25 August 2023: Clinical Research
Exploring the Impact of Positional Sleep Apnea in a Turkish Population: Unveiling the Untold Story
Makbule Ozlem AkbayDOI: 10.12659/MSM.941425
Med Sci Monit 2023; 29:e941425
Abstract
BACKGROUND: The present study aimed to investigate the risk factors associated with demographical, clinical and polysomnographic features of positional sleep apnea through different criterion of positional sleep apnea (POSA vs e-POSA) in a large patient cohort from a tertiary referral center
MATERIAL AND METHODS: A total of 782 OSA patients who were further diagnosed with POSA (total: n=470, e-POSA: n=204) or non-POSA (n=312) based on apnea-hypopnea index (AHI) events by overnight polysomnography were included. Demographical, clinical, and polysomnographic characteristics were recorded, while independent predictors of POSA and e-POSA were determined via linear regression analysis.
RESULTS: Severe OSA (AHI ≥30/h) was less common in the POSA (33.4% vs 71.5%, P<0.001) and e-POSA (9.8% vs 62.3%, P<0.001) groups than in the non-POSA and non-e-POSA groups, respectively. For POSA and e-POSA, male sex (OR 2.195, P<0.001 and OR 2.021, P=0.004, respectively), low body mass index (BMI; OR 0.932, P<0.001 and OR 0.948, P=0.006), low AHI (OR 0.954 and OR 0.902, P<0.001 for each), and less desaturation (T90%, OR 0.972 and OR 0.968, P<0.001 for each) were the common statistically significant predictors. Younger age was an independent predictor of POSA (OR 0.97, P=0.003). POSA (median 20.4 s) and e-POSA (20.5 s) groups demonstrated similar apnea-hypopnea durations (min) as the non-POSA (median 21.1 s) group.
CONCLUSIONS: Our findings revealed that male sex and lower values of BMI, AHI, and desaturation were common determinants of POSA and e-POSA, while younger age independently predicted POSA. POSA and e-POSA had similar clinical and polysomnographic characteristics and shared the unvaried hypoxic burden.
Keywords: Sleep Apnea Syndromes, Humans, Male, Hypoxia, Linear Models, Polysomnography, Ubiquitin-Protein Ligases, Sleep apnea, obstructive
Background
Obstructive sleep apnea (OSA) is a common but often underdiagnosed sleep disorder, which leads to a significant health burden when left untreated through its associations with cardiometabolic diseases and impaired quality of life [1–4].
Patients with suspected OSA usually present with excessive daytime sleepiness, loud snoring, gasping, choking, or breathing cessation during sleep. The STOP-BANG questionnaire is one of the most widely accepted screening tools for OSA, while nighttime in-laboratory level 1 polysomnography (PSG) is the criterion standard test for the diagnosis [5].
Sleeping position has been defined as an important factor affecting the severity of OSA, and a specific clinical definition for positional obstructive sleep apnea (POSA) has been developed, which considers the different characteristics and need for different treatment strategies for this patient group [6–10].
Different definitions of positional sleep apnea have been developed in regards to the severity of OSA, as defined by the apnea-hypopnea index (AHI) in supine and non-supine positions [2,11]. Accordingly, 3 distinct clinical types of OSA have been defined, namely POSA, exclusive POSA (e-POSA), and non-positional OSA (non-POSA) [2,9,12,13]. POSA is OSA with at least double frequency of breathing abnormalities during supine than during the lateral position, OSA occurring exclusively in the supine position (normalized AHI in non-supine positions) is defined as e-POSA, and non-POSA is defined as a high frequency of breathing abnormalities in all positions [2,12–16].
Continuous positive airway pressure (CPAP) is considered the most effective treatment for eliminating respiratory events in adults with moderate-severe OSA, while its effectiveness is lessened by the decreased use of treatment during sleep and inadequate patient adherence [5]. Cardiovascular comorbidities, excessive daytime sleepiness, and adherence to treatment are the factors that play a significant role in treatment decisions. Lifestyle changes, positional treatment, oral appliances, and surgical treatment are other treatment options. The treatment of OSA is a multi-pronged approach and should be individualized to each patient [5]. The clinical type of OSA considerably alters the selected treatment. Patients with POSA significantly benefit from positional therapy (PT), which is based on the avoidance of sleeping in the supine position via new-generation PT devices, whereas CPAP is the mainstay therapy for those with non-POSA [6,7,16,17].
CPAP has a greater effect than PT on improving AHI in POSA, while PT is better than inactive control for improving Epworth Sleepiness Scale scores and AHI and may have better adherence than CPAP. There are no significant differences between the 2 treatment modalities in terms of other clinically relevant outcomes, such as quality of life or cognitive function [10].
In addition, since the POSA definition addresses a large OSA patient population, the e-POSA definition has been widely preferred for defining patients that would particularly benefit from PT devices and automatic PAP devices [13,14,16]. In this regard, given that PAP adherence remains a major problem in OSA patients, POSA patients treated with CPAP can also have low adherence, and thus may be confronting unwanted cardiometabolic consequences [13,18,19]. In contrast, although they represent young populations with mild OSA severity, e-POSA patients that are exposed to significant hypoxic burden may be underestimated and undertreated [2,7,9,12,13]. Despite the significant differences between positional and non-positional OSA types in terms of clinical manifestations and treatment options, few studies investigated the differences between clinical types of OSA and the risk factors that incite the emergence of POSA, e-POSA, or non-POSA, and findings were often contradictory [2,7–9,12,13,20].
We hypothesized that patients with e-POSA and POSA have similar risk factors and demographical, clinical, and polysomnographic characteristics, including extensive measures of hypoxic burden. Therefore, this retrospective study from a tertiary center in Turkey aimed to evaluate the demographic, clinical, and polysomnographic findings in patients with OSA and to identify factors associated with POSA, e-POSA, and non-POSA.
