13 September 2023: Clinical Research
The Impact of Nasal Carriage on Surgical-Site Infections after Immediate Breast Reconstruction: Risk Factors and Biofilm Formation Potential
Maria SzymankiewiczDOI: 10.12659/MSM.940898
Med Sci Monit 2023; 29:e940898
Abstract
BACKGROUND: Despite the benefits of implant-based breast reconstruction in patients with breast cancer, the procedure can be complicated by surgical site infections (SSI). This study aimed to evaluate the association between nasal carriage of Staphylococcus aureus strains and the incidence of SSI among patients who underwent reconstructive procedures. We also assessed the ability of colonizing S. aureus strains to form biofilm.
MATERIAL AND METHODS: Medical data from 124 patients with 132 post-mastectomy breast reconstructions performed at the Oncology Center in Bydgoszcz, Poland, between June 2020 and August 2021 were analyzed. A 90-day incidence of SSI was found in 7/132 reconstructions (5.3%). The study group included 132 reconstructions, and was divided into those with infection (n=7) and without infection (n=125). Between-group differences were assessed using the t test for continuous variables and chi-square test for categorical variables. Biofilm formation among 32 S. aureus strains was determined by using quantitative and qualitative assays.
RESULTS: There were no significant differences in relation to the patients’ S. aureus colonization status. Infections occurred both in patients colonized and not colonized with S. aureus. S. aureus nasal carriage did not affect the rate of SSI at 90 days after surgery. About 97.0% of the strains had a strong capacity for biofilm formation.
CONCLUSIONS: There was no association between nasal carriage of strains of S. aureus and the incidence of SSI. However, further investigations on a larger group of patients and longer observation time are needed to investigate this potential risk factor in detail.
Keywords: Biofilms, Breast Implantation, Breast Neoplasms, Mupirocin, Humans, Female, Surgical Wound Infection, Staphylococcus aureus, Mastectomy, Mammaplasty, Risk Factors, Staphylococcal Infections
Background
In patients with breast cancer, post-mastectomy breast reconstruction is part of the patient’s treatment and is associated with higher patient satisfaction and psychological benefits [1]. The rate of breast reconstruction surgery has increased in recent years, with implants being used in 50.0% of the procedures performed [2]. Despite the undoubted benefits of implant-based reconstruction, the procedure can be complicated by implant infections, which affect up to 15.0% of patients undergoing reconstruction [3–5]. The most common causative microorganism is
Therefore, we aimed to evaluate the association between nasal carriage of strains of
Material and Methods
ETHICS STATEMENT:
Samples from nasal vestibule mucosa were submitted to the clinical microbiology laboratory as a part of routine clinical care for screening of MSSA and MRSA. This test was performed per standard of care and was reported in the patients’ medical records. This study was retrospective and used the medical records of patients who underwent mastectomy and breast reconstruction. The study was approved by the Bioethics Committee at L. Rydygier Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University in Toruń, Poland (approval No. 578/2021, date of approval: November 16, 2021).
STUDY POPULATION:
This was a retrospective study of patients who underwent reconstructive procedures between June 2020 and August 2021 at the Oncology Center – Prof. Franciszek Łukaszczyk Memorial Hospital in Bydgoszcz. All patients underwent mastectomy and either 1-stage or 2-stage implant-based breast reconstruction for breast cancer. All patients were screened for S. aureus nasal carriage prior to admission to hospital. The preparation of the patient for surgery proceeded under the standards of nursing practice, according to the World Health Organization guidelines [18]. All patients received parenteral antimicrobial prophylaxis, cephazolin i.v. 2.0 g or 1.0 g, in a single dose (depending on body weight and age). In the case of severe beta-lactam allergy, 1 dose of clindamycin i.v. 0.6 g was administered as an alternative to cephalosporin.
POLYMERASE CHAIN REACTION XPERT MRSA/MSSA NASAL ASSAY:
Nasal colonization with
STANDARD MICROBIOLOGICAL CULTURE, STRAIN IDENTIFICATION, ANTIBIOTIC SUSCEPTIBILITY TESTING:
In the case of a positive PCR Xpert MRSA/MSSA nasal test, standard culture methods for S. aureus were followed. The samples were processed using culture-based methods on Staph/Strep Selective Medium (Oxoid Deutschland GmbH, Wesel, Germany) under aerobic conditions at 35±2°C. After 24 h, suspected isolates of S. aureus were identified using a coagulase test, the commercial Dry Spot Staphytect Plus (Oxoid Limited, Basingstoke, Hampshire, England), and matrix-assisted laser desorption/ionisation-time of flight mass spectrometry (IVD MALDI Biotyper Smart System, microflex LT/SH smart, Bruker Daltonik, Bremen, Germany). Culture-negative plates were further incubated and examined after 48 h. The screening swabs taken from patients were also inoculated on Brilliance MRSA 2 agar (Fisher Scientific, Loughborough, Leicestershire, UK) for MRSA detection. All agar plates were read after 18 to 24 h of incubation at 35±2 °C, according to the manufacturer’s instructions. If, after this time, no denim blue colonies were observed, the specimen was considered negative for MRSA and the plates were discarded. Susceptibility to antibiotics was determined by the disk diffusion method on Mueller-Hinton E agar (BioMérieux SA, Marcy l’Etoile, France), as recommended by the European Committee on Antimicrobial Susceptibility Testing [19]. The antibiotics used were cefoxitin (disk content 30 μg) and mupirocin (disk content 200 μg; Oxoid Limited). The results were interpreted in accordance with the European Committee on Antimicrobial Susceptibility Testing breakpoint tables v. 11, valid from January 1, 2021 [20]. S. aureus strain ATCC 29213 was used for quality control.
