27 August 2023: Database Analysis
The Impact of Visceral Adiposity on Testosterone Levels in American Adult Men: A Cross-Sectional Analysis
Mingqin Su1ABCDEF, Hongquan Wei1ABCD, Lijun Chen1BC, Yuxiang Guan1BD, Wei Dong1B, Min Zhao1AG*DOI: 10.12659/MSM.941394
Med Sci Monit 2023; 29:e941394
Abstract
BACKGROUND: Testosterone decline and deficiency importantly affect men’s health, and may be associated with excessive deposition of visceral adipose tissue. This study was conducted to explore the association between visceral adiposity index (VAI) and testosterone level.
MATERIAL AND METHODS: A total of 1551 participants from the NHANES 2013-2013 cycle and 2015-2016 cycle were selected for our analyses. The VAI index was calculated based on waist circumference (WC), body mass index (BMI), triglyceride (TG), and high-density lipoprotein cholesterol (HDL-c), and serum testosterone was measured by isotope dilution liquid chromatography tandem mass spectrometry. Multivariable adjusted linear and logistic regression were utilized to investigate the associations between VAI index and testosterone level and testosterone deficiency, respectively. Additionally, subgroup analyses were performed to identify sensitive populations.
RESULTS: A total of 1551 participants with mean VAI index of 1.95±0.08 were eligible for our analysis. After adjusting for all potential cofounders, men with higher VAI index displayed a lower level of total testosterone level (β: -11.74, 95% CI: -17.33, -6.15, P<0.0001), and higher risk of testosterone deficiency (OR: 1.24, 95% CI: 1.09, 1.40, P=0.0022). Comparing to VAI quartile 1, quartile 4 showed the most decreased testosterone level (β: -94.59, 95% CI: -130.04, -59.14, P<0.0001), and highest risk of testosterone deficiency (OR: 5.07, 95% CI: 2.41,10.63, P<0.0001). Subgroup analysis demonstrated that VAI index was strongly related to testosterone level and testosterone deficiency in aged and obese men.
CONCLUSIONS: Men with higher VAI index displayed lower testosterone levels and higher risk of testosterone deficiency, especially in aged men and obese men.
Keywords: Intra-Abdominal Fat, Nutrition Surveys, Testosterone, Hypogonadism, Male, Humans, Adult, Aged, Cross-Sectional Studies, Adiposity, Obesity
Background
Testosterone is an indispensable steroid hormone in men, mainly produced and secreted by Leydig cells in the testes. The numerous sexual and non-sexual physiological processes of males are regulated by the normal circulatory testosterone, including erectile function, sexual libido, reproduction, cardiovascular health, poor cognition, and psychological health [1–4]. However, approximate 30% of adult men aged 40–79 years are affected by decreased testosterone level [5], diagnosed as testosterone deficiency with a cut-off value (300 ng/dl) of at least 2 measurements [6]. The rapidly increased prevalence of testosterone deficiency worldwide is associated with the prevalence of aging, obesity, diabetes, and metabolic syndrome [7]. Furthermore, men with testosterone deficiency are more susceptible to sexual dysfunction, poor reproduction, cardiovascular disease, and psychological disorders [8–10]. Testosterone deficiency has become a prevalent issue in adult men, and needs to receive increased public attention.
Presently, there is evidence that metabolic syndrome (Mets) is a combination of risk factors for testosterone deficiency, which included dyslipidemia, hypertension, insulin resistance, and obesity [11]. Among these risk factors of Mets, obesity is considered as the core manifestation, and has attracted more attention from researchers [12,13]. Several well-designed studies have reported the high prevalence of testosterone deficiency in men with obesity. For instance, Zumoff et al reported an inverse correlation between free testosterone and body mass index (BMI) in adult men with a BMI ranging from 21 to 95 kg/m2[14]. Another study, by Hofstra et al, also reached similar conclusions, and reported that about 35% of men with obesity had testosterone level under 300 ng/dl, diagnosed as testosterone deficiency [15]. Studies have verified the strong association between obesity and insulin resistance [16], which could decrease the production and secretion of testosterone from Leydig cells in the testes [17]. Additionally, accumulation of adipose tissue can release pro-inflammatory cytokines, which can also decrease the secretion of testosterone by exerting inhibitory effects on the hypothalamic-pituitary-gonadal axis [18]. Traditionally, BMI was used to evaluate obesity-related sex hormone disorders, while it disregarded body fat distribution [19]. Then, waist circumference (WC) was used to assess obesity-related sex hormone disorders, as it can easily evaluate abdominal obesity [20]. Intriguingly, recent research has recognized that adipose tissue has complicated functions and not all adipose tissue is harmful to human health. Consequently, it is helpful to precisely quantify adipose tissue in specific settings to better understand the role of adipose tissue in the pathophysiological process of disease. However, these traditional indicators of obesity failed to accomplish these goals [21].
Excessive deposition of visceral fat is associated with higher circulatory pro-inflammatory cytokines and worsen insulin resistance [22]. However, there have been few studies concentrating on the association between visceral obesity and testosterone deficiency, owing to the lack of reliable indicators to evaluate the relationship between excessive deposition of visceral fat and testosterone deficiency. Amato et al proposed a novel indicator named visceral adiposity index (VAI) to directly evaluate the distribution of abdominal fat and to indirectly assess the function of adipose tissue [23]. The VAI is calculated based on the anthropometric indicators (BMI, WC), and the biochemical metabolic indicators (triglyceride [TG], high-density lipoprotein cholesterol [HDL-c]) [23]. It has been identified as a potential risk factors for many clinical diseases, including cardiovascular disease, stroke, and even depression [24–26]. Because the metrics and metabolisms for VAI calculation are strongly associated with testosterone deficiency, it is important to explore the association between VAI and testosterone level in men. Additionally, we also compared the ability of VAI to predict testosterone deficiency with another 2 indicators – the triglyceride-glucose (TyG) index, and homeostasis model assessment of insulin resistance (HOMA-IR) – finding that all 3 are reliable indicators of testosterone deficiency [27].
Material and Methods
STUDY POPULATION:
The National Health and Nutrition Examination Survey (NHANES) is a continuous national survey conducted every other year by the National Center for Health Statistics (NCHS) at the U.S. Centers for Disease Control and Prevention (CDC). The data were collected by personal interviews, standardized physical examinations, laboratory tests, demographic data, dietary data, examination data, and questionnaire data. Every survey participant was assigned a multistage sample weight to clearly represent the nutritional and health condition of noninstitutionalized U.S. civilians. All NHANES processes were permitted by the NAHNES Institutional Review Board (IRB)/NCHS Research Ethics Review Board (ERB), and all participants have signed the written informed consents. The NHANES data are available at
For our study analysis, we selected a total of 20 146 participants from the NHANES 2013–2014 cycle and 2015–2016 cycle. First, we excluded all female participants (10 251). Then, all the participants aged younger than 20 years were also excluded from our study (4390). Additionally, another 3954 participants were excluded for the following reasons: participants without testosterone values (505), participants without data for VAI calculation (2810), participants without data for covariates (624), and participants receiving sex hormone medication (15). Finally, 1551 eligible participants were included for our final analysis. The detailed sample selection process is shown in Figure 1.
