10 September 2023: Database Analysis
Enhanced Detection of Suspicious Breast Lesions: A Comparative Study of Full-Field Digital Mammography and Automated Breast Ultrasound in 117 Patients with Core Needle Biopsy
Marta Pawlak1ABCDEF, Wojciech RudnickiDOI: 10.12659/MSM.941072
Med Sci Monit 2023; 29:e941072
Abstract
BACKGROUND: This retrospective study from a single center aimed to compare the performance of full-field digital mammography (FFDM) vs automated breast ultrasound (ABUS) in the identification and characterization of suspicious breast lesions in 117 patients who underwent core-needle biopsy (CNB) of the breast.
MATERIAL AND METHODS: The study involved a group of 301 women. Every patient underwent FFDM followed by ABUS, which were assessed in concordance with BI-RADS (Breast Imaging Reporting and Data System) classification.
RESULTS: No focal lesions were found in 168 patients. In 133 patients, 117 histopathologically verified focal lesions were found. Among them, 78% appeared to be malignant and 22% benign. ABUS detected 246 focal lesions, including 115 classified as BI-RADS 4 or 5 and submitted to verification, while FFDM revealed 122 lesions, including 75 submitted to verification. The analysis revealed that combined application of both methods caused sensitivity to increase to 100, and improved accuracy improvement. Margin assessments in these examinations are consistent (P<0.00), the lesion’s margin type with both methods depends on its malignant or benign character (P<0.03), lesion margins distribution on ABUS depends on estrogen receptor presence (P=0.033), and there was significant correlation between malignant character of the lesion and retraction phenomenon sign (P=0.033). ABUS obtained higher compliance between the size of the lesion in histopathology compared to FFDM (P>0.05).
CONCLUSIONS: The results shows that ABUS is comparable to FFDM, and even outperforms it in a few of the analyzed categories, suggesting that the combination of these 2 methods may have an important role in breast cancer detection.
Keywords: Breast Neoplasms, Mammography, Radiology, ultrasonic waves, Humans, Female, Biopsy, Large-Core Needle, Retrospective Studies, Breast
Background
Breast cancer is the most frequent malignant cancer in women, characterized by high mortality and one of the highest disability-adjusted life-years (DALYs) numbers among all cancers [1]. Breast cancer treatment can be highly effective, particularly with early-stage diagnosis. Therefore, screening examinations enabling lesion detection in asymptomatic patients are very important. At present, mammography is the only accepted diagnostic method used among 50–69-year-old women within screening programs for early breast cancer detection, performed every 2 years [2–4]. The American College of Radiology (ACR) recommends beginning annual mammography screening at age 40, which should continue past age 74 years, with no upper age limit unless severe comorbidities limit life expectancy.
BI-RADS is a system created by ACR to standardize reporting of breast lesions visualized in mammography, ultrasound, and magnetic resonance imaging of the breast. In each method, morphologic features of the lesion are assessed to classify it into a specific category: BI-RADS 0, 1, 2, 3, 4a, 4b, 4c, 5, and 6. Each category corresponds to the probability of malignancy and recommendation concerning follow-up or histopathological verification. BI-RADS 2 lesions require a standard fallow-up, BI-RADS 3 requires short-term follow-up, BI-RADS 4 and 5 require biopsy, and BI-RADS 6 is histopathologically proven cancer. BI-RADS 1 indicates no lesion was detected.
Classic mammography (FFDM – full-field digital mammography) is a basic diagnostic tool used for detection of clinically asymptomatic cancers. FFDM is an X-ray imaging method. Each examination consists of 4 images (2 for each breast) of compressed breasts using 2 different projections – craniocaudal (CC) and mediolateral oblique (MLO). MLO projection should visualize the pectoral muscle, a part of the axilla, and the inframammary fold. In CC projection nipple should be positioned on the midline. Despite its proven ability to reduce mortality due to breast cancer women 50–69 years old, FFDM has some limitations, especially for dense breasts (categories C and D according to ACR, showing much lower sensitivity than in other breast density categories [5]. This limitation results from the fact that FFDM is a summation examination, which in case of dense glandular breast tissue, which absorbs ionizing radiation more strongly than fat tissue, can obscuring an underlying focal lesion of similar density. Additionally, dense glandular breasts are much more difficult to compress, which is vital for partial distribution of glandular tissue – the thinner the breast during FFDM, the better the visualization of potential focal lesions, due to a smaller amount of overlapping glandular tissue. Many women have dense breast anatomy (about 43% of women aged 40–74), which results not only from age or genetic predisposition, but also from hormonal treatment [6]. Dense glandular breast anatomy is associated with increased risk of breast cancer, about 4–6 times higher than in other patients, increased mortality due to breast cancer, higher interval cancers incidence (cancers developed between a performed screening examination and a recommended follow-up examination), and higher-stage cancers at the time of diagnosis [7,8]. Due to the above-mentioned limitations, the sensitivity and specificity of FFDM in this age group are unsatisfactory (Figure 1).
Classic ultrasonography HHUS (hand-held ultrasound) was supposed to be a solution to the above problems. Undoubtedly, its advantages are wide availability, low costs, lack of exposure to ionizing radiation, and lack of influence of breast density. It was shown that HHUS applied as a complementary tool both in screening and everyday clinical practice significantly improved cancer detection in breasts of density category C and D according to ACR, which enabled detection of approximately 1.8 to 4.6 times more cancers per 1000 patients with dense breast anatomy, and caused a drop in the incidence of interval cancers [9–17]. Nevertheless, hand-held ultrasound has many limitations: lack of standardization, numerous false-positive results, the necessity of long-term training, result dependence of interpreter experience, small field of view (FOV), impossible recreation of the examination and its assessment by other readers at different times, a time-consuming procedure performed by a physician, and performance time exceeding the interpretation time of the obtained images.
Consequently, because of the limitations of mammography and hand-held ultrasound, new diagnostic methods to facilitate detection of a more cases of cancers, especially among women with dense breasts, are still being researched.
Automated breast ultrasound (ABUS) is an ultrasound technique allowing visualization of the whole breast, which was accepted and introduced by the US FDA in 2008. It is composed of a net of linear transducers producing frequencies about 6–14 MHZ, which are connected with a rigid compressive plate. The examination is performed by an electro-radiologist or a trained technician. During the examination, the patient is in supine position. Before the procedure, the nipples must be marked and the depth of the imaging must be determined, which depends on the breasts’ size (up to 5 centimeters of depth). Then, gel must be applied on the breasts to enable proper ultrasound transmission. The patient is asked not to move and to breathe calmly, because excessive movement during the procedure can cause artifacts. The transducers move automatically along the breasts, obtaining thin-layer images in axial views including tissues from the surface of the skin to the chest wall. Three projections are typically performed: anteroposterior AP (breast nipple in the center of the obtained image), lateral (breast nipple visible in medial lower part of the image), and medial (breast nipple in lateral upper part of the image). For large breasts, 2 additional acquisitions are obtained covering the lower outer and upper inner quadrants.
ABUS is has the advantage of a wide field of view, which enables obtaining multiplanar reconstructions, mainly in coronal (so-called “surgical view,” resembling breast position during surgical procedures), and sagittal projections, making precise localization of the focal lesion possible. The coronal view has great importance in assessing mammary ducts ectasia and retraction phenomenon, which are commonly observed in malignant lesions. Using ABUS also makes it possible to mark the lesion and to assess it in all types of reconstructions provided. The software automatically describes localization of the lesion (distance from the nipple, from the skin and chest wall, clockface localization), which limits incompatibility between different readers’ assessments compared to HUSS, and has a major role in comparing the lesions’ characteristics between various modalities, where the breasts’ position during the examination is different. Individual examinations are recorded and sent to the radiologic stations; therefore, the time between the examination performance and its interpretation is separated in comparison to HHUS. It also facilitates evaluation by different readers at different times and enables AI application. The technique itself is operator-independent and time-saving for doctors, as the examination is performed by technicians.
However, ABUS is not entirely free from limitations. Scanning does not cover the whole axilla and in case of large breasts and in the periphery of external quadrants, which can lead to missing lesions located in these areas, vascularity assessment is not possible and neither is elastography or biopsy of the identified lesions [18–20]. Moreover, during ABUS performance, characteristic artifacts may appear, connected both with patient movement, inappropriately covering the skin with gel, with skin folding, or presence of a palpable focal lesion in the breast, changing its consistency and structure, which hinders proper adhering of the compression plate to the breast. The most common artifacts are: folding, which is a result of chest wall movement during acquisition; shadowing, which occurs when the surface of the skin in the examined area is not entirely and properly covered with the gel, leading to blockage of the ultrasound transmission by gas particles; and artifacts caused by insufficient compression of the transducer, which result in posterior shadowing caused by Cooper ligaments [21] (Figure 2).
A lack of direct contact between doctor and patient may be considered as another drawback of the method, especially in case of patient anxiety pertaining to the particular area of the breast; there were attempts to address the problem with application of skin markers not interfering with image interpretation, but preventing significant clinical data loss in case of emerging symptoms [22]. The examination is well tolerated by women and lasts about 15 min.