SSI IDENTIFICATIONS:
SSI were diagnosed if clinical signs of infection occurred within 90 days after the operation and diagnosis was made by a surgeon, according to the European Centre for Disease Prevention and Control for superficial and deep incisional infections [21].
STUDY GROUPS:
All cases of patients with immediate post-mastectomy reconstruction were analyzed. A total of 124 patients with 133 reconstructive surgery procedures for breast cancer were identified. Among them, 115 patients underwent unilateral reconstruction, 3 patients underwent bilateral surgery, and 6 patients underwent 2 reconstructions. One of these reconstructions (patient with 2 reconstructions) was excluded from further analysis because they did not meet the adopted criteria, owing to treatment with mupirocin during hospitalization. The patients were stratified into 2 groups according to occurrence of infection. The differences between the groups were evaluated in relation to the possible risk factors for SSI after the placement: age, body mass index (BMI), length of hospital stay, histological form of cancer, American Society of Anesthesiologists (ASA) grading, type of implant, treatment with adjuvant or neoadjuvant chemotherapy (CHTH,) occurrence of risk factors, and number of risk factors. Moreover, according to the PCR result and mupirocin treatment status, patients were separated as follows: PCR negative for MSSA/MRSA, PCR positive for MSSA/MRSA, PCR positive for MSSA/MRSA with mupirocin treatment, PCR positive for MSSA/MRSA without mupirocin treatment.
COLONIZING STRAINS:
In total, 36
DETERMINATION OF THE BIOFILM FORMATION:
The qualitative determination of the biofilm formation was provided by the colorimetric assay with crystal violet staining, as previously described [22,23]. Briefly, fresh bacterial suspensions were prepared in Luria-Bertani broth (LB, Merck KGaA, Darmstadt, Germany), supplemented with 2% glucose (Merck KGaA) from overnight cultures and adjusted to 106 colony forming units (CFU)/mL. The wells of a sterile 96-well flat-bottomed polystyrene microplate (Kartell S.p.a., Medlab) were filled with 200 μL of the overnight bacterial culture into each well. The negative control wells contained LB broth only. After 48 h of incubation in 35±2°C, the medium was gently removed and the microtiter plate wells were washed 3 times with 150 μL sterile 1% phosphate-buffered saline (PBS; Thermo Fisher Scientific, Loughborough, Leicestershire, UK). The temperature-fixed (10 min at 60°C,) biofilms were stained with 100 μL 0.15% crystal violet (Merck KGaA) and stored for 15 min at room temperature. The unbound crystal violet stain was removed, and the wells were washed gently 3 times with 150 μL 1% PBS. The wells were air-dried for 15 min and the crystal violet in each well was solubilized by adding 200 μL 96% ethyl alcohol (Chempur, Piekary Śląskie, Poland). The absorbance (A) of each well with alcohol extracts was measured in new flat-bottomed microtiter plate at 570 nm using an automated Synergy HTX multi-mode reader (Biotek Instruments, Inc., USA). Results were analyzed as biofilm biomass in negative and positive biofilm productions in comparison to the control without bacteria, and the classes were defined as follows: A isolate ≤A control=non-biofilm producer; A control <A isolate <2×A control=weak producer; 2×A control <A isolate <4×A control=moderate; 4×A control <A isolate=strong-producer. Each experiment was performed 3 times in 3 replications, and means and standard deviations were calculated and the 1-way ANOVA test was performed. In the quantitative determination of biofilm formation, the strains of the S. aureus were grown in LB broth, as in the qualitative methods described. After incubation in the microplate, the supernatants were removed and the biofilms were carefully washed 3 times with 150 μL PBS. The biofilms were resuspended in 150 μL PBS by scraping them from the cavities and pipetting up and down several times. From each cavity, an aliquot of 200 μL of cell suspensions was transferred into a 1.5-mL Eppendorf tube and serially diluted in a logarithmic manner up to 10−5. From selected dilutions, 10 μL were dropped on LB agar plates, and the CFU/mL were determined after incubation at 35±2°C overnight. Each experiment was independently performed 2 times, and the results are expressed as mean and standard deviation.
STATISTICAL ANALYSIS:
Statistical analysis was conducted using Statistica (data analysis software system, TIBCO Software Inc.,
Results
CHARACTERISTICS OF STUDY GROUPS:
A total of 124 patients who had 132 reconstructions were analyzed. The mean age of patients was 45.41 years (±7.21 SD), and the mean BMI was 25.16 (±3.76 SD). Risk factors were present in 79 of 132 patient cases (59.8%). Twenty-two of 132 the analyzed patient cases (16.7%) had 2 risk factors. Characteristics of the whole group are presented in Table 1.
:
The overall, preoperative nasal
BIOFILM FORMATION ASSAYS:
Qualitative results of the biofilm biomass in absorbance (A) of the strains were presented in the following classes: A ≤0.165=non-biofilm producer; 0.165 <A <0.329=weak producer; 0.329 <A <0.659=moderate; and 0.659 <A=strong producer. A total of 31 (96.9%) clinical S. aureus strains had a strong biofilm formation capacity, and 1 strain (3.1%), number 29, showed moderate ability (A=0.482). The maximal absorbance (A=3.046) was observed for strain 3 (Figure 1). The 1-way ANOVA test showed significant differences in the amount of biofilm produced in replicated groups of the experiment (P<0.0001). In the quantitative in vitro test with log10 CFU/mL, measurements of the average viable cells counts ranged between 6.826 (strain number 17) and 9.435 (strain number 20; Figure 1). For most strains, a 2-log increase in viable cells in the CFU biofilm compared with the control inoculation was observed. In 8 cases (25%), the biofilm mass in the crystal violet was higher than CFU assay, which probably can be associated with aggregation of extracellular substances in the biofilm matrix and not with the number of viable S. aureus cells. The Mann-Whitney test showed statistical significance in the amount of biofilm analyzed by crystal violet test and CFU method (P<0.0001).
