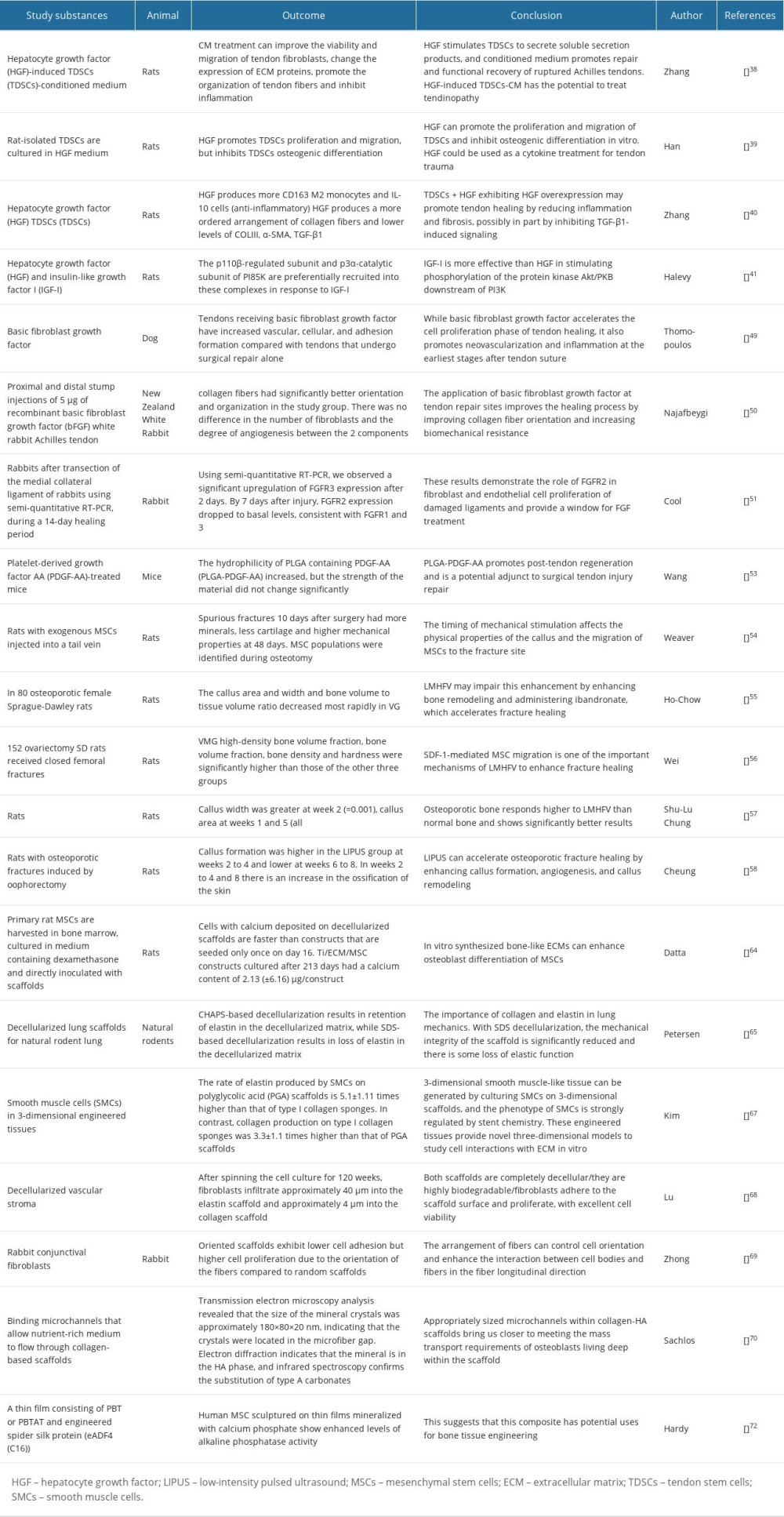
In addition to regulating many biological processes, hepatocyte growth factor (HGF) exerts multiple effects on several types of cells, There are a range of activities involved in these processes, including inflammation, tissue repair, morphogenesis, angiogenesis, tumor reproduction, and viral immunomodulation [31]. The liver, kidneys, digestive system, and tumors are all repaired and regenerate by HGF, according to research [32–36], and numerous studies have shown HGF binds to its receptor, MET, and promotes pleiotropic signaling. Therefore, injuries to the tendon interface might be repaired and regenerated by HGF [37]. Zhang et al [38] investigated the decellularization therapy composed of TDSCs conditioned medium induced by HGF, and the results showed that HGF stimulates TDSCs to secrete soluble secretion products. It is therefore possible that HGF-induced TDSC-conditioned medium could be used therapeutically for the treatment of Achilles tendon rupture. HGF promotes proliferating and migrating TDSCs and inhibits osteogenic differentiation in vitro, according to Han et al, who suggested that HGF could be used as a cytokine treatment for tendon injuries [39]. Zhang et al [40] showed that HGF can promote tendon healing by reducing inflammation and fibrosis, or partly promote healing by inhibiting TGF-β1-induced signaling. Halevy et al [41] found that HGF can promote the proliferation of adult TDSCs and inhibit their differentiation, and studies have shown that HGF promotes its proliferation by activating PI28K, MAPK, and ERK in myogenic cells. There are currently no studies showing the therapeutic efficacy of HGF in clinical trials, and further research is pending. In addition, FGF is an active substance present in tissue extracts of the brain and pituitary gland that promotes fibroblast growth. It is possible to regulate various biological and pathophysiological processes, including embryo development, angiogenesis, and cell migration [42]. In recent years, ligament/tendon and cartilage injuries have been repaired with exogenous FGF2, which has a synergistic effect with endogenous FGF2. Therefore, FGF2 is a promising biomolecule for speeding up tendon-bone healing after injury, as well as cartilage repair [48]. Thomopoulos et al [49] found that FGF accelerates the cell proliferation phase of tendon healing, but it also promotes new blood vessels and inflammation in the early stages after tendon suture. Najafbeygi et al [50] found the effect of basic FGF on Achilles tendon healing in rabbits, and applied basic FGF to sites of tendon repair to enhance the healing process by improving the orientation of collagen fibers and