01 August 2023: Database Analysis
Impact of Pre-Transplant Parathyroidectomy on Graft Survival: A Comparative Study of Renal Transplant Patients (2005–2015)
Ming-Hsien Tsai12ADEF, Mingchih ChenDOI: 10.12659/MSM.940959
Med Sci Monit 2023; 29:e940959
Abstract
BACKGROUND: Hyperparathyroidism poses significant risks for patients prior to kidney transplantation. However, the outcomes of patients who undergo parathyroidectomy before renal transplantation compared to those without such a procedure remain uncertain. This real-world data study aimed to examine the clinical outcomes of both patient groups.
MATERIAL AND METHODS: Using the Taiwan National Health Insurance Research Database, we conducted a retrospective cohort study on patients who underwent renal transplantation between January 2005 and December 2015. The patients were divided into two groups: a case group (n=294) with parathyroidectomy and a control group (n=588) without the need for parathyroidectomy before kidney transplantation. The groups were matched based on age, sex, dialysis vintage, and baseline characteristics at a 1:2 ratio. Hazard ratios (HR) were estimated using the Cox regression model. The main outcomes assessed were graft failure, mortality, and major adverse cardiovascular events (MACE) recorded until December 2019.
RESULTS: During a mean follow-up period of 6 years, a significant difference was observed in graft failure (HR 1.40; 95% confidence interval 1.10-1.79, p=0.007) between the two groups. After further adjustment, graft failure remained significant (HR 1.52; 95% CI 1.07-2.15, p=0.019). Additionally, machine learning-based feature selection identified the importance of parathyroidectomy (ranked 9 out of 11) before kidney transplantation in predicting subsequent graft failure.
CONCLUSIONS: Our study demonstrates that severe hyperparathyroidism requiring parathyroidectomy before kidney transplantation may contribute to poor post-transplant graft outcomes compared to patients who do not require parathyroidectomy.
Keywords: Cardiovascular Diseases, Dialysis, Graft Survival, Mortality, Parathyroidectomy, Humans, Kidney Transplantation, Retrospective Studies, Hyperparathyroidism, Renal Dialysis
Background
Hyperparathyroidism, which is defined as elevated parathyroid hormone levels, is a common complication of chronic kidney disease (CKD). Being a major feature of CKD–mineral and bone disorder, especially in patients with longer dialysis vintage, it results from derangements in the homeostasis of calcium, phosphate, and vitamin D [1]. Starting from CKD stage 3 with an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2, the prevalence increases as the kidney function declines and peaks after dialysis initiation [2]. Major etiologies included phosphate retention with low calcium concentration, decreased 1,25-dihydroxyvitamin D production, and increased fibroblast growth factor 23 (FGF23) levels [3].
As a non-traditional risk factor of cardiovascular disease in dialysis, serum parathyroid hormone (PTH) levels were associated with mortality, as revealed by the Dialysis Outcomes and Practice Patterns Study (DOPPS) [4]. The DOPPS showed PTH levels of 101–300 pg/mL are associated with the lowest mortality risk, whereas PTH levels >600 pg/mL are associated with the greatest mortality risk [4]. In the CORES Study, over 22 000 patients on hemodialysis with dialysis vintage >18 years were recruited; both high and low quantile levels of PTH were strongly correlated with all-cause and cardiovascular mortalities [5].
Since the prevalence of secondary hyperparathyroidism increased with dialysis vintage, by the time of renal transplantation, patients already suffered from various degrees of hyperparathyroidism. Usually, mild hyperparathyroidism spontaneously resolves in the post-kidney transplantation stage because the renal function is restored [6–8]. In more severe stages, hyperparathyroidism persists in 15–50% of patients [8–10]. This may be due to structural changes in PTH, with the formation of hyperplasia or adenoma, making it difficult to control even if the initial stimuli have disappeared [11,12]. The effect of persistent hyperparathyroidism after kidney transplantation is well documented in many aspects. Previous studies have shown its association with decreased bone density, leading to an increased risk of osteoporosis and fractures [13,14]. In addition, hypercalcemia and hypophosphatemia can occur following PTH oversecretion. As consequence, soft-tissue calcification [15,16] and graft dysfunction [3,17] can be observed in this high-risk group.
Parathyroidectomy is a surgical option for refractory and persistent hyperparathyroidism. Following total or partial removal of the parathyroid gland, serum PTH levels can be effectively lowered and associated complications reduced [18]. In patients with severe hyperparathyroidism awaiting renal transplantation, no consensus has been established regarding the timing of parathyroidectomy [19]. Callender et al reported that in the severe hyperparathyroidism group, those undergoing parathyroidectomy before transplantation had better clinical outcomes [20]. However, whether the effects of hyperparathyroidism on post-parathyroidectomy clinical outcomes can be eliminated to the same level as the normal parathyroid gland is unclear.
Therefore, this study aimed to compare outcomes between patients with and without need for parathyroidectomy prior to kidney transplantation in terms of graft failure, major adverse cardiovascular events (MACE), and mortality at a mean 6-year follow-up, using data from the Taiwan National Health Insurance Research Database (NHIRD).
Material and Methods
ETHICS STATEMENT:
In conducting our study, we adhered to all relevant ethical guidelines and regulations set forth by institutional and governmental bodies regarding the appropriate use of data obtained from human subjects. All beneficial claims records were encrypted and anonymized before analysis; thus, the requirement for informed consent was waived by the Institutional Review Board of Shin Kong Wu Ho-Su Memorial Hospital (IRB approval no. 20200804R).
DATA SOURCES:
This retrospective cohort study was based on the NHIRD in 2002–2019. On March 1, 1995, Taiwan introduced a universal single-payer National Health Insurance (NHI) program. By 2014, approximately 99.9% of Taiwan’s population had enrolled in this program [21]. Notably, even foreigners residing in Taiwan are eligible for coverage under this program. The database associated with the NHI program includes registration files and original claim data, which are utilized for reimbursement purposes. The National Health Insurance Administration (formerly the Bureau of National Health Insurance, BNHI) under the Ministry of Health and Welfare (formerly the Department of Health, DOH) in Taiwan manages these large, computerized databases. The databases are maintained by the National Health Research Institutes, Taiwan, and are made accessible to researchers in Taiwan for scientific research endeavors.