Therefore, this retrospective study from a single center aimed to compare the performance of FFDM vs ABUS in the identification and characterization of suspicious breast lesions in 117 patients who underwent CNB of the breast.
Material and Methods
ETHICS:
This study was performed according to the guidelines of the Declaration of Helsinki and was approved by the Institutional Bioethical Committee operating at the Regional Medical Chamber in Cracow). The approval number is 293/KBL/OIL/2020.
PATIENTS:
The study involved a group of 301 women presented at the Breast Diagnostic Imaging Laboratory from 2020 to 2022 to undergo FFDM. The mean age of the women was 53.2 years, with the youngest patient 29 years old and the oldest 77 years old.
IMAGING EXAMINATIONS:
Every patient underwent FFDM followed by ABUS. FFDM was performed according to a standard protocol in oblique and cranio-caudal projections for each breast. ABUS was carried out by a trained electro-radiologist in basic projections – anteroposterior, medial, and lateral, and in case of large breasts, also in additional projections. Both examinations were assessed in concordance with BI-RADS classification (Breast Imaging Reporting and Data System) independently by 2 radiologists with 5 and 30 years of experience in breast diagnostics. The subject matter of examinations was focal lesion presence or absence, described using the BI-RADS scale. In FFDM, the BI-RADS classification was applied to characterize breast anatomy type, and in case of a focal lesion, its shape, margin, and density, as well as microcalcifications and architectural distortion. Lesion margins were divided into well-defined circumscribed, indistinct (ill-defined), and to deepen the analysis, the category of lesions with indistinct margin was complemented with spiculated margins. In ABUS breast anatomy type was characterized, presence of focal lesions and their characteristics such as their shape, orientation, margin, echo pattern, enhancement, or acoustic shadow behind the back wall presence and calcifications, and microlobulated and blurred margin. Moreover, retraction phenomenon visible in ABUS was interpreted (most often seen on coronal plane unique for ABUS), as well as acoustic shadow effect behind the lesion. Each FFDM and ABUS examination was assigned BI-RADS category 1–5. Lesion margin type and appearance in both methods are shown in Figure 3.
Further patient management depended on focal lesions presence (in either examination), their character, and assigned BI-RADS category. In all women with detected focal lesions classified as BI-RADS 4 and 5 by either method, a hand-held ultrasound was performed, and core-needle biopsy or vacuum-needle biopsy was performed to verify the findings. Following the histopathological diagnosis, the patients were subjected to surgical treatment or further evaluation (follow-up).
HISTOPATHOLOGICAL EXAMINATION:
The material obtained in biopsy was subjected to detailed histopathological analysis performed by a pathologist specialized in breast cancer. The examinations were carried out at a single pathomorphology laboratory. All samples were fixed in 10% neutral buffered formalin and subsequently paraffin-embedded. After standard hematoxylin and eosin staining, the samples were microscopically assessed. Each sample had its character determined as benign or malignant according to the B1–B5 scale. Histopathological findings were divided into 2 main groups – infiltrating cancers or benign lesions. All lesions described as category lower than B4 and B5 on histopathology were included into the group of benign lesions. If a malignant cancer was determined, its histopathological type, stage, ER, PR, and HER2 receptors expression, and Ki67 index were evaluated. For ER and PR receptors, the following categories were applied: negative, ≤80% and >80%. For HER2, the 2 categories were negative and positive. Cancers were additionally divided into those with lower and higher proliferation index, taking into consideration expression level of Ki67 receptor using the values ≤20% and >20% as cut-off criteria. We assessed possible correlations between focal lesion margin type on ABUS and FFDM and cancer grade, applying the following categories: benign lesions, DCIS, and infiltrating cancers. The group of infiltrating cancers also included invasive carcinoma of no special type co-existing with any other type of lesions, invasive lobular carcinoma, micropapillary, and mucinous, with apocrine differentiation, in any combination. The remaining divisions were formed as a result of grouping (Table 1).
STATISTICAL METHODS:
Obtained numerical data were submitted to statistical analysis using the most appropriate methods for the searched correlation. McNemar’s test was applied for dependent tests and the Z-test was used for 2 independent ratios. ROC curves were drawn and ROC analysis performed, as well as kappa conformity coefficients, the chi-square test of independence and the non-parametric Mann-Whitney U test.
Results
DETECTED LESIONS:
FFDM and ABUS were performed in 301 patients. No focal lesions were found in 168 patients and a routine follow-up was recommended for them. In 133 patients, 117 histopathologically verified focal lesions were found in total at 1 or both examinations. Among them, 78% appeared to be malignant and 22% benign. ABUS detected 246 focal lesions, including 115 classified as BI-RADS 4 or 5 and submitted to verification, while FFDM revealed 122 lesions, including 75 submitted to verification.
MULTIPLE LESIONS DETECTION:
Among the 301 examined patients, 69 had multiple lesions. The number of focal lesions in particular patients and number of patients whose lesions were found on ABUS were significantly higher than those on FFDM – in total, 160 lesions vs 84 lesions and 65 patients vs 48 patients – which means that ABUS has an advantage over FFDM in revealing additional, particularly tiny lesions. Detailed quantitative data are presented in Table 2.
BI-RADS ASSESSMENT IN ABUS AND FFDM COMPARED TO HISTOPATHOLOGY AS A CRITERION STANDARD:
On FFDM, 91 histopathologically verified cancers were diagnosed, including 82 (90.11%) previously classified as category BI-RADS 4 or BI-RADS 5, and 9 (9.89%) to category BI-RADS 1 or BI-RADS 2. Among 26 histopathologically verified benign lesions, 22 (84.62%) were classified as BI-RADS 1 or 2, while 4 lesions (15.38%) were BI-RADS 4 or 5 (Table 3).
The next table presents distribution of benign and malignant lesions in relation to BI-RADS on ABUS examination (Table 4).
On ABUS, 91 histopathologically verified cancers were diagnosed, including 73 (80.22%) previously classified to category BI-RADS 4 or BI-RADS 5, and 18 (19.78%) to category BI-RADS 1 or BI-RADS 2. Among 26 histopathologically verified benign lesions, 18 (69.23%) were classified as BI-RADS 1 or 2, and 8 (30.77%) were BI-RADS 4 or 5.
BI-RADS categories allocated by ABUS and FFDM appeared to be consistent, with 48% compatibility. In total, 62% of lesions were assessed as benign or a lack of visible lesions in a particular method (BI-RADS 1 or BI-RADS 2 on FFDM and ABUS). The largest group of lesions – 30% – were assessed on ABUS as BI-RADS 1, and on mammography as BI-RADS 2, which in most analyzed cases resulted from frequently present benign calcifications on FFDM (calcifications in the skin, vascular, pop-corn-like type, post-inflammatory, round, ring, dystrophic, milk of calcium, in a scar). The second largest group (19%) was category BI-RADS 2, both on FFDM and ABUS, included mainly cysts and lymph nodes.
Consistent assessment BI-RADS 5 was allocated to 14% of lesions, and BI-RADS 4 to 5% of lesions. In total, 19% of lesions required biopsy (BI-RADS 4 or BI-RADS 5 on both methods). There were also 5% of lesions assessed as Bi-RADS 1 or BI-RADS 2 on ABUS and BI-RADS 4 or 5 on FFDM, which in all analyzed cases resulted exclusively from presence of microcalcifications of suspicious morphology (amorphous, coarse heterogeneous, pleomorphic, fine linear, or fine-linear branching), usually invisible on ultrasound. In 4% of the examined lesions, the reverse situation appeared – Bi-RADS 1 or BI-RADS 2 were assigned on FFDM, while category BI-RADS 4 or 5 were assigned on ABUS, due to the masking effect of focal lesions by glandular tissue in dense glandular breasts (subtype C and D according to ACR).
HISTOPATHOLOGICAL DIAGNOSES:
More lesions were found on ABUS than FFDM (246 on ABUS and 122 on FDMM). Among them, 54% visible on FFDM and only 35% on ABUS were submitted to histopathological verification, because most of the lesions determined on ABUS showed benign morphology (mostly simple and complex cysts, or simple cysts conglomerations). Lesions visible on FFDM were verified with VABB more often than those found on ABUS (12% vs 7%), due to findings exclusive for FFDM, mainly microcalcifications of suspicious morphology. Histopathological diagnoses of all lesions verified in patients subjected to FFDM and ABUS examinations are presented in Table 1.
Invasive cancers are the largest group among the verified lesions (81 in total), the most frequent being invasive carcinoma of no special type (NST) with ductal carcinoma in situ (DCIS), followed by invasive lobular carcinoma co-existing with lobular carcinoma in situ (LCIS), and the third largest group was invasive carcinoma of no special type and invasive lobular carcinoma. There were only 5% of B3 cases and 17% of benign lesions. In the B3 group, radial scar and ADH were dominant. Among benign lesions, 1 case of amyloidosis was encountered. Preview of histopathology results is presented in Figure 4A–4D.
ASSOCIATION BETWEEN LESIONS’ MARGIN ON ABUS AND FFDM AND ITS MALIGNANCY STATUS:
The analysis revealed that a lesion’s margin type, both on FFDM and ABUS, depends on its malignant or benign character (
It was determined that lesion’s margin type depends on its type – the correlation is presented in Figure 5.