increasing biomechanical strength. Cool et al [51] found that FGFs activate FGFR2 after medial collateral ligament transection, and that FGFR-2 expression is significantly upregulated at 3 days and drops to basal levels after 7 days; these results suggest FGFR2 plays a role in fibroblast and endothelial cell proliferation of damaged ligaments. In addition, there are many growth factors that play a role in tendon-bone healing, and after comparing the healing process of tendons with human recombinant epidermal growth factor (hrEGF) and platelet-rich plasma, Sarkaya et al concluded that hrEGF is less effective at healing tendons than is platelet-rich plasma. The results showed hrEGF causes an increase in healing tissue at the levels of neovascular, tendon cells, fibroblasts, collagen, and tissue macrophages, and higher levels of TNF-α, IL-6, IL8, and CXCR2 [52]. Wang et al [53] found that platelet-derived growth factor AA-modified electrospun fiber (PLGA-PDGF-AA) effectively promoted tendon healing, and in summary, PLGA-PDGF-AA promoted regeneration after tendon-bone injury. PLGA-PDGF-AA is a potential adjunct to the repair of surgical tendon injuries.
Mechanical Stimulation
Growth factors play a major role in the proliferation and differentiation of stem cells. External factors also have a certain impact on stem cells, and studies have shown that the availability of the local mechanical environment and MSCs is an important factor in fracture healing. The results of Weaver et al suggest that the timing of mechanical stimulation affects the physical properties of calluses and the migration of MSCs to the fracture site [54]. Osteoporotic fractures can be healed with low-amplitude high-frequency vibration (LMHFV) [55]. In MSCs, mechanical stimulation enhances SDF-1/CXCR4 signaling. Wei et al [56] found that LMHFV enhances MSC migration through the SDF-1/CXCR4 pathway in osteoporotic fracture healing, LMHFV promotes MSC migration and fracture healing in osteoporotic rats, and CXCR4 inhibitors attenuate this effect, providing strong evidence that LMHFV improves anabolic and osteogenic responses during the healing process of intact bones with osteoporosis and osteoporotic fractures. However, the molecular response to the healing process of osteoporotic fractures is inconclusive. Chung et al [57] showed that LMHFV enhances callus formation and accelerates mineralization and remodeling by upregulating gene expression in Col-2, Col-1, and RANKL/OPG. Cheung et al found that low-intensity pulsed ultrasound can accelerate the healing of osteoporotic fractures by regulating Col-1 and bone morphogenetic protein-2 to enhance callus formation, angiogenesis, and callus remodeling [58].