Material and Methods
ETHICS CONSIDERATIONS:
This study was conducted in accordance with the ethics principles stated in the Declaration of Helsinki and was approved by the University of Health Sciences Istanbul Sureyyapasa Chest Diseases and Thoracic Surgery Training and Research Hospital Ethics Committee (date of approval: 11/08/2022, protocol no: 116.2017.R-249). Written informed consent was obtained from each participant, following a detailed explanation of the objectives and protocol of the study.
STUDY DESIGN AND POPULATION:
A total of 782 patients with OSA (median [min-max] age: 52 [17–87] years, 66.2% male) diagnosed by overnight polysomnography (PSG), based on presence of more than 5 predominantly obstructive sleep AHI events per hour (AHI ≥5/h) during sleep were included in this retrospective study conducted between January 2020 and July 2022. Although 809 patients with suspected OSA were initially enrolled, the final study population included 782 patients, with the exclusion of 27 patients due to incomplete PSG data.
ASSESSMENTS:
The following data were recorded for each patient: data on prevalence of POSA, e-POSA, and non-POSA, patient demographics (age, sex), body mass index (BMI; kg/m2), neck circumference (cm), comorbid diseases, and PSG findings, including Epworth Sleepiness Scale scores, total sleep time (TST; min, total, supine, and lateral), AHI (1/h; total, supine, non-supine, rapid eye movement [REM], non-REM), sleep efficiency (%), sleep onset latency, total wake time, sleep proportion (%; stage 1, stage 2, stage 3, stage REM), heart rate (bpm), minimum oxygen saturation (SaO2,%), mean SaO2 (%), percentage of time spent at oxygen saturation below 90% in TST (T90%), oxygen desaturation index (ODI, 1/h), and mean apnea-hypopnea duration (MAD, s). Baseline characteristics and PSG findings were compared in POSA vs non-POSA groups, as well as in patients with vs without e-POSA. Factors independently associated with POSA and e-POSA were also determined via multivariate logistic regression analysis.
DEFINITIONS USED FOR OSA CLINICAL TYPES:
POSA was defined by OSA with supine/non-supine AHI ratio ≥2, in accordance with Cartwright ’s definition [14]. e-POSA was defined by supine/non-supine AHI ratio ≥2 and non-supine AHI <5/h, in accordance with the definition of Mador et al [15]. As recommended by the American Academy of Sleep Medicine, OSA was classified as mild (AHI: 5–14.9/h), moderate (AHI: 15–29.9/h) and severe (AHI: ≥30/h), based on AHI values [11].
POLYSOMNOGRAPHY:
The full-night PSG (Comet Grass; Astro-Med, Inc., West Warwick, RI, USA) was performed with standard electrodes and sensors, and respiratory events (apnea, hypopnea, AHI) were scored according to the 2017 American Academy of Sleep Medicine criteria [11] by an experienced sleep investigator. Oxygen saturation parameters included ODI, SAT 90%, and minimum and mean values of SaO2 (%). TST, sleep proportion, sleep efficiency, and AHI in the supine and non-supine positions in stages 1, 2, and 3, REM, and non-REM sleep were also collected from PSG. Non-REM AHI was the average apnea and/or hypopnea respiratory events per hour during total NREM sleep. Sleep efficiency was calculated as TST divided by total recording time.
STATISTICAL ANALYSIS:
Statistical analysis was done using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, USA). The chi-square test was used for the comparison of categorical data. The
Results
BASELINE CHARACTERISTICS OVERALL:
In the overall study population, the median patient age was 52 years (range, 17 to 87 years), and 66.2% of patients with OSA were male. Obesity (BMI >30 kg/m2) was evident in 61.9% of patients, while 48.6% of patients had severe OSA (AHI ≥30/h) (Table 1).
Of 782 patients overall, POSA was defined in 470 patients and non-POSA in 312 patients, while 204 patients in the POSA group were considered to have e-POSA. Accordingly, the prevalence of POSA, e-POSA, and non-POSA was determined to be 60.1%, 26.1%, and 39.9%, respectively (Table 1).
BASELINE CHARACTERISTICS IN POSA AND NON-POSA GROUPS:
Compared with the non-POSA group, the POSA group had significantly younger patient age (49(17–78) vs 54(25–87) years, P<0.001), higher percentage of male patients (72.6 vs 56.7%, P<0.001), lower values for neck circumference (41.0 (25.0–50.0) vs 42.0 (27.0–55.0) cm, P=0.019), and BMI (30.1 (19.8–54.7) vs 35.0 (21.6–64.3) kg/m2, P<0.001), as well as a lower rate of class 2–3 obesity (20.1 vs 50.9%, P<0.001) (Table 1).
Mild OSA (AHI 5–14.9/h) was significantly more common (30.6 vs 13.8%, P<0.001) in the POSA group than in the non-POSA group; however, severe OSA (AHI ≥30/h, 33.4 vs 71.5%, P<0.001), co-morbid hypertension (32.2 vs 46.0%, P<0.001), diabetes (16.0 vs 26.3%, P<0.001) and chronic heart failure (CHF, 1.1 vs 3.2%, P=0.034) were less common in the POSA group than in the non-POSA group (Table 1).
BASELINE CHARACTERISTICS IN PATIENTS WITH VS WITHOUT E-POSA:
Patients with e-POSA vs those without e-POSA were significantly younger (48 (17–74) vs 53 (24–87) years, P=0.001) and had significantly lower values for neck circumference (40.0 (31.0–50.0) vs 41.8 (25.0–55.0) cm, P<0.001) and BMI (29.0 (21.1–52.1) vs 33.0 (19.8–64.3) kg/m2, P<0.001), and lower rate of class 2–3 obesity (14.3 vs 38.6%, P<0.001) (Table 2).