Figure 5A shows visible gradation of well-defined lesions to ill-defined lesions ratio in relation to lesion grade on ABUS. The proportion of ell-defined margins decreases with increased cancer grade – 46% among benign lesions, 18% among DCIS, and 6% among invasive cancers. On FFDM, well-defined margins are present exclusively in cases with of benign lesions and this feature is dominant within the whole group, at 63%. All DCIS lesions and invasive cancers appeared to have ill-defined margins on FFDM. Figure 6A and 6B show correlations between invasive cancer’s character (NST and other invasive cancers) and focal lesions’ margin on ABUS and FFDM.
Figure 6A and 6B show that invasive cancers other than NST were more likely to have well-defined lesions, both on ABUS and FFDM, which suggests that the presence of a well-defined circumscribed margin of a focal lesion being an invasive cancer allows exclusion of NST with high probability.
Association Between Lesions’ Margin on ABUS and FFDM and Its Receptor and Ki67 Status
A similar comparison of lesion margin types on ABUS and FFDM for malignant lesions in relation to their receptor status was performed to search for a correlation between a focal lesion margin on a given imaging modality and its receptor status, taking into consideration estrogen, progesterone, and HER2 receptors. We found that lesion margin distribution on ABUS depends on estrogen receptor presence (P=0.033, chi-square test of independence). Blurred (indistinct) margins were more frequent lesions whose receptor expression value was less than 80% or negative, while well-defined margins were found only in lesions with receptor expression value above 80% were absent among lesions with values >80%. No correlation was determined between progesterone receptor expression level, HER2, or Ki-67 protein and focal lesion margin on ABUS (P>0.05). The correlations are presented in Figure 7.
All lesions well-defined on FFDM appeared to be benign on histopathology or were not subjected to verification due to cyst presence. In the remaining cases of the analyzed margins (spiculated, ill-defined, lobulated), no correlations were found between lesion margin and receptor status on FFDM.
THE COMPLIANCE OF FOCAL LESIONS’ MARGIN ASSESSMENT ON ABUS AND FFDM:
The compliance of focal lesions’ margin assessment on ABUS and FFDM was also compared and was found to be 77%. Kappa conformity coefficient for these parameters was 70% [52%, 87%], showing that margin assessments on these examinations were consistent (
RETRACTION PHENOMENON AND POSTERIOR SHADOWING:
Coronal view image reconstruction unique for ABUS enables visualization of the retraction phenomenon sign, which is perfectly seen in that projection – a stellate pattern in which white spicules are caused by the desmoplastic reaction of the tissue surrounding a malignant lesion, which is the most reliable sign for the differentiation of focal lesions in comparison to solely transverse images evaluation [23]. Retraction features among malignant lesions (B5) were present in 48% of focal lesions. This is significantly more frequent than in benign lesions, with a statistically significant correlation between malignant character of the lesion and retraction phenomenon sign (P=0.033, chi-square test of independence). This feature was also present in 10% of benign lesions, which in some cases were histopathologically diagnosed radial scars or lesions localized within a scar. There was no statistically significant correlation between benign or malignant character of the lesion and an acoustic shadow behind the lesion. The retraction phenomenon is seen in Figure 8.
MICROCALCIFICATIONS:
Microcalcifications without a measurable focal lesion on FFDM were encountered in 14 cases (11%), which was observed on ABUS in 5 cases (33%). In this study, microcalcifications presence in histopathologically diagnosed DCIS and LCIS was compared on FFDM and on ABUS, showing that the frequency of microcalcifications on FFDM and ABUS depends on lesion type (LCIS and DCIS) and is higher for DCIS than LCIS (P<0.007). However, both LCIS and DCIS lesions contained microcalcifications more often on FFDM than ABUS, suggesting higher sensitivity of FFDM for their evaluation. No correlation between focal lesion grade and microcalcifications presence on FFDM and ABUS was noted. This relation is shown in Figure 9A and 9B.
LESION SIZE ON FFDM AND ABUS IN COMPARISON TO HISTOPATHOLOGY AS THE CRITERION STANDARD:
The study also evaluated the size of focal lesions on FFDM and ABUS and compared the data with histopathological examination as the criterion standard. The median difference of lesion sizes appeared to be smaller for ABUS than for FFDM (−3.00 vs 5.90), which implies higher compliance between values obtained on ABUS compared to FFDM, as statistical testing does not determine if the obtained differences between lesion sizes differ significantly from 0 (
DIAGNOSTIC PERFORMANCE OF FFDM AND ABUS:
ABUS sensitivity was 80.22 and FFDM sensitivity was 90.11, while ABUS specificity was 30.77 and FFDM specificity was 15.38. Combined application of both methods caused sensitivity increase to 100 and also improved the accuracy. We wondered whether it is possible to determine the likelihood of malignancy incidence based on BI-RADS category specified on ABUS and FFDM using ROC analysis, with BI-RADS 4 as a cut-off point. Both methods appeared to be effective (AUC significantly above 0.5). The values are presented in Figure 10.
Discussion
ABUS detected more lesions than FFDM and also detected more lesions requiring biopsy, but the percentage of lesions histopathologically verified was lower for ABUS than for FFDM because of visualization of many lesions with benign imaging characteristics in ABUS (eg, cysts). ABUS enabled visualization of more lesions in patients with multiple lesions. BI-RADS categories allocated both on ABUS and FFDM appeared to be consistent. In this analysis the high compliance of focal lesions’ margin assessment on ABUS and FFDM was demonstrated. The margin of the lesion depends on its malignant or benign character FFDM and ABUS. The relation of the lesions margin in ABUS and estrogen status of the lesion has been noticed. Retraction phenomenon (visualized in the unique for ABUS coronal plane) was observed significantly more frequently in malignant lesions. Microcalcifications were noticed more often in FFDM than in ABUS, and lesions containing microcalcifications were most frequently DCIS. The study revealed higher compliance between lesions’ size measured on histopathology as a criterion standard and ABUS compared to FFDM. The specificity of ABUS was higher than FFDM, the sensitivity of ABUS was lower than FFDM, but the combination of these 2 methods improved accuracy up to 100%.
High compliance in BI-RADS categories allocated on ABUS and FFDM was achieved (48%, while in He Chen et al, only 32.22%), as well as in determining the lesion localization and defining its margins [24]. Focal lesions margins are dependent on their benign or malignant character, both on ABUS and FFDM. The added value of ABUS is gradation of well-defined and ill-defined lesions ratio in relation to their grade, which was not observed on FFDM. It was also determined that invasive cancers other than NST were well-defined both on ABUS and FFDM more often than NST (on ABUS, the difference in margin distribution was more substantial), while cancers with low estrogen receptor expression were more often ill-defined on ABUS.
Microcalcifications presence is often associated with DCIS [25]. The study confirmed that frequency of microcalcifications presence on FFDM and ABUS is higher in DCIS than LCIS. Both types of lesions were more often manifested as microcalcifications on FFDM than on ABUS, which proves that FFDM provides superior detection.
ABUS has a distinct advantage over FFDM in detection of further foci in multifocal processes, as well as numerous additional tiny lesions in patients with multiple lesions in breasts. In most cases, lesions subsequently detected on ABUS were not subjected to verification as their appearance was considered benign, not requiring histological evaluation. Nevertheless, it may be assumed that using ABUS together with FFDM will enable detection of further, potentially malignant lesions, which is crucial for diagnosis and treatment, as it drastically changes in case of diagnosis of multiple malignancies. Similar conclusions may be drawn based on studies carried out previously. Wilczek et al showed that performing ABUS in a group of 1668 asymptomatic women with dense breasts facilitated diagnosis of 2.4 additional cancers per 1000 patients in comparison to examination only with FFDM [26].
The possibility to create multiplanar image reconstructions, which is a great advantage of ABUS, provides exceptionally useful coronal view images, perfectly visualizing the retraction phenomenon sign present in numerous malignant cases. Moreover, those images being “surgical views” constitute an invaluable tool for surgeons, who can assess a focal lesion in a position similar to the position on the operating table. Suzuki et al, in their retrospective study including 100 focal lesions, confirmed the importance of the coronal view and demonstrated that interpretation time of these images is significantly lower than for axial images, which may provide additional important information facilitating interpretation [27].
ABUS determines focal lesion size more precisely than FFDM (in comparison to histopathological examination as the criterion standard). Similar conclusions were obtained by He Chen et al in a study of 344 verified malignant focal lesions – the compliance of foci sizes in comparison to histopathology results as the criterion standard was 52.91% for ABUS and 43.87% for FFDM [23]. Despite the correction of foci sizes on FFDM with a coefficient resulting from compression, FFDM overestimates their size in relation to the actual status.
Although the above study demonstrated lower sensitivity and accuracy of ABUS in comparison to FFDM, it exceeds the other method in terms of positive predictive value, and the combination of both methods increased the negative predictive value and sensitivity to 100%, but reducing its specificity. The lower sensitivity and accuracy of ABUS in some cases resulted from the presence of malignant lesions undetectable on ultrasound and visible only on FFDM, such as microcalcifications without a distinguished tumor mass. Brem et al examined a group of 15318 women with dense glandular breast anatomy and found sensitivity increase by 26.7% when applying the combined methods, and enriching FFDM diagnostics with ABUS allowed detection of 30 additional cancers [28]. Gatta et al proved that combined FFDM and ABUS caused a rise in deepened diagnostic referrals by 12.2 per 1000 women with dense breasts [29].
