12 August 2023: Clinical Research
Transgastric Jejunostomy (PEG-J) for Continuous Infusion of Levodopa-Carbidopa Intestinal Gel: An Approach for Parkinson’s Disease Treatment
Yusuke Nomoto1ABCDEF*, Makoto Furihata1ABCDEF, Haruka Hagiwara1F, Hirotaka Ishino1B, Shintaro Yano1D, Hiroki Okawa1B, Yoichi Nakatsu1B, Kumiko Noda1B, Shinjiro Nishi1F, Shingo Ogiwara1AB, Tsuneo Kitamura1D, Taro Osada1AGDOI: 10.12659/MSM.941285
Med Sci Monit 2023; 29:e941285
Abstract
BACKGROUND: Parkinson’s disease (PD) is a neurodegenerative disorder that often requires long-term management of motor symptoms. Continuous infusion of levodopa-carbidopa intestinal gel (LCIG) has shown promising results in alleviating motor fluctuations and improving quality of life. This study aimed to evaluate the efficacy and safety of transgastric jejunostomy (PEG-J) as a delivery method for LCIG in a cohort of 43 PD patients.
MATERIAL AND METHODS: Forty-three PD patients who were candidates for LCIG therapy underwent transgastric jejunostomy to facilitate continuous infusion of LCIG. The primary outcomes assessed were motor symptom improvement, reduction in motor fluctuations, and medication-related adverse events. Secondary outcomes included changes in quality of life, dyskinesia severity, and healthcare resource utilization.
RESULTS: The results of this study demonstrated significant improvements in motor symptoms, reduction in motor fluctuations, and enhanced quality of life following PEG-J for LCIG infusion. The treatment was generally well-tolerated, with a low incidence of procedure-related complications. Notably, the use of PEG-J allowed for precise and continuous delivery of LCIG, minimizing variations in drug absorption and ensuring consistent therapeutic levels.
CONCLUSIONS: Transgastric jejunostomy (PEG-J) offers an effective approach for the continuous infusion of LCIG in Parkinson’s disease treatment. This method provides a stable and reliable delivery system, leading to improved symptom control and enhanced quality of life for PD patients.
Keywords: Carbidopa, Levodopa Drug Combination, endoscopy, Gastrostomy, Jejunostomy, Parkinson Disease, Humans, Carbidopa, Levodopa, Antiparkinson Agents, Quality of Life, Drug Combinations, Gels
Background
Parkinson’s disease (PD) is a chronic progressive neurodegenerative disorder characterized by motor and nonmotor symptoms that impair patient autonomy and quality of life (QoL). PD places considerable burdens on patients and their caregivers as a consequence [1]. It is generally believed that the efficacy of oral levodopa medication is affected by gastric emptying and diet-related competition for intestinal uptake [1,2]. Oral levodopa efficacy decreases as PD progresses. Overall, 50% of patients with PD tend to feel a sense of motor complications, such as wearing-off, dyskinesis, and on-off fluctuations, within 2–5 years after starting oral levodopa, reaching approximately 90% after 9 years. This is mainly due to the progressive loss of pulsate stimulation at the striatal dopamine nerve terminal receptors, short levodopa half-life, delayed gastric emptying, and irregular intestinal absorption. Gastric emptying can lead to undesirable fluctuations in serum concentration of levodopa, as this agent is prone to instability in the presence of gastric factors [1,3–5]. To overcome this shortcoming, device-aided treatments include chronic infusion of the dopamine agonist apomorphine by subcutaneous pump [6,7] deep brain stimulation of the subthalamic or internal globus palidus nuclei [6,8], levodopa/carbidopa/entacapone gel administration, or levodopa/carbidopa intestinal gel (LCIG) [6,9–11]. LCIG use started in 2014–2015 [1,12–14]. Randomized controlled trials have confirmed that this treatment reduces OFF time and increases ON time without increasing troublesome dyskinesia and improves QoL. Thus, LCIG (Duodopa®; AbbVie, North Chicago, USA) has been developed as a new system of continuous drug delivery via the small bowel [1,15]. RYTARY (carbidopa and levodopa) improved symptoms in patients with both early and advanced PD and offered significantly improved MDS-UPDRS and ‘ON-times’, without worsening troublesome dyskinesias, when compared to other levodopa formulas [16]. LCIG has been approved and widely adopted since 2016 in Japan. Percutaneous endoscopic gastrojejunostomy (PEG-J) tube has also been used in clinical practice for the efficient delivery of LCIG. LCIG markedly improved QoL and led to significant and sustained reductions in dyskinesia time, severity, and associated pain in advanced PD patients with high baseline dyskinesia burden [17]. Zafer et al referred to LCIG therapy as a continuous gastrointestinal pump infusion form of levodopa in the current guidelines of PD [18]. Nevertheless, procedure- and device-related AEs (PrAEs and DrAEs, respectively) are a serious issue with the introduction of PEG-J, hampering LCIG treatment. Therefore, this retrospective study aimed to evaluate the effects of PEG-J for continuous infusion of LCIG in the treatment of 43 patients with chronic PD and to evaluate whether LCIG via the PEG-J procedure is safe, focusing on the pros and cons, which can bring clinical benefits to patients with advanced PD.
Material and Methods
PATIENT CHARACTERISTICS:
A total of 45 patients diagnosed with advanced PD and motor fluctuations resistant to standard oral medications underwent PEG-J placement at Juntendo Urayasu Hospital (Chiba, Japan) between January 2016 and May 2020. The exclusion criteria for the patients were decided individually by an engaging endoscopist, judging mainly from the general status, abdominal conditions by computed tomography (CT), and the capability of resting body movement during the endoscopic procedure. One patient who could not stop body movements due to PD during endoscopy and another patient who had aspiration pneumonia before the procedure could not undergo the PEG-J procedure.
ETHICS APPROVAL:
All procedures performed were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments. The Institutional Review Board of our institution approved this study (approval number: U21-0005). Written informed consent for PEG-J was obtained from all patients prior to the procedure.