Mild OSA (AHI 5–14.9/h) was significantly more common (57.4 vs 12.1%, P<0.001), but severe OSA (AHI values ≥30/h, 9.8 vs 62.3%, P<0.001), co-morbid hypertension (25.5 vs 42.0%, P<0.001), and diabetes (14.3 vs 22.1%, P=0.016) were less common in patients with vs those without e-POSA (Table 2).
PSG FINDINGS IN POSA VS NON-POSA GROUPS:
AHI values, including total, supine, non-supine, REM, and non-REM measurements, were all significantly lower in the POSA group than in the non-POSA group (P<0.001 for each) (Table 3).
Compared with the non-POSA group, the POSA group had significantly higher sleep efficiency (86.2 (47.0–99.5) vs 84.3 (45.0–99.4)%, P=0.034) and higher values for minimum SaO2 (79.0 (32.0–92.0) vs 70.0 (30.0–89.0)%, P<0.001) and mean SaO2 (94.0 (80.0–98.0) vs 93.0 (73.0–99.0)%, P<0.001), and the POSA group had lower heart rate (68.0 (45.0–151.0) vs 72.0 (41.0–160.0) bpm, P=0.001), T90% (6.9 (0.0–100.0) vs 29.4 (0.1–100.0)%, P<0.001), and ODI (23.2 (0.3–197.0 vs 54.4 (4.81–127.6)/h, P<0.001) (Table 3).
The MAD was similar between the non-POSA and POSA groups (median(min–max) 21.1 (1.6–54.5) vs 20.4 (12.2–39.7) s, P=0.107) (Table 3).
PSG FINDINGS IN PATIENTS WITH VS WITHOUT E-POSA:
In patients with e-POSA vs those without e-POSA, total TST (406.8 (246.0–508.0) vs 395.0 (205.0–519.5) min, P=0.007) and TST in supine position (218.00 (32.00–426.50) vs 184.50 (33.50–423.00 min, P<0.001) were significantly higher, whereas TST in the lateral position (191.50 (40.00–420.00) vs 203.75 (33.00–469.50) min, P=0.016) was significantly lower, respectively (Table 4).
AHI values, including total, supine, non-supine, REM, and non-REM measurements, were significantly lower in patients with e-POSA than in those without e-POSA (P<0.001 for each) (Table 4).
Patients with e-POSA vs those without e-POSA had significantly higher sleep efficiency (87.3 (48.0–98.5) vs 84.9 (45.0–99.5)%, P=0.032) and higher values for minimum SaO2 (82.0 (32.0–92.0) vs 72.0 (30.0–91.0)%, P<0.001) and mean SaO2 (95.0 (85.0–98.0) vs 94.0 (73.0–99.0)%, P<0.001), whereas, in patients with e-POSA, there was a lower heart rate (68.0 (48.0–150.0) vs 70.0 (41.0–160.0) bpm, P=0.014), T90% (3.3 (0.0–100.0)% vs 16.9 (0.1–100.0)%, P<0.001) and ODI (13.7 (3.5–197.0) vs 42.6 (0.26–127.6)/h, P<0.001) (Table 4).
The MAD was similar between patients with and without e-POSA (median (min–max) 20.5 (14.2–36.9) vs 20.7 (1.6–54.5) s, P=0.458), respectively (Table 4).
LOGISTIC REGRESSION ANALYSIS FOR FACTORS INDEPENDENTLY ASSOCIATED WITH POSA AND E-POSA:
Male sex (OR 2.195, 95% CI 1.484–3.248, P<0.001), younger age (OR 0.976, 95% CI 0.960–0.991, P=0.003), lower BMI (OR 0.932, 95% CI 0.904–0.961, P<0.001), lower AHI-total (OR 0.954, 95% CI 0.946–0.962, P<0.001), and lower T90% (OR 0.972, 95% CI 0.965–0.979, P<0.001) were found to be independently associated with POSA (Table 5).
Male sex (OR 2.021, 95% CI 1.253–3.261, P=0.004), increase in TST in supine position (OR 1.005, 95% CI 1.002–1.008, P<0.001), lower BMI (OR 0.948, 95% CI 0.913–0.984, P=0.006), lower AHI-total (OR 0.902, 95% CI 0.884–0.9191, P<0.001), and lower T90% (OR 0.968, 95% CI 0.955–0.980, P<0.001) were found to be independently associated with e-POSA (Table 5).
Discussion
Our findings in Turkish patients with OSA revealed that POSA and e-POSA had a prevalence of 60.1% and 26.1%, respectively, and both were associated with younger age, lower rates of obesity, hypertension, and diabetes, and a lower rate of severe OSA (AHI ≥30/h). On PSG, POSA and e-POSA were associated with lower AHI (total, supine, non-supine, REM, non-REM), increased sleep efficiency, higher SaO2, lower heart rate, lower T90%, and lower ODI. The multivariate logistic regression analysis revealed that male sex, lower BMI, lower AHI, and lower T90% were independently associated with POSA and e-POSA, while younger age predicted POSA, and increase in TST in the supine position predicted e-POSA.
Using the definitions of POSA and e-POSA previously defined by Cartwright [12] and Mador et al [15], respectively, POSA and e-POSA were found to have a prevalence of 60.1% and 26.1% in our study population. Other studies across many countries using the similar definitions also reported the prevalence of POSA and e-POSA to range from 53% to 62.3% and from 20.1% to 33.8%, respectively [2,9,12,13,21,22]. In a study by Sabil et al in a French population of OSA patients (n=6437), the prevalence of POSA and e-POSA were reported to be 53.5% and 20.1%, respectively, and both were independently associated with time in supine position, male sex, younger age, lower AHI, and lower BMI [12]. Also, in a study by Heinzer et al in a Swiss population of OSA patients (n=1224), prevalence of POSA and e-POSA was reported to be 75% and 36%, respectively, and both were independently associated with lower AHI and lower BMI [2].