Biological Scaffold
The biological scaffold can mimic the structure, function, and biomechanical properties of the tendon interface mechanical biomimetic properties, formability, biodegradability, and biocompatible (non-cytotoxic or inflammatory) [59–63]. Biological scaffolds are mainly divided into decellularization scaffolds, collagen scaffolds, and silk scaffolds. Decellularization scaffolds are further divided into autologous attachment point scaffolds, allogeneic attachment point scaffolds, and allogeneic attachment point scaffold. The main advantage is that the decellularized attachment point scaffolds are easy to obtain and ensure tissue activity. Datta et al [64] investigated the effect of extracellular matrix formation by osteoblasts on the differentiation of MSCs into osteoblasts. Petersen et al [65] demonstrated the feasibility of producing decellulated lung scaffolds through mechanical tests, and they found that decellulated scaffolds retain tensile strength and elastic properties that can be used to study lung matrix biology and mechanics independent of cellular components. Hussein et al [66] describe the different methods involved in cytotoxicity, pathogenicity, immunogenicity, and biodegradability testing for assessing the biocompatibility of various decellularized matrices obtained from humans or animals. Collagen scaffolds are based on collagen biomaterials, that is, the main components of the attachment point extracellular matrix; therefore, they have good biocompatibility and are widely used in the medical field. Kim et al [67] investigated whether the scaffold material chemically regulates the phenotype of smooth muscle cells. Lu et al [68] studied novel porous aortic elastin and collagen scaffolds for tissue engineering nanofiber scaffolds with well-defined structures that mimic native extracellular matrix analogues and have great potential in many specific tissue engineering and organ regeneration applications. Zhong et al [69] described the preparation of arranged collagen nanofiber scaffolds by electrospinning. To this end, Sahlos et al [70] developed a method to bind microchannels to allow the flow of nutrient-rich medium through the collagen scaffold. Natural silk scaffolds contain natural proteins produced by insects, primarily silkworms, and have ideal mechanical properties because they are biocompatible [71]. Hardy et al showed that thin-film human MSCs composed of butylene glycol benzenedicarboxylate (PBT) or polybutylene terephthalate (PBTAT) and engineered spider silk protein (eADF4(C16)) showed enhanced levels of alkaline phosphatase activity. This suggests that this composite has potential uses for bone tissue engineering protein-based material, binding to proteins found in the extracellular matrix to provide a biomimetic coating on the film, thereby improving cell adhesion to the surface of the engineered spider silk film [72,73]. This silk-based material has the potential to be used as a coating for degradable implantable devices. Many studies show that cell scaffolds have a promoting effect on tendon-bone healing and have great potential in clinical treatment (Table 2).
The Combination of Multiple Strategies
The combination of multiple strategies may be the most effective method, in which stem cells combine with growth factors to form cell complexes, so that cells can better perform biological functions and functions. The cell scaffold is equipped with a cell complex that can play a functional role with exogenous cells to promote cell proliferation and differentiation. Zhu et al found that the application of a composite scaffold composed of polyL-lactic acid/polylactic acid-coglycolic acid/poly ɛ-caprolactone (PLLA/PLGA/PCL) with low-intensity pulsed ultrasound combined with bone morphogenetic protein-2 (BMP-2) loading was able to improve the healing of steroid-induced osteonecrosis of the femoral head (ONFH) in a rat model. The results showed that the application could improve the healing of steroid-induced osteonecrosis of the femoral head (ONFH) in a rat model of low-intensity pulsed ultrasound combined with the placement of a composite PLLA/PLGA/PCL scaffold with BMP-2 microspheres. This protected the rats from steroid-induced ONFH and improved the carrying capacity, bone formation, angiogenesis, and differentiation, making this treatment a potential alternative therapeutic solution for the treatment of bone diseases [74]. In a study of bone tissue regeneration using porous nanofiber scaffolds, Wang et al studied BMP-2 local delivery from poly(lactic acid-glycolic acid) microspheres, and these composite scaffolds loaded with BMP-2 had high bone inductance, suggesting that the constructed BMP-2-loaded composite scaffold has the potential to be used as bone implants, and the extended release of BMP-2 promotes the osteogenic capacity of bone implants [75]. The observations of Lu et al [76] show that injectable hydrogels can be developed to deliver immobilized human bone morphogenetic protein-2 (rhBMP-2) and MSCs derived from rat bone marrow (rBMSC), with superior encapsulation efficiency, compatibility, and osteogenic differentiation. Moreover, in vivo studies show that injectable hydrogel scaffolds induce bone formation, bone density, and mineral density. According to Heo et al, collagen/fibrin hydrogels can be used to encapsulate human MSCs and human umbilical vein endothelial cell spheroids, and these cells contain hydrogels that provide a highly suitable 3D microenvironment for the formation of bone tissues [77] (Figure 2).
Future Directions
The role of TDSCs in tendon-bone healing is a promising area, and although there are still some challenges and limitations, the future direction is very promising. Improving the viability and function of TDSCs is the main direction of future research, including finding suitable growth factors and delivery methods to promote the proliferation, differentiation, and migration ability of stem cells, so as to improve the healing effect of tendons and bones. In this study, we have analyzed the advantages and application prospects of stem cells and growth factors; however, the adverse reactions of single growth factors and delivery methods also greatly limit the application of TDSCs. Also, direct application of attachment point-related cells to injury site tissue will result in a decrease in the number of these units and the associated effects of healing, with the possibility of ectopic differentiation and blood vessels being clogged. Gleeson et al [78] showed that the addition of MSCs to whole blood enhanced the deposition of platelet thrombus in the arteries. Coppin et al [79] found that procoagulant activity is expressed in MSCs, which initiates blood clotting when they are in contact with blood. Hu et al and Chen et al [80,81] showed that inflammatory conditions can lead to cartilage/osteogenic differentiation of TDSCs, proliferating to re-repair damaged tissue, although some of them exhibit osteogenic differentiation in the local pathological microenvironment and eventually lead to ectopic calcification of tendons. However, local infusion of growth factors can not only lead to a surge in acute blood indicators, but is also unpredictable, while increased blood circulation of growth factors has an effect on the molecules of normal tissues. Li et al [82] showed that local injection of vascular endothelial growth factor will form a local granulation tissue layer and neovascularization. Therefore, TDSCs and growth factors alone still have many drawbacks in treating tendon-bone injuries. In general, the future direction of TDSCs in tendon-bone healing will be the main development direction of improving the vitality and function of stem cells, optimizing delivery methods, and reducing adverse reactions. Combination therapy with TDSCs and growth factors on cell scaffolds can address these issues simultaneously, improve tendon healing, speed up the recovery process, and provide better treatment options.