SSI WITHIN 90 DAYS AFTER RECONSTRUCTION:
The infection rate following reconstruction procedures was 5.3% (7/132). In all cases, we identified deep incisional SSI. Infections occurred both in patients colonized (n=1) and non-colonized with S. aureus (n=6). Among the patients who were PCR positive for MSSA/MRSA without treatment with mupirocin, one 44-year-old S. aureus carrier, after CHTH treatment, developed an infection with S. aureus 70 days after reconstruction. The colonizing S. aureus strain (strain number 19) had a high ability to form biofilm, confirmed by qualitative and quantitative assays, as presented in Figure 1. The mean time to onset of infection was 64.63 (±16.90) days. There was no significant difference between study groups, except regarding the type of implant. The use of an expander tended to be higher in the group with infections (85.7% vs 14.3%, P=0.0312). Comparisons between groups are shown in Table 1. BMI data are presented as a categorized histogram and are shown in Figure 2. The length of hospital stay in the 2 groups was similar, being 8.8 (±2.9) days for cases without infection vs 8.9 (±3.9) days (P=0.9328) for cases with infections.
RISK FACTOR ANALYSIS FOR IMPLANT-BASED BREAST RECONSTRUCTION INFECTIONS:
Patients treated with CHTH had around a 4-fold higher risk (95% CI 0.75, 21.5; P=0.1049) for developing infection. Using an expander led to an almost 8-fold higher risk (95% CI 0.89, 65.32; P=0.0634), and there was an almost 5-times higher risk when 2 risk factors were present (95% CI 0.43, 57.59; P=0.2012). However, none of the analyzed risk factors had a statistically significant influence on the occurrence of implant-based breast reconstruction infections. The risk of infection in univariate analysis is presented in Table 2. Time-dependent risk of infection for number of risk factors, type of implant, PCR status of MSSA/MRSA, and treatment with CHTH is shown in Figure 3A–3D. The cumulative infection probability was higher for the use of an expander than for a prosthesis (P=0.031), and for treatment with CHTH than without CHTH treatment (P=0.089); however, the differences in relation to CHTH were not statistically significant.
Discussion
STRENGTHS AND LIMITATIONS OF THE STUDY:
The limitations of this work include the relatively small group of patients with infections, the retrospective nature of the work, and the lack of in-depth microbiological analysis determining the causative agents of infection among non
Conclusions
There was no association between nasal carriage of strains of
Figures
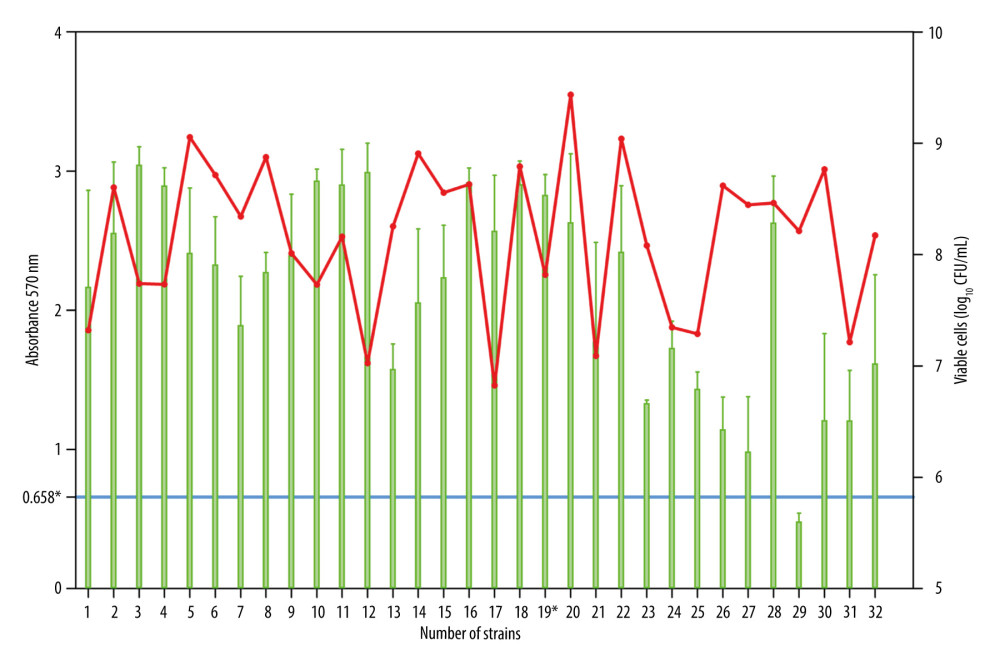 Figure 1. Estimation of biofilm biomass for 32 clinical S. aureus strains by crystal violet (CV) staining and viable cells analysis by colony counting (log10 CFU/mL). The Mann-Whitney test showed statistical significance in the amount of biofilm analyzed by CV test and CFU method (P<0.0001). *0.658=the cut-off for the moderate and strong biofilm mass producer; 19*, strain isolated from patient with S. aureus infection; red point, qualitative biofilm biomass test (CV); green point, the quantitative in vitro biofilm test (log10 CFU/mL).