After combining FFDM and ABUS, the sensitivity increased by about 10% in comparison to FFDM’s sensitivity, which is lower than in the previous publications and may result from the higher initial sensitivity of FFDM and including women with different breast anatomy types, not only glandular breasts, in which FFDM shows much lower sensitivity.
The major limitation of FFDM is its lower sensitivity in the patients with dense breasts and the fact that false-positive findings are more often observed on the first mammogram because the comparison of the previous mammogram has a crucial role in diagnosis. ABUS mainly detects benign lesions and creates many specific artifacts and thus is less specific. The low availability of ABUS results in low numbers of radiologists experienced in this method.
Limitations of this study include its single-center design and relatively small sample size, which consisted of asymptomatic and symptomatic patients from different age groups and with different breast density type. It would be beneficial to conduct a similar study in a larger group of patients with dense breasts, where FFDM has much lower sensitivity.
Conclusions
The results obtained in this study suggest that ABUS is a fast method, imposing no time or effort burden on a physician; is not only comparable to FFDM in many aspects, but even outperforms it in a few of the analyzed categories. Numerous benign lesions are detected on ABUS, but its specificity is lower than FFDM. Using combined ABUS and FFDM causes sensitivity increase in both methods separately and significantly improves breast cancer detection; therefore, it seems reasonable to use ABUS, which has great potential to be applied in screening, as an additional method to FFDM.
Figures
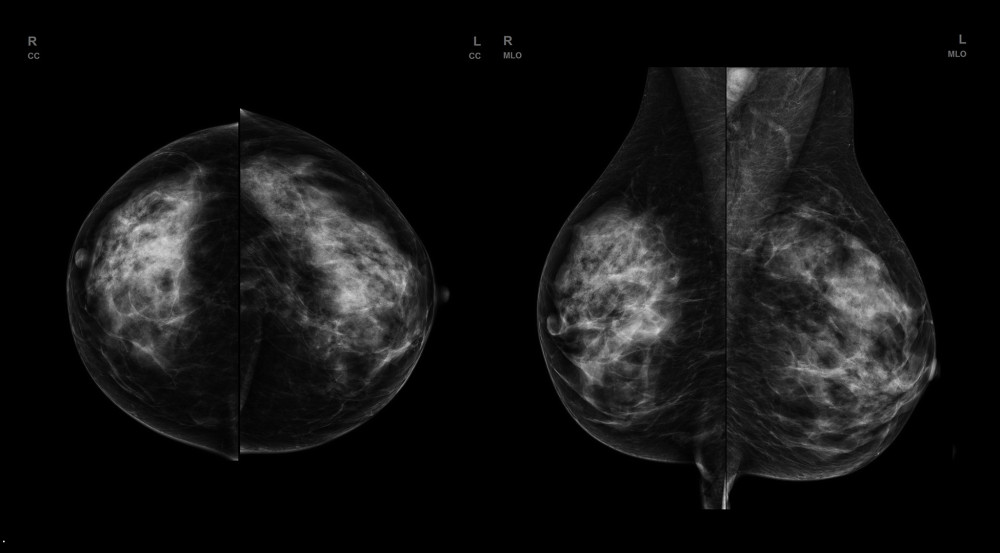 Figure 1. FFDM (full-filled digital mammography) of a patient with dense, glandular breast type. No evident lesion is seen.
Figure 1. FFDM (full-filled digital mammography) of a patient with dense, glandular breast type. No evident lesion is seen. 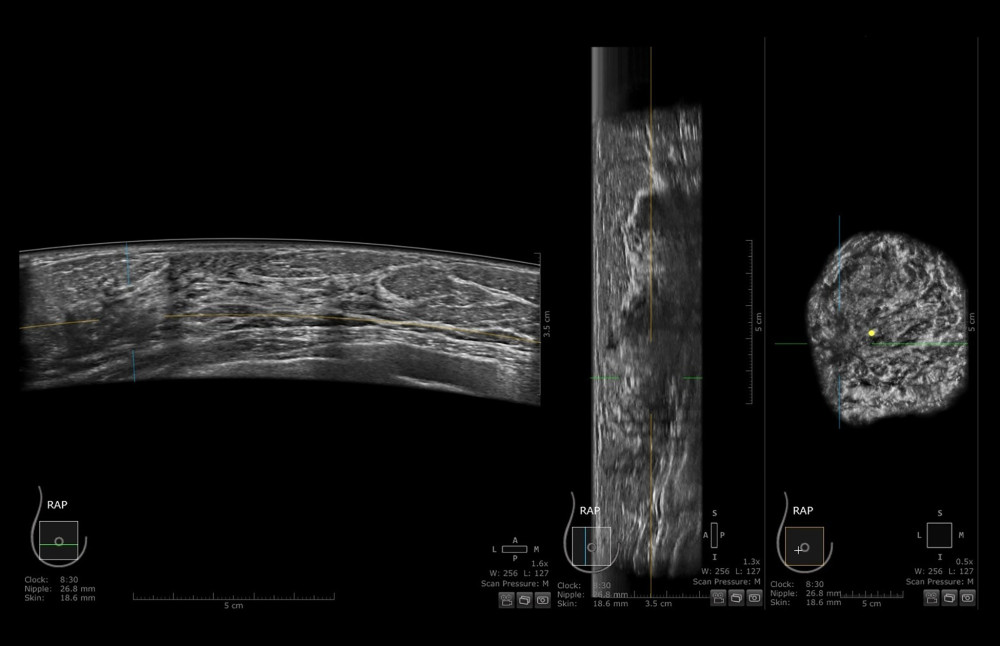 Figure 2. ABUS (automated breast ultrasound) scan of patient from Figure 1. Right breast in axial, sagittal, and coronal views. An ill-defined, hypoechogenic mass is visible at 9 o’clock. After core-needle biopsy, a lobular carcinoma was diagnosed.
Figure 2. ABUS (automated breast ultrasound) scan of patient from Figure 1. Right breast in axial, sagittal, and coronal views. An ill-defined, hypoechogenic mass is visible at 9 o’clock. After core-needle biopsy, a lobular carcinoma was diagnosed. 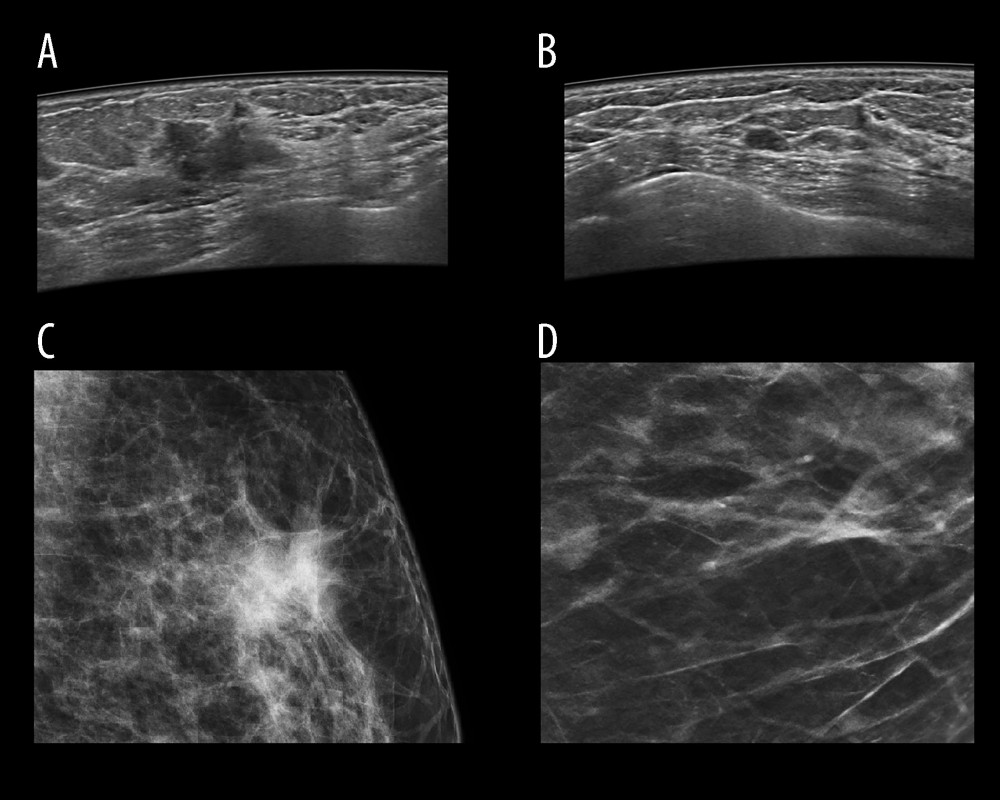 Figure 3. Image of focal lesion’s margin on ABUS and FFDM depending on its type. Figure A shows ABUS image and C shows FFDM image of the same malignant lesion with ill-defined margin. Figure B shows ABUS image of benign lesion with well-defined margin. Figure B shows ABUS image and Figure D shows FFDM image of the same benign lesion with well-defined margin.