Conclusions
TDSCs have the potential for multidirectional differentiation. Current research shows that TDSCs are an ideal cell type that exhibits a tendon-like phenotype, compared with MSCs from other sources, and expresses the highest levels of tendon-related markers for tendon regeneration. Although the use of TDSCs showed good results, the morbidity of donor site, the need for prolonged cell culture, and the phenotype of expansion deflection in vitro limit the use of TDSCs. Therefore, growth factors and stem cells should also be used in combination with cell scaffolds, which can not only improve their localization ability, but can also regulate the release of growth factors and regulate stem cell proliferation and differentiation to achieve a better result in clinical practice. However, research on determining how to solve the limitations of the use of TDSCs and how to use combination therapy for the best treatment effect is still in its infancy, and further research is needed.
Figures
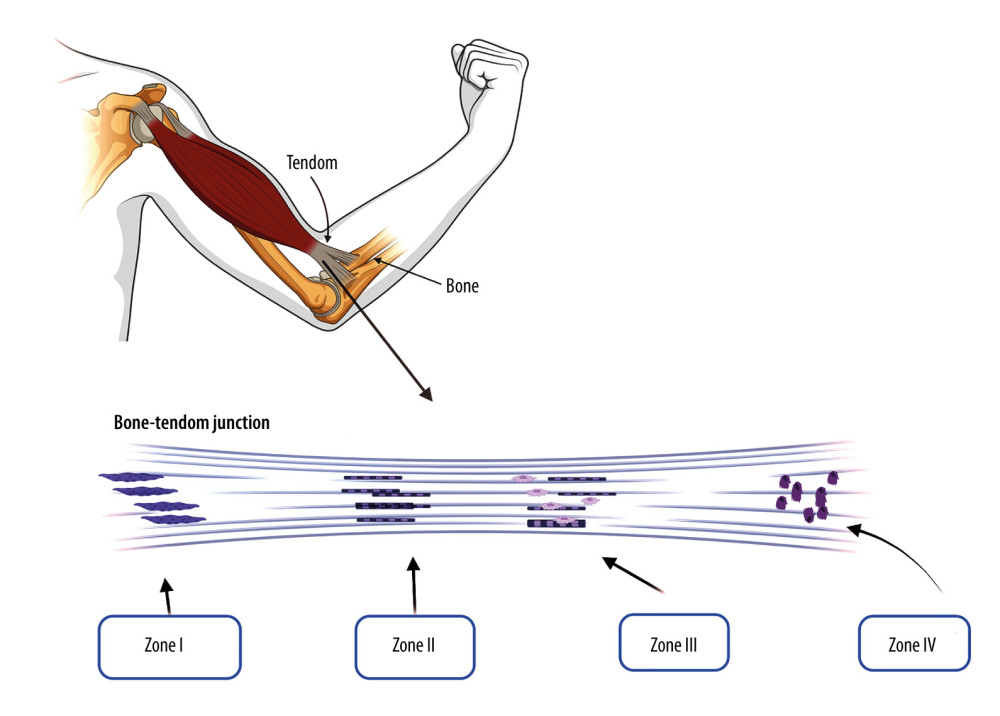 Figure 1. The physiological structure of tendon bone tissue: zone I-dense connective tissue, zone II-uncalcified fibrocartilage, zone III-calcified fibrocartilage, and zone IV-bone. Created by Biorender.com.