EXPOSURE AND OUTCOME DEFINITIONS:
The VAI, was calculated utilizing the gender-specific equations:
TG and HDL-C were calculated in mmol/L, and WC was calculated in cm in the formulas, and the TyG index was calculated as Ln (fasting triglycerides [mg/dl] × fasting blood glucose [mg/dl]/2) [29]. Furthermore, the HOMA-IR was calculated as (fasting glucose [mg/dl] × fasting insulin [μU/ml]/405) [30]. The testosterone level and testosterone deficiency were considered as outcome indicators. According to the American Urological Association, the testosterone deficiency was diagnosed as total testosterone level <300 ng/dl [6].
The physical examinations were performed at the mobile examination center, requiring the participants to fast at least 9 h. The BMI was calculated by dividing the weight (kg) by square of the height (m), rounding to the nearest 1/10 cm. In order to measure the WC, the participants were advised to stand naturally with legs about 25–30 cm apart. Measurement was conducted at the end of a normal exhalation, using an inelastic ruler with a minimum scale of 1 mm. The ruler was placed at the midpoint of the connecting line between the upper edge of the top of the iliac crest and the lower edge of the 12th rib (often the natural narrowest part of the waist), and then horizontally circled the abdomen. The results were rounded to 0.1 cm, as in BMI [31].
Triglycerides (TG) and fasting blood glucose (FBG) were measured via enzymatic assay, utilizing the Roche Modular P and Roche Cobas 6000 chemistry analyzers and the hexokinase-mediated reaction on Roche/Hitachi Cobas C 501 chemistry analyzers, respectively. The HDL-c levels were measured enzymatically with a Hitachi 704 Analyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN, USA). Total testosterone was measured through isotope dilution liquid chromatography tandem mass spectrometry (ID-LC-MS/MS) following the National Institute for Standards and Technology’s (NIST) reference method. All detailed information about the analyzer and corresponding methods were accessible on the NHANES website with a document titled “Laboratory Procedures”.
COVARIATES:
The potential confounding variables for the association between VAI index and testosterone level were included for the covariates. For demographic characteristics, the covariates included age, race, educational level, and family-to-poverty ratio (PIR: <1, ≥1). BMI was also included, and categorized into 3 groups: normal (BMI <25 kg/m2), overweight (25≤BMI ≤30 kg/m2), and obese (BMI >30 kg/m2) [32]. Based on the self-administrated survey, the smoking status was divided into never, former, and current, and the alcohol status was categorized into yes and no. Additionally, a set of dietary data were also extracted, including total energy, total fat, and total protein. Diabetes was diagnosed when they had a self-reported diabetes diagnosis, a plasma glucose level ≥200 mg/dl at 2 h after oral glucose tolerance test (OGTT), HbAlc ≥6.5%, a fasting glucose level ≥126 mg/dl, or use of oral hypoglycemic agents. Borderline diabetes was diagnosed when participants had abnormal results of these glucose related tests less than the standard of diabetes. Hypertension was defined as a diastolic blood pressure ≥90 mmHg and a systolic blood pressure ≥140 mmHg, using antihypertensive medications, or a self-reported hypertension diagnosis.
STATISTICAL ANALYSIS:
All the statistical analyses were conducted following the CDC guidelines for NHANES statistical analyses. We applied a suitable sample weight of each participant from the complex multistage cluster survey design of NHANES [33]. Percentages were used for categorical variables and mean and standard deviation (SD) were used for continuous variables. When comparing the difference of categorical and continuous variables among different VAI index quartiles, the chi-square test and analysis of variance (ANOVA) were utilized, respectively. The association between VAI index and total testosterone level was analyzed by linear regression, while the association between VAI index and testosterone deficiency was analyzed by logistic regressions. The former results are presented as β and 95% confidential interval (95% CI), while the latter results are reported as odds ratio (OR) and 95% CI. A total of 3 different models were included in the present analysis to explore the potential covariate impact on these associations. No covariates were adjusted in the crude model. Then, age, race, and educational level were adjusted in model 1. Finally, all potential covariates, including age, race, education, diabetes, hypertension, smoking, alcohol use, and BMI, were adjusted in model 2. The subgroup analyses were performed based on age group, BMI group, alcohol status, hypertension status, and diabetes status, intending to explore the special populations. Interaction terms were also used to evaluate heterogeneity among these subgroups. Heterogeneity was confirmed when the P value for interaction was <0.05. The receiver operating characteristic (ROC) curves were graphed and the corresponding areas under the curve (AUCs) were calculated to compare the predictive performance of VAI index, TyG index, HOMA-IR index, FBG, and TG for predicting testosterone deficiency. Z tests were utilized to assess the differences between AUCs. We also used the non-parameter method of DeLong et al to compare AUCs for TyG with other indicators. Statistical significance was defined as P<0.05. All statistical analyses were performed using R version 4.0.5 and EmpowerStats (www.empowerstats.com; X & Y solutions, Inc., Boston MA) software.
Results
The baseline characteristics of the sample are displayed in Table 1. The mean VAI index was 1.95±0.08, and the ranges of VAI index quartiles were quartile 1: 0.10~0.75, quartile 2: 0.75~1.26, quartile 3: 1.26~2.27, and quartile 4 greater than 2.27. The mean age of the participants increased with the increase of the VAI index quartiles. A higher prevalence of hypertension and diabetes were also reported in VAI quartile 4 compared with quartile 1. The participants with higher VAI were more likely to be obese and less likely to have low educational level. The total testosterone level in VAI quartile 4 was lower than that in quartile 1 (385.34±14.74 mg/dl vs 534.96±13.51 mg/dl), while the prevalence of testosterone deficiency in VAI quartile 4 was higher than that in quartile 1 (33.99% vs 5.97%). As the VAI index increased, the HOMA-IR and TyG values were also increased gradually.
The associations between VAI index and total testosterone level and testosterone deficiency are shown in Table 2. Multivariable logistic regression analyses were performed to explore the association between VAI index and testosterone deficiency. Our results showed that the VAI index was positively associated with testosterone deficiency in all models (crude model: OR: 1.31, 95% CI 1.17, 1.47, P<0.0001; model 1: OR: 1.33, 95% CI: 1.19, 1.48, P<0.0001; model 2: OR: 1.24, 95% CI: 1.09, 1.40, P=0.0022). Multivariable linear regression analyses were conducted to explore the association between VAI index and total testosterone level, showing a negative association between them in all models (crude model: β: −19.31, 95% CI: −26.16, −12.46, P<0.0001; model 1: β: −19.09, 95% CI: −25.62, −12.57, P<0.0001; model 2: β: −11.74, 95% CI: −17.33, −6.15, P<0.0001). All the results in the 3 models remained significant when we grouped the VAI index into 4 quartiles. The smooth-curve fittings were also performed to characterize the nonlinear associations between VAI index and total testosterone level and testosterone deficiency. As shown in Figure 2, the total testosterone level and testosterone deficiency were linearly corelated with the VAI index.