Figure 3. Image of focal lesion’s margin on ABUS and FFDM depending on its type. Figure A shows ABUS image and C shows FFDM image of the same malignant lesion with ill-defined margin. Figure B shows ABUS image of benign lesion with well-defined margin. Figure B shows ABUS image and Figure D shows FFDM image of the same benign lesion with well-defined margin. 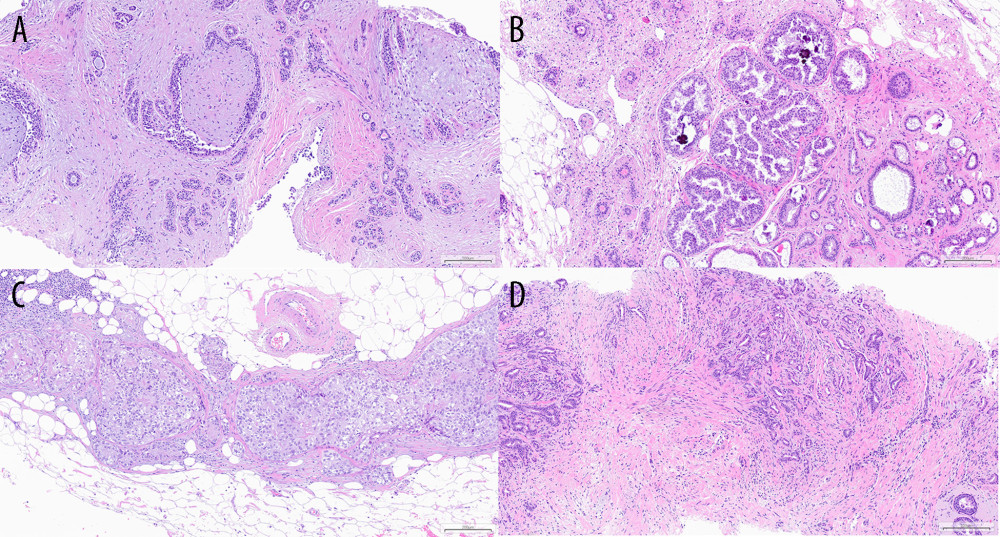 Figure 4. Examples of histopathology specimens at 10× magnification. Figure A shows fibroadenoma. Figure B shows ADH. Figure C shows DCIS G2. Figure D shows NST cancer G1.
Figure 4. Examples of histopathology specimens at 10× magnification. Figure A shows fibroadenoma. Figure B shows ADH. Figure C shows DCIS G2. Figure D shows NST cancer G1. 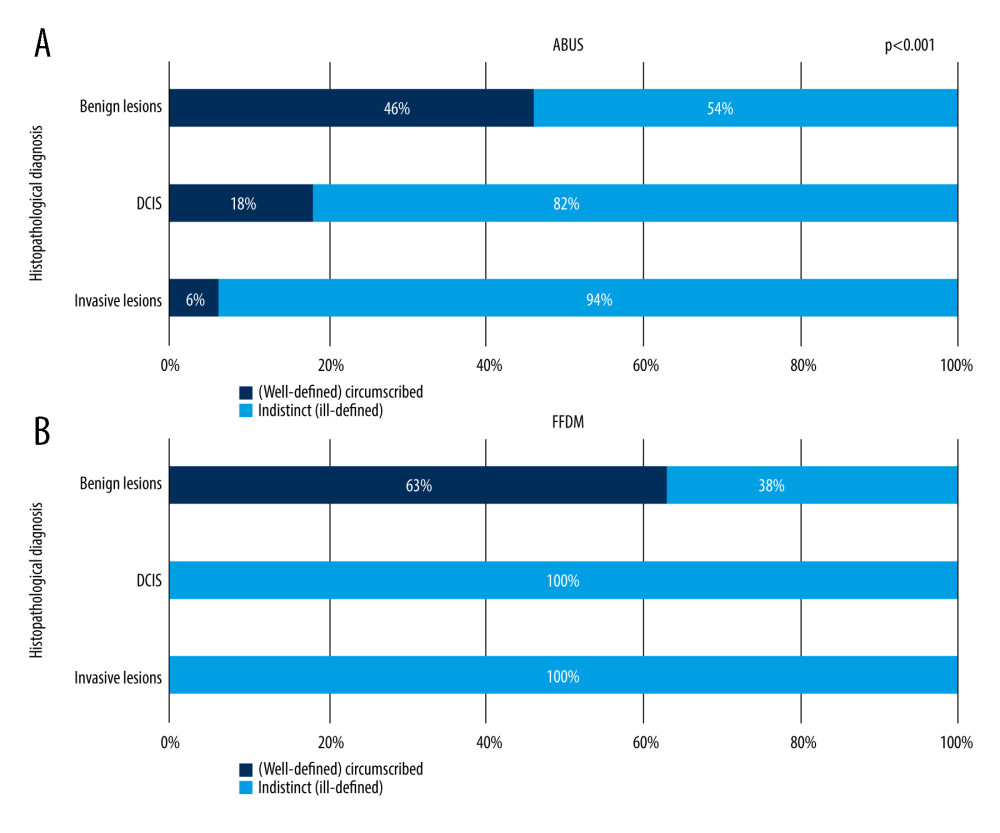 Figure 5. (A, B) Correlation between focal lesion’s margin on ABUS and FFDM and its grade.
Figure 5. (A, B) Correlation between focal lesion’s margin on ABUS and FFDM and its grade. 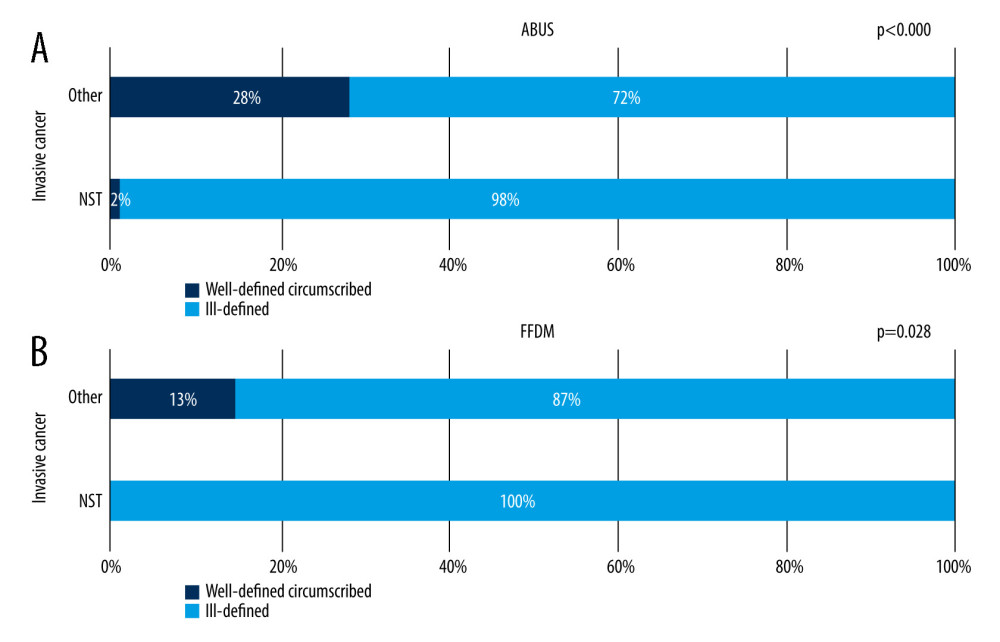 Figure 6. (A, B) Correlation between focal lesion’s margin on ABUS and FFDM and invasive cancer’s character.
Figure 6. (A, B) Correlation between focal lesion’s margin on ABUS and FFDM and invasive cancer’s character. 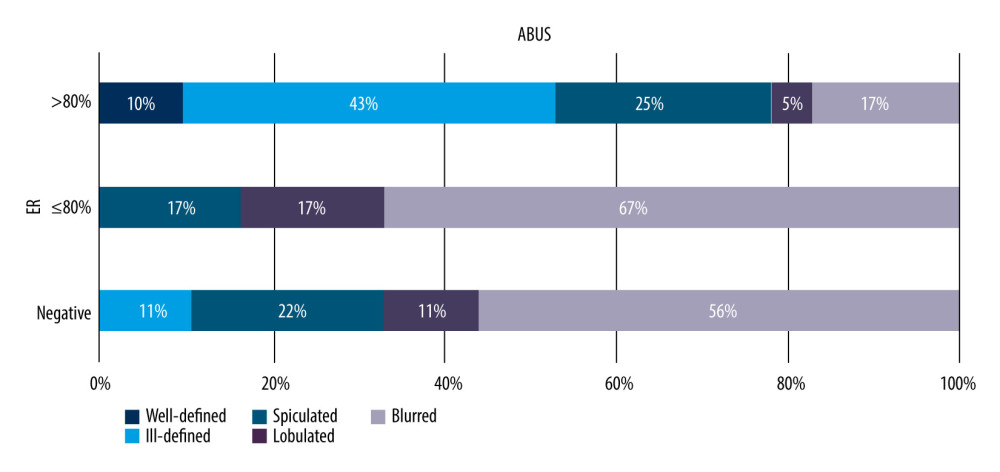 Figure 7. Distribution of correlation between focal lesion margin and estrogen receptor expression level on ABUS, p=0.033.