DESIGN AND STUDY PARTICIPANTS:
The study was designed as a retrospective longitudinal cohort study. As shown in Figure 1, this study analyzed patients who underwent kidney transplantation (n=8538) from NHIRD between January 1, 2005, and December 31, 2015, and followed up until December 31, 2019. The exclusion criteria were as follows: (1) aged ≤18 years (n=226), (2) had cancer diagnosis before 2005 (n=157), (3) had kidney transplantation before 2005 (n=3048), (4) had missing information (n=42), and (5) had parathyroidectomy after kidney transplantation (n=263). Finally, the patients were divided into 2 groups: patients who underwent parathyroidectomy (case group; n=393) and patients who did not undergo parathyroidectomy before kidney transplantation (control group; n=4409).
The date on kidney transplantation were denoted as the index date, and the follow-up period ended until event occurrence, death, or end date of database use (December 31, 2019). To reduce confounding factors, the propensity-score method [22] was used to match the case and control groups by sex, age, dialysis vintage, and baseline characteristics. After 1: 2 ratio matching, the final case and control groups included 294 and 588 patients, respectively.
COVARIATES:
The variables collected were as follows: sex, age, dialysis vintage, the time from parathyroidectomy to kidney transplantation, dialysis modality before kidney transplantation, comorbidities (hypertension, diabetes mellitus [DM], myocardial infarction [MI], congestive heart failure [CHF], atrial fibrillation [Af], peripheral vascular disease [PVD], chronic obstructive pulmonary disease [COPD], stroke, hyperlipidemia, gout, and liver cirrhosis), Charlson comorbidity index [CCI] scores, history of receiving cardiovascular intervention, and kidney transplantation with complications. The detail codes of baseline are shown in Supplementary Table 1.
The medication history was also collected, including angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, b-blockers, calcium channel blockers (CCB), diuretics, insulin, lipid-lowering agents, oral hypoglycemic agents (OHA), benzodiazepine, uric acid-lowering agent, anticoagulants, and non-steroidal anti-inflammatory drugs (NSAID). The codes of drugs are shown in Supplementary Table 2.
CLINICAL OUTCOMES DEFINITION:
The study outcomes were graft failure (return to dialysis again) defined as unsuccessful functioning of a transplanted organ, leading to the recipient needing to resume dialysis treatment, all-cause mortality, MACE, new-onset stroke, and fracture. However, MACE is a composite endpoint consisting of myocardial infarction, cerebrovascular disease, heart failure, and arrhythmia [23]. If patients do not have any of the aforementioned outcomes, they were followed up until death or the end of the study (31 December 2019). The codes of outcomes are provided in Supplementary Table 1.
FEATURE SELECTION:
Feature selection, also known as dimensionality reduction, is a preprocessing step that can identify which variables could obviously affect the desired outcomes, and it has been applied in medical informatics and research [24,25]. Through feature selection, we can easily understand which parameters can make predictions more accurate from the importance ranking of predictors. Therefore, in this study, feature selection was used to estimate the importance of parameters on graft failure of patients on dialysis undergoing kidney transplantation. Five machine learning models were used to evaluate which variables can affect the outcomes more: logistic regression (LGR) [26], random forest (RF) [27], K-nearest neighbor (KNN), and eXtreme gradient boosting (XGB) [28]. Finally, the top-ranked important variables were selected based on the average ranking of each method.
STATISTICAL ANALYSIS:
Demographic distributions of age, sex, and comorbidities are denoted as the number of patients and percentage [numbers (%)]. Categorical variables were compared by the chi-square test. Continuous variables denoted as mean with a standard deviation were compared by the
All analyses were performed by SAS version 9.4 (SAS Institute, Cary, NC, USA). A 2-tailed
Results
STUDY COHORT AND BASELINE CHARACTERISTICS:
This study included a total of 4802 patients who received their first kidney transplant between January 2005 and December 2015. After propensity score matching with 1: 2 ratio, 294 patients who underwent parathyroidectomy (case group) were matched with 588 patients (control group) who did not undergo parathyroidectomy before their kidney transplant (Figure 1). As shown in Table 1, The mean age of case group was 46.9±10.2 years, of whom 42.7% were men, 60.3% had hypertension, 33.6% had diabetes mellitus history. Moreover, the mean dialysis vintage of the case group was 5.7±2.9 years and the mean time from parathyroidectomy to kidney transplantation was 3.3±2.6 years.
Before matching, the case group was predominantly women, with a longer dialysis vintage, with lower CCIS, non-diabetic, and with a history of CHF, COPD, and kidney transplantation with complication history. The case group was less likely to be prescribed ACEI/ARB, b-blockers, CCB, diuretic, insulin, OHA, and uric acid-lowering agent. However, after matching, there were still some variables (MI, COPD, kidney transplant with complication, CCIS, CCB, insulin, and OHA) that had significant differences (all P<0.05) (Table 1).
COMPARISON OF OUTCOMES:
In Table 2, 60 patients with graft failure in the kidney transplant patients with parathyroidectomy (case group) were compared with 77 patients with graft failure in the kidney transplant patients without parathyroidectomy (control group) during a mean observation period of 6 years. The incidence rate of graft failure was higher in the case group than in the control group (33 vs 22 per 1000 person-years). In a crude analysis after the propensity-score matching, the case group had a significantly increased risk of graft failure (HR 1.40; 95% CI 1.10–1.79; P<0.007).
After further multivariable adjusting, the case group still maintained a significantly higher risk of graft failure (HR 1.52; 95% CI 1.07–2.15; P<0.019). The risks of all-cause mortality (HR 0.88; 95% CI 0.63–1.21; P=0.419), MACEs (HR 1.15; 95% CI 0.74–1.79; P=0.536), new-onset stroke (HR 1.13; 95% CI 0.71–1.81; P=0.616), and fractures (HR 1.91; 95% CI 0.91–4.03; P=0.088) did not differ significantly between the case and control groups in the multivariable adjusted model. Furthermore, the Kaplan-Meier plot in Figure 2 shows that there was a significance difference of event-free curves in graft failure between groups (P=0.008) but not in all-cause mortality (P=0.276).