Table 3 displays the subgroup analysis of the association between total testosterone level and VAI index. The negative association between them remained stable among men with (β: −10.578, 95% CI: −17.246, −3.910, P=0.004) or without hypertension (β: −11.845, 95% CI: −20.470, −3.221, P=0.010). Additionally, the relationship also remained stable among men with different BMI status. However, this negative association was only significant in participants without diabetes (β: −9.621, 95% CI: −17.073, −2.170, P=0.014). Figure 3 displays the subgroup analysis of the association between testosterone level and VAI index. The VAI index was positively associated with testosterone deficiency in men without diabetes (OR: 1.247, 95% CI: 1.055–1.476, P=0.013), with hypertension (OR: 1.24, 95% CI: 1.095–1.408, P=0.002), or aged older than 60 years (OR: 1.555, 95% CI: 1.130–2.139).
The ROC analyses are shown in Figure 4 and Table 4. The AUCs (95% CI) for the HOMA-IR, TyG, VAI, FBG, and TG, were 0.74 (0.70–0.77), 0.67 (0.64–0.71), 0.66 (0.63–0.70), 0.63 (0.59–0.67), and 0.64 (0.60–0.67), respectively. Compared to TyG for predicting testosterone deficiency, HOMA-IR showed the highest predictive ability, while VAI had comparable performance similar to TyG (P=0.2700) and better than FBG and TG.
Discussion
To the best of our knowledge, this is the first nationally representative study to explore the associations between VAI index (reflecting the distribution of abdominal fat, and the function of adipose tissue) and total testosterone level and testosterone deficiency in men. Our study revealed that men with higher VAI index displayed decreased total testosterone level (β: −11.74, 95% CI: −17.33, −6.15, P<0.0001) and higher risk of testosterone deficiency (OR: 1.24, 95% CI: 1.09, 1.40, P=0.0022). Additionally, the result remained stable when we categorized VAI index into quartiles. During our subgroup analysis, the association was stronger among aged men, obese men, or men without diabetes. Furthermore, the VAI displayed comparable predictive performance to TyG index (0.66 vs 0.67, P=0.27) for testosterone deficiency, but not better than that of the HOMA-IR index.
Generally, obesity can be divided into overall and abdominal obesity [34]. Different obesity phenotypes can lead to different clinical diseases [35]. Previous studies indicated that obesity was negatively related to testosterone deficiency by BMI [36]. However, BMI can only be used to reflect overall obesity, and was not a better indicator for participants with simple abdominal obesity, considering the phenomenon of “normal-weight obesity”. Therefore, the WC indicator was used to evaluate the effects of abdominal obesity on the sex hormone, while the results were inconsistent for adult and adolescents[20,37]. The controversial outcomes may result from the inability of WC to discriminate between visceral and subcutaneous fat in the abdominal region [38]. A recent study reported that approximate 51% of overweight and 32% of obese adults are metabolically healthy, and adults with metabolic disturbance are not generally obese [39]. As a result, it is confusing for andrologists to use these simple indicators to explore the associations between obesity and testosterone deficiency, and it is better to accurately assess visceral adiposity because visceral fat is more metabolically deleterious than subcutaneous fat [40,41]. Before the VAI index for visceral adipose, magnetic resonance imaging (MRI) and computed tomography (CT) were used to precisely measure visceral fat, as recommended by the International Diabetes Federation [42]. However, these imaging methods were not universally utilized in clinics owing to high cost and low efficacy.
The VAI index, calculated based on anthropometric indicators (BMI and WC) and biochemical metabolic indicators (TG, HDL-c) has been considered as a surrogate marker of both visceral fat distribution and function, and was positively associated with the visceral adipose tissue measured by MRI [23]. Another study showed that the VAI could replace visceral CT scanning for visceral adiposity [43]. Additionally, many studies have been performed to explore the associations between VAI index and clinical diseases, including cardiovascular diseases, diabetes, chronic kidney disease, and even hepatic steatosis [23,44–46]. For urological diseases, the VAI index was used as an indicator of erectile dysfunction, with a cut-off value of 4.33 (AUC=0.695, 95% CI: 0.622–0.767, Specificity: 73.8%, Sensitivity: 61.2%), and it performed better than BMI and WC alone [47]. A man with VAI index above 4.33 had a higher risk of erectile dysfunction (OR: 1.299,95% CI: 1.123–1.503, P=0.001). A higher VAI index was also related to higher prevalence of kidney stone (OR: 1.13, 95% CI: 1.08–1.19)[48]. Additionally, Cai et al reported that the VAI index might indicate poor prognosis after radical prostatectomy, mainly due to the serum testosterone level [49]. In our study, the ROC result displayed that the VAI index had good predictive performance for testosterone deficiency, which agrees with other published studies [50,51].
A cross-sectional study that enrolled 355 patients with type 2 diabetes found that the testosterone level in men was significantly negatively associated with BMI and waist circumference [52]. They also concluded that the visceral obesity, evaluated by BMI and waist circumference, was an important cause of insulin resistance, and their results are in agreement with other studies in men [53]. Another study, conducted by Liu et al, found that the lipid accumulation products (LAP) index was related to testosterone deficiency [54]. Actually, the LAP index was also an indicator of excessive abdominal fat accumulation combining the TG with WC; their result showed that with each unit increase in LAP, testosterone levels decreased by 0.49 (β=−0.49, 95% CI [−0.77, −0.22],
The potential mechanisms underlying the association between VAI index and testosterone deficiency are as follows. Firstly, most circulating estradiol is derived from testosterone via aromatase [55], and a substantial amount of aromatase located in visceral adipose tissue may ultimately catalyze the aromatization of testosterone into estradiol. Then, elevated estradiol may activate the hypothalamic estrogen receptors to inhibit the HPG axis [56]. Treatment with aromatase inhibitors may normalize the serum total testosterone in obese men [57]. Secondly, visceral adipocytes secret adipose-specific cytokines, such as leptin, IL-6, and TNF-α, and these cytokines may increase insulin resistance [58], and the insulin resistance would induce DAX-1 expression in Leydig cells and inhibit steroidogenesis, ultimately resulting in reduced secretion of testosterone [59]. Thirdly, a higher VAI index is also an indicator of adipose tissue dysfunction and dyslipidemia, which also could promote secretion of the aforementioned pro-inflammatory cytokines [11]. Additionally, increased expression and secretion of adipokine in adipocytes also participates in the process of decreasing testosterone secretion [60].
When comparing the predictive performance of different indicators, we found that the VAI index showed comparable ability to the TyG index for testosterone deficiency (0.66 vs 0.67,
Interestingly, the positive association between VAI index and testosterone deficiency remained significant only in men aged than 60 years (OR: 1.56, 95% CI: 1.13–2.14,
Our study demonstrated that the VAI index reliably predicted testosterone deficiency, as did the TyG index. Compared to the TyG index derived from fasting glucose and triglyceride, the VAI index combines anthropometric indicators (BMI, WC) with lipid profiles, so we should not ignore use of the VAI index in predicting testosterone deficiency. Our study has several limitations that limit interpretation of our results. Firstly, owing to the cross-sectional nature of the NHANES, it is impossible to conclude that there is a causal association between VAI index and testosterone deficiency. Second, testosterone deficiency was diagnosed only based on serum testosterone. Actually, testosterone deficiency, also named hypogonadism, should include serum total testosterone, combined with a series of related symptoms (eg, depression, diminished libido) [6]. Lastly, not all participants had VAI index values available, which may have caused selection bias. However, our study also has several strengths. Firstly, this study included the largest number of samples on the present topic. Secondly, we adjusted for confounding covariables to reduce confounding bias, and also simultaneously conducted linear regression and logistic regression analyses. Furthermore, subgroup analyses were also conducted to identify sensitive populations. Our results should be confirmed by further well-designed studies. In the future, randomized controlled studies should be conducted to evaluate the effect of weight loss on testosterone level, and a basic study should be conducted to explore potential mechanisms underlying the association between visceral adiposity and testosterone deficiency.