Consistent with previous reports, the presence of POSA and e-POSA in our study was associated with milder OSA (lower AHI), lower neck circumference, lower BMI, and lower rate of obesity, as well as with male sex and younger age [2,7–9,12,16,21–24]. Hence, given its association with younger age, lower BMI, and milder OSA severity, POSA is suggested to represent an early stage of the OSA natural history that can develop in non-POSA with increasing age and weight, emphasizing the loss of the positional variability of the obstructive events as disease severity progresses [2,7,9,12,17,25]. Supporting the likelihood of POSA patients to become non-POSA with increasing BMI [7], reduction in weight after bariatric surgery was reported to be associated with an increase in the prevalence of POSA at the expense of non-POSA [25].
In our patients, both POSA and e-POSA were independently associated with lower AHI (total, supine, non-supine, REM, and non-REM). Higher AHI (total, supine, non-supine, REM, non-REM) in non-POSA than in POSA patients has also been reported in other studies, and is suggested to indicate a higher tendency of airway collapse and a more severe disease among non-POSA patients, regardless of the body position during sleep [7,24,26]. Moreover, our patients in the e-POSA group had significantly higher TST in the supine position and lower time in the lateral position, while an increase in TST in supine position was also independently associated with e-POSA. Likewise, Sabil et al reported that while both POSA and e-POSA patients spent more time on their back than non-POSA patients, a more significant difference was evident for those with e-POSA, indicating that the more time e-POSA patients sleep in the supine position, the more likely they have positional OSA [12]. Hence, our results seem to agree with results that show TST in the supine position is an important determining factor of the overall AHI, suggesting the potential use of PT devices as a therapeutic alternative to CPAP, at least for patients with e-POSA [12,27]. In fact, time spent sleeping in the supine position was also reported to be independently associated with POSA, and PT is considered an effective alternative in the management of POSA, by reducing the TST in supine position [7,13,20]. Individualizing the treatment by differentiating between POSA and non-POSA patients is considered the most effective approach for OSA, while use of the AHI percentage is considered important in finding true POSA patients who can be treated by PT alone, particularly those with mild OSA, or those who can benefit from PT as an adjunctive therapy by decreasing the OSA severity [7,28]. While CPAP adherence is a challenge for all patients with OSA, patients with POSA and e-POSA were found to be significantly less adherent to their CPAP treatment than patients with non-POSA [12,13]. Indeed, both the POSA and e-POSA groups were associated with factors related to poor treatment adherence, such as younger age and low Epworth Sleepiness Scale scores, which further emphasizes the likelihood of these patients not receiving appropriate therapy and thus the consideration of individualized treatment approaches. The efficacy of postural therapy even in severe cases of POSA indicates the importance of determining the phenotype status of OSA, particularly in those who cannot tolerate CPAP therapy [13]. Nonetheless, despite its importance, non-CPAP PT is currently relatively underutilized in clinical practice [7,13,20].
Notably, in a recent study by Oksenberg et al on the conversion of POSA to non-POSA, after an average of 6.6 years, 30% of patients with POSA were reported to have significant changes in BMI and AHI and became non-POSA, while those with no significant changes in these parameters continued to have POSA (70%) [6]. In a study from Switzerland in 292 patients with severe OSA (AHI ≥30), more than one-third of patients were found to be positional patients, and 75.7% of them obtained significant improvement of their OSA severity, while 8.7% became non-POSA after adopting the lateral posture via PT. The remaining 24.3% still had severe OSA, but with decreased severity [8]. Nonetheless, patients with mild-to-moderate POSA using PT should be frequently monitored in terms of treatment adherence and OSA severity, given that their benefit from PT depends on maintenance of good adherence and continues as long as their OSA remains positional [12].
In our POSA and e-POSA groups, patients had higher mean SaO2, lower T90%, and lower ODI, while a lower AHI and T90% were also independently associated with both POSA and e-POSA. These findings are in line with the oxygen saturation findings from previous studies, indicating less severe OSA and nocturnal hypoxia (lower AHI, ODI, T90%, and a higher mean SaO2) in patients with POSA [7,23,28,29]. Moreover, similar to previous studies, sleep efficiency and non-REM stage 3 were significantly higher among our POSA and e-POSA patients, along with higher total TST in e-POSA patients, indicating better sleeping quality and better sleeping quantity as a reflection of milder disease severity among those patients [7,9,12,24].
Positional patients are suggested to have higher parasympathetic activity than non-positional patients, and therefore to have a lower risk for cardiovascular morbidity [30,31]. In our study, hypertension and diabetes were less common in patients with POSA and e-POSA, and heart failure was less common in those with POSA, which is consistent with the previously reported lower prevalence of hypertension and diabetes in POSA [7,26]. Nonetheless, smoking and COPD had no significant impact on POSA or e-POSA, and none of the comorbidities were independently associated with POSA or e-POSA in the multivariate regression analysis. Similarly, some authors also reported no link between POSA and comorbidities, emphasizing that POSA is more likely to be present in relatively less severe OSA [12,13].
The MAD values were similar in our POSA and non-POSA groups as well as in patients with or without e-POSA, which seems to contradict the idea that both POSA and e-POSA are characterized by significantly shorter apneas but a longer duration of all respiratory events, [12]. In fact, while some authors suggested that the association of MAD with the severity of hemodynamic changes is due to apnea-hypopnea [32,33], others suggested that duration of apnea episodes may not be reflected in the AHI and ODI scores, and the lengthening of apnea and hypopnea events may even lead to a decrease in AHI and ODI indices [32]. Given the likelihood that OSA severity differs among patients with similar AHIs, use of novel parameters, such as the MAD, in PSG are suggested to enable more individualized estimations of the severity of OSA [32,34]. Our findings support that the MAD is a valuable tool, reflecting the hypoxic load more accurately than other tools (ie, AHI and ODI). Notably, MAD values in the e-POSA group were similar to those of other groups, emphasizing the likelihood that these patients were also at risk of significant hypoxic load despite concomitantly low values for AHI and ODI. Accordingly, use of the MAD can enable identification of e-POSA patients with significant hypoxic load who would be prone to cardiometabolic hazards of OSA in case of poor treatment adherence [18].