ENDOSCOPIC PROCEDURES OF PEG-J PLACEMENT:
Three experienced endoscopists who had performed 1000 upper endoscopies conducted the procedure. During each procedure session, vital signs such as blood pressure, oxygen saturation, heart rate, consciousness, and blood temperature were continuously monitored in all patients immediately before procedure. All patients were placed in the supine position. For premedication for PEG-J, all patients were administered a topical pharyngeal spray with 10% lidocaine and sedated using 2–3 mg of midazolam as an intravenous sedative, with 35 mg of pethidine hydrochloride as an initial dosage, according to the latest Japanese guidelines for sedation in gastroenterological endoscopy [15]. Additional midazolam (1 mg each) was administered if sedation was considered inadequate, at the discretion of the endoscopist. Agents used for duodenal relaxation were not applied. A PEG-J tube placement was performed using endoscopy for the large bowel (PCF Q260; Olympus Optical Co., Ltd., Tokyo, Japan) in all patients. First, we inserted an endoscope while the patient was in the supine position. Then, an adequate puncture site was determined by pushing the abdomen, where our fingers were directly transmitted to the endoscope, and the transmitted light from the endoscope was observed just beneath the epigastric region. Second, the abdomen was sterilized using isodine®. Before an actual puncture, lidocaine was administered as local anesthesia for an exploratory puncture using a 22-gauge needle, and it was assured that it was an adequate puncture site to avoid mispuncture of non-intended organs such as the intestine and liver. Gastropexy was not performed before puncture. We used a PEG tube kit (AbbVie, Inc., North Chicago, IL, USA) (Figure 1A, 1B). We inserted the introducer needle into the air-insufflated stomach, with only the outer sheath left (Figure 2A). A loop thread was then inserted through the sheath (Figure 2B). A standby endoscopist skillfully used snare forceps to capture the loop thread and gently pulled it out through the working channel of the endoscope, resulting in a loop thread left via the mouth by removing the endoscope. The gastrostomy device was attached to the loop thread and anchored to the gastric wall by pulling the loop thread (Figure 2C). Thus, gastrostomy was completed in the first step (Figure 2D). The jejunal tube (AbbVie. J tube 9Fr; Inc., North Chicago, IL, USA) was inserted through the gastrostomy device (Figure 3A), and a standby endoscopist grasped the catheter with grasping forceps (Figure 3B) and positioned it to the jejunum neighboring the ligament of Treitz. To proceed with this step, the endoscope was ordinarily pushed to the farther side of the second portion of the duodenum, accompanying the tube. Thereafter, we drew the endoscope to the stomach, pushing the grasping forceps conversely to the jejunum to prevent the catheter from slipping away from the optimal position (Figure 3C). Finally, a PEG-J tube was placed in a designated part of the upper jejunum (Figure 3D).
PROCEDURES TIME OF PEG-J PLACEMENT:
The procedure time was measured from the time of endoscope insertion to the time of completion of PEG-J placement.
OBSERVATIONAL PERIOD:
We observed patient conditions and collected data from January 2017 to May 2020. The median observation period was 19 months (range, 3–40 months).
LABORATORY AND RADIOLOGICAL EXAMINATIONS BEFORE AND AFTER PEG-J PLACEMENT:
CT was routinely performed to determine the inappropriate abdominal conditions for PEG-J placement preoperatively. On postoperative day (POD) 1, CT was routinely performed to determine the presence of intra-abdominal free gas (IAFG), bleeding, or ascites due to procedure-induced peritonitis. We evaluated the degree of IAFG by CT and classified them into 3 groups: A, B, and C, in descending order, depending on the amount of IAFG on day 1. We statistically evaluated the BMI levels among the 3 groups. As for hematological parameters, white blood cells (WBC), hemoglobin (Hb), and C-reactive protein (CRP) were routinely evaluated on preoperative day and POD1 and POD7 to acquire information about inflammation or progressive anemia in the early postoperative days compared with those on preoperative data. Hematological parameters were statistically compared over time.
ADVERSE EVENTS (AES) BY PEG-J PLACEMENT:
AEs were classified into PrAEs and DrAEs. PrAEs were defined as a direct complication of an endoscopic procedure. DrAEs were defined as complications due to the device after placement of the PEG-J tube. For PrAEs, events were counted in 44 procedures. Regarding DrAEs, repetition was allowed for cumulative patients and frequencies during the observation period. Acute AEs were defined as AEs occurring within 30 days, and late AEs were defined as those occurring after 30 days. AEs occurring outside the observation period were described if they were important for the PEG-J procedure.
DOSING OF LCIG:
LCIG contains 20 mg/ml of levodopa and 5 mg/ml of carbidopa monohydrates. It was supplied in cassettes containing 100 ml of gel solution delivered directly into the small intestine via a modified CADD®-Legacy PCA (Smiths Medical, St. Paul, MN, USA) infusion pump to a PEG-J catheter. The individual optimized dose of LCIG was administered as a morning bolus infusion, followed by a continuous infusion for 16 hours in total. Extra doses based on patient’ symptoms were intermittently administered if needed. Levodopa equivalent daily dose (mg) and the kinds and dose of additional therapy at discharge were examined.
LCIG TREATMENT EVALUATION IN NEUROLOGICAL ASPECT AND LENGTH OF HOSPITAL STAY:
We estimated the utility of LCIG treatment using PEG-J by scoring UPDRS at the time of admission and discharge, with ON-OFF time alteration before (on admission) and after LCIG treatment (at discharge). The scores were determined by an attending neurologist. Length of hospital stay was calculated from the day of admission for PEG-J placement to the day of discharge after initiating LCIG treatment. The data of 1 patient who had critical cardiorespiratory trouble independent of the PEG-J procedure and required a markedly long hospitalization (545 days) were excluded from the analysis.
STATISTICAL ANALYSES:
The JMP 14 statistical software (SAS Institute, Japan) was used for all analyses. UPDRS motor scores were compared over time using the paired
Results
PATIENT BACKGROUNDS AND DEMOGRAPHIC DATA:
PEG-J placement was performed in 45 patients. The data of 2 patients were excluded from the preoperative data because of the impossibility of resting body motion during endoscopy. Data were collected from 43 patients, and 44 procedures were performed. Patient backgrounds and demographic data are summarized in Table 1. The mean age of the patients was 68.1±8.0 years (range: 46–79 years). The enrolled patients comprised 21 males and 22 females with a mean BMI of 20.5±3.3 kg/m2 (range: 14.4–27.3 kg/m2). Commencing with metabolic and cardiovascular diseases, 39 of the 43 patients (90.7%) had comorbidities. The mean duration of PD was 11.0±6.0 years (range: 2–27 years) with a UPDRS motor score of 26.4±13.3 (range: 4–59) before treatment. Levodopa was the most frequently used prior treatment (100%), followed by dopamine agonists (93%) and monoamine oxidase inhibitors (34.9%).