Conclusions
The present study indicated there was a strong association between VAI index and testosterone level in U.S. noninstitutionalized adult men. Men with a higher VAI index have a higher risk of declined testosterone level, or even testosterone deficiency, especially in aged men, or men without diabetes. The predictive performance of VAI index for testosterone deficiency was comparable to the TyG index but worse than the HOMA-IR index. In the future, more well-designed clinical studies should be performed to validate this association, and basic studies should be conducted to explore the underlying mechanisms between them.
Figures
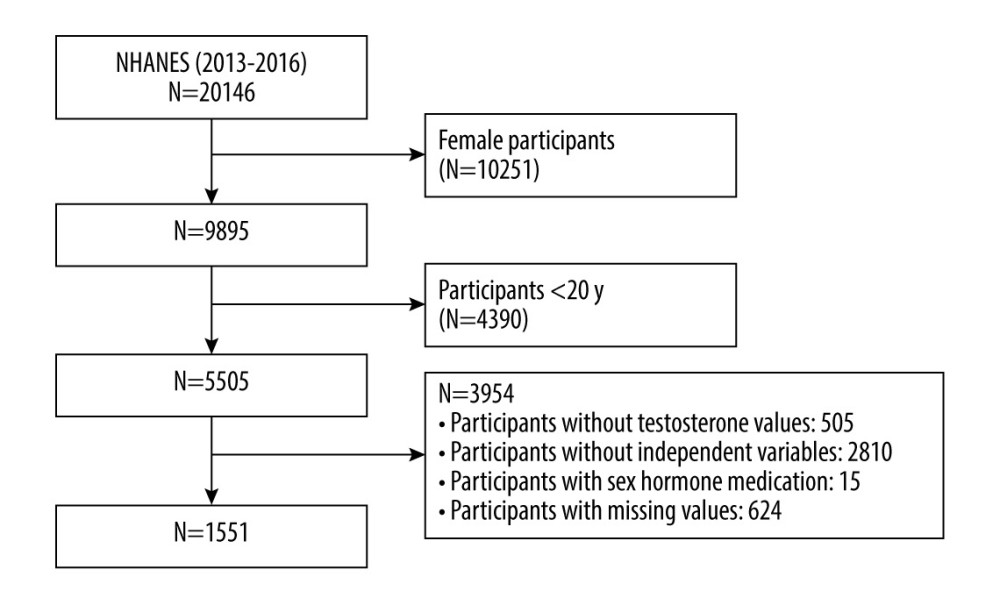 Figure 1. Flow chart of the participants selection process. The figure was created using Word 2010 and Adobe Photoshop 2020 software.
Figure 1. Flow chart of the participants selection process. The figure was created using Word 2010 and Adobe Photoshop 2020 software. 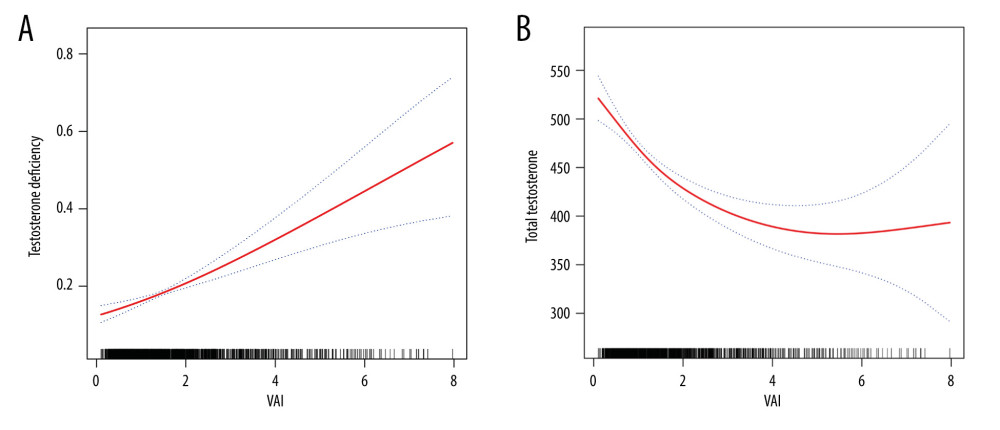 Figure 2. (A, B) Smooth curve fittings between the VAI index and testosterone level and testosterone deficiency. The figure was created using EmpowerStats (www.empowerstats.com; X & Y solutions, Inc., Boston MA) software and Adobe Photoshop 2020 software.
Figure 2. (A, B) Smooth curve fittings between the VAI index and testosterone level and testosterone deficiency. The figure was created using EmpowerStats (www.empowerstats.com; X & Y solutions, Inc., Boston MA) software and Adobe Photoshop 2020 software. 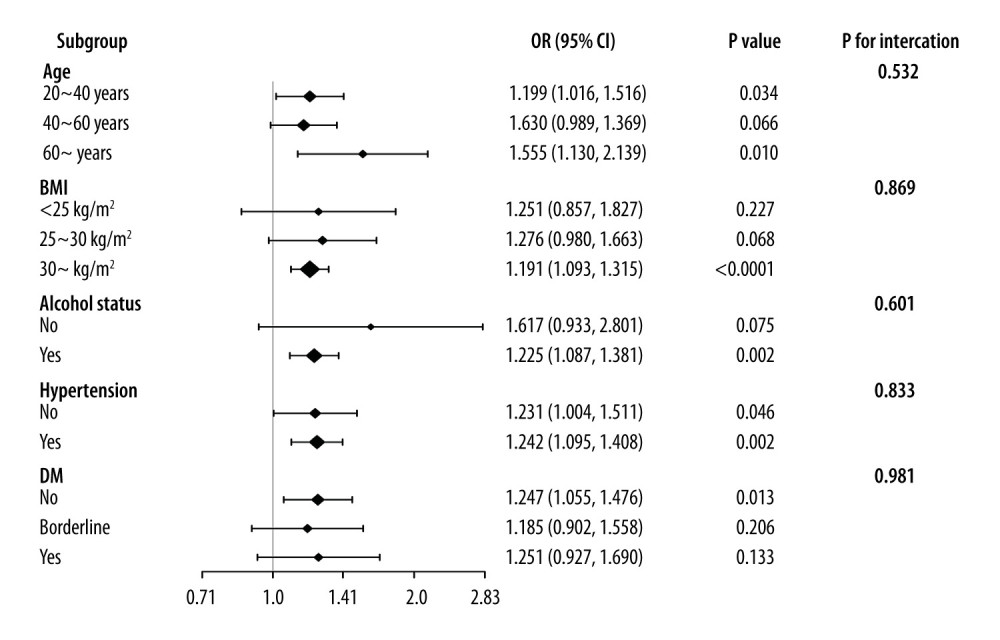 Figure 3. Subgroup analysis of the associations between VAI index and testosterone deficiency. The figure was created using EmpowerStats (www.empowerstats.com; X & Y solutions, Inc., Boston MA) software.