IDENTIFYING THE IMPORTANT PARAMETERS FOR GRAFT FAILURE:
The importance ranking of parameters for graft failure after kidney transplantation by machine learning methods is shown in Supplementary Table 3. Figure 3 shows the ranking by the average scores of LGR, RF, KNN, and XGB, which comorbidities in kidney transplantation with complications, PVD, liver cirrhosis, Af, CHF, stroke, and CCI scores, and MI), parathyroidectomy, medications in anticoagulants, and age were the most important features. The parameter, whether to undergo parathyroidectomy before kidney transplantation or not, ranked 9 of 11 for graft failure.
Discussion
In this study, we found the incidence rate of graft failure was higher in the parathyroidectomy before transplantation group than in the control group. This finding remained significant in a crude analysis after propensity-score matching and further multivariable adjustment. Although the severity of hyperparathyroidism before kidney transplantation may have hazardous effects on graft function and survival even in patients who underwent parathyroidectomy compared with those who did not require parathyroidectomy, the cardiovascular events and overall mortality rates were not significantly different. Preventing hyperparathyroidism in patients on dialysis before kidney transplantation might be more important than removing the parathyroid gland to restore its normal biochemical level.
The management of post-transplant hyperparathyroidism has been challenging. According to a previous study, persistent hyperparathyroidism after kidney transplantation can increase mortality and graft dysfunction. In a pilot study of the Assessment of Lescol in Renal Transplantation (ALERT) trial, 1600 kidney transplantation recipients were recruited. High serum levels of PTH showed a strong association with all-cause mortality and graft failure [29]. In a retrospective study conducted by Littbarski et al, parathyroidectomy after renal transplantation was associated with increased rates of worsening graft function then before transplantation [30]. Another study, by Jeon et al, had the same conclusion, finding the initial 5-year levels of eGFR after renal transplantation were significantly lower in the patients who underwent parathyroidectomy less than 1 year after transplantation than in the pre-transplant parathyroidectomy patients [31].
In addition to the ALERT trial, a recent study investigated the association between high PTH levels after kidney transplantation and graft failure. Hyperparathyroidism in this stage has an independent role in graft function [32]. Although the exact mechanism of how hyperparathyroidism contributes to graft failure remains unknown, hypercalcemia-induced acute kidney injury should be considered. In the glomerulus, PTH can induce vasodilation in afferent arterioles and vasoconstriction in efferent arterioles, leading to glomerular hyperfiltration and proteinuria [33]. Furthermore, high PTH levels can lead to immune deficiency and anemia, which can reduce renal function [34,35]. In the present study, the effect of parathyroidectomy on mortality was not significantly different. This may be due to the short observation time; a 3-year observation may not reveal such an endpoint.
The timing of parathyroidectomy for kidney transplantation recipients remained inconclusive because most hyperparathyroidism cases reached spontaneous resolution when renal function is restored by successful transplantation [7,36,37]. To date, there are no consensus guidelines regarding this issue [19]. The 2015 European Renal Best Practice Guideline suggested management of concomitant hyperparathyroidism for patients on the waiting list [38]. The 2017 KDIGO CKD-MBD guidelines suggest parathyroidectomy should be considered in G3a–G5D patients with severe HPT refractory to medical treatment but do not mention transplant candidates [39,40]. The 2020 KDIGO clinical practice guidelines recommended aggressive treatment of severe HPT before transplantation. Pre-transplantation parathyroidectomy should be considered when medical therapy is unsuccessful [41]. However, the latest National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI) US Commentary had no comment on this [42].
Persistent hyperparathyroidism after kidney transplantation has been regarded as an independent risk with graft dysfunction [3,43,44], which made parathyroidectomy before transplantation reasonable. However, in our population-based study, no benefit was observed on graft function, and this may be related to hyperparathyroidism-induced secretion of FGF23 from the bone. FGF23, as a phosphaturic hormone, is primarily secreted by osteocytes to increase urinary phosphate excretion and suppress renal vitamin D synthesis [45]. In CKD, serum FGF23 levels started to rise since the eGFR is <90 mL/min/1.73 m2 to maintain phosphate homeostasis [46,47]. In addition to its effects on the bones, FGF23 is associated with increased rates of cardiovascular disease, renal anemia, left ventricular hypertrophy, and mortality in both non-dialysis and dialysis CKD groups [48–51]. In fact, serum FGF23 levels after kidney transplantation showed a strong relationship with cardiovascular and graft mortality [52,53]. Although PTH can induce FGF23 production, parathyroidectomy has no effects on its reduction, which is further proved by meta-analyses [54–56].
The present study has several strengths. First, the results of this nationwide population-based cohort study can be generalized. Second, the sample size and follow-up duration were adequate to examine the primary outcomes. Third, we employed data-driven machine learning techniques to identify the top 11 factors that play a critical role in determining graft failure after kidney transplantation, and these findings have significant value for future discussions and research in this field. Despite these strengths, some limitations remain. First, information regarding potential confounding factors that are associated with graft failure after renal transplantation, including body mass index, blood pressure, socioeconomic status, laboratory data, and lifestyle, were not available in the database. Second, the PTH level could not be determined in the database, which might have biased our inferences. However, we utilized propensity score matching to address baseline imbalances and we excluded individuals who underwent parathyroidectomy after kidney transplantation, as these patients may have had severe hyperparathyroidism but declined parathyroidectomy.
Conclusions
This study provides valuable insights indicating that patients on dialysis who require parathyroidectomy before kidney transplantation because of severe hyperparathyroidism experience poorer graft survival compared to those who do not require this procedure. As a result, it is highly recommended to focus on preventing the progression of hyperparathyroidism to the extent that surgery becomes necessary in patients undergoing dialysis before receiving a kidney transplantation.