PROCEDURE-RELATED AES:
The AEs associated with procedure and device are listed in Tables 2 and 3, respectively. In total, 7 PrAEs in were classified as inevitable or incident AEs and early or late AEs, according to our definition. One case each of pneumonia, peritonitis, gastric ulcer, skin trouble or granulation, and hemorrhage (2.32%) was observed as inevitable AEs. We could not determine the cause of the hemorrhage using esophagogastroduodenoscopy. However, we believe that the hemorrhage was caused by a postoperative bleeding originating from gastrostomy penetrating site. These AEs were probably inevitable and attributable to the PEG-J procedure. All PrAEs, except 1 hemorrhage and 1 acute pneumonia, were late AEs. No organ injury, pneumoperitoneum, or buried bumper syndrome was observed. One case of intestinal volvulus during the follow-up period and 1 of ileal pulling syndrome were also observed after the observation period, both of which were late AEs; however, the intestinal volvulus might have been incidental, and the ileal pulling syndrome has been identified in the literature [17].
DEVICE-RELATED AES:
There were 22 patients who developed DrAEs, and the total frequency was 44. The most common AEs associated with the device were inflection (n=12, out of 43 patients (27.9%), frequency=28 out of 44 procedures (63.6%)), followed by inadvertent removal (n=8 (18.6%), frequency=9 (20.5%)), breakage (n=6 (14.0%), frequency=7 (15.9%)), catheter migration (n=0), and lumen occlusion (n=0). Migration of the catheter and lumen occlusion were observed; however, they occurred due to inflection, inadvertent removal of the catheter, or breakage of particles. DrAEs were more frequent than PrAEs. All DrAEs were late AEs.
COUNTERMEASURES FOR AES:
Table 4 lists the countermeasures used for DrAEs/PrAEs, showing that we could obtain good outcomes for each AE using less invasive countermeasures. Surgery was not required in any cases. DrAEs resolved spontaneously or with radioscopy or endoscopy.
THERAPEUTIC OUTCOMES OF LCIG ON NEUROLOGICAL ASPECTS:
Regarding neurological aspects, UPDRS significantly decreased after initiating LCIG therapy (MDS-UPDRS motor score; before vs after: 26.4±13.3 vs 17.6 ±12.1; P<0.0001), which was evaluated using Wilcoxon’s signed-rank test, meaning that LCIG treatment was significantly effective. Moreover, the ON and OFF ratio was improved by LCIG therapy (ON: OFF ratio; before vs after: 24: 18 vs 42: 0; P<0.0001), which was evaluated using the chi-square test. The therapeutic outcomes of LCIG on neurological aspects are summarized in Table 5.
THE OUTCOMES OF PEG-J PROCEDURES:
The outcomes of the PEG-J procedures are summarized in Table 6. All PEG-J trials were completed, and LCIG treatment was initiated successfully. The mean procedure time was 21.7±12.5 min (range: 8–51 min). All patients achieved LCIG treatment (n=43, 43/43, success rate=100%) without mortality using less invasive countermeasures, as shown in Table 4. No surgical procedures were required due to any complications. The mean length of hospital stay was 27.8±15.8 days (range: 13–98 days). There were 7 dropouts (dropout rate 7/43; 16.3%); reasons for dropouts from the LCIG treatment were hospital change (n=3), intractable skin problems, cancer-related death, worsening by composite disease, and reduction of LCIG effectiveness (n=1, each). One patient with ileal pulling syndrome, although outside of the observation period, was forced to cancel LCIG treatment.
LABORATORY DATA:
As for laboratory data, the serum levels of WBC significantly increased on POD1 and POD7 compared with those preoperatively (preoperative vs POD1 vs POD7: 5.169 vs 8.165 vs 5.406 (×103/mm3);
BODY MASS INDEX AND INTRA-ABDOMINAL FREE GAS:
BMI was classified according to the degree of IAFG as follows: Group A (23.8 kg/m2, n=2), Group B (21.9 kg/m2, n=18), and Group C (19.1 kg/m2, n=23). Patients with a higher BMI tended to have higher IAFG scores. Among the 3 groups, only Group B had a significantly higher BMI than Group C based on Tukey’s honestly significant difference test (
Discussion
LIMITATIONS OF THE STUDY:
This study was limited to retrospective data from a single institution, and focused mainly on the safety of the process in PEG-J placement. Although the PEG-J technique has been shown to be safe in patients with advanced PD, a prospective study observing how LCIG treatment affects neurological aspects has not been conducted. A larger number of patients and better outcomes are needed to confirm the effectiveness of PEG-J in both gastroenterological and neurological aspects. Further research is needed to compare the efficacy of LCIG treatment via PEG-J and the newly marketed combination of subcutaneous infusion of levodopa and carbidopa.
Conclusions
The findings supported those from previous studies that LCIG treatment by PEG-J is an effective and safe treatment method for patients with chronic PD. Cooperation between neurologists and gastroenterologists is necessary to prevent complications related to LCIG therapy.
Figures
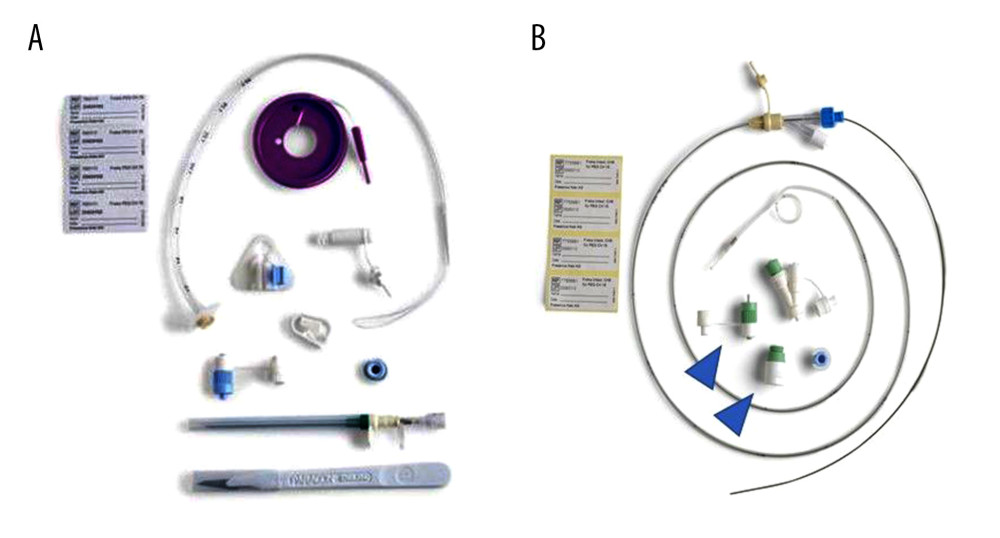 Figure 1. Percutaneous endoscopic transgastric jejunostomy (PEG-J) kit used in the procedures. Particles which are necessary for establishing gastrostomy are shown (A). A catheter and an accessory metallic guidewire are utilized for catheter placement (B). These particles are all included in a set.