Figure 7. Distribution of correlation between focal lesion margin and estrogen receptor expression level on ABUS, p=0.033. 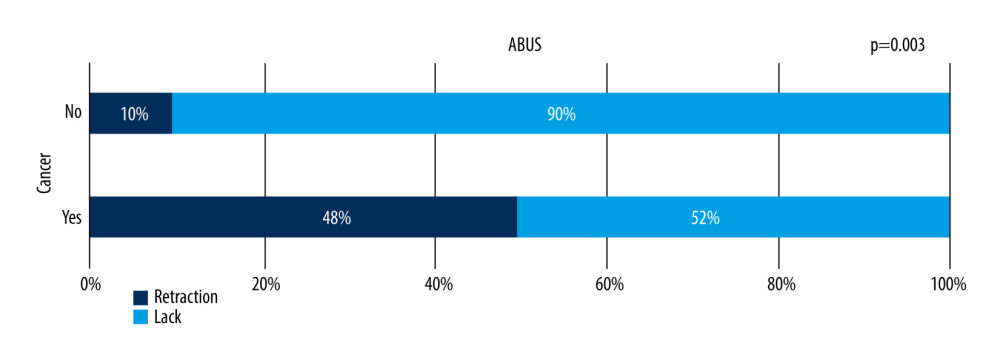 Figure 8. Presence of the retraction phenomenon on ABUS in benign and malignant lesions.
Figure 8. Presence of the retraction phenomenon on ABUS in benign and malignant lesions. 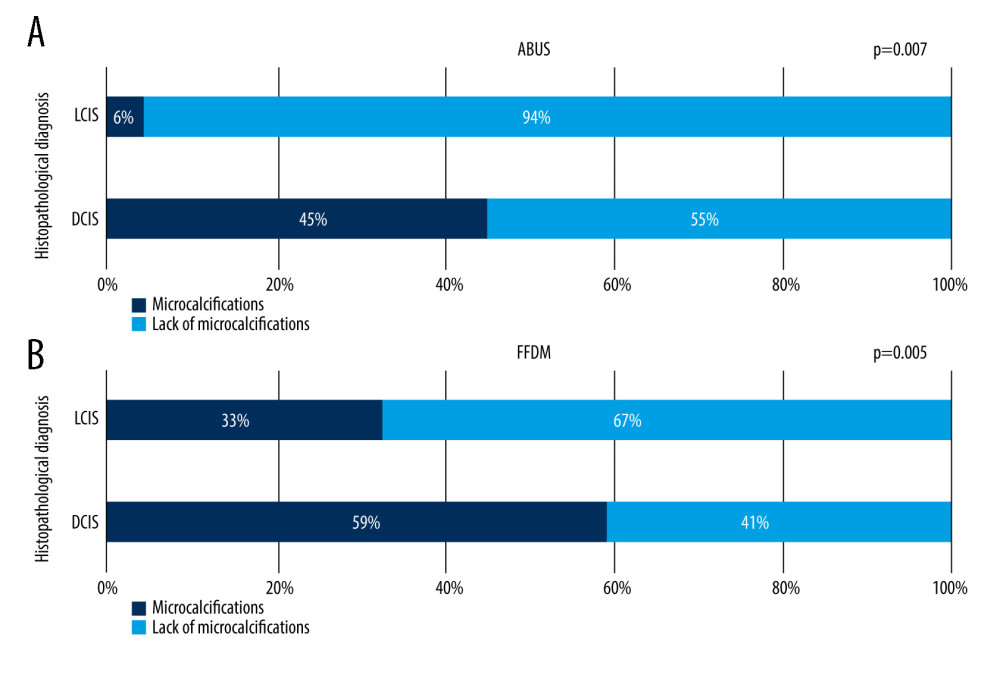 Figure 9. (A, B) Frequency of microcalcifications presence on ABUS and FFDM in lesions diagnosed histopathologically as DCIS (ductal carcinoma in situ) and LCIS (lobular carcinoma in situ).
Figure 9. (A, B) Frequency of microcalcifications presence on ABUS and FFDM in lesions diagnosed histopathologically as DCIS (ductal carcinoma in situ) and LCIS (lobular carcinoma in situ). 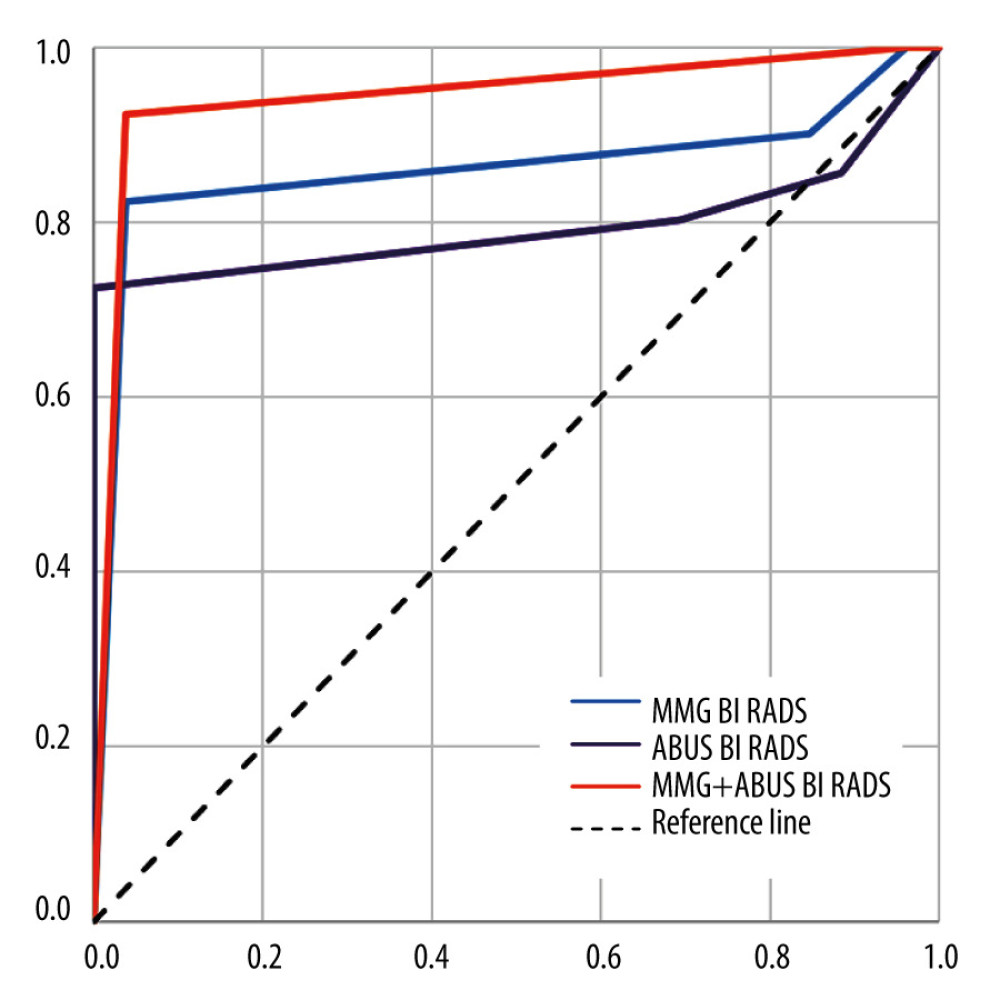 Figure 10. ABUS, FFDM, and combined ABUS and FFDM ROC curves.
Figure 10. ABUS, FFDM, and combined ABUS and FFDM ROC curves. Tables
Table 1. Histopathological diagnoses of all focal lesions in patients included in the study, their percentage and grouping model.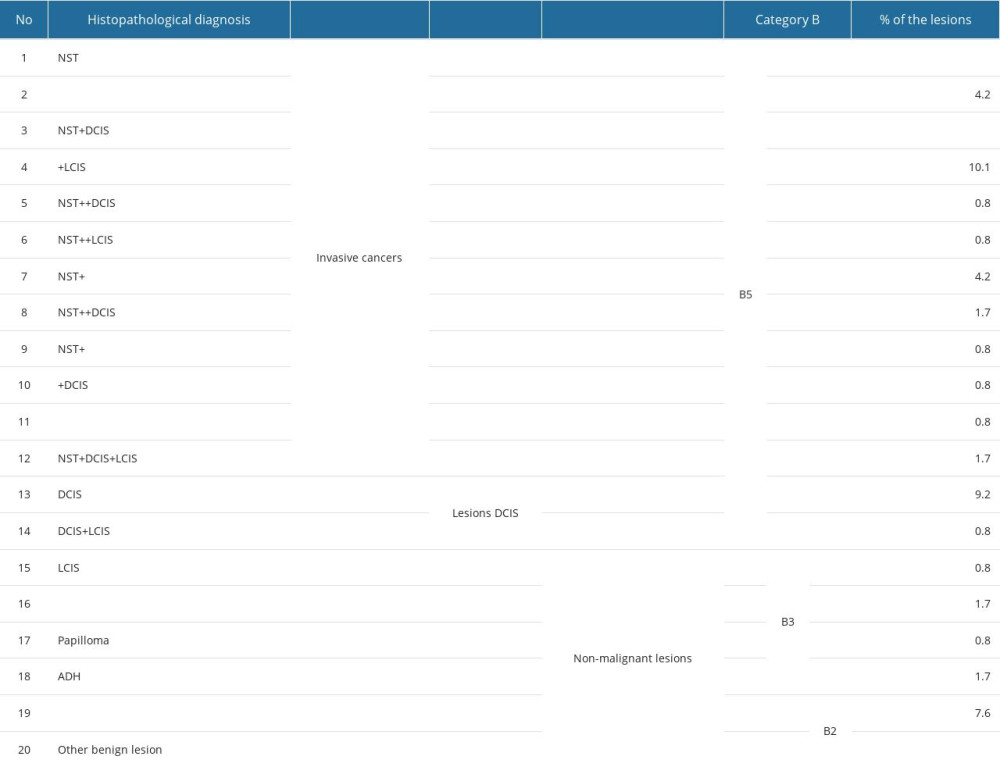 Table 2. Patients with multiple focal lesions – distribution of focal lesions number visible on FFDM and ABUS.