Figure 1. Estimation of biofilm biomass for 32 clinical S. aureus strains by crystal violet (CV) staining and viable cells analysis by colony counting (log10 CFU/mL). The Mann-Whitney test showed statistical significance in the amount of biofilm analyzed by CV test and CFU method (P<0.0001). *0.658=the cut-off for the moderate and strong biofilm mass producer; 19*, strain isolated from patient with S. aureus infection; red point, qualitative biofilm biomass test (CV); green point, the quantitative in vitro biofilm test (log10 CFU/mL). 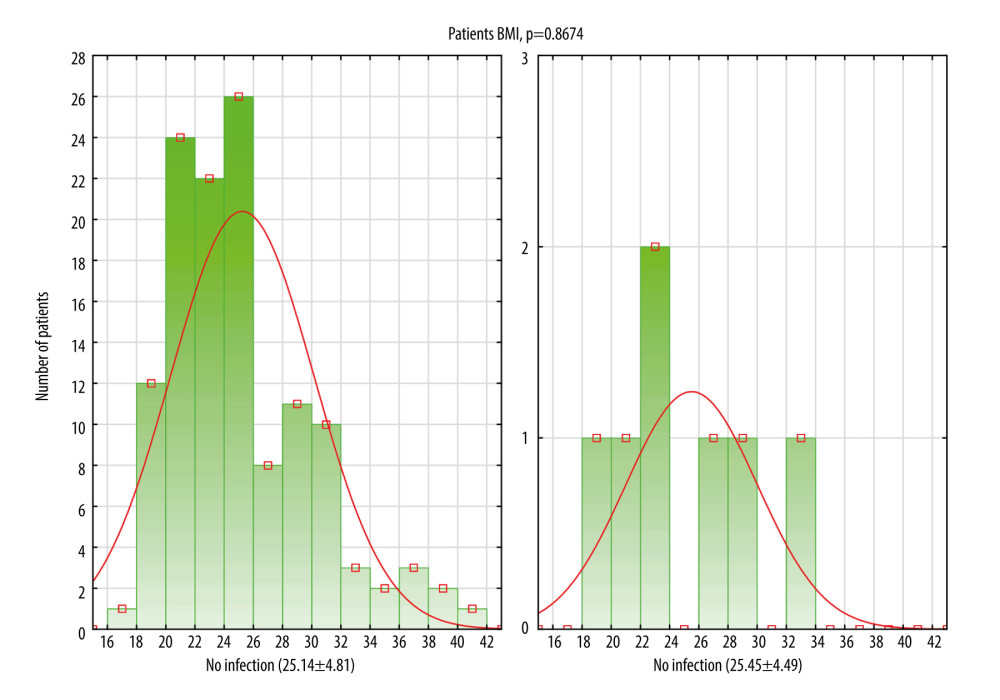 Figure 2. Histogram showing comparison between 2 groups of patients: without infection and with infection in relation to body mass index (BMI) distribution. Data are presented as a mean±standard deviation. This figure was created using Statistica software (version 13, TIBCO Software Inc.).
Figure 2. Histogram showing comparison between 2 groups of patients: without infection and with infection in relation to body mass index (BMI) distribution. Data are presented as a mean±standard deviation. This figure was created using Statistica software (version 13, TIBCO Software Inc.). 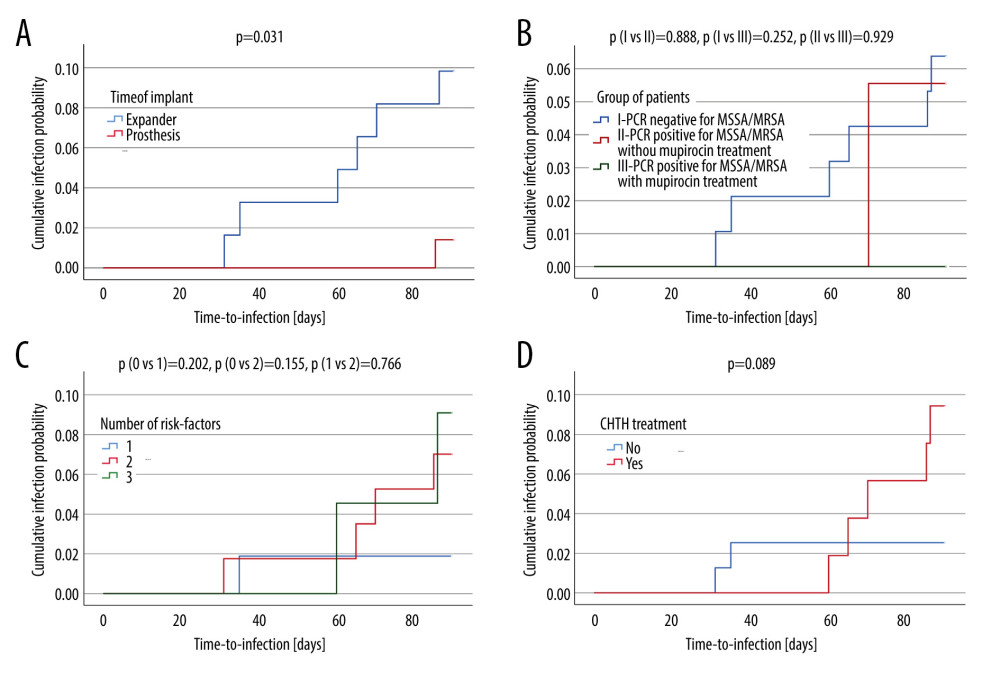 Figure 3. Kaplan-Meier curves displaying the estimated infection probability for different groups of patients. Time-dependent risk of infection was assessed using the log-rank test. Infection probability was calculated as one minus cumulative survival probability. (C) Shows infection probability for 3 groups. Patients with 0 or 1 or 2 risk-factors. (A) Shows infection probability for 2 groups. Patients with expander or with prosthesis. The cumulative infection probability was higher for the use of an expander compared with a prosthesis (P=0.031). (B) Shows infection probability for 3 groups. Patients with PCR negative for MSSA/MRSA, with PCR positive for MSSA/MRSA who did and who did not perioperatively receive mupirocin. (D) Shows infection probability for 2 groups. Patients either did or did not receive treatment with CHTH. The cumulative infection probability was higher for treatment with CHTH than without CHTH treatment. However, the differences were not statistically significant (P>0.05). PCR – polymerase chain reaction; MSSA – methicillin-susceptible S. aureus; MRSA – methicillin-resistant S. aureus; CHTH – chemotherapy (adjuvant or neoadjuvant). These figures were created using IBM SPSS Statistics (version 29, IBM Corp, Armonk, NY, USA).