The main strengths of our study were the inclusion of a large population of OSA patients with complete PSG recordings, use of the MAD as a valuable tool reflecting hypoxic load, and multivariate analysis with detailed clinical subtyping in POSA, non-POSA, and e-POSA groups. Major limitations of this study included the large age range (17–87 years) of the patients studied, the cross-sectional and retrospective design, and the lack of information regarding the clinical history of the study participants. The diagnosis of POSA, based on a single-night PSG recoding, seems to be another limitation, given that it is unknown whether or not POSA is a stable night-to-night phenotype. The lack of data on PT interventions was another limitation, which if available, would extend the knowledge achieved in the present study.
Conclusions
In conclusion, our findings revealed that male sex and lower values of BMI, AHI, and desaturation were common determinants of POSA and e-POSA, while younger age independently predicted POSA. POSA, having a high frequency among OSA patients, presents similar clinical and polysomnographic characteristics and associations with e-POSA; thus, a personalized treatment approach in terms of positional therapies or different PAP modalities is required to increase treatment adherence. Accordingly, our findings emphasize the importance of improved awareness of clinicians of POSA and e-POSA subtypes in the setting of OSA, given that patients with POSA and e-POSA demonstrate different clinical characteristics than those with non-POSA and are considered likely to benefit from a personalized approach for alternative therapies, such as PT. Additionally, patients in the e-POSA group, sharing the unvaried hypoxic burden of POSA, should be closely monitored for effective treatment in order to prevent unfavorable cardiometabolic consequences of OSA. Future prospective studies performing serial PSG recording are needed to reach a consensus to determine the best definition for POSA and e-POSA phenotypes and thus to better characterize these patients and address the potential utility of PT as an effective cost-efficient and noninvasive treatment option in these patients.
Tables
Table 1. Baseline characteristics overall and in POSA vs non-POSA groups.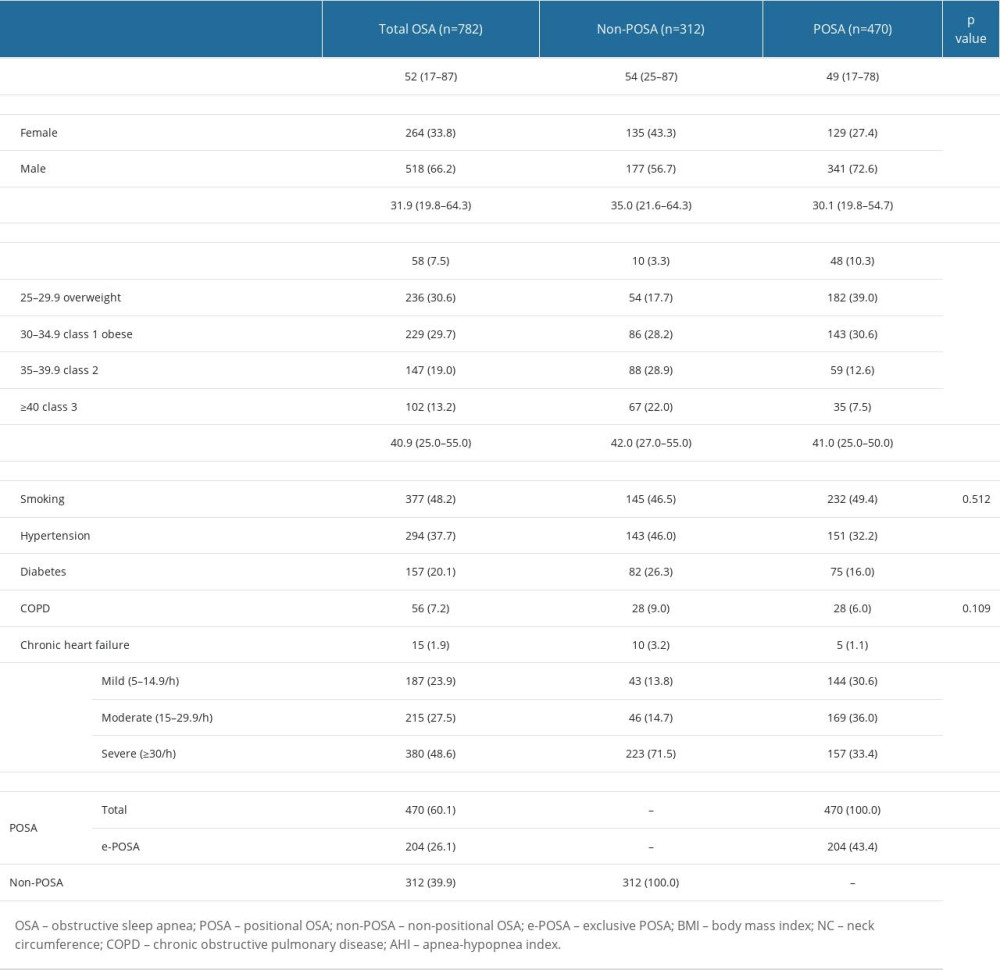 Table 2. Baseline characteristics in OSA patients with vs without e-POSA.