Table 2. Patients with multiple focal lesions – distribution of focal lesions number visible on FFDM and ABUS.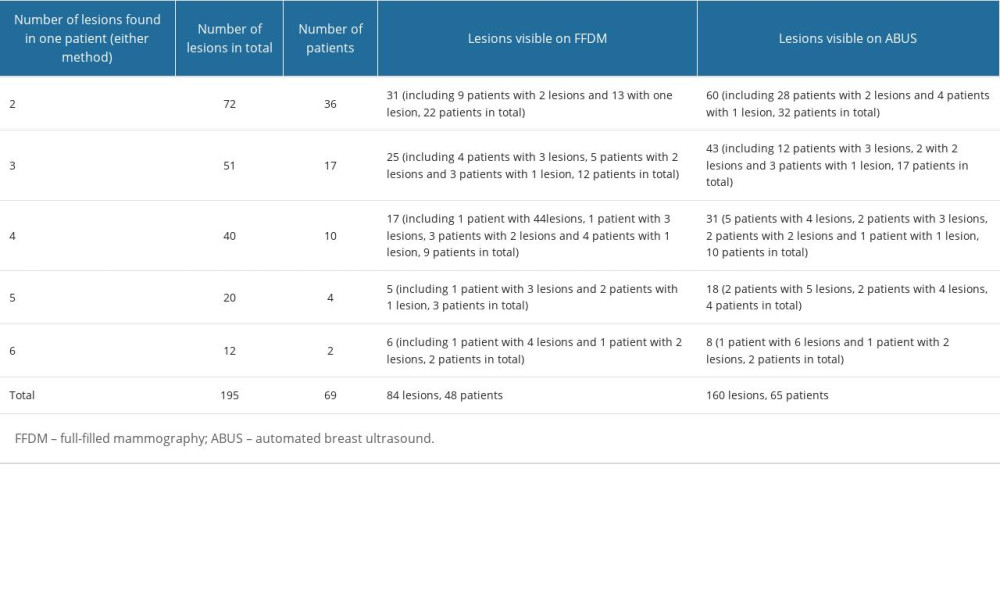 Table 3. Distribution of benign and malignant lesions in relation to BI-RADS category allocated on FFDM examination.
Table 3. Distribution of benign and malignant lesions in relation to BI-RADS category allocated on FFDM examination.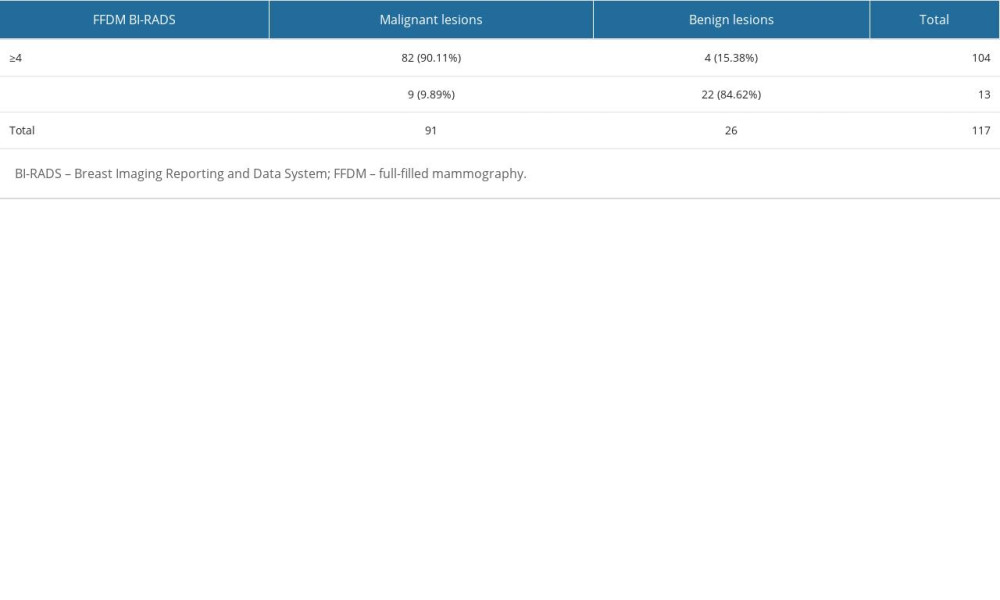 Table 4. Distribution of benign and malignant lesions in relation to BI-RADS category allocated on ABUS examination.
Table 4. Distribution of benign and malignant lesions in relation to BI-RADS category allocated on ABUS examination.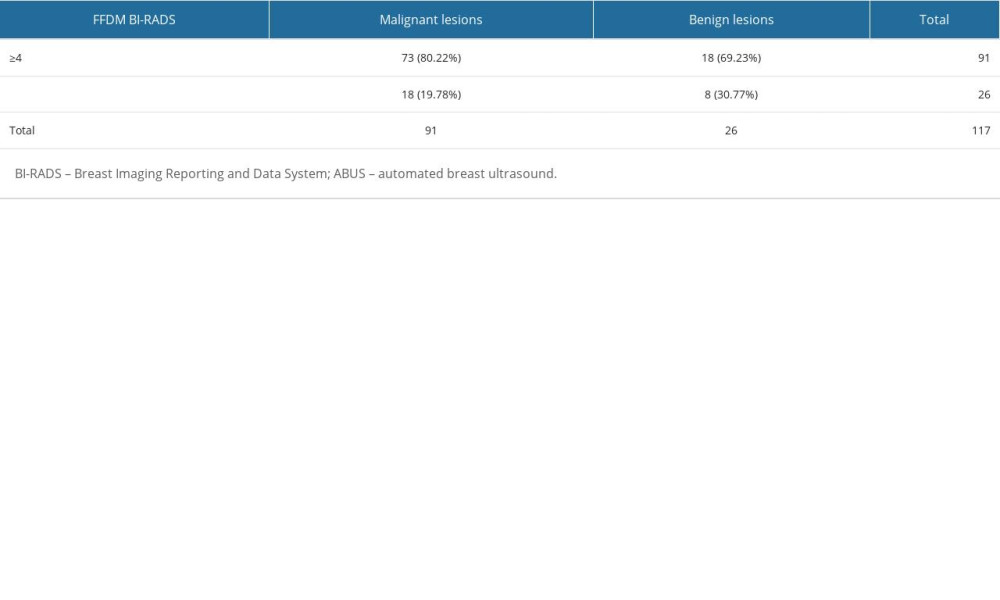
References
1. Kocarnik JM, Compton K, Dean FE, Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: A systematic analysis for the global burden of disease study 2019: JAMA Oncol, 2022; 8(3); 420-44
2. : Strategia walki z rakiem w Polsce 2015–2025 [Internet] czerwca 14, 2014 [cited 2015, November 16]. Available from: [on Polish]http://walkazrakiem.pl/sites/default/files/library/files/strategia_walki_z_rakiem_w_polsce_2015_2024.pdf
3. Kolak A, Kamińska M, Sygit K, Primary and secondary prevention of breast cancer: Ann Agric Environ Med, 2017; 24(4); 549-53
4. Niell BL, Freer PE, Weinfurtner RJ, Screening for breast cancer: Radiol Clin North Am, 2017; 55(6); 1145-62
5. Vourtsis A, Berg WA, Breast density implications and supplemental screening: Eur Radiol, 2019; 29(4); 1762-77
6. Sprague BL, Gangnon RE, Burt V, Prevalence of mammographically dense breasts in the United States: J Natl Cancer Inst, 2014; 106(10); dju255
7. McCormack VA, dos Santos Silva I, Breast density and parenchymal patterns as markers of breast cancer risk: A meta-analysis: Cancer Epidemiol Biomarkers Prev, 2006; 15(6); 1159-69
8. Chiu SY, Duffy S, Yen AM, Effect of baseline breast density on breast cancer incidence, stage, mortality, and screening parameters: 25-year follow-up of a Swedish mammographic screening: Cancer Epidemiol Biomarkers Prev, 2010; 19(5); 1219-28
9. Yuan WH, Hsu HC, Chen YY, Wu CH, Supplemental breast cancer-screening ultrasonography in women with dense breasts: A systematic review and meta-analysis: Br J Cancer, 2020; 123(4); 673-88
10. Corsetti V, Houssami N, Ferrari A, Breast screening with ultrasound in women with mammography-negative dense breasts: Evidence on incremental cancer detection and false positives, and associated cost: Eur J Cancer, 2008; 44(4); 539-44
11. Scheel JR, Lee JM, Sprague BL, Lee CI, Lehman CD, Screening ultrasound as an adjunct to mammography in women with mammographically dense breasts: Am J Obstet Gynecol, 2015; 212(1); 9-17
12. Berg WA, Blume JD, Cormack JB, Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer: JAMA, 2008; 299(18); 2151-63
13. Berg WA, Zhang Z, Lehrer D, Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk: JAMA, 2012; 307(13); 1394-404
14. Buchberger W, Niehoff A, Obrist P, Clinically and mammographically occult breast lesions: Detection and classification with high-resolution sonography: Semin Ultrasound CT MR, 2000; 21(4); 325-36
15. Sprague BL, Stout NK, Schechter C, Benefits, harms, and cost-effectiveness of supplemental ultrasonography screening for women with dense breasts: Ann Intern Med, 2015; 162(3); 157-66
16. Ohuchi N, Suzuki A, Sobue T, Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-cancer Randomized Trial (J-START): A randomised controlled trial: Lancet, 2016; 387(10016); 341-48
17. Corsetti V, Houssami N, Ghirardi M, Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: Interval breast cancers at 1 year follow-up: Eur J Cancer, 2011; 47(7); 1021-26
18. Vourtsis A, Three-dimensional automated breast ultrasound: Technical aspects and first results: Diagn Interv Imaging, 2019; 100(10); 579-92
19. Karst I, Henley C, Gottschalk N, Three-dimensional automated breast US: Facts and artifacts: Radiographics, 2019; 39(4); 913-31
20. Nicosia L, Ferrari F, Bozzini AC, Automatic breast ultrasound: State of the art and future perspectives: Ecancermedicalscience, 2020; 14; 1062
21. Łuczyńska E, Pawlak M, Popiela T, Rudnicki W, The role of ABUS in the diagnosis of breast cancer: J Ultrason, 2022; 22(89); 76-85
22. de Jong L, Welleweerd MK, van Zelst JCM, Production and clinical evaluation of breast lesion skin markers for automated three-dimensional ultrasonography of the breast: A pilot study: Eur Radiol, 2020; 30(6); 3356-62
23. Van Zelst JC, Platel B, Karssemeijer N, Mann RM, Multiplanar reconstructions of 3D automated breast ultrasound improve lesion differentiation by radiologists: Acad Radiol, 2015; 22(12); 1489-96
24. Chen H, Han M, Jing H, Dependability of Automated Breast Ultrasound (ABUS) in Assessing Breast Imaging Reporting and Data System (BI-RADS) category and size of malignant breast lesions compared with Handheld Ultrasound (HHUS) and Mammography (MG): Int J Gen Med, 2021; 14; 9193-202
25. Bent CK, Bassett LW, D’Orsi CJ, Sayre JW, The positive predictive value of BI-RADS microcalcification descriptors and final assessment categories: Am J Roentgenol, 2010; 194(5); 1378-83
26. Wilczek B, Wilczek HE, Rasouliyan L, Leifland K, Adding 3D automated breast ultrasound to mammography screening in women with heterogeneously and extremely dense breasts: Report from a hospital-based, high-volume, single-center breast cancer screening program: Eur J Radiol, 2016; 85(9); 1554-63
27. Suzuki M, Nakayama R, Namba K, Diagnostic performance of coronal view in comparison with transverse view of three-dimensional automated breast ultrasound: Acta Radiol, 2021; 62(1); 27-33