Figure 1. Percutaneous endoscopic transgastric jejunostomy (PEG-J) kit used in the procedures. Particles which are necessary for establishing gastrostomy are shown (A). A catheter and an accessory metallic guidewire are utilized for catheter placement (B). These particles are all included in a set. 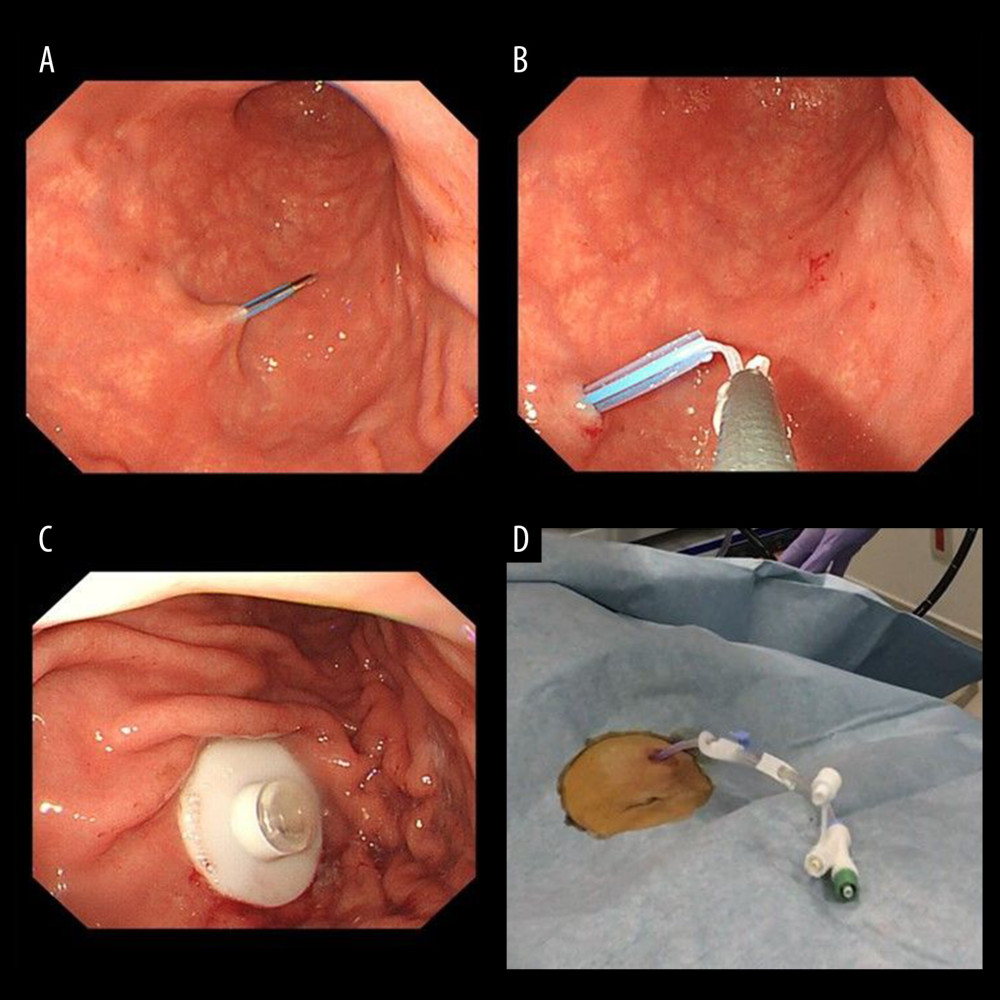 Figure 2. Endoscopic procedure for establishing a gastrostomy. The procedural sequence from an endoscopy to the placement of gastrostomy is shown in figure A–D).
Figure 2. Endoscopic procedure for establishing a gastrostomy. The procedural sequence from an endoscopy to the placement of gastrostomy is shown in figure A–D). 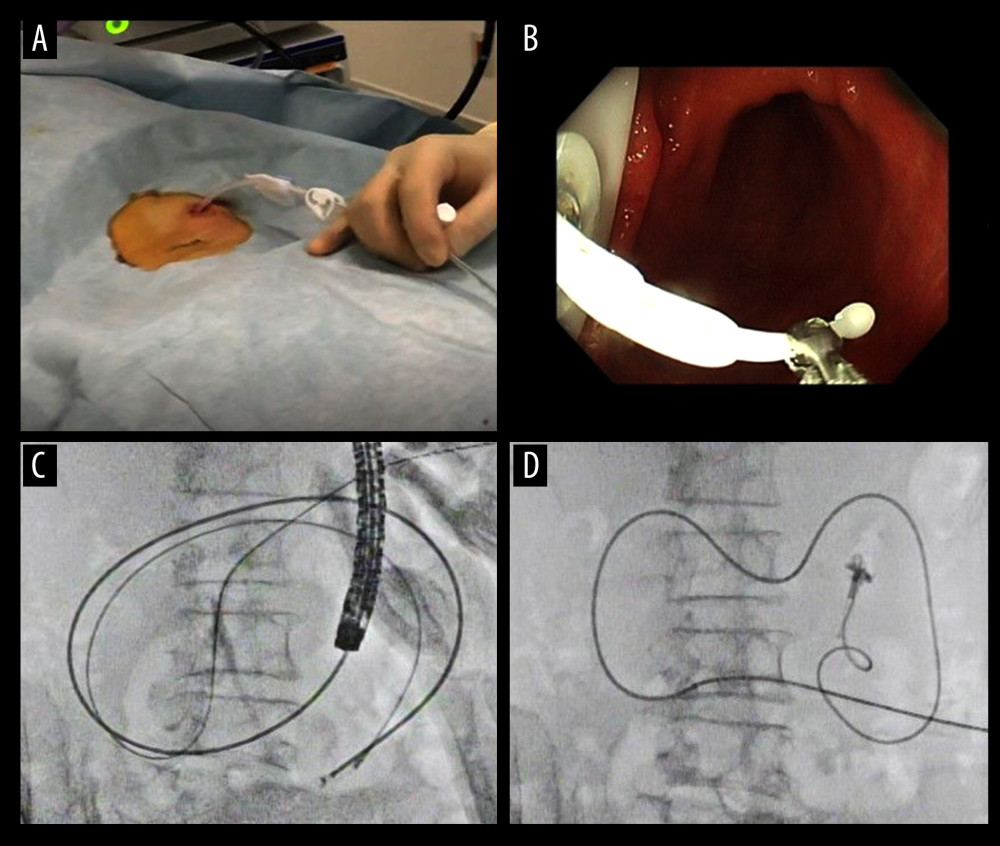 Figure 3. An appearance of endoscopic procedure for placing PEG-J tube under radioscopy. The procedural sequence from a gastrostomy to the placement of the PEG-J tube is shown in figure A–D).