Table 2. Baseline characteristics in OSA patients with vs without e-POSA.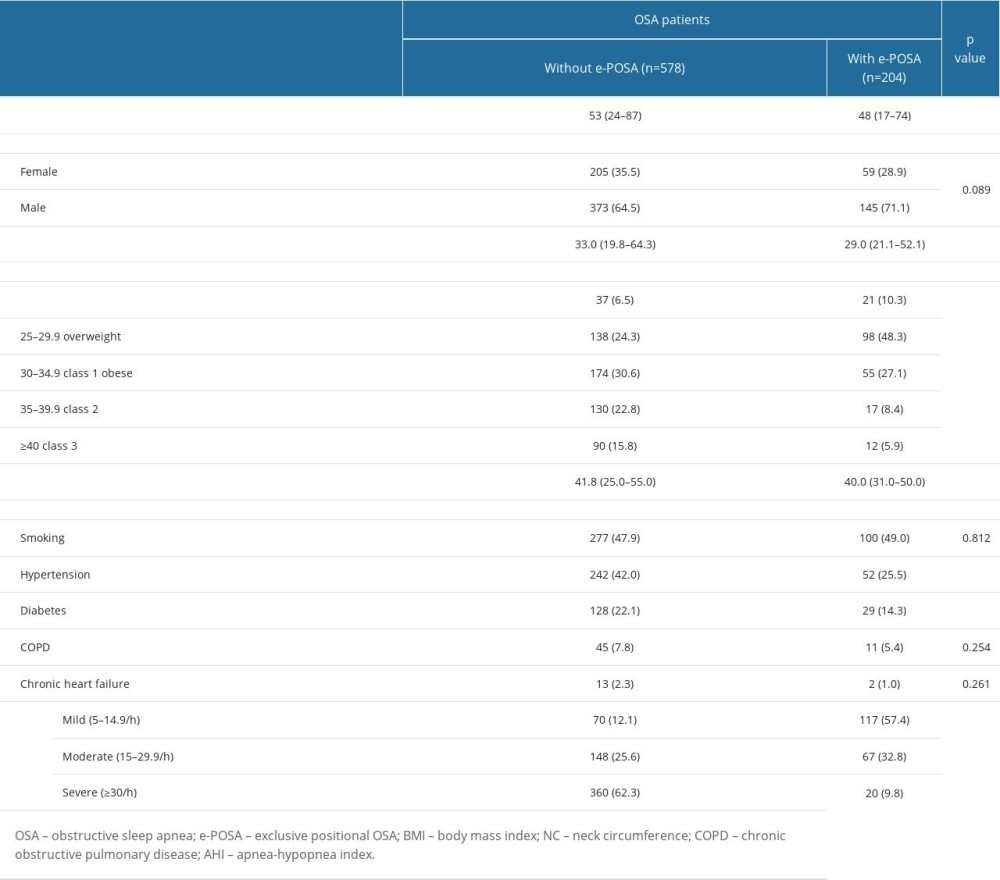 Table 3. Polysomnography findings overall and in POSA vs non-POSA groups.
Table 3. Polysomnography findings overall and in POSA vs non-POSA groups.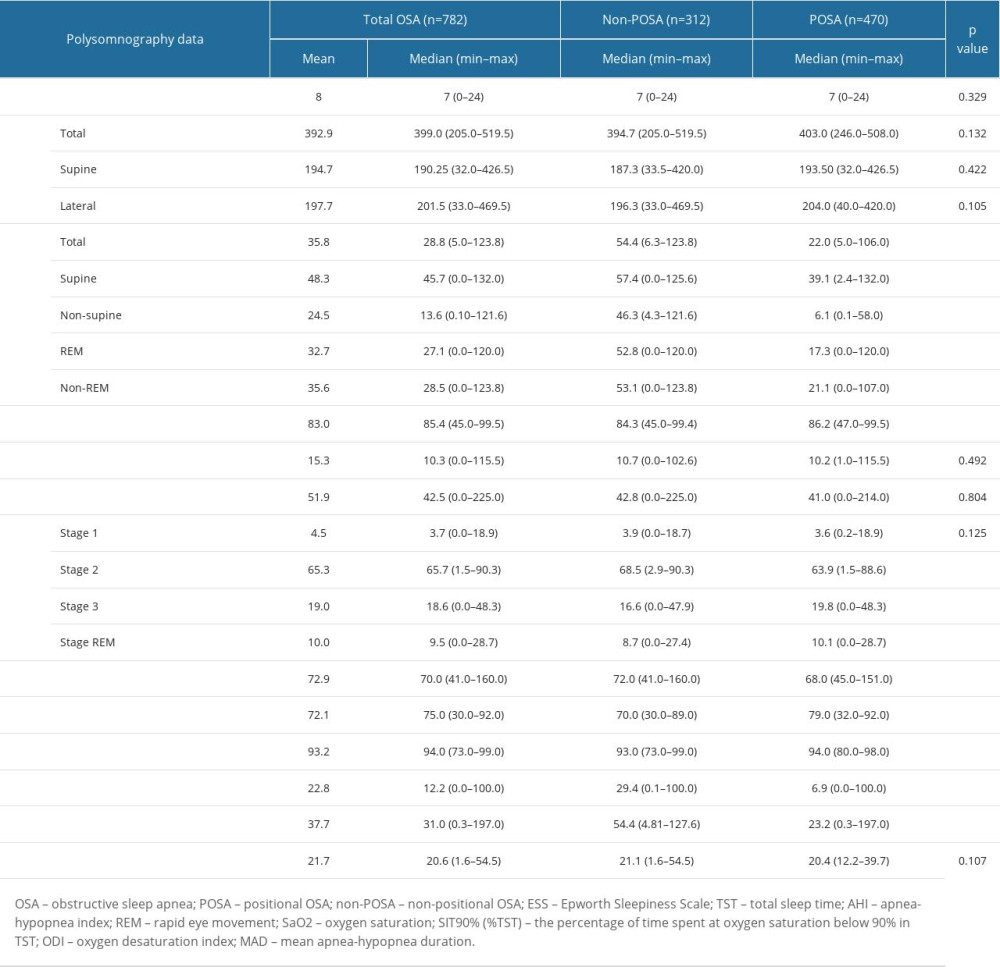 Table 4. Polysomnography findings in OSA patients with vs without e-POSA.