Figure 3. Kaplan-Meier curves displaying the estimated infection probability for different groups of patients. Time-dependent risk of infection was assessed using the log-rank test. Infection probability was calculated as one minus cumulative survival probability. (C) Shows infection probability for 3 groups. Patients with 0 or 1 or 2 risk-factors. (A) Shows infection probability for 2 groups. Patients with expander or with prosthesis. The cumulative infection probability was higher for the use of an expander compared with a prosthesis (P=0.031). (B) Shows infection probability for 3 groups. Patients with PCR negative for MSSA/MRSA, with PCR positive for MSSA/MRSA who did and who did not perioperatively receive mupirocin. (D) Shows infection probability for 2 groups. Patients either did or did not receive treatment with CHTH. The cumulative infection probability was higher for treatment with CHTH than without CHTH treatment. However, the differences were not statistically significant (P>0.05). PCR – polymerase chain reaction; MSSA – methicillin-susceptible S. aureus; MRSA – methicillin-resistant S. aureus; CHTH – chemotherapy (adjuvant or neoadjuvant). These figures were created using IBM SPSS Statistics (version 29, IBM Corp, Armonk, NY, USA). References
1. Matthews H, Carroll N, Renshaw D, Predictors of satisfaction and quality of life following post-mastectomy breast reconstruction: Psychooncology, 2017; 26(11); 1860-65
2. Chang JM, Kosiorek HE, Dueck AC, Trends in mastectomy and reconstruction for breast cancer; A twelve year experience from a tertiary care center: Am J Surg, 2016; 212(6); 1201-10
3. Olsen MA, Nickel KB, Fox IK, Comparison of wound complications after immediate, delayed, and secondary breast reconstruction procedures: JAMA Surg, 2017; 152(9); e172338
4. Szymankiewicz M, Nowikiewicz T, Biedka M, Significance of infections in implant loss after breast reconstruction in the course of breast cancer treatment: Pol J Microbiol, 2019; 68(3); 343-51
5. Palubicka A, Jaworski R, Wekwejt M, Surgical site infection after breast surgery:A retrospective analysis of 5-year postoperative data from a single center in Poland: Medicina (Kaunas), 2019; 55(9); 512
6. Chidester JR, Danci I, Lewis P, Antibiogram for periprosthetic infections:A tool for better informed selection of empiric antibiotics for surgical site infections: Ann Plast Surg, 2016; 76(Suppl 3); S158-61
7. Piper ML, Roussel LO, Koltz PF, Characterizing infections in prosthetic breast reconstruction:A validity assessment of national health databases: J Plast Reconstr Aesthet Surg, 2017; 70(10); 1345-53
8. Seng P, Bayle S, Alliez A, The microbial epidemiology of breast implant infections in a regional referral centre for plastic and reconstructive surgery in the south of France: Int J Infect Dis, 2015; 35; 62-66
9. Viola GM, Baumann DP, Mohan K, Improving antimicrobial regimens for the treatment of breast tissue expander-related infections: Plast Reconstr Surg Glob Open, 2016; 4(5); e704
10. Perl TM, Cullen JJ, Wenzel RP: N Engl J Med, 2002; 346(24); 1871-77
11. González-García SHA, Bustos-Hamdan A, Bustos Martinez J: Pharynx – Diagnosis and Treatment, 2021, London, IntechOpen Available from:https://www.intechopen.com/chapters/74905
12. Cho OH, Baek EH, Bak MH: Am J Infect Control, 2016; 44(5); 533-38
13. Saraswat MK, Magruder JT, Crawford TC: Ann Thorac Surg, 2017; 104(4); 1349-56
14. Kluytmans J, van Belkum A, Verbrugh H: Clin Microbiol Rev, 1997; 10(3); 505-20
15. Sakr A, Bregeon F, Mege JL: Front Microbiol, 2018; 9; 2419
16. Hrynyshyn A, Simoes M, Borges A, Biofilms in surgical site infections:Recent advances and novel prevention and eradication strategies: Antibiotics (Basel), 2022; 11(1); 69
17. Oliva A, Miele MC, Al Ismail D, Challenges in the microbiological diagnosis of implant-associated infections:A summary of the current knowledge: Front Microbiol, 2021; 12; 750460
18. : Global Guidelines for the Prevention of Surgical Site Infection, 2018, Geneva, World Health Organization Web Appendix 3, Summary of a systematic review on decolonization with mupirocin ointment with or without chlorhexidine gluconate body wash for the prevention of infection in nasal carriers undergoing surgery. Available from:https://www.ncbi.nlm.nih.gov/books/NBK536421/
19. The European Committee on Antimicrobial Susceptibility Testing: Disk diffusion method for antimicrobial susceptibility testing. Version 9.0, 2021 Available from:http://www.eucast.org
20. The European Committee on Antimicrobial Susceptibility Testing: Breakpoint tables for interpretation of MICs and zone diameters Version 11.0, 2021 Available from:http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_11.0_Breakpoint_Tables.pdf