Figure 3. Subgroup analysis of the associations between VAI index and testosterone deficiency. The figure was created using EmpowerStats (www.empowerstats.com; X & Y solutions, Inc., Boston MA) software. 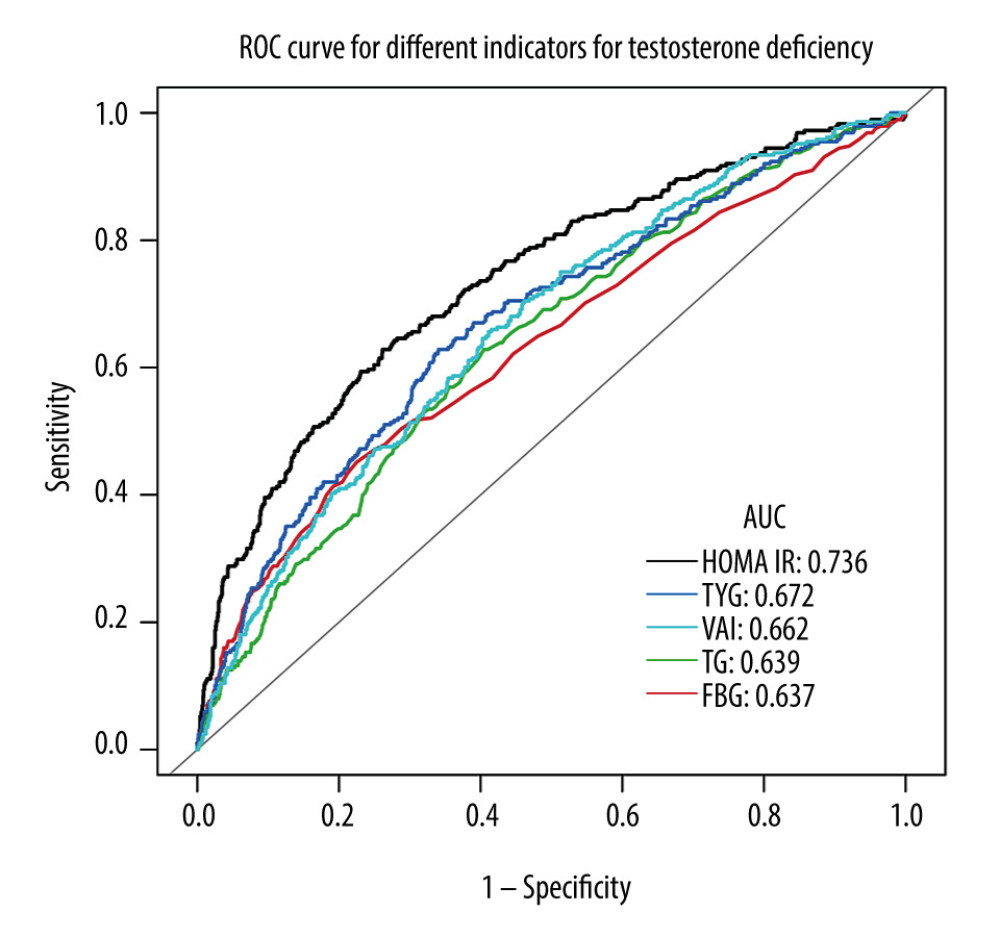 Figure 4. ROC curve analysis for different indicators in predicting testosterone deficiency. The figure was created using EmpowerStats (www.empowerstats.com; X & Y solutions, Inc., Boston MA) software.
Figure 4. ROC curve analysis for different indicators in predicting testosterone deficiency. The figure was created using EmpowerStats (www.empowerstats.com; X & Y solutions, Inc., Boston MA) software. Tables
Table 1. Baseline characteristics of enrolled participants classified by VAI quartiles.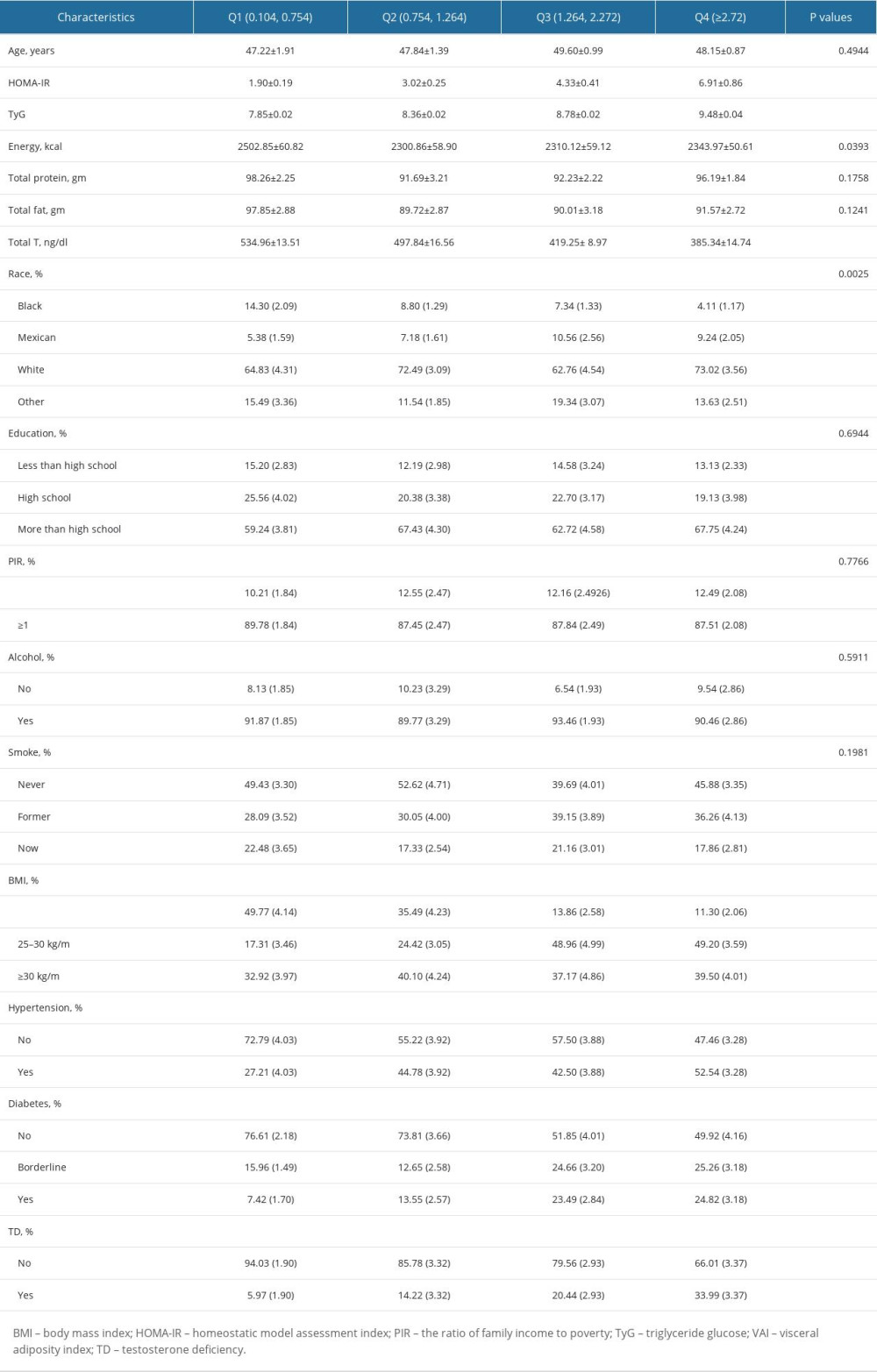 Table 2. Association between VAI index and testosterone level and testosterone deficiency.