Figures
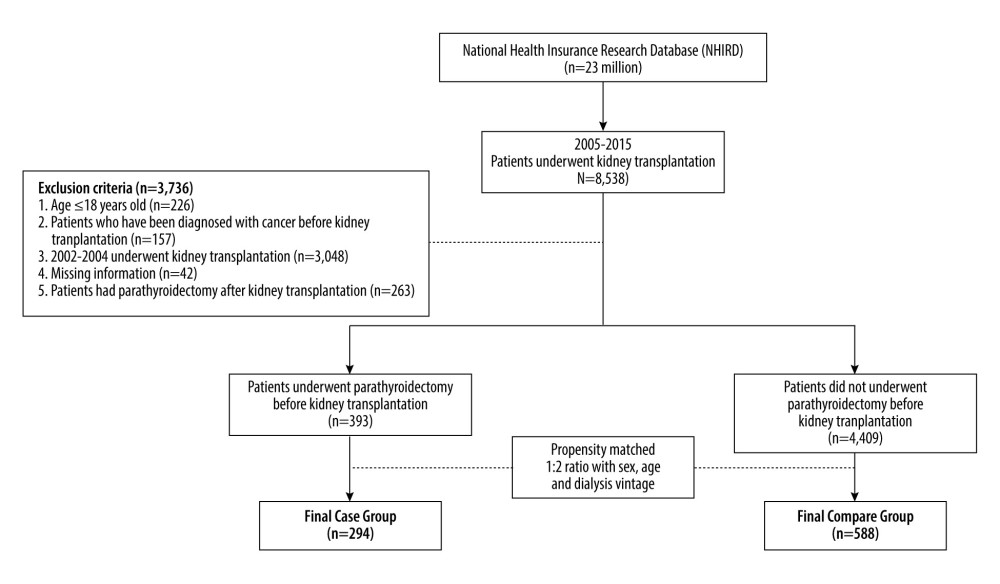 Figure 1. Schema of patient enrollment in the study.
Figure 1. Schema of patient enrollment in the study. 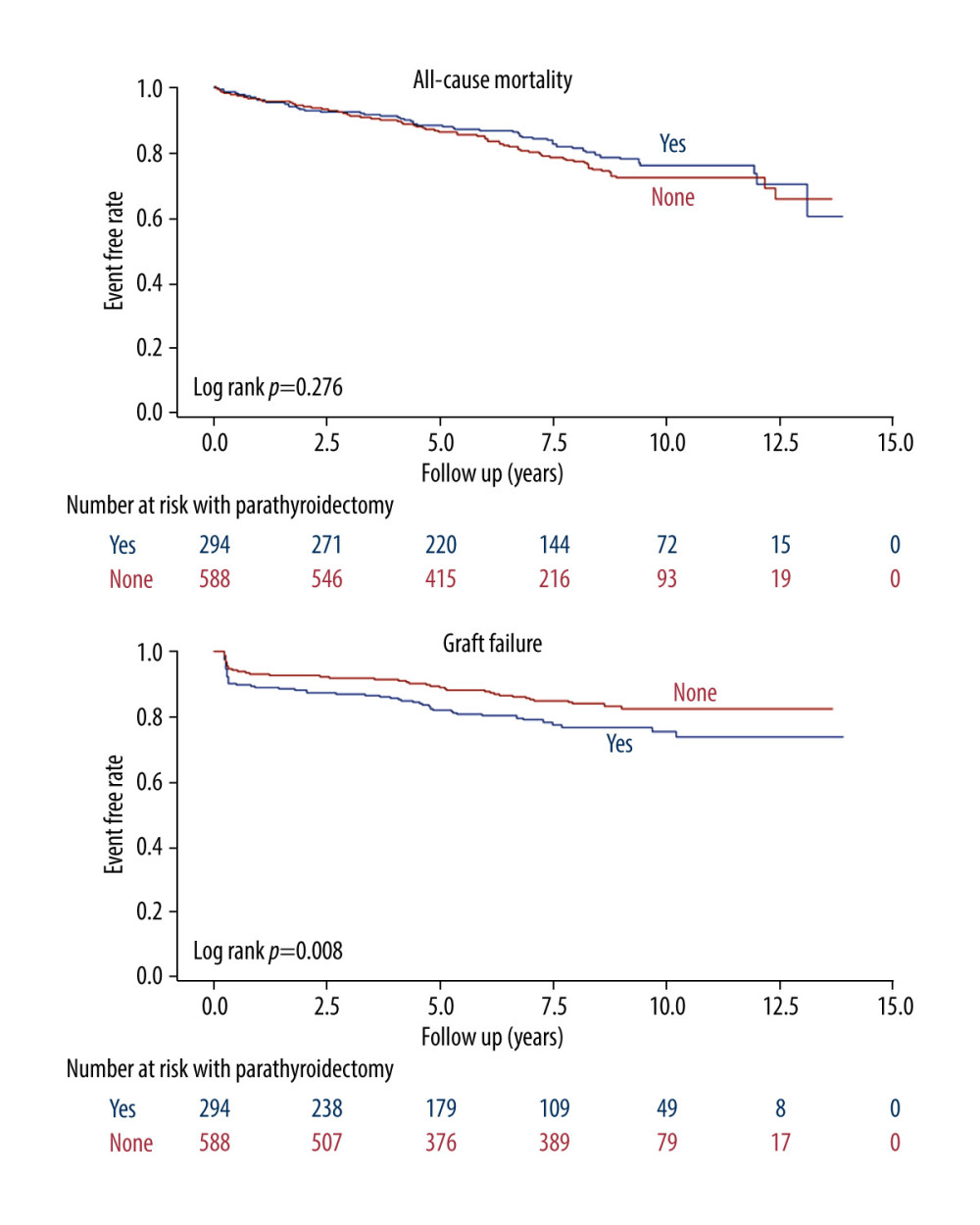 Figure 2. Kaplan-Meier cumulative event-free plots of (A) all-cause mortality (B) graft failure in the study population according to whether parathyroidectomy was performed before kidney transplantation.