Figure 3. An appearance of endoscopic procedure for placing PEG-J tube under radioscopy. The procedural sequence from a gastrostomy to the placement of the PEG-J tube is shown in figure A–D). Tables
Table 1. Patient background and demographic data.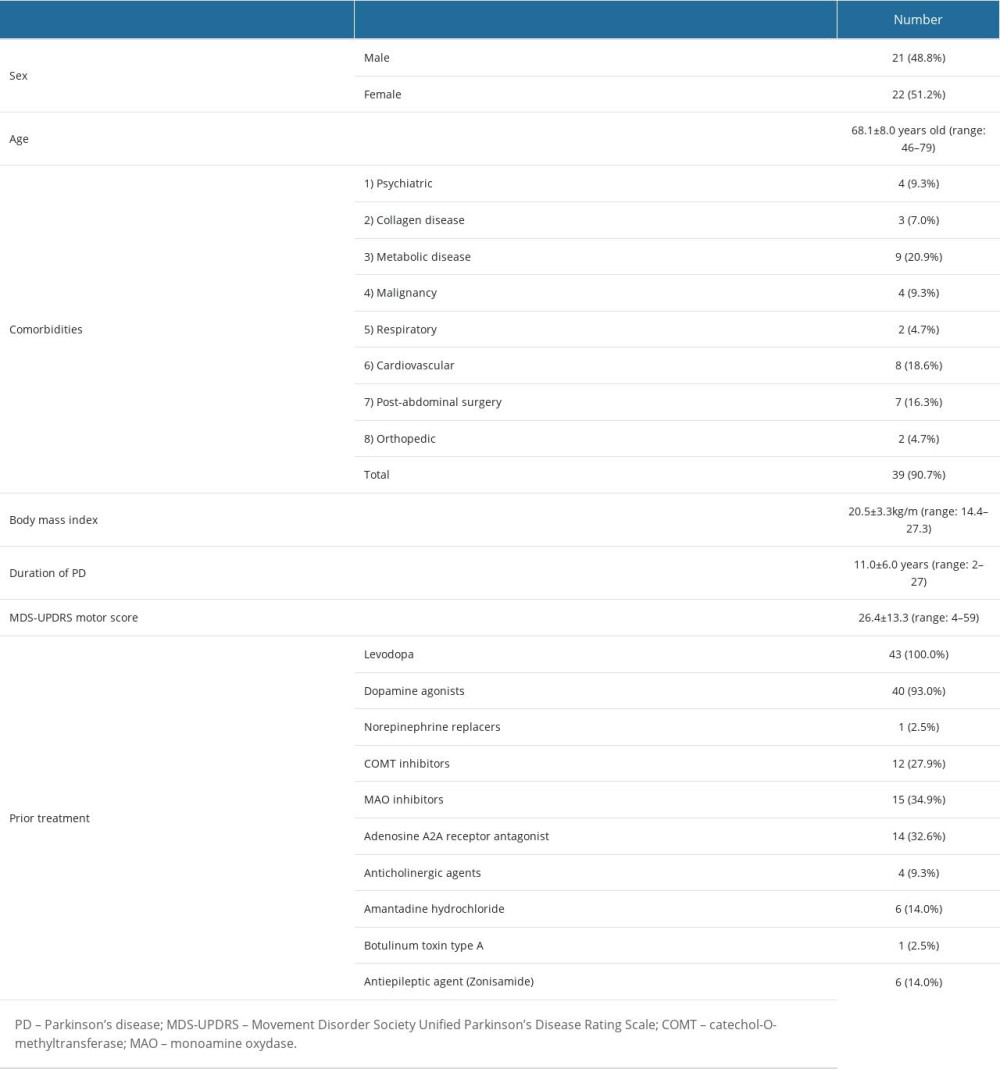 Table 2. Procedure-related adverse events.
Table 2. Procedure-related adverse events.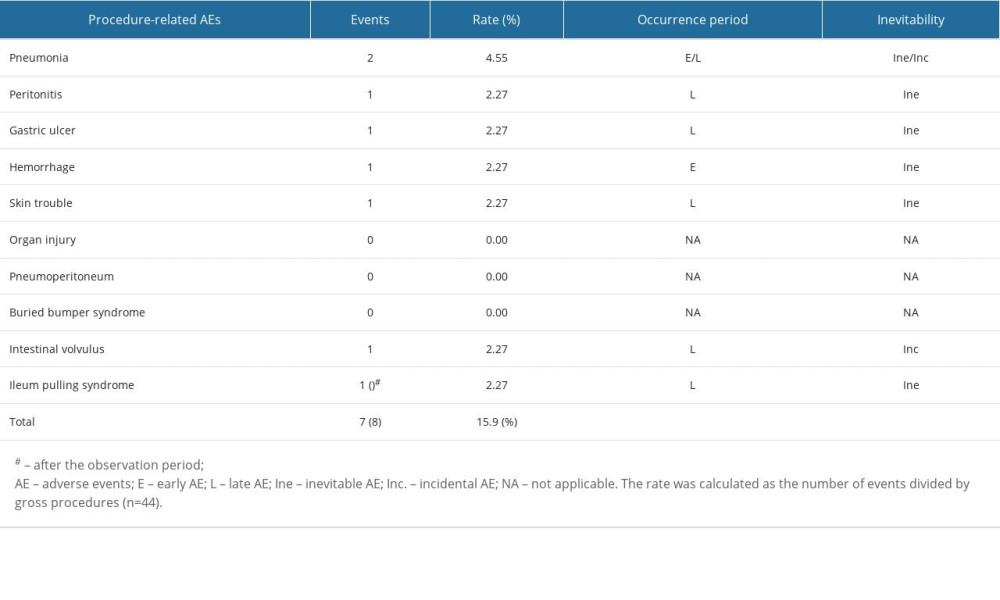 Table 3. Device-related adverse events.