Figure 1. The physiological structure of tendon bone tissue: zone I-dense connective tissue, zone II-uncalcified fibrocartilage, zone III-calcified fibrocartilage, and zone IV-bone. Created by Biorender.com. 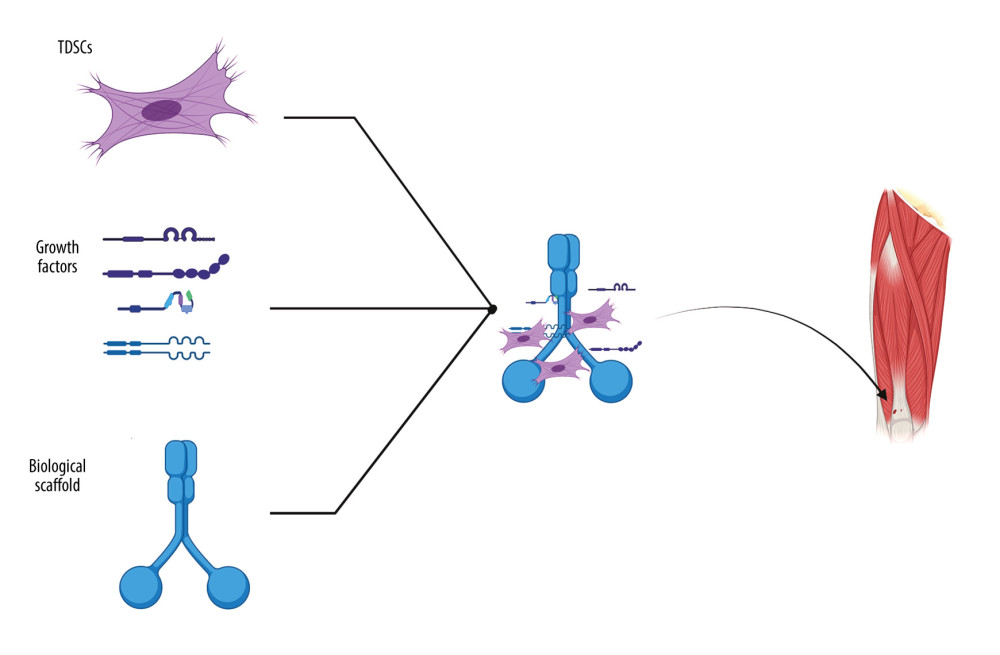 Figure 2. Cell scaffolds are equipped with TDSCs and growth factors that act on tendon bone injuries. Created by Biorender.com.
Figure 2. Cell scaffolds are equipped with TDSCs and growth factors that act on tendon bone injuries. Created by Biorender.com. References
1. Musahl V, Karlsson J, Anterior cruciate ligament tear: N Engl J Med, 2019; 380(24); 2341-48
2. Xu Y, Zhang WX, Wang LN, Stem cell therapies in tendon-bone healing: World J Stem Cells, 2021; 13(7); 753-75
3. Rui YF, Lui PP, Li G, Isolation and characterization of multipotent rat tendon-derived stem cells: Tissue Eng Part A, 2010; 16(5); 1549-58
4. Wang S, Ying JH, Xu H, Identification of diagnostic biomarkers associated with stromal and immune cell infiltration in fatty infiltration after rotator cuff tear by integrating bioinformatic analysis and machine-learning: Int J Gen Med, 2022; 15; 1805-19
5. Gallagher BP, Bishop ME, Tjoumakaris FP, Freedman KB, Early versus delayed rehabilitation following arthroscopic rotator cuff repair: A systematic review: Phys Sportsmed, 2015; 43(2); 178-87
6. Zou J, Yang W, Cui W, Therapeutic potential and mechanisms of mesenchymal stem cell-derived exosomes as bioactive materials in tendon-bone healing: J Nanobiotechnology, 2023; 21(1); 14
7. Xu Y, Zhang WX, Wang LN, Stem cell therapies in tendon-bone healing: World J Stem Cells, 2021; 13(7); 753-75
8. Yang C, Teng Y, Geng B, Strategies for promoting tendon-bone healing: Current status and prospects: Front Bioeng Biotechnol, 2023; 11; 1118468
9. Tian B, Zhang M, Kang X, Strategies to promote tendon-bone healing after anterior cruciate ligament reconstruction: Present and future: Front Bioeng Biotechnol, 2023; 11; 1104214
10. Lee KJ, Clegg PD, Comerford EJ, Canty-Laird EG, A comparison of the stem cell characteristics of murine tenocytes and tendon-derived stem cells: BMC Musculoskelet Disord, 2018; 19(1); 116
11. Zhang J, Wang JH, Characterization of differential properties of rabbit tendon stem cells and tenocytes: BMC Musculoskelet Disord, 2010; 11; 10
12. Rajpar I, Barrett JG, Multi-differentiation potential is necessary for optimal tenogenesis of tendon stem cells: Stem Cell Res Ther, 2020; 11(1); 152
13. Shojaee A, Parham A, Strategies of tenogenic differentiation of equine stem cells for tendon repair: current status and challenges: Stem Cell Res Ther, 2019; 10(1); 181
14. Randelli P, Conforti E, Piccoli M, Isolation and characterization of 2 new human rotator cuff and long head of biceps tendon cells possessing stem cell-like self-renewal and multipotential differentiation capacity: Am J Sports Med, 2013; 41(7); 1653-64
15. Yin Z, Chen X, Chen JL, The regulation of tendon stem cell differentiation by the alignment of nanofibers: Biomaterials, 2010; 31(8); 2163-75
16. Chartier C, ElHawary H, Baradaran A, Tendon: Principles of healing and repair: Semin Plast Surg, 2021; 35(3); 211-15
17. Tabuchi K, Soejima T, Kanazawa T, Chronological changes in the collagen-type composition at tendon-bone interface in rabbits: Bone Joint Res, 2012; 1(9); 218-24
18. Chen CH, Chen WJ, Shih CH, Enveloping the tendon graft with periosteum to enhance tendon-bone healing in a bone tunnel: A biomechanical and histologic study in rabbits: Arthroscopy, 2003; 19(3); 290-96
19. Mohamed-Ahmed S, Fristad I, Lie SA, Adipose-derived and bone marrow mesenchymal stem cells: A donor-matched comparison: Stem Cell Res Ther, 2018; 9(1); 168
20. Harvey T, Flamenco S, Fan CM, A Tppp3(+)Pdgfra(+) tendon stem cell population contributes to regeneration and reveals a shared role for PDGF signalling in regeneration and fibrosis: Nat Cell Biol, 2019; 21(12); 1490-503
21. Ray SK, Mukherjee S, Clinical practice of umbilical cord blood stem cells in transplantation and regenerative medicine – prodigious promise for imminent times: Recent Pat Biotechnol, 2022; 16(1); 16-34