Figure 2. Kaplan-Meier cumulative event-free plots of (A) all-cause mortality (B) graft failure in the study population according to whether parathyroidectomy was performed before kidney transplantation. 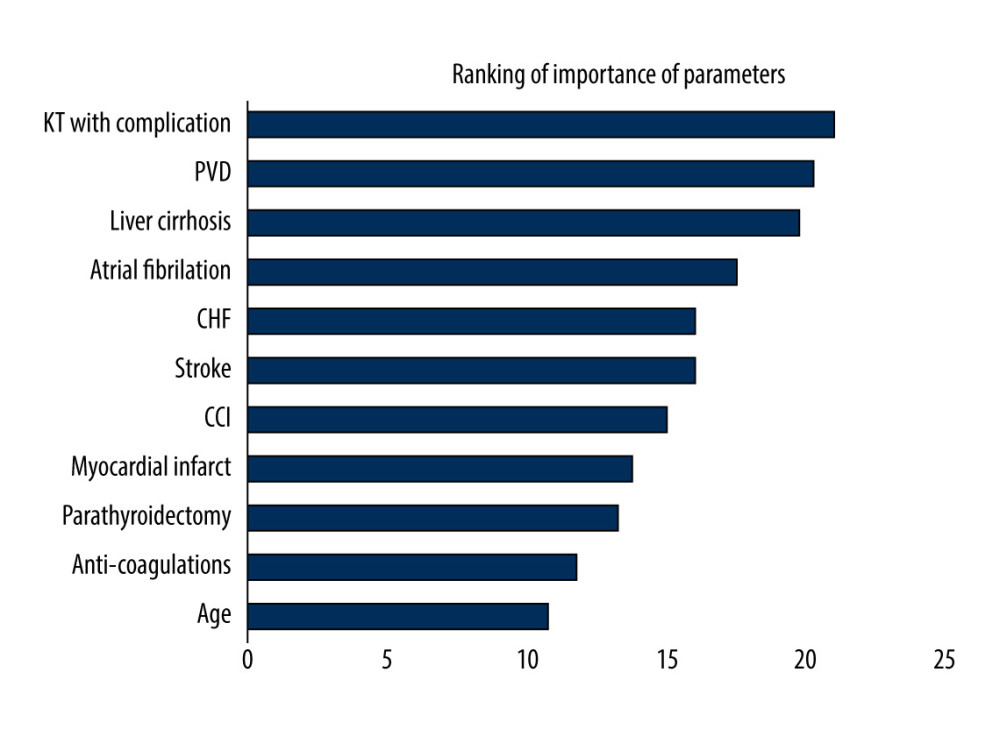 Figure 3. Ranking of importance of parameters for graft failure using machine learning method. KT – kidney transplantation; PVD – peripheral vascular disease; CHF – congestive heart failure; CCI – Charlson comorbidity index.
Figure 3. Ranking of importance of parameters for graft failure using machine learning method. KT – kidney transplantation; PVD – peripheral vascular disease; CHF – congestive heart failure; CCI – Charlson comorbidity index. Tables
Table 1. Baseline characteristics between those with/without parathyroidectomy prior kidney transplantation.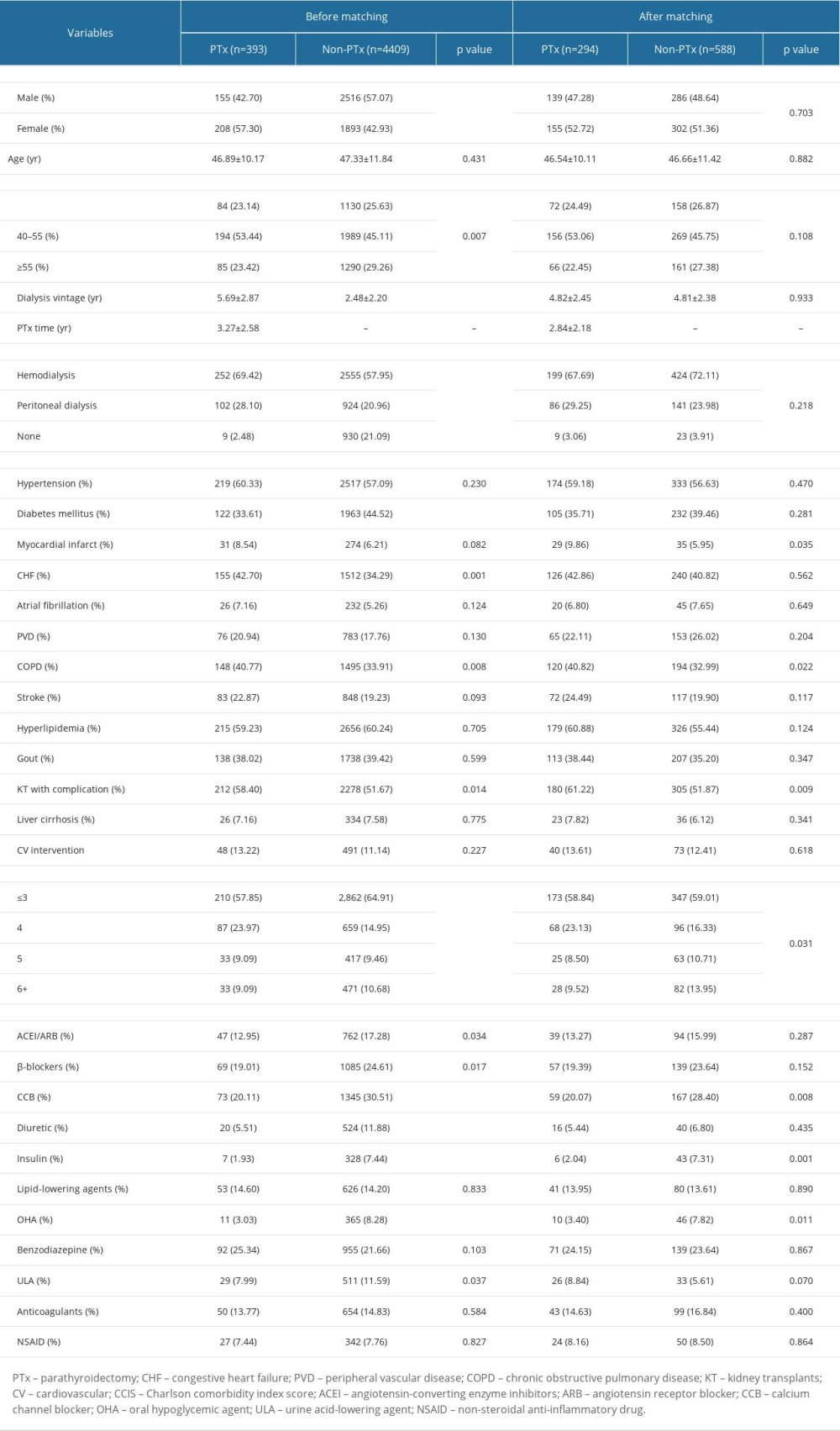 Table 2. Comparison of outcomes between those with/without parathyroidectomy prior kidney transplantation.