Table 3. Device-related adverse events.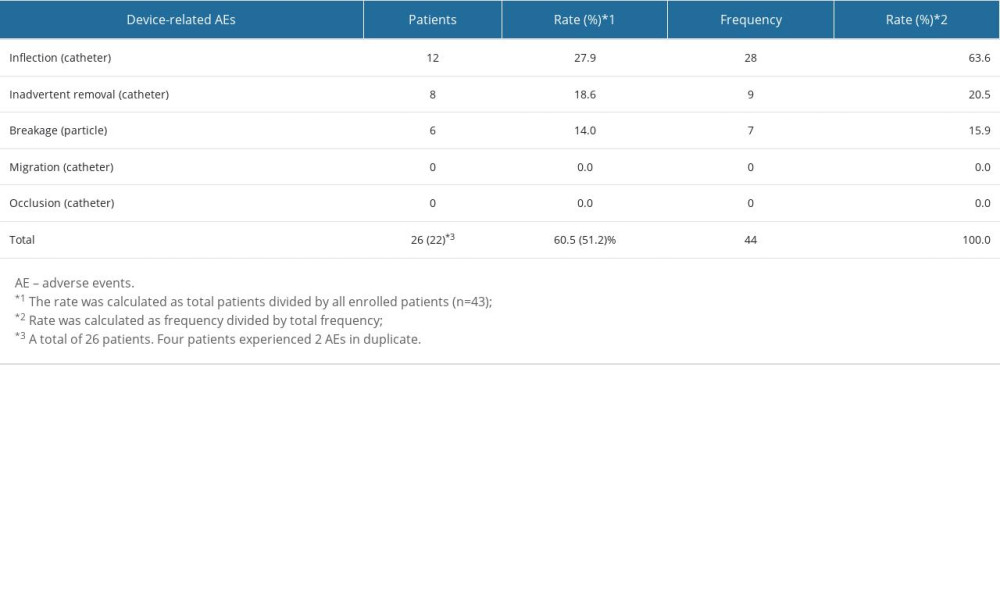 Table 4. Countermeasures for procedure-/device-related adverse events.
Table 4. Countermeasures for procedure-/device-related adverse events.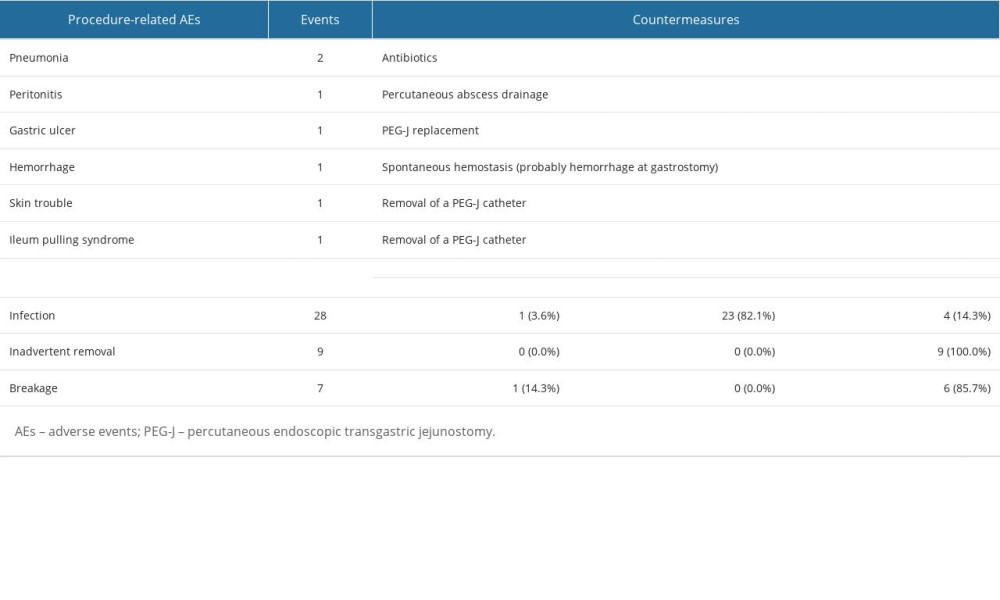 Table 5. Therapeutic outcomes of LCIG on neurological aspects.
Table 5. Therapeutic outcomes of LCIG on neurological aspects.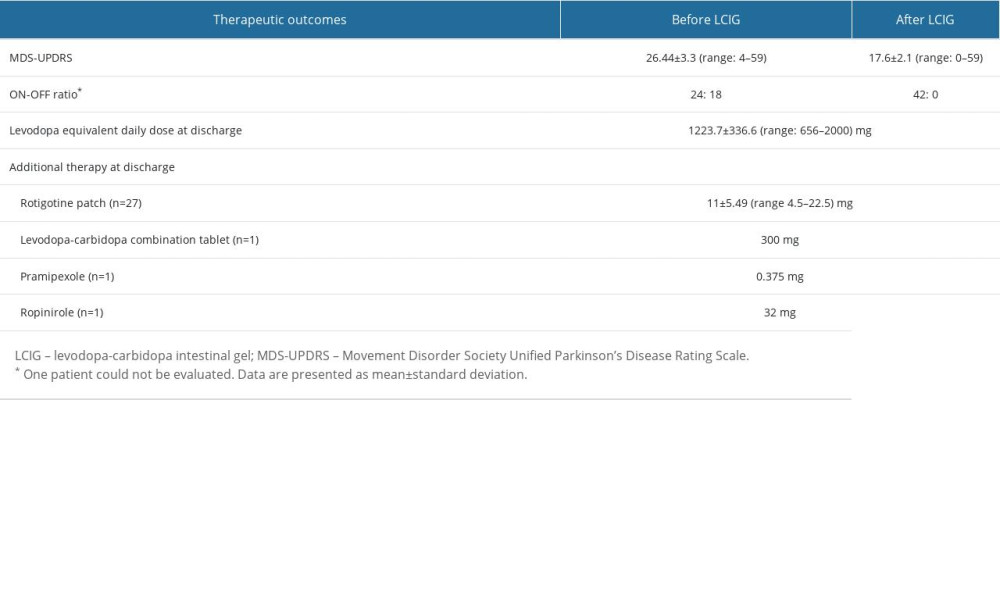 Table 6. Outcomes of PEG-J procedures.