22. Zhai Q, Dong Z, Wang W, Li B, Jin Y, Dental stem cell and dental tissue regeneration: Front Med, 2019; 13(2); 152-59
23. Lyu K, Liu T, Chen Y, A “cell-free treatment” for tendon injuries: adipose stem cell-derived exosomes: Eur J Med Res, 2022; 27(1); 75
24. Festa E, Fretz J, Berry R, Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling: Cell, 2011; 146(5); 761-71
25. Rodas G, Soler-Rich R, Rius-Tarruella J, Effect of autologous expanded bone marrow mesenchymal stem cells or leukocyte-poor platelet-rich plasma in chronic patellar tendinopathy (with gap >3 mm): Preliminary outcomes after 6 months of a double-blind, randomized, prospective study: Am J Sports Med, 2021; 49(6); 1492-504
26. Choi JH, Shim IK, Shin MJ, Stem cell sheet interpositioned between the tendon and bone would be better for healing than stem cell sheet overlaid above the tendon-to-bone junction in rotator cuff repair of rats: PLoS One, 2022; 17(3); e0266030
27. Bi Y, Ehirchiou D, Kilts TM, Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche: Nat Med, 2007; 13(10); 1219-27
28. Xu Y, Zhang WX, Wang LN, Stem cell therapies in tendon-bone healing: World J Stem Cells, 2021; 13(7); 753-75
29. Chen W, Sun Y, Gu X, Conditioned medium of human bone marrow-derived stem cells promotes tendon-bone healing of the rotator cuff in a rat model: Biomaterials, 2021; 271; 120714
30. Voss A, McCarthy MB, Allen D, Fibrin scaffold as a carrier for mesenchymal stem cells and growth factors in shoulder rotator cuff repair: Arthrosc Tech, 2016; 5(3); e447-51
31. Libetta C, Esposito P, Martinelli C, Hepatocyte growth factor (HGF) and hemodialysis: physiopathology and clinical implications: Clin Exp Nephrol, 2016; 20(3); 371-78
32. Matsumoto K, Nakamura T, Roles of HGF as a pleiotropic factor in organ regeneration: EXS, 1993; 65; 225-49
33. Tahara Y, Ido A, Yamamoto S, Hepatocyte growth factor facilitates colonic mucosal repair in experimental ulcerative colitis in rats: J Pharmacol Exp Ther, 2003; 307(1); 146-51
34. Mammen JM, Matthews JB, Mucosal repair in the gastrointestinal tract: Crit Care Med, 2003; 31(8 Suppl); S532-37
35. Matsumoto K, Umitsu M, De Silva DM, Hepatocyte growth factor/MET in cancer progression and biomarker discovery: Cancer Sci, 2017; 108(3); 296-307
36. Mueller KL, Madden JM, Zoratti GL, Fibroblast-secreted hepatocyte growth factor mediates epidermal growth factor receptor tyrosine kinase inhibitor resistance in triple-negative breast cancers through paracrine activation of Met: Breast Cancer Res, 2012; 14(4); R104
37. Fukushima T, Uchiyama S, Tanaka H, Kataoka H, Hepatocyte growth factor activator: A proteinase linking tissue injury with repair: Int J Mol Sci, 2018; 19(11); 3435
38. Zhang Z, Li Y, Zhang T, Hepatocyte growth factor-induced tendon stem cell conditioned medium promotes healing of injured achilles tendon: Front Cell Dev Biol, 2021; 9; 654084
39. Han P, Cui Q, Lu W, Hepatocyte growth factor plays a dual role in tendon-derived stem cell proliferation, migration, and differentiation: J Cell Physiol, 2019; 234(10); 17382-91
40. Zhang M, Liu H, Shi M, Potential mechanisms of the impact of hepatocyte growth factor gene-modified tendon stem cells on tendon healing: Front Cell Dev Biol, 2021; 9; 659389
41. Halevy O, Cantley LC, Differential regulation of the phosphoinositide 3-kinase and MAP kinase pathways by hepatocyte growth factor vs. insulin-like growth factor-I in myogenic cells: Exp Cell Res, 2004; 297(1); 224-34
42. Turner N, Grose R, Fibroblast growth factor signalling: From development to cancer: Nat Rev Cancer, 2010; 10(2); 116-29
43. Tanner Y, Grose RP, Dysregulated FGF signalling in neoplastic disorders: Semin Cell Dev Biol, 2016; 53; 126-35
44. Chen H, Cui Y, Zhang D, The role of fibroblast growth factor 8 in cartilage development and disease: J Cell Mol Med, 2022; 26(4); 990-99
45. Itoh N, Ornitz DM, Evolution of the Fgf and Fgfr gene families: Trends Genet, 2004; 20(11); 563-69