Table 2. Association between VAI index and testosterone level and testosterone deficiency.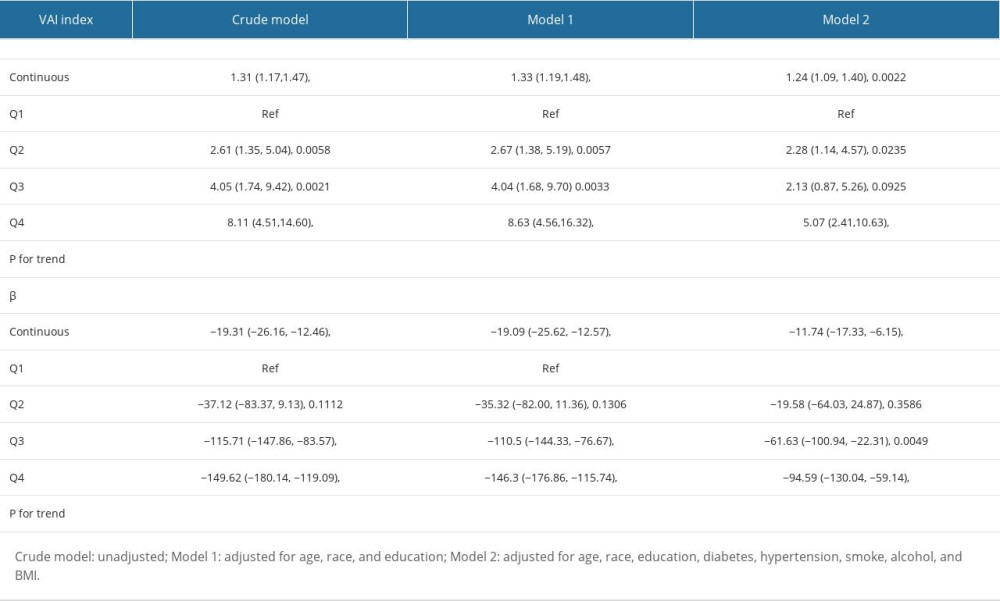 Table 3. Subgroup analysis of the association between VAI index and testosterone level.
Table 3. Subgroup analysis of the association between VAI index and testosterone level.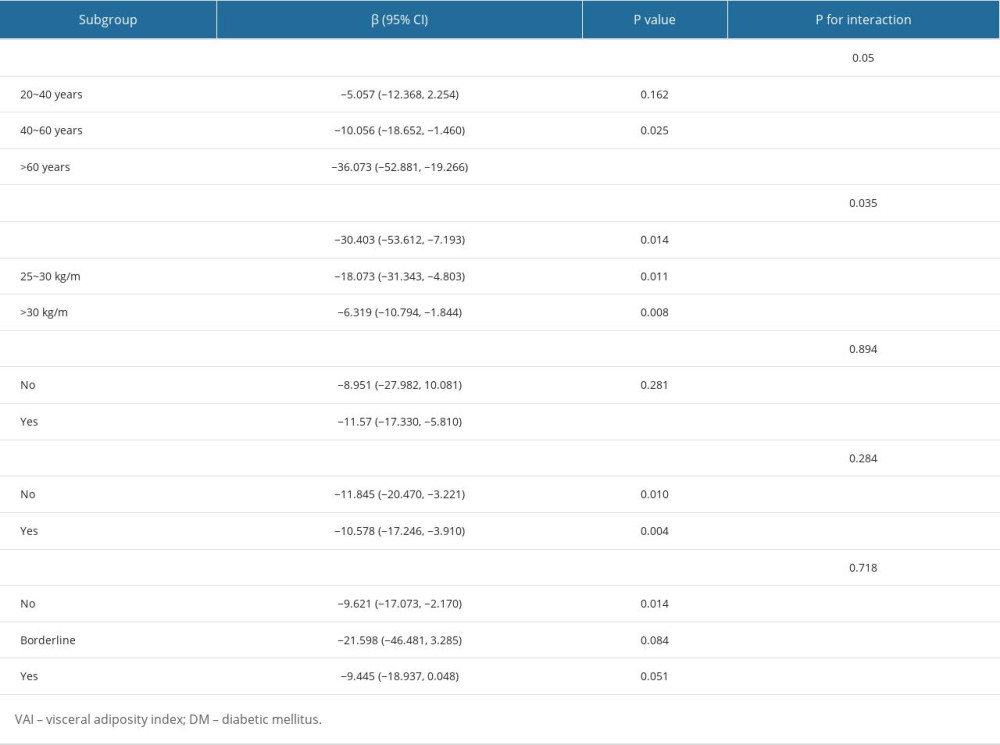 Table 4. Comparison of different indicators for predicting testosterone deficiency.