Table 6. Outcomes of PEG-J procedures.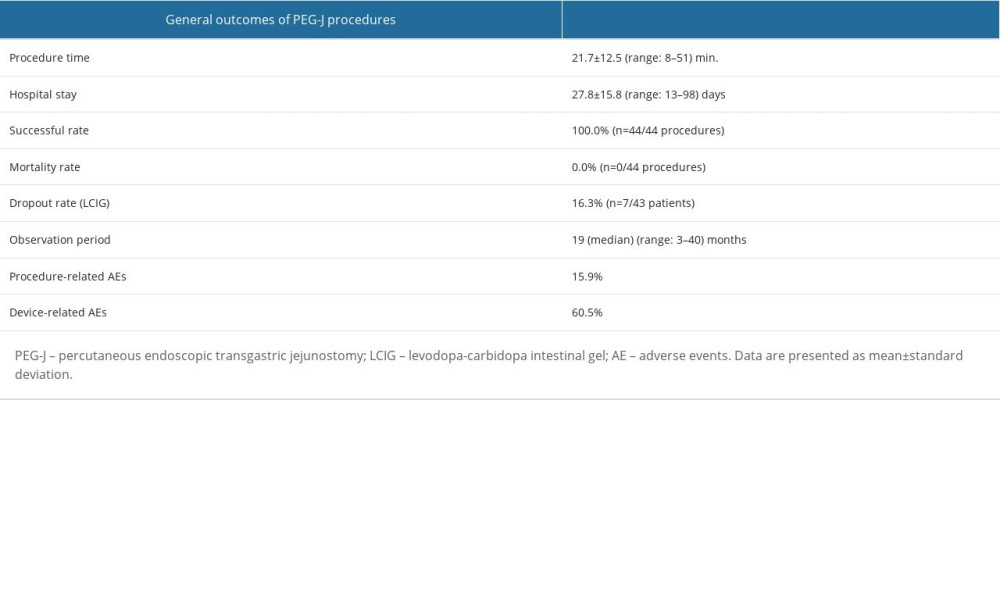
References
1. Lopiano L, Modugno N, Marano P, Motor and non-motor outcomes in patients with advanced Parkinson’s disease treated with levodopa/carbidopa intestinal gel: Final results of the GREENFIELD observational study: J Neurol, 2019; 266; 2164-76
2. Ishibashi Y, Shimo Y, Yube Y, Technique and outcome of percutaneous endoscopic transgastric jejunostomy for continuous infusion of levodopa-carbidopa intestinal gel for treatment of Parkinson’s disease: Scand J Gastroenterol, 2019; 54; 787-92
3. Freitas ME, Hess CW, Fox SH, Motor complications of dopaminergic medications in Parkinson’s disease: Semin Neurol, 2017; 37; 147-57
4. Altavista MC, Cassetta E, Brusa L, Wearing-off detection in clinical practice: The wearing of real practice key (WORK-PD) study in Parkinson’s disease: Parkinsonism Relat Disord, 2015; 21; 95-100
5. Antonini A, Moro E, Godeiro C, Reichmann H, Medical and surgical management of advanced Parkinson’s disease: Mov Disord, 2018; 33; 900-8
6. Rus T, Premzl M, Križnar NZ, Adverse effects of levodopa/carbidopa intrajejunal gel treatment: A single-center long-term follow-up study: Acta Neurol Scand, 2022; 146; 537-44
7. Katzenschlager R, Poewe W, Rascol O, Apomorphine subcutaneous infusion in patients with Parkinson’s disease with persistent motor fluctuations (TOLEDO): A multicentre, double-blind, randomised, placebo-controlled trial: Lancet Neurol, 2018; 17; 749-59
8. Artusi CA, Lopiano L, Morgante F, Deep brain stimulation selection criteria for Parkinson’s disease: time to go beyond CAPSIT-PD: J Clin Med, 2020; 9; 1-14
9. Tsunemi T, Oyama G, Saiki S, Intrajejunal infusion of levodopa/carbidopa for advanced Parkinson’s disease: A systematic review: Mov Disord, 2021; 36; 1759-71
10. Senek M, Nielsen EI, Nyholm D, Levodopa-entacapone-carbidopa intestinal gel in Parkinson’s disease: A randomized crossover study: Mov Disord, 2017; 32; 283-86
11. Öthman M, Widman E, Nygren I, Nyholm D, Initial experience of the levodopa-entacapone-carbidopa intestinal gel in clinical practice: J Pers Med, 2021; 11; 254
12. Olanow CW, Kieburtz K, Odin P, Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: A randomised, controlled, double-blind, double-dummy study: Lancet Neurol, 2014; 13; 141-49
13. Fernandez HH, Standaert DG, Hauser RA, Levodopa-carbidopa intestinal gel in advanced Parkinson’s disease: Final 12-month, open-label results: Mov Disord, 2015; 30; 500-9
14. Slevin JT, Fernandez HH, Zadikoff C, Long-term safety and maintenance of efficacy of levodopa-carbidopa intestinal gel: An open-label extension of the double-blind pivotal study in advanced Parkinson’s disease patients: J Parkinsons Dis, 2015; 5; 165-74
15. Nyholm D, Odin P, Johansson A, Pharmacokinetics of levodopa, carbidopa, and 3-O-methyldopa following 16-hour jejunal infusion of levodopa-carbidopa intestinal gel in advanced Parkinson’s disease patients: AAPS J, 2013; 15; 316-23
16. Dhall R, Kreitzman DL, Advances in levodopa therapy for Parkinson disease: Review of RYTARY (carbidopa and levodopa) clinical efficacy and safety: Neurology, 2016; 86(14 Suppl 1); S13-24
17. Poewe W, Chaudhuri KR, Bergmann L, Levodopa-carbidopa intestinal gel in a subgroup of patients with dyskinesia at baseline from the GLORIA Registry: Neurodegener Dis Manag, 2019; 9; 39-46
18. Zafer S, Yaddanapudi SS, Parkinson Disease. [Updated 2022 Aug 8]: StatPearls [Internet] Jan, 2023, Treasure Island (FL), StatPearls Publishing Available from: https://www.ncbi.nlm.nih.gov/books/NBK470193/
19. Obara K, Haruma K, Irisawa A, Guidelines for sedation in gastroenterological endoscopy: Dig Endosc, 2015; 27; 435-49
20. Marano M, Pizzicannella M, di Biase L, Jejunal pulling syndrome: A peculiar LCIG complication: Parkinsonism Relat Disord, 2018; 52; 113-14
21. Nyholm D, Nilsson Remahl AI, Dizdar N, Duodenal levodopa infusion monotherapy vs oral polypharmacy in advanced Parkinson disease: Neurology, 2005; 64; 216-23
22. Olanow CW, Kieburtz K, Odin P, Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: A randomised, controlled, double-blind, double-dummy study: Lancet Neurol, 2014; 13; 141-49
23. Antonini A, Poewe W, Chaudhuri KR, Levodopa-carbidopa intestinal gel in advanced Parkinson’s: Final results of the GLORIA registry: Parkinsonism Relat Disord, 2017; 45; 13-20