Table 4. Polysomnography findings in OSA patients with vs without e-POSA.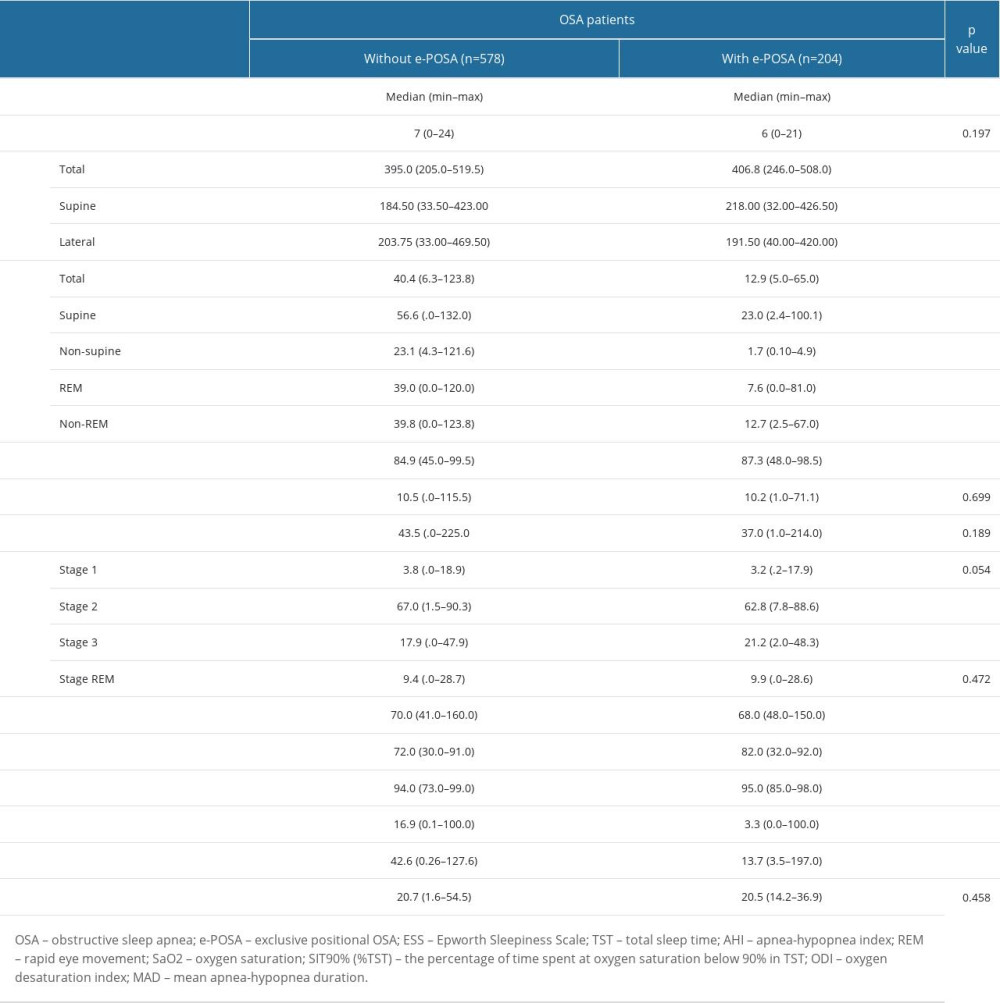 Table 5. Multivariate logistic regression analysis for factors independently associated with POSA and e-POSA.
Table 5. Multivariate logistic regression analysis for factors independently associated with POSA and e-POSA.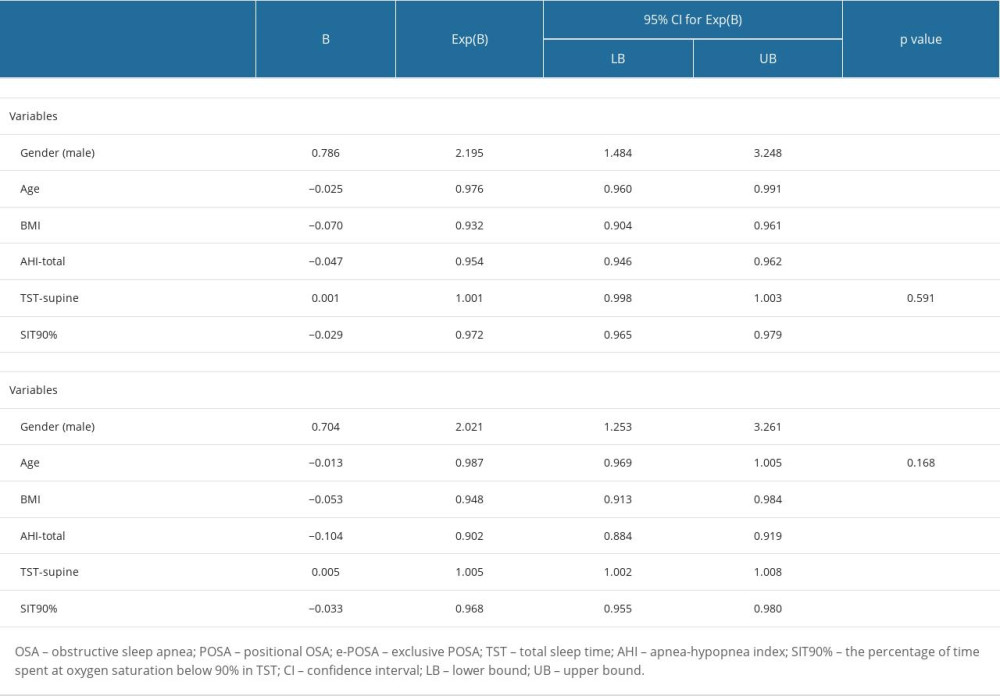
References
1. Veasey SC, Rosen IM, Obstructive sleep apnea in adults: N Eng J Med, 2019; 380(15); 1442-49
2. Heinzer R, Petitpierre NJ, Marti-Soler H, Haba-Rubio J, Prevalence and characteristics of positional sleep apnea in the HypnoLaus population-based cohort: Sleep Med, 2018; 48; 157-62
3. Sánchez-de-la-Torre M, Campos-Rodriguez F, Barbé F, Obstructive sleep apnoea and cardiovascular disease: Lancet Respir Med, 2013; 1(1); 61-72
4. Charčiūnaitė K, Gauronskaitė R, Šlekytė G, Evaluation of obstructive sleep apnea phenotypes treatment effectiveness: Medicina (Kaunas), 2021; 57(4); 335
5. Slowik JM, Sankari A, Collen JF, Obstructive sleep apnea: StatPearls [Internet], 2023, Treasure Island (FL), StatPearls Publishing Updated 2022 Dec 11
6. Oksenberg A, Goizman V, Eitan E, Obstructive sleep apnea: Do positional patients become nonpositional patients with time?: Laryngoscope, 2020; 130(9); 2263-68
7. Oweidat KA, Toubasi AA, Albtoosh AS, Comparing the characteristics of positional and nonpositional sleep apnea patients among the Jordanian population: Ann Thorac Med, 2022; 17(4); 207-13
8. Oksenberg A, Gadoth N, Töyräs J, Leppänen T, Prevalence and characteristics of positional obstructive sleep apnea (POSA) in patients with severe OSA: Sleep Breath, 2020; 24(2); 551-59
9. Garg H, Er XY, Howarth T, Heraganahally SS, Positional sleep apnea among regional and remote australian population and simulated positional treatment effects: Nat Sci Sleep, 2020; 12; 1123-35
10. Srijithesh PR, Aghoram R, Goel A, Dhanya J, Positional therapy for obstructive sleep apnoea: Cochrane Database Syst Rev, 2019; 5(5); CD010990
11. Kapur VK, Auckley DH, Chowdhuri S, Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American Academy of Sleep Medicine clinical practice guideline: J Clin Sleep Med, 2017; 13(3); 479-504
12. Sabil A, Blanchard M, Trzepizur W, Positional obstructive sleep apnea within a large multicenter French cohort: Prevalence, characteristics, and treatment outcomes: J Clin Sleep Med, 2020; 16(12); 2037-46
13. Wali SO, AlQassas I, Qanash S, The prevalence of positional obstructive sleep apnoea in a sample of the Saudi population: J Epidemiol Glob Health, 2023; 13(1); 129-39
14. Cartwright RD, Effect of sleep position on sleep apnea severity: Sleep, 1984; 7(2); 110-14
15. Mador MJ, Kufel TJ, Magalang UJ, Prevalence of positional sleep apnea in patients undergoing polysomnography: Chest, 2005; 128(4); 2130-37