Table 4. Comparison of different indicators for predicting testosterone deficiency.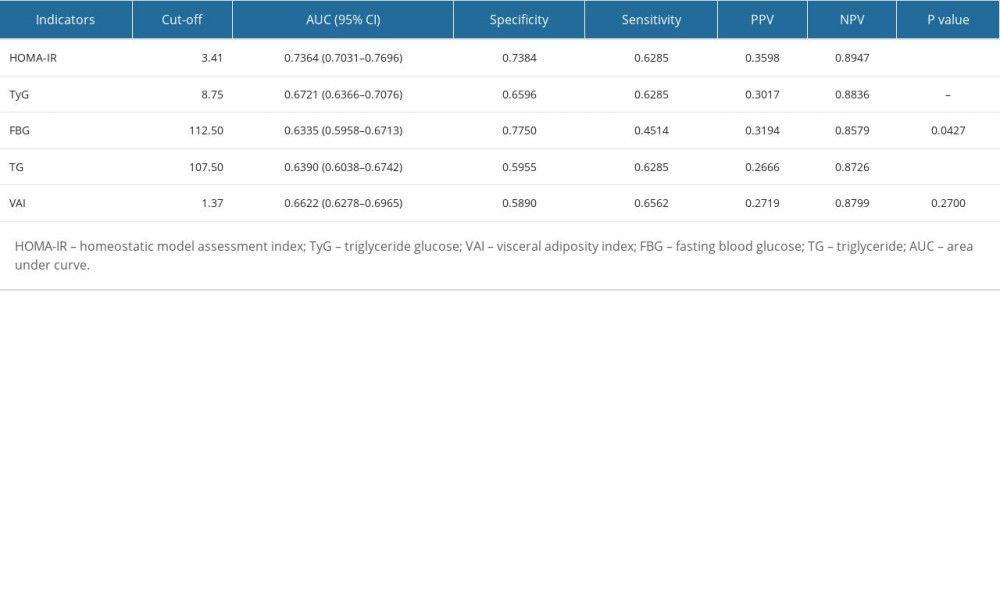
References
1. Muller MN, Testosterone and reproductive effort in male primates: Horm Behav, 2017; 91; 36-51
2. Iliescu R, Reckelhoff JF, Testosterone and vascular reactivity: Clin Sci (Lond), 2006; 111(4); 251-52
3. Kaufman JM, Lapauw B, Role of testosterone in cognition and mobility of aging men: Andrology, 2020; 8(6); 1567-79
4. Zito S, Nosari G, Pigoni A, Association between testosterone levels and mood disorders: A minireview: J Affect Disord, 2023; 330; 48-56
5. Traish AM, Miner MM, Morgentaler A, Zitzmann M, Testosterone deficiency: Am J Med, 2011; 124(7); 578-87
6. Mulhall JP, Trost LW, Brannigan RE, Evaluation and management of testosterone deficiency: AUA Guideline: J Urol, 2018; 200(2); 423-32
7. Fernandez CJ, Chacko EC, Pappachan JM, Male obesity-related secondary hypogonadism – pathophysiology, clinical implications and management: Eur Endocrinol, 2019; 15(2); 83-90
8. Morgentaler A, Testosterone deficiency and cardiovascular mortality: Asian J Androl, 2015; 17(1); 26-31
9. Kruljac M, Finnbogadóttir H, Bobjer J, Symptoms of sexual dysfunction among men from infertile couples: Prevalence and association with testosterone deficiency: Andrology, 2020; 8(1); 160-65
10. Pintana H, Chattipakorn N, Chattipakorn S, Testosterone deficiency, insulin-resistant obesity and cognitive function: Metab Brain Dis, 2015; 30(4); 853-76
11. Zitzmann M, Testosterone deficiency, insulin resistance and the metabolic syndrome: Nat Rev Endocrinol, 2009; 5(12); 673-81
12. Kelly DM, Jones TH, Testosterone and obesity: Obes Rev, 2015; 16(7); 581-606
13. Carrageta DF, Oliveira PF, Alves MG, Monteiro MP, Obesity and male hypogonadism: Tales of a vicious cycle: Obes Rev, 2019; 20(8); 1148-58
14. Zumoff B, Strain GW, Miller LK, Plasma free and non-sex-hormone-binding-globulin-bound testosterone are decreased in obese men in proportion to their degree of obesity: J Clin Endocrinol Metab, 1990; 71(4); 929-31
15. Hofstra J, Loves S, van Wageningen B, High prevalence of hypogonadotropic hypogonadism in men referred for obesity treatment: Neth J Med, 2008; 66(3); 103-9
16. Tong Y, Xu S, Huang L, Chen C, Obesity and insulin resistance: Pathophysiology and treatment: Drug Discov Today, 2022; 27(3); 822-30
17. Pitteloud N, Hardin M, Dwyer AA, Increasing insulin resistance is associated with a decrease in Leydig cell testosterone secretion in men: J Clin Endocrinol Metab, 2005; 90(5); 2636-41
18. Pivonello R, Menafra D, Riccio E, Metabolic disorders and male hypogonadotropic hypogonadism: Front Endocrinol (Lausanne), 2019; 10; 345
19. Jensen TK, Andersson AM, Jørgensen N, Body mass index in relation to semen quality and reproductive hormones among 1,558 Danish men: Fertil Steril, 2004; 82(4); 863-70
20. Wang ZY, Li BC, Xing JJ, Associations of waist circumference with sex steroid hormones among 4031 USA children and adolescents: Asian J Androl, 2022 Online ahead of print
21. Omran F, Christian M, Inflammatory signaling and brown fat activity: Front Endocrinol (Lausanne), 2020; 11; 156
22. Yang SJ, Li HR, Zhang WH, Visceral Fat Area (VFA) superior to BMI for predicting postoperative complications after radical gastrectomy: A prospective cohort study: J Gastrointest Surg, 2020; 24(6); 1298-306
23. Amato MC, Giordano C, Galia M, Visceral adiposity index: A reliable indicator of visceral fat function associated with cardiometabolic risk: Diabetes Care, 2010; 33(4); 920-22
24. Kouli GM, Panagiotakos DB, Kyrou I, Visceral adiposity index and 10-year cardiovascular disease incidence: The ATTICA study: Nutr Metab Cardiovasc Dis, 2017; 27(10); 881-89
25. Yu Y, Zhang FL, Yan XL, Visceral adiposity index and cervical arterial atherosclerosis in northeast China: A population based cross-sectional survey: Eur J Neurol, 2021; 28(1); 161-71
26. Lei J, Luo Y, Xie Y, Wang X, Visceral adiposity index is a measure of the likelihood of developing depression among adults in the United States: Front Psychol, 2022; 13; 772556
27. Liu N, Luo X, Li P, Xiong W, The triglycerides and glucose index is not superior to HOMA-IR in predicting testosterone deficiency among adult males: Andrology, 2023; 11(2); 215-24
28. Amato MC, Giordano C, Visceral adiposity index: An indicator of adipose tissue dysfunction: Int J Endocrinol, 2014; 2014; 730827
29. Sánchez-García A, Rodríguez-Gutiérrez R, Mancillas-Adame L, Diagnostic accuracy of the triglyceride and glucose index for insulin resistance: A systematic review: Int J Endocrinol, 2020; 2020; 4678526
30. Matthews DR, Hosker JP, Rudenski AS, Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man: Diabetologia, 1985; 28(7); 412-19
31. Bawadi H, Abouwatfa M, Alsaeed S, Body shape index is a stronger predictor of diabetes: Nutrients, 2019; 11(5); 1018
32. Liu N, Ma F, Feng Y, Ma X, The association between the dietary inflammatory index and thyroid function in U.S. adult males: Nutrients, 2021; 13(10); 3330
33. Johnson CL, Paulose-Ram R, Ogden CL, National health and nutrition examination survey: Analytic guidelines, 1999-2010: Vital Health Stat, 2013; 2(161); 1-24
34. Zhang X, Sun Y, Li Y, Association between visceral adiposity index and heart failure: A cross-sectional study: Clin Cardiol, 2023; 46(3); 310-19
35. Després JP, Lemieux I, Abdominal obesity and metabolic syndrome: Nature, 2006; 444(7121); 881-87
36. Gurayah AA, Mason MM, Masterson JM, U-shaped association between prevalence of secondary hypogonadism and body mass index: A retrospective analysis of men with testosterone deficiency: Int J Impot Res, 2023; 35(4); 374-77
37. Seyfart T, Friedrich N, Kische H, Association of sex hormones with physical, laboratory, and imaging markers of anthropometry in men and women from the general population: PLoS One, 2018; 13(1); e0189042
38. Kelley DE, Thaete FL, Troost F, Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance: Am J Physiol Endocrinol Metab, 2000; 278(5); E941-48
39. Wildman RP, Muntner P, Reynolds K, The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: Prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004): Arch Intern Med, 2008; 168(15); 1617-24
40. Smith JD, Borel AL, Nazare JA, Visceral adipose tissue indicates the severity of cardiometabolic risk in patients with and without type 2 diabetes: Results from the INSPIRE ME IAA study: J Clin Endocrinol Metab, 2012; 97(5); 1517-25
41. Liu J, Fox CS, Hickson DA, Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: The Jackson Heart Study: J Clin Endocrinol Metab, 2010; 95(12); 5419-26
42. Alberti KG, Zimmet P, Shaw J, Metabolic syndrome – a new world-wide definition. A consensus statement from the International Diabetes Federation: Diabet Med, 2006; 23(5); 469-80