28. Brem RF, Tabár L, Duffy SW, Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: The SomoInsight Study: Radiology, 2015; 274(3); 663-73
29. Gatta G, Cappabianca S, La Forgia D, Second-generation 3D automated breast ultrasonography (Prone ABUS) for dense breast cancer screening integrated to mammography: effectiveness, performance and detection rates: J Pers Med, 2021; 11(9); 875
Figures
 Figure 1. FFDM (full-filled digital mammography) of a patient with dense, glandular breast type. No evident lesion is seen.
Figure 1. FFDM (full-filled digital mammography) of a patient with dense, glandular breast type. No evident lesion is seen. Figure 2. ABUS (automated breast ultrasound) scan of patient from Figure 1. Right breast in axial, sagittal, and coronal views. An ill-defined, hypoechogenic mass is visible at 9 o’clock. After core-needle biopsy, a lobular carcinoma was diagnosed.
Figure 2. ABUS (automated breast ultrasound) scan of patient from Figure 1. Right breast in axial, sagittal, and coronal views. An ill-defined, hypoechogenic mass is visible at 9 o’clock. After core-needle biopsy, a lobular carcinoma was diagnosed. Figure 3. Image of focal lesion’s margin on ABUS and FFDM depending on its type. Figure A shows ABUS image and C shows FFDM image of the same malignant lesion with ill-defined margin. Figure B shows ABUS image of benign lesion with well-defined margin. Figure B shows ABUS image and Figure D shows FFDM image of the same benign lesion with well-defined margin.
Figure 3. Image of focal lesion’s margin on ABUS and FFDM depending on its type. Figure A shows ABUS image and C shows FFDM image of the same malignant lesion with ill-defined margin. Figure B shows ABUS image of benign lesion with well-defined margin. Figure B shows ABUS image and Figure D shows FFDM image of the same benign lesion with well-defined margin. Figure 4. Examples of histopathology specimens at 10× magnification. Figure A shows fibroadenoma. Figure B shows ADH. Figure C shows DCIS G2. Figure D shows NST cancer G1.
Figure 4. Examples of histopathology specimens at 10× magnification. Figure A shows fibroadenoma. Figure B shows ADH. Figure C shows DCIS G2. Figure D shows NST cancer G1. Figure 5. (A, B) Correlation between focal lesion’s margin on ABUS and FFDM and its grade.
Figure 5. (A, B) Correlation between focal lesion’s margin on ABUS and FFDM and its grade. Figure 6. (A, B) Correlation between focal lesion’s margin on ABUS and FFDM and invasive cancer’s character.
Figure 6. (A, B) Correlation between focal lesion’s margin on ABUS and FFDM and invasive cancer’s character. Figure 7. Distribution of correlation between focal lesion margin and estrogen receptor expression level on ABUS, p=0.033.
Figure 7. Distribution of correlation between focal lesion margin and estrogen receptor expression level on ABUS, p=0.033. Figure 8. Presence of the retraction phenomenon on ABUS in benign and malignant lesions.
Figure 8. Presence of the retraction phenomenon on ABUS in benign and malignant lesions. Figure 9. (A, B) Frequency of microcalcifications presence on ABUS and FFDM in lesions diagnosed histopathologically as DCIS (ductal carcinoma in situ) and LCIS (lobular carcinoma in situ).
Figure 9. (A, B) Frequency of microcalcifications presence on ABUS and FFDM in lesions diagnosed histopathologically as DCIS (ductal carcinoma in situ) and LCIS (lobular carcinoma in situ). Figure 10. ABUS, FFDM, and combined ABUS and FFDM ROC curves.
Figure 10. ABUS, FFDM, and combined ABUS and FFDM ROC curves. Tables
 Table 1. Histopathological diagnoses of all focal lesions in patients included in the study, their percentage and grouping model.
Table 1. Histopathological diagnoses of all focal lesions in patients included in the study, their percentage and grouping model. Table 2. Patients with multiple focal lesions – distribution of focal lesions number visible on FFDM and ABUS.
Table 2. Patients with multiple focal lesions – distribution of focal lesions number visible on FFDM and ABUS. Table 3. Distribution of benign and malignant lesions in relation to BI-RADS category allocated on FFDM examination.
Table 3. Distribution of benign and malignant lesions in relation to BI-RADS category allocated on FFDM examination. Table 4. Distribution of benign and malignant lesions in relation to BI-RADS category allocated on ABUS examination.
Table 4. Distribution of benign and malignant lesions in relation to BI-RADS category allocated on ABUS examination. Table 1. Histopathological diagnoses of all focal lesions in patients included in the study, their percentage and grouping model.
Table 1. Histopathological diagnoses of all focal lesions in patients included in the study, their percentage and grouping model. Table 2. Patients with multiple focal lesions – distribution of focal lesions number visible on FFDM and ABUS.
Table 2. Patients with multiple focal lesions – distribution of focal lesions number visible on FFDM and ABUS. Table 3. Distribution of benign and malignant lesions in relation to BI-RADS category allocated on FFDM examination.
Table 3. Distribution of benign and malignant lesions in relation to BI-RADS category allocated on FFDM examination. Table 4. Distribution of benign and malignant lesions in relation to BI-RADS category allocated on ABUS examination.
Table 4. Distribution of benign and malignant lesions in relation to BI-RADS category allocated on ABUS examination. In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