21. European Centre for Disease Prevention and Control: Surveillance of surgical site infections and prevention indicators in European hospitals-HAI-Net SSI protocol. Version 2,2 2017 Available from: https://www.ecdc.europa.eu/en
22. Stepanovic S, Vukovic D, Dakic I, A modified microtiter-plate test for quantification of staphylococcal biofilm formation: J Microbiol Methods, 2000; 40(2); 175-79
23. Merritt JH, Kadouri DE, O’Toole GA, Growing and analyzing static biofilms: Curr Protoc Microbiol, 2005; Chapter 1(Unit 1B); 1
24. Franchelli S, Vassallo F, Porzio C, Breast implant infections after surgical reconstruction in patients with breast cancer:Assessment of risk factors and pathogens over extended post-operative observation: Surg Infect (Larchmt), 2012; 13(3); 154-58
25. Papa G, Frasca A, Renzi N, Protocol for prevention and monitoring of surgical site infections in implant-based breast reconstruction:Preliminary results: Medicina (Kaunas), 2021; 57(2); 151
26. Nishibayashi A, Tomita K, Sugio Y: Plast Reconstr Surg Glob Open, 2016; 4(10); e1076
27. Ooi A, Song DH, Reducing infection risk in implant-based breast-reconstruction surgery:Challenges and solutions: Breast Cancer (Dove Med Press), 2016; 8; 161-72
28. Brand JS, Colzani E, Johansson ALV, Infection-related hospitalizations in breast cancer patients:Risk and impact on prognosis: J Infect, 2016; 72(6); 650-58
29. Fairchild B, Ellsworth W, Selber JC, Safety and efficacy of smooth surface tissue expander breast reconstruction: Aesthet Surg J, 2020; 40(1); 53-62
30. Hanwright PJ, Davila AA, Hirsch EM, The differential effect of BMI on prosthetic versus autogenous breast reconstruction:A multivariate analysis of 12,986 patients: Breast, 2013; 22(5); 938-45
31. Kato H, Nakagami G, Iwahira Y, Risk factors and risk scoring tool for infection during tissue expansion in tissue expander and implant breast reconstruction: Breast J, 2013; 19(6); 618-26
32. Bode LG, van Rijen MM, Wertheim HF: Ann Surg, 2016; 263(3); 511-15
33. Langenberg JC, Thomas AR, Donker JM: PLoS One, 2016; 11(8); e0161058
34. Lelieveld-Vroom MZA, Neijenhuis PA: Br J Med Health Res, 2018; 5(3); 26-34
35. Reish RG, Damjanovic B, Austen WG, Infection following implant-based reconstruction in 1952 consecutive breast reconstructions:Salvage rates and predictors of success: Plast Reconstr Surg, 2013; 131(6); 1223-30
36. Jung SM, Jeon BJ, Woo J, Does chemotherapy or radiotherapy affect the postoperative complication in breast cancer patients who underwent immediate breast reconstruction with tissue expander?: BMC Cancer, 2021; 21(1); 88
37. Price CK, Bexley A, Kostiou Vthe Modernising Medical Microbiology Informatics Group: Lancet Infect Dis, 2017; 17(2); 207-14
38. Barbieri R, Pesce M, Franchelli S, Baldelli I, Phenotypic and genotypic characterization of Staphylococci causing breast peri-implant infections in oncologic patients: BMC Microbiol, 2015; 15; 26
39. Sharabiani HR, Sadeghi J, Pirzade T: Microb Pathog, 2021; 154; 104860
40. Whittard E, Redfern J, Xia G: Front Cell Infect Microbiol, 2021; 11; 698909
41. Nguyen HTT, Nguyen TH, Otto M, The staphylococcal exopolysaccharide PIA – biosynthesis and role in biofilm formation, colonization, and infection: Comput Struct Biotechnol J, 2020; 18; 3324-34
42. Sritharadol R, Hamada M, Kimura S: Microb Drug Resist, 2018; 24(9); 1249-58
Figures
 Figure 1. Estimation of biofilm biomass for 32 clinical S. aureus strains by crystal violet (CV) staining and viable cells analysis by colony counting (log10 CFU/mL). The Mann-Whitney test showed statistical significance in the amount of biofilm analyzed by CV test and CFU method (P<0.0001). *0.658=the cut-off for the moderate and strong biofilm mass producer; 19*, strain isolated from patient with S. aureus infection; red point, qualitative biofilm biomass test (CV); green point, the quantitative in vitro biofilm test (log10 CFU/mL).
Figure 1. Estimation of biofilm biomass for 32 clinical S. aureus strains by crystal violet (CV) staining and viable cells analysis by colony counting (log10 CFU/mL). The Mann-Whitney test showed statistical significance in the amount of biofilm analyzed by CV test and CFU method (P<0.0001). *0.658=the cut-off for the moderate and strong biofilm mass producer; 19*, strain isolated from patient with S. aureus infection; red point, qualitative biofilm biomass test (CV); green point, the quantitative in vitro biofilm test (log10 CFU/mL). Figure 2. Histogram showing comparison between 2 groups of patients: without infection and with infection in relation to body mass index (BMI) distribution. Data are presented as a mean±standard deviation. This figure was created using Statistica software (version 13, TIBCO Software Inc.).