43. Oh JY, Sung YA, Lee HJ, The visceral adiposity index as a predictor of insulin resistance in young women with polycystic ovary syndrome: Obesity (Silver Spring), 2013; 21(8); 1690-94
44. Wang J, Jin X, Chen K, Visceral adiposity index is closely associated with urinary albumin-creatinine ratio in the Chinese population with prediabetes: Diabetes Metab Res Rev, 2021; 37(7); e3424
45. Murai J, Nishizawa H, Otsuka A, Low muscle quality in Japanese type 2 diabetic patients with visceral fat accumulation: Cardiovasc Diabetol, 2018; 17(1); 112
46. Choi MH, Choi JI, Park MY, Validation of intimate correlation between visceral fat and hepatic steatosis: Quantitative measurement techniques using CT for area of fat and MR for hepatic steatosis: Clin Nutr, 2018; 37(1); 214-22
47. Bolat MS, Kocamanoglu F, Ozbek ML, Can high visceral adiposity index be a risk factor for sexual dysfunction in sexually active men?: J Sex Med, 2020; 17(10); 1926-33
48. Hou B, Shen X, He Q, Is the visceral adiposity index a potential indicator for the risk of kidney stones?: Front Endocrinol (Lausanne), 2022; 13; 1065520
49. Qu YY, Dai B, Kong YY, Influence of obesity on localized prostate cancer patients treated with radical prostatectomy: Asian J Androl, 2013; 15(6); 747-52
50. Rotter I, Rył A, Grzesiak K, Cross-sectional inverse associations of obesity and fat accumulation indicators with testosterone in non-diabetic aging men: Int J Environ Res Public Health, 2018; 15(6); 1207
51. Gouda SI, Aboelnaga MM, Elbeltagy AMG, Elbaz A, Testosterone deficiency in non-obese type 2 diabetic male patients: Arch Ital Urol Androl, 2022; 94(4); 464-69
52. Kapoor D, Aldred H, Clark S, Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: Correlations with bioavailable testosterone and visceral adiposity: Diabetes Care, 2007; 30(4); 911-17
53. Svartberg J, Jenssen T, Sundsfjord J, Jorde R, The associations of endogenous testosterone and sex hormone-binding globulin with glycosylated hemoglobin levels, in community dwelling men. The Tromsø Study: Diabetes Metab, 2004; 30(1); 29-34
54. Liu Q, Huang Y, Wang M, Association of lipid accumulation products with testosterone deficiency in adult American men: A cross-sectional study: Andrology, 2023; 11(3); 551-60
55. Hemsell DL, Grodin JM, Brenner PF, Plasma precursors of estrogen. II. Correlation of the extent of conversion of plasma androstenedione to estrone with age: J Clin Endocrinol Metab, 1974; 38(3); 476-79
56. Dursun M, Besiroglu H, Cakir SS, Increased visceral adiposity index associated with sexual dysfunction in men: Aging Male, 2018; 21(3); 187-92
57. Loves S, Ruinemans-Koerts J, de Boer H, Letrozole once a week normalizes serum testosterone in obesity-related male hypogonadism: Eur J Endocrinol, 2008; 158(5); 741-47
58. Makki K, Froguel P, Wolowczuk I, Adipose tissue in obesity-related inflammation and insulin resistance: Cells, cytokines, and chemokines: ISRN Inflamm, 2013; 2013; 139239
59. Ahn SW, Gang GT, Kim YD, Insulin directly regulates steroidogenesis via induction of the orphan nuclear receptor DAX-1 in testicular Leydig cells: J Biol Chem, 2013; 288(22); 15937-46
60. Lanfranco F, Zitzmann M, Simoni M, Nieschlag E, Serum adiponectin levels in hypogonadal males: Influence of testosterone replacement therapy: Clin Endocrinol (Oxf), 2004; 60(4); 500-7
61. Jiang K, Luan H, Pu X, Association between visceral adiposity index and insulin resistance: A cross-sectional study based on US Adults: Front Endocrinol (Lausanne), 2022; 13; 921067
62. Giagulli VA, Castellana M, Lisco G, Triggiani V, Critical evaluation of different available guidelines for late-onset hypogonadism: Andrology, 2020; 8(6); 1628-41
Figures
 Figure 1. Flow chart of the participants selection process. The figure was created using Word 2010 and Adobe Photoshop 2020 software.
Figure 1. Flow chart of the participants selection process. The figure was created using Word 2010 and Adobe Photoshop 2020 software. Figure 2. (A, B) Smooth curve fittings between the VAI index and testosterone level and testosterone deficiency. The figure was created using EmpowerStats (www.empowerstats.com; X & Y solutions, Inc., Boston MA) software and Adobe Photoshop 2020 software.
Figure 2. (A, B) Smooth curve fittings between the VAI index and testosterone level and testosterone deficiency. The figure was created using EmpowerStats (www.empowerstats.com; X & Y solutions, Inc., Boston MA) software and Adobe Photoshop 2020 software. Figure 3. Subgroup analysis of the associations between VAI index and testosterone deficiency. The figure was created using EmpowerStats (www.empowerstats.com; X & Y solutions, Inc., Boston MA) software.
Figure 3. Subgroup analysis of the associations between VAI index and testosterone deficiency. The figure was created using EmpowerStats (www.empowerstats.com; X & Y solutions, Inc., Boston MA) software. Figure 4. ROC curve analysis for different indicators in predicting testosterone deficiency. The figure was created using EmpowerStats (www.empowerstats.com; X & Y solutions, Inc., Boston MA) software.
Figure 4. ROC curve analysis for different indicators in predicting testosterone deficiency. The figure was created using EmpowerStats (www.empowerstats.com; X & Y solutions, Inc., Boston MA) software. Tables
 Table 1. Baseline characteristics of enrolled participants classified by VAI quartiles.
Table 1. Baseline characteristics of enrolled participants classified by VAI quartiles. Table 2. Association between VAI index and testosterone level and testosterone deficiency.
Table 2. Association between VAI index and testosterone level and testosterone deficiency. Table 3. Subgroup analysis of the association between VAI index and testosterone level.
Table 3. Subgroup analysis of the association between VAI index and testosterone level. Table 4. Comparison of different indicators for predicting testosterone deficiency.
Table 4. Comparison of different indicators for predicting testosterone deficiency. Table 1. Baseline characteristics of enrolled participants classified by VAI quartiles.
Table 1. Baseline characteristics of enrolled participants classified by VAI quartiles. Table 2. Association between VAI index and testosterone level and testosterone deficiency.
Table 2. Association between VAI index and testosterone level and testosterone deficiency. Table 3. Subgroup analysis of the association between VAI index and testosterone level.
Table 3. Subgroup analysis of the association between VAI index and testosterone level. Table 4. Comparison of different indicators for predicting testosterone deficiency.
Table 4. Comparison of different indicators for predicting testosterone deficiency. In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