24. Okumura N, Tsuji N, Ozaki N, Percutaneous endoscopic gastrostomy with Funada-style gastropexy greatly reduces the risk of peristomal infection: Gastroenterol Rep, 2015; 3; 69-74
25. Epstein M, Johnson DA, Hawes R, Long-term PEG-J tube safety in patients with advanced Parkinson’s disease: Clin Transl Gastroenterol, 2016; 7; e159
26. Karadağ YS, Saltoğlu T, Küçükdağli FE, Comprehensive assessment of levodo pa-carbidopa intestinal gel for Turkish advanced Parkinson’s disease patients: Turk J Med Sci, 2021; 51; 84-89
27. Yamashita K, Yube Y, Yamazaki Y, The impact of tube replacement timing during LCIG therapy on PEG-J associated adverse events: A retrospective multicenter observational study: BMC Neurol, 2021; 21; 242
28. Giladi N, Gurevich T, Djaldetti R, ND0612 (levodopa/carbidopa for subcutaneous infusion) in patients with Parkinson’s disease and motor response fluctuations: A randomized, placebo-controlled phase 2 study: Parkinsonism Relat Disord, 2021; 91; 139-45
Figures
 Figure 1. Percutaneous endoscopic transgastric jejunostomy (PEG-J) kit used in the procedures. Particles which are necessary for establishing gastrostomy are shown (A). A catheter and an accessory metallic guidewire are utilized for catheter placement (B). These particles are all included in a set.
Figure 1. Percutaneous endoscopic transgastric jejunostomy (PEG-J) kit used in the procedures. Particles which are necessary for establishing gastrostomy are shown (A). A catheter and an accessory metallic guidewire are utilized for catheter placement (B). These particles are all included in a set. Figure 2. Endoscopic procedure for establishing a gastrostomy. The procedural sequence from an endoscopy to the placement of gastrostomy is shown in figure A–D).
Figure 2. Endoscopic procedure for establishing a gastrostomy. The procedural sequence from an endoscopy to the placement of gastrostomy is shown in figure A–D). Figure 3. An appearance of endoscopic procedure for placing PEG-J tube under radioscopy. The procedural sequence from a gastrostomy to the placement of the PEG-J tube is shown in figure A–D).
Figure 3. An appearance of endoscopic procedure for placing PEG-J tube under radioscopy. The procedural sequence from a gastrostomy to the placement of the PEG-J tube is shown in figure A–D). Tables
 Table 1. Patient background and demographic data.
Table 1. Patient background and demographic data. Table 2. Procedure-related adverse events.
Table 2. Procedure-related adverse events. Table 3. Device-related adverse events.
Table 3. Device-related adverse events. Table 4. Countermeasures for procedure-/device-related adverse events.
Table 4. Countermeasures for procedure-/device-related adverse events. Table 5. Therapeutic outcomes of LCIG on neurological aspects.
Table 5. Therapeutic outcomes of LCIG on neurological aspects. Table 6. Outcomes of PEG-J procedures.
Table 6. Outcomes of PEG-J procedures. Table 1. Patient background and demographic data.
Table 1. Patient background and demographic data. Table 2. Procedure-related adverse events.
Table 2. Procedure-related adverse events. Table 3. Device-related adverse events.
Table 3. Device-related adverse events. Table 4. Countermeasures for procedure-/device-related adverse events.
Table 4. Countermeasures for procedure-/device-related adverse events. Table 5. Therapeutic outcomes of LCIG on neurological aspects.
Table 5. Therapeutic outcomes of LCIG on neurological aspects. Table 6. Outcomes of PEG-J procedures.
Table 6. Outcomes of PEG-J procedures. In Press
12 Mar 2024 : Clinical Research
Metabolomic Alterations in Methotrexate Treatment of Moderate-to-Severe PsoriasisMed Sci Monit In Press; DOI: 10.12659/MSM.943360
14 Mar 2024 : Clinical Research
Renal Dysfunction Increases Risk of Adverse Cardiovascular Events in 5-Year Follow-Up Study of Intermediate...Med Sci Monit In Press; DOI: 10.12659/MSM.943956
15 Mar 2024 : Clinical Research
Impact of One-Lung Ventilation on Oxygenation and Ventilation Time in Thoracoscopic Heart Surgery: A Compar...Med Sci Monit In Press; DOI: 10.12659/MSM.943089
14 Mar 2024 : Clinical Research
Differential DHA and EPA Levels in Women with Preterm and Term Births: A Tertiary Hospital Study in IndonesiaMed Sci Monit In Press; DOI: 10.12659/MSM.943895
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