Table 2. Comparison of outcomes between those with/without parathyroidectomy prior kidney transplantation.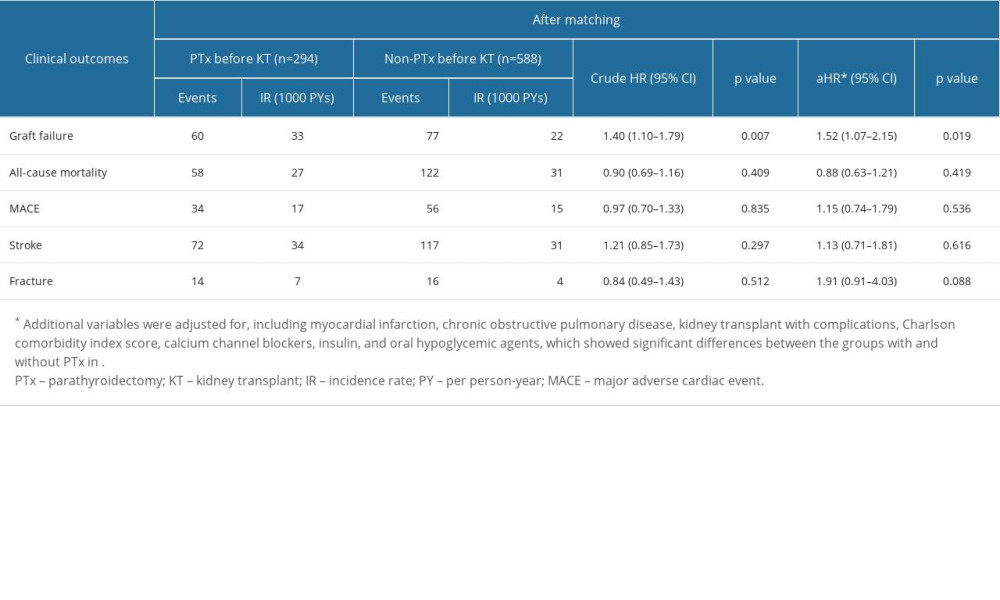 Supplementary Table 1. Code for diseases.
Supplementary Table 1. Code for diseases. Supplementary Table 2. Code for drugs.
Supplementary Table 2. Code for drugs. Supplementary Table 3. Ranking of feature selection on graft failure in the patients receiving kidney translation.
Supplementary Table 3. Ranking of feature selection on graft failure in the patients receiving kidney translation.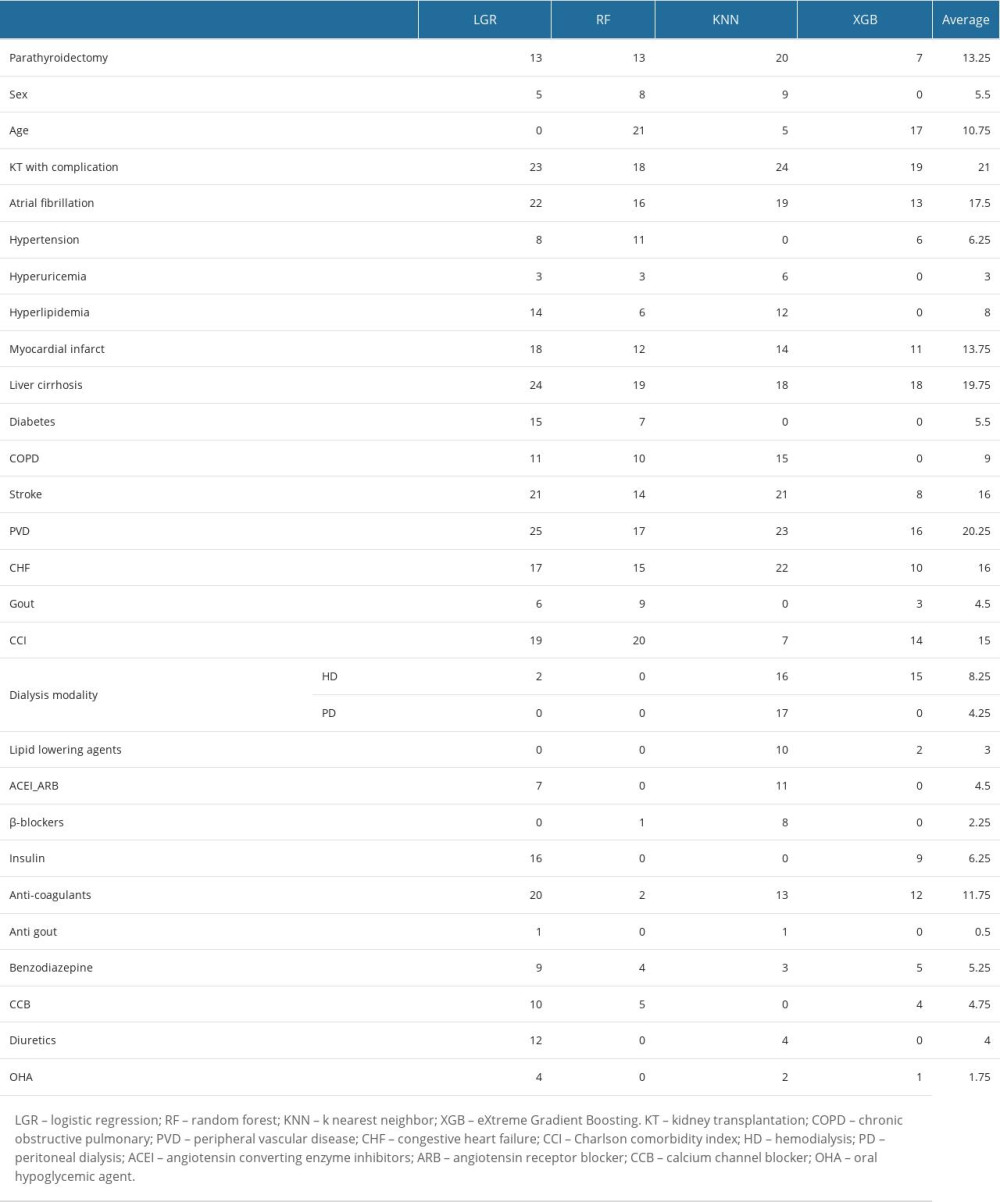
References
1. Habas E, Eledrisi M, Khan F, Elzouki AY, Secondary hyperparathyroidism in chronic kidney disease: Pathophysiology and management: Cureus, 2021; 13(7); e16388
2. Levin A, Bakris GL, Molitch M, Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease: Kidney Int, 2007; 71(1); 31-38
3. Gwinner W, Suppa S, Mengel M, Early calcification of renal allografts detected by protocol biopsies: causes and clinical implications: Am J Transplant, 2005; 5(8); 1934-41
4. Tentori F, Blayney MJ, Albert JM, Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS): Am J Kidney Dis, 2008; 52(3); 519-30
5. Naves-Díaz M, Passlick-Deetjen J, Guinsburg A, Calcium, phosphorus, PTH and death rates in a large sample of dialysis patients from Latin America. The CORES Study: Nephrol Dial Transplant, 2011; 26(6); 1938-47
6. Heaf JG, Bone disease after renal transplantation: Transplantation, 2003; 75(3); 315-25
7. Evenepoel P, Claes K, Kuypers D, Natural history of parathyroid function and calcium metabolism after kidney transplantation: A single-centre study: Nephrol Dial Transplant, 2004; 19(5); 1281-87
8. Sprague SM, Belozeroff V, Danese MD, Abnormal bone and mineral metabolism in kidney transplant patients – a review: Am J Nephrol, 2008; 28(2); 246-53
9. Isaksson E, Sterner G, Early development of secondary hyperparathyroidism following renal transplantation: Nephron Clin Pract, 2012; 121(1–2); c68-72
10. Wolf M, Weir MR, Kopyt N, A prospective cohort study of mineral metabolism after kidney transplantation: Transplantation, 2016; 100(1); 184-93
11. Drüeke TB, The pathogenesis of parathyroid gland hyperplasia in chronic renal failure: Kidney Int, 1995; 48(1); 259-72
12. Tillmann FP, Wächtler C, Hansen A, Vitamin D and cinacalcet administration pre-transplantation predict hypercalcaemic hyperparathyroidism post-transplantation: A case-control study of 355 deceased-donor renal transplant recipients over 3 years: Transplant Res, 2014; 3(1); 21
13. Moe S, Drüeke T, Cunningham J, Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO): Kidney Int, 2006; 69(11); 1945-53
14. Giannini S, D’Angelo A, Carraro G, Persistently increased bone turnover and low bone density in long-term survivors to kidney transplantation: Clin Nephrol, 2001; 56(5); 353-63
15. Mazzaferro S, Pasquali M, Taggi F, Progression of coronary artery calcification in renal transplantation and the role of secondary hyperparathyroidism and inflammation: Clin J Am Soc Nephrol, 2009; 4(3); 685-90
16. Nguyen PT, Coche E, Goffin E, Prevalence and determinants of coronary and aortic calcifications assessed by chest CT in renal transplant recipients: Am J Nephrol, 2007; 27(4); 329-35
17. Ozdemir FN, Afsar B, Akgul A, Persistent hypercalcemia is a significant risk factor for graft dysfunction in renal transplantation recipients: Transplant Proc, 2006; 38(2); 480-82
18. Lau WL, Obi Y, Kalantar-Zadeh K, Parathyroidectomy in the management of secondary hyperparathyroidism: Clin J Am Soc Nephrol, 2018; 13(6); 952-61
19. Cianciolo G, Tondolo F, Barbuto S, A roadmap to parathyroidectomy for kidney transplant candidates: Clin Kidney J, 2022; 15(8); 1459-74
20. Callender GG, Malinowski J, Javid M, Parathyroidectomy prior to kidney transplant decreases graft failure: Surgery, 2017; 161(1); 44-50
21. Hsieh CY, Su CC, Shao SC, Taiwan’s National Health Insurance Research Database: Past and future: Clin Epidemiol, 2019; 11; 349-58
22. McMurry TL, Hu Y, Blackstone EH, Kozower BD, Propensity scores: Methods, considerations, and applications in the Journal of Thoracic and Cardiovascular Surgery: J Thorac Cardiovasc Surg, 2015; 150(1); 14-19