Figure 2. Histogram showing comparison between 2 groups of patients: without infection and with infection in relation to body mass index (BMI) distribution. Data are presented as a mean±standard deviation. This figure was created using Statistica software (version 13, TIBCO Software Inc.). Figure 3. Kaplan-Meier curves displaying the estimated infection probability for different groups of patients. Time-dependent risk of infection was assessed using the log-rank test. Infection probability was calculated as one minus cumulative survival probability. (C) Shows infection probability for 3 groups. Patients with 0 or 1 or 2 risk-factors. (A) Shows infection probability for 2 groups. Patients with expander or with prosthesis. The cumulative infection probability was higher for the use of an expander compared with a prosthesis (P=0.031). (B) Shows infection probability for 3 groups. Patients with PCR negative for MSSA/MRSA, with PCR positive for MSSA/MRSA who did and who did not perioperatively receive mupirocin. (D) Shows infection probability for 2 groups. Patients either did or did not receive treatment with CHTH. The cumulative infection probability was higher for treatment with CHTH than without CHTH treatment. However, the differences were not statistically significant (P>0.05). PCR – polymerase chain reaction; MSSA – methicillin-susceptible S. aureus; MRSA – methicillin-resistant S. aureus; CHTH – chemotherapy (adjuvant or neoadjuvant). These figures were created using IBM SPSS Statistics (version 29, IBM Corp, Armonk, NY, USA).
Figure 3. Kaplan-Meier curves displaying the estimated infection probability for different groups of patients. Time-dependent risk of infection was assessed using the log-rank test. Infection probability was calculated as one minus cumulative survival probability. (C) Shows infection probability for 3 groups. Patients with 0 or 1 or 2 risk-factors. (A) Shows infection probability for 2 groups. Patients with expander or with prosthesis. The cumulative infection probability was higher for the use of an expander compared with a prosthesis (P=0.031). (B) Shows infection probability for 3 groups. Patients with PCR negative for MSSA/MRSA, with PCR positive for MSSA/MRSA who did and who did not perioperatively receive mupirocin. (D) Shows infection probability for 2 groups. Patients either did or did not receive treatment with CHTH. The cumulative infection probability was higher for treatment with CHTH than without CHTH treatment. However, the differences were not statistically significant (P>0.05). PCR – polymerase chain reaction; MSSA – methicillin-susceptible S. aureus; MRSA – methicillin-resistant S. aureus; CHTH – chemotherapy (adjuvant or neoadjuvant). These figures were created using IBM SPSS Statistics (version 29, IBM Corp, Armonk, NY, USA). Tables
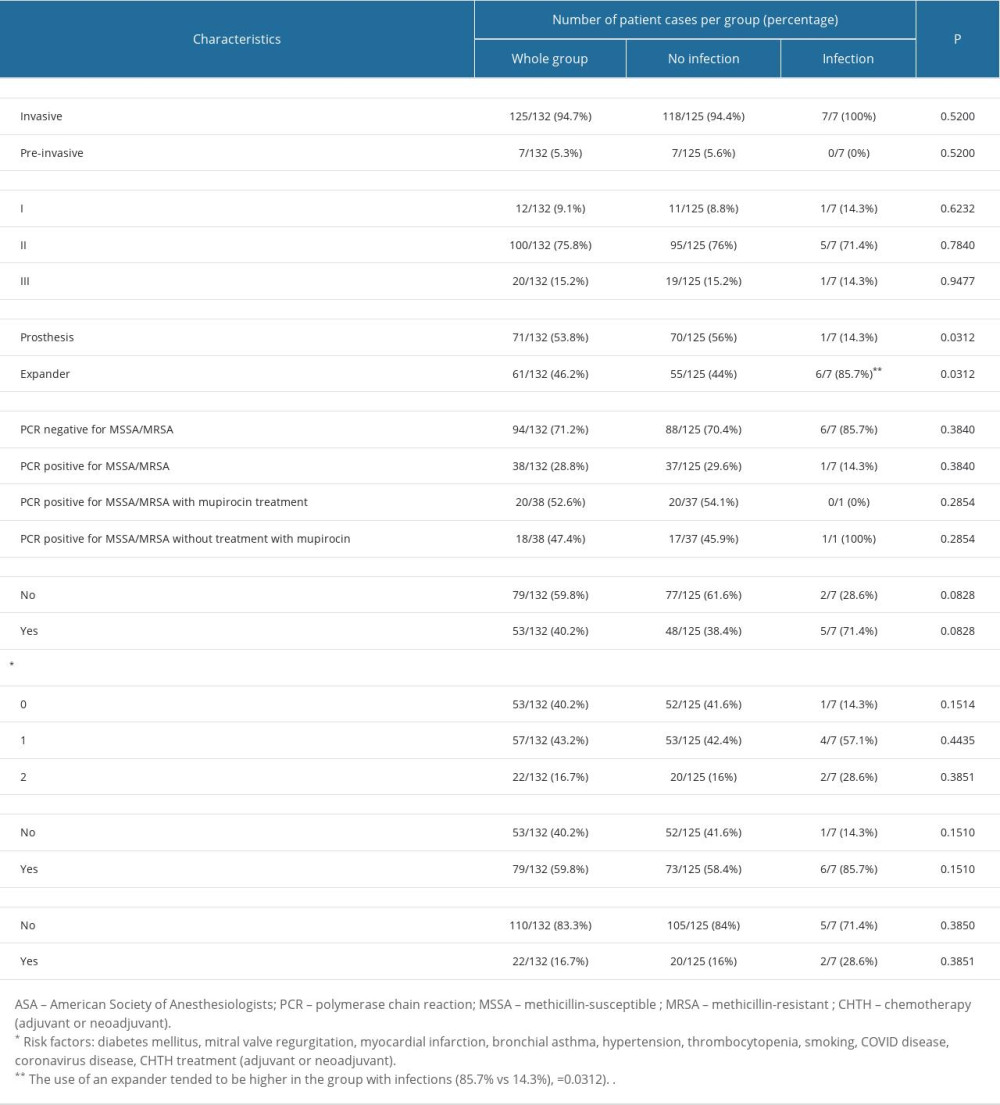 Table 1. Characteristics influencing the development of implant infections in breast cancer reconstructive surgery: Comparison of the studied groups.