46. Marie PJ, Miraoui H, Severe N, FGF/FGFR signaling in bone formation: progress and perspectives: Growth Factors, 2012; 30(2); 117-23
47. Fakhry A, Ratisoontorn C, Vedhachalam C, Effects of FGF-2/-9 in calvarial bone cell cultures: Differentiation stage-dependent mitogenic effect, inverse regulation of BMP-2 and noggin, and enhancement of osteogenic potential: Bone, 2005; 36(2); 254-66
48. Zhang J, Liu Z, Li Y, FGF2: A key regulator augmenting tendon-to-bone healing and cartilage repair: Regen Med, 2020; 15(9); 2129-42
49. Thomopoulos S, Kim HM, Das R, The effects of exogenous basic fibroblast growth factor on intrasynovial flexor tendon healing in a canine model: J Bone Joint Surg Am, 2010; 92(13); 2285-93
50. Najafbeygi A, Fatemi MJ, Lebaschi AH, Effect of basic fibroblast growth factor on achilles tendon healing in rabbit: World J Plast Surg, 2017; 6(1); 26-32
51. Cool SM, Snyman CP, Nurcombe V, Forwood M, Temporal expression of fibroblast growth factor receptors during primary ligament repair: Knee Surg Sports Traumatol Arthrosc, 2004; 12(5); 490-96
52. Sarıkaya B, Yumuşak N, Yigin A, Comparison of the effects of human recombinant epidermal growth factor and platelet-rich plasma on healing of rabbit patellar tendon: Eklem Hastalik Cerrahisi, 2017; 28(2); 92-99
53. Wang L, Yang T, Ding L, Platelet-derived growth factor AA-modified electrospun fibers promote tendon healing: J Biomater Appl, 2023; 37(6); 1018-28
54. Weaver AS, Su YP, Begun DL, The effects of axial displacement on fracture callus morphology and MSC homing depend on the timing of application: Bone, 2010; 47(1); 41-48
55. Chow DH, Leung KS, Qin L, Low-magnitude high-frequency vibration (LMHFV) enhances bone remodeling in osteoporotic rat femoral fracture healing: J Orthop Res, 2011; 29(5); 746-52
56. Wei FY, Chow SK, Leung KS, Low-magnitude high-frequency vibration enhanced mesenchymal stem cell recruitment in osteoporotic fracture healing through the SDF-1/CXCR4 pathway: Eur Cell Mater, 2016; 31; 341-54
57. Chung SL, Leung KS, Cheung WH, Low-magnitude high-frequency vibration enhances gene expression related to callus formation, mineralization and remodeling during osteoporotic fracture healing in rats: J Orthop Res, 2014; 32(12); 1572-79
58. Cheung WH, Chow SK, Sun MH, Low-intensity pulsed ultrasound accelerated callus formation, angiogenesis and callus remodeling in osteoporotic fracture healing: Ultrasound Med Biol, 2011; 37(2); 231-38
59. Luo W, Wang Y, Han Q, Advanced strategies for constructing interfacial tissues of bone and tendon/ligament: J Tissue Eng, 2022; 13; 20417314221144714
60. Font TS, Bonani W, Balmayor ER, Fabrication and characterization of biphasic silk fibroin scaffolds for tendon/ligament-to-bone tissue engineering: Tissue Eng Part A, 2017; 23(15–16); 859-72
61. Puetzer JL, Ma T, Sallent I, Driving hierarchical collagen fiber formation for functional tendon, ligament, and meniscus replacement: Biomaterials, 2021; 269; 120527
62. Rossbach BP, Gulecyuz MF, Kempfert L, Rotator cuff repair with autologous tenocytes and biodegradable collagen scaffold: A histological and biomechanical study in sheep: Am J Sports Med, 2020; 48(2); 450-59
63. Sensini A, Cristofolini L, Biofabrication of electrospun scaffolds for the regeneration of tendons and ligaments: Materials (Basel), 2018; 11(10); 1963
64. Datta N, Holtorf HL, Sikavitsas VI, Effect of bone extracellular matrix synthesized in vitro on the osteoblastic differentiation of marrow stromal cells: Biomaterials, 2005; 26(9); 971-77
65. Petersen TH, Calle EA, Colehour MB, Niklason LE, Matrix composition and mechanics of decellularized lung scaffolds: Cells Tissues Organs, 2012; 195(3); 222-31
66. Hussein KH, Park KM, Kang KS, Woo HM, Biocompatibility evaluation of tissue-engineered decellularized scaffolds for biomedical application: Mater Sci Eng C Mater Biol Appl, 2016; 67; 766-78
67. Kim BS, Nikolovski J, Bonadio J, Engineered smooth muscle tissues: Regulating cell phenotype with the scaffold: Exp Cell Res, 1999; 251(2); 318-28