23. Bosco E, Hsueh L, McConeghy KW, Major adverse cardiovascular event definitions used in observational analysis of administrative databases: A systematic review: BMC Med Res Methodol, 2021; 21(1); 241
24. Huang YC, Li SJ, Chen M, Machine-learning techniques for feature selection and prediction of mortality in elderly CABG patients: Healthcare (Basel), 2021; 9(5); 547
25. Huang YC, Li SJ, Chen M, Lee TS, The prediction model of medical expenditure appling machine learning algorithm in CABG patients: Healthcare (Basel), 2021; 9(6); 710
26. Stoltzfus JC, Logistic regression: A brief primer: Acad Emerg Med, 2011; 18(10); 1099-104
27. Rigatti SJ, Random forest: J Insur Med, 2017; 47(1); 31-39
28. Casillas N, Torres AM, Moret M, Mortality predictors in patients with COVID-19 pneumonia: A machine learning approach using eXtreme Gradient Boosting model: Intern Emerg Med, 2022; 17(7); 1929-39
29. Pihlstrøm H, Dahle DO, Mjøen G, Increased risk of all-cause mortality and renal graft loss in stable renal transplant recipients with hyperparathyroidism: Transplantation, 2015; 99(2); 351-59
30. Littbarski SA, Kaltenborn A, Gwiasda J, Timing of parathyroidectomy in kidney transplant candidates with secondary hyperparathryroidism: Effect of pretransplant versus early or late post-transplant parathyroidectomy: Surgery, 2018; 163(2); 373-80
31. Jeon HJ, Kim YJ, Kwon HY, Impact of parathyroidectomy on allograft outcomes in kidney transplantation: Transpl Int, 2012; 25(12); 1248-56
32. Okada M, Tominaga Y, Sato T, Elevated parathyroid hormone one year after kidney transplantation is an independent risk factor for graft loss even without hypercalcemia: BMC Nephrol, 2022; 23(1); 212
33. Massfelder T, Parekh N, Endlich K, Effect of intrarenally infused parathyroid hormone-related protein on renal blood flow and glomerular filtration rate in the anaesthetized rat: Br J Pharmacol, 1996; 118(8); 1995-2000
34. Trunzo JA, McHenry CR, Schulak JA, Wilhelm SM, Effect of parathyroidectomy on anemia and erythropoietin dosing in end-stage renal disease patients with hyperparathyroidism: Surgery, 2008; 144(6); 915-18 discussion 919
35. Yasunaga C, Nakamoto M, Matsuo K, Effects of a parathyroidectomy on the immune system and nutritional condition in chronic dialysis patients with secondary hyperparathyroidism: Am J Surg, 1999; 178(4); 332-36
36. Schmid T, Müller P, Spelsberg F, Parathyroidectomy after renal transplantation: A retrospective analysis of long-term outcome: Nephrol Dial Transplant, 1997; 12(11); 2393-96
37. Ferreira GF, Montenegro FL, Machado DJ, Parathyroidectomy after kidney transplantation: Short-and long-term impact on renal function: Clinics (Sao Paulo), 2011; 66(3); 431-35
38. Abramowicz D, Cochat P, Claas FH, European Renal Best Practice Guideline on kidney donor and recipient evaluation and perioperative care: Nephrol Dial Transplant, 2015; 30(11); 1790-97
39. , KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD): Kidney Int Suppl (2011), 2017; 7(1); 1-59
40. Burton JO, Goldsmith DJ, Ruddock N, Renal association commentary on the KDIGO (2017) clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of CKD-MBD: BMC Nephrol, 2018; 19(1); 240
41. Chadban SJ, Ahn C, Axelrod DA, KDIGO clinical practice guideline on the evaluation and management of candidates for kidney transplantation: Transplantation, 2020; 104(4S1 Suppl 1); S11-S103
42. Puttarajappa CM, Schinstock CA, Wu CM, KDOQI US commentary on the 2020 KDIGO clinical practice guideline on the evaluation and management of candidates for kidney transplantation: Am J Kidney Dis, 2021; 77(6); 833-56
43. Messa P, Cafforio C, Alfieri C, Clinical impact of hypercalcemia in kidney transplant: Int J Nephrol, 2011; 2011; 906832
44. Egbuna OI, Taylor JG, Bushinsky DA, Zand MS, Elevated calcium phosphate product after renal transplantation is a risk factor for graft failure: Clin Transplant, 2007; 21(4); 558-66
45. Perwad F, Zhang MY, Tenenhouse HS, Portale AA, Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro: Am J Physiol Renal Physiol, 2007; 293(5); F1577-83