16. Oksenberg A, Gadoth N, Are we missing a simple treatment for most adult sleep apnea patients? The avoidance of the supine sleep position: J Sleep Res, 2014; 23(2); 204-10
17. Levendowski DJ, Oksenberg A, Vicini C, A systematic comparison of factors that could impact treatment recommendations for patients with Positional Obstructive Sleep Apnea (POSA): Sleep Med, 2018; 50; 145-51
18. Shaukat R, Gamal Y, Ali A, Mohamed S, Adherence to positive airway pressure therapy in patients with obstructive sleep apnea: Cureus, 2022; 14(6); e25946
19. Weaver TE, Grunstein RR, Adherence to continuous positive airway pressure therapy: The challenge to effective treatment: Proc Am Thorac Soc, 2008; 5(2); 173-78
20. Ravesloot MJ, van Maanen JP, Dun L, de Vries N, The undervalued potential of positional therapy in position-dependent snoring and obstructive sleep apnea – a review of the literature: Sleep Breath, 2013; 17(1); 39-49
21. Oulhaj A, Al Dhaheri S, Su BB, Al-Houqani M, Discriminating between positional and non-positional obstructive sleep apnea using some clinical characteristics: Sleep Breath, 2017; 21(4); 877-84
22. Laub RR, Mikkelsen KL, Ønnesen P, Prevalence of positional obstructive sleep apnea and patients characteristics using various definitions: Eur Respir J, 2015; 46(Suppl 59); PA2372
23. Jiao X, Zou J, Meng L, Risk factors for non-positional obstructive sleep apnea-hypopnea syndrome: Sleep Breath, 2022; 26(2); 675-80
24. Kim KT, Cho YW, Kim DE, Two subtypes of positional obstructive sleep apnea: Supine-predominant and supine-isolated: Clin Neurophysiol, 2016; 127(1); 565-70
25. Joosten SA, Khoo JK, Edwards BA, Improvement in obstructive sleep apnea with weight loss is dependent on body position during sleep: Sleep, 2017; 40(5); zsx047
26. Beyhan Sagmen S, Cömert S, Polysomnographic and clinical characteristics of positional obstructive sleep apnea patients: Egypt J Bronchol, 2021; 15; 1-6
27. Dieltjens M, Vroegop AV, Verbruggen AE, A promising concept of combination therapy for positional obstructive sleep apnea: Sleep Breath, 2015; 19(2); 637-44
28. Wang X, Luo J, Huang R, Yi X, Preliminary study on clinical characteristics of Chinese patients with positional obstructive sleep apnea: Sleep Breath, 2022; 26(1); 67-74
29. Park MW, Cho JH, Park WK, Comparison of positional and non-positional obstructive sleep apnea patients by nocturnal polysomnography: J Oral Med Pain, 2009; 34(4); 371-77
30. Byun JI, Shin YY, Hwang KJ, Comparison of cardiac autonomic activity between positional and nonpositional obstructive sleep apnea using heart rate variability: Sleep Med, 2019; 64; 101-5
31. Álvarez D, Arroyo CA, de Frutos JF, Assessment of nocturnal autonomic cardiac imbalance in positional