68. Lu Q, Ganesan K, Simionescu DT, Vyavahare NR, Novel porous aortic elastin and collagen scaffolds for tissue engineering: Biomaterials, 2004; 25(22); 5227-37
69. Zhong S, Teo WE, Zhu X, An aligned nanofibrous collagen scaffold by electrospinning and its effects on in vitro fibroblast culture: J Biomed Mater Res A, 2006; 79(3); 456-63
70. Sachlos E, Gotora D, Czernuszka JT, Collagen scaffolds reinforced with biomimetic composite nano-sized carbonate-substituted hydroxyapatite crystals and shaped by rapid prototyping to contain internal microchannels: Tissue Eng, 2006; 12(9); 2479-87
71. Han F, Liu S, Liu X, Woven silk fabric-reinforced silk nanofibrous scaffolds for regenerating load-bearing soft tissues: Acta Biomater, 2014; 10(2); 921-30
72. Hardy JG, Torres-Rendon JG, Leal-Egana A, Biomineralization of engineered spider silk protein-based composite materials for bone tissue engineering: Materials (Basel), 2016; 9(7); 560
73. Hardy JG, Pfaff A, Leal-Egana A, Glycopolymer functionalization of engineered spider silk protein-based materials for improved cell adhesion: Macromol Biosci, 2014; 14(7); 936-42
74. Zhu H, Shi Z, Cai X, The combination of PLLA/PLGA/PCL composite scaffolds integrated with BMP-2-loaded microspheres and low-intensity pulsed ultrasound alleviates steroid-induced osteonecrosis of the femoral head: Exp Ther Med, 2020; 20(6); 126
75. Wang W, Miao Y, Zhou X, Local delivery of BMP-2 from Poly(lactic-co-glycolic acid) microspheres incorporated into porous nanofibrous scaffold for bone tissue regeneration: J Biomed Nanotechnol, 2017; 13(11); 1446-56
76. Lu X, Guo H, Li J, Sun T, Xiong M, Recombinant human bone morphogenic protein-2 immobilized fabrication of magnesium functionalized injectable hydrogels for controlled-delivery and osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells in femoral head necrosis repair: Front Cell Dev Biol, 2021; 9; 723789
77. Heo DN, Hospodiuk M, Ozbolat IT, Synergistic interplay between human MSCs and HUVECs in 3D spheroids laden in collagen/fibrin hydrogels for bone tissue engineering: Acta Biomater, 2019; 95; 348-56
78. Gleeson BM, Martin K, Ali MT, Bone marrow-derived mesenchymal stem cells have innate procoagulant activity and cause microvascular obstruction following intracoronary delivery: Amelioration by antithrombin therapy: Stem Cells, 2015; 33(9); 2726-37
79. Coppin L, Sokal E, Stéphenne X, Thrombogenic risk induced by intravascular mesenchymal stem cell therapy: current status and future perspectives: Cells, 2019; 8(10); 1160
80. Chen Y, Xie Y, Liu M, Controlled-release curcumin attenuates progression of tendon ectopic calcification by regulating the differentiation of tendon stem/progenitor cells: Mater Sci Eng C Mater Biol Appl, 2019; 103; 109711
81. Hu JJ, Yin Z, Shen WL, Pharmacological regulation of in situ tissue stem cells differentiation for soft tissue calcification treatment: Stem Cells, 2016; 34(4); 1083-96
82. Lee J, Lee KY, Local and sustained vascular endothelial growth factor delivery for angiogenesis using an injectable system: Pharm Res, 2009; 26(7); 1739-44
Figures
 Figure 1. The physiological structure of tendon bone tissue: zone I-dense connective tissue, zone II-uncalcified fibrocartilage, zone III-calcified fibrocartilage, and zone IV-bone. Created by Biorender.com.
Figure 1. The physiological structure of tendon bone tissue: zone I-dense connective tissue, zone II-uncalcified fibrocartilage, zone III-calcified fibrocartilage, and zone IV-bone. Created by Biorender.com. Figure 2. Cell scaffolds are equipped with TDSCs and growth factors that act on tendon bone injuries. Created by Biorender.com.
Figure 2. Cell scaffolds are equipped with TDSCs and growth factors that act on tendon bone injuries. Created by Biorender.com. In Press
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
12 Mar 2024 : Clinical Research
Comparing Neuromuscular Blockade Measurement Between Upper Arm (TOF Cuff®) and Eyelid (TOF Scan®) Using Miv...Med Sci Monit In Press; DOI: 10.12659/MSM.943630
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952