46. Isakova T, Wahl P, Vargas GS, Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease: Kidney Int, 2011; 79(12); 1370-78
47. Ix JH, Shlipak MG, Wassel CL, Whooley MA, Fibroblast growth factor-23 and early decrements in kidney function: The Heart and Soul Study: Nephrol Dial Transplant, 2010; 25(3); 993-97
48. Gutiérrez OM, Mannstadt M, Isakova T, Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis: N Engl J Med, 2008; 359(6); 584-92
49. Scialla JJ, Xie H, Rahman M, Fibroblast growth factor-23 and cardiovascular events in CKD: J Am Soc Nephrol, 2014; 25(2); 349-60
50. Tsai MH, Leu JG, Fang YW, Liou HH, High fibroblast growth factor 23 levels associated with low hemoglobin levels in patients with chronic kidney disease stages 3 and 4: Medicine (Baltimore), 2016; 95(11); e3049
51. Coe LM, Madathil SV, Casu C, FGF-23 is a negative regulator of prenatal and postnatal erythropoiesis: J Biol Chem, 2014; 289(14); 9795-810
52. Wolf M, Molnar MZ, Amaral AP, Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality: J Am Soc Nephrol, 2011; 22(5); 956-66
53. Baia LC, Humalda JK, Vervloet MG, Fibroblast growth factor 23 and cardiovascular mortality after kidney transplantation: Clin J Am Soc Nephrol, 2013; 8(11); 1968-78
54. Takahashi H, Komaba H, Takahashi Y, Impact of parathyroidectomy on serum FGF23 and soluble Klotho in hemodialysis patients with severe secondary hyperparathyroidism: J Clin Endocrinol Metab, 2014; 99(4); E652-58
55. Liao SC, Moi SH, Chou FF, Changes in serum concentrations of fibroblast growth factor 23 and soluble Klotho in hemodialysis patients after total parathyroidectomy: Biomed Res Int, 2016; 2016; 6453803
56. Takkavatakarn K, Wuttiputhanun T, Phannajit J, Effectiveness of fibroblast growth factor 23 lowering modalities in chronic kidney disease: A systematic review and meta-analysis: Int Urol Nephrol, 2022; 54(2); 309-21
Figures
 Figure 1. Schema of patient enrollment in the study.
Figure 1. Schema of patient enrollment in the study. Figure 2. Kaplan-Meier cumulative event-free plots of (A) all-cause mortality (B) graft failure in the study population according to whether parathyroidectomy was performed before kidney transplantation.
Figure 2. Kaplan-Meier cumulative event-free plots of (A) all-cause mortality (B) graft failure in the study population according to whether parathyroidectomy was performed before kidney transplantation. Figure 3. Ranking of importance of parameters for graft failure using machine learning method. KT – kidney transplantation; PVD – peripheral vascular disease; CHF – congestive heart failure; CCI – Charlson comorbidity index.
Figure 3. Ranking of importance of parameters for graft failure using machine learning method. KT – kidney transplantation; PVD – peripheral vascular disease; CHF – congestive heart failure; CCI – Charlson comorbidity index. Tables
 Table 1. Baseline characteristics between those with/without parathyroidectomy prior kidney transplantation.
Table 1. Baseline characteristics between those with/without parathyroidectomy prior kidney transplantation. Table 2. Comparison of outcomes between those with/without parathyroidectomy prior kidney transplantation.
Table 2. Comparison of outcomes between those with/without parathyroidectomy prior kidney transplantation. Table 1. Baseline characteristics between those with/without parathyroidectomy prior kidney transplantation.
Table 1. Baseline characteristics between those with/without parathyroidectomy prior kidney transplantation. Table 2. Comparison of outcomes between those with/without parathyroidectomy prior kidney transplantation.
Table 2. Comparison of outcomes between those with/without parathyroidectomy prior kidney transplantation. Supplementary Table 1. Code for diseases.
Supplementary Table 1. Code for diseases. Supplementary Table 2. Code for drugs.
Supplementary Table 2. Code for drugs. Supplementary Table 3. Ranking of feature selection on graft failure in the patients receiving kidney translation.
Supplementary Table 3. Ranking of feature selection on graft failure in the patients receiving kidney translation. In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