Table 1. Characteristics influencing the development of implant infections in breast cancer reconstructive surgery: Comparison of the studied groups.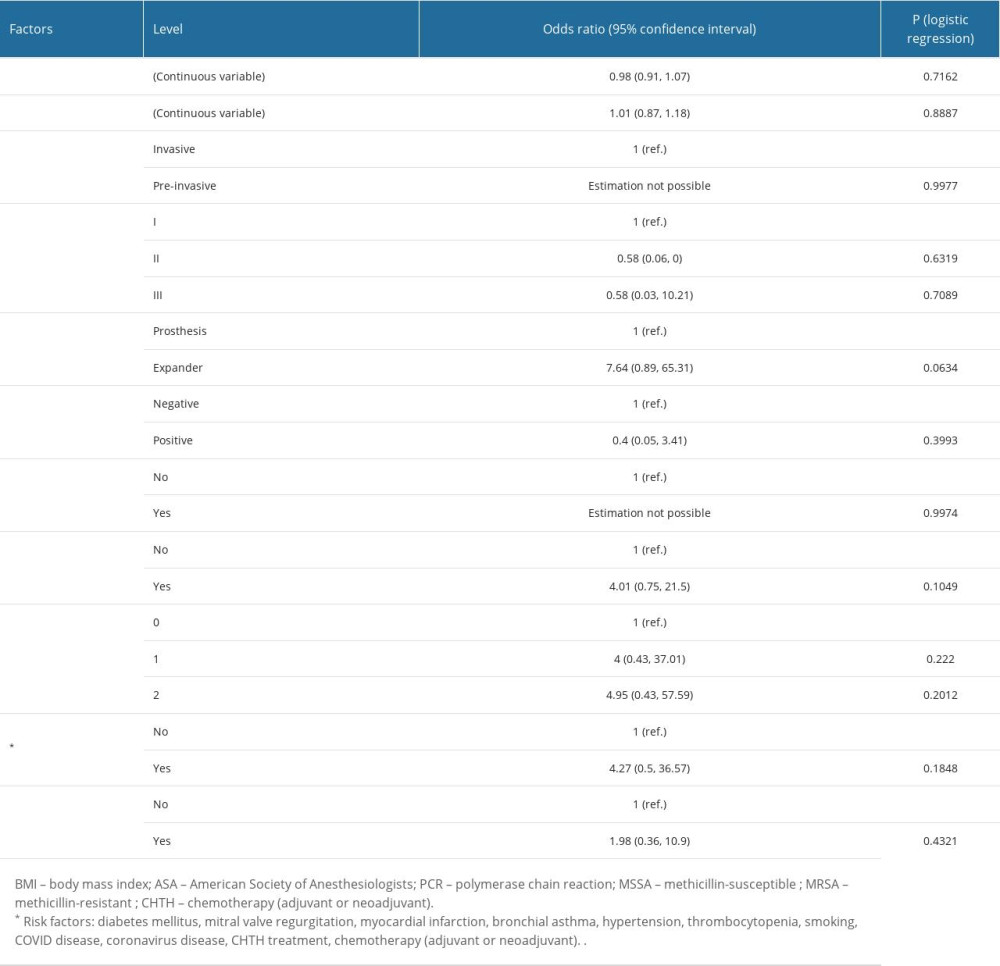 Table 2. The influence of analyzed factors on the risk of infection: univariate analysis.
Table 2. The influence of analyzed factors on the risk of infection: univariate analysis. Table 1. Characteristics influencing the development of implant infections in breast cancer reconstructive surgery: Comparison of the studied groups.
Table 1. Characteristics influencing the development of implant infections in breast cancer reconstructive surgery: Comparison of the studied groups. Table 2. The influence of analyzed factors on the risk of infection: univariate analysis.
Table 2. The influence of analyzed factors on the risk of infection: univariate analysis. In Press
12 Mar 2024 : Clinical Research
Metabolomic Alterations in Methotrexate Treatment of Moderate-to-Severe PsoriasisMed Sci Monit In Press; DOI: 10.12659/MSM.943360
14 Mar 2024 : Clinical Research
Renal Dysfunction Increases Risk of Adverse Cardiovascular Events in 5-Year Follow-Up Study of Intermediate...Med Sci Monit In Press; DOI: 10.12659/MSM.943956
15 Mar 2024 : Clinical Research
Impact of One-Lung Ventilation on Oxygenation and Ventilation Time in Thoracoscopic Heart Surgery: A Compar...Med Sci Monit In Press; DOI: 10.12659/MSM.943089
14 Mar 2024 : Clinical Research
Differential DHA and EPA Levels in Women with Preterm and Term Births: A Tertiary Hospital Study in IndonesiaMed Sci Monit In Press; DOI: 10.12659/MSM.943895
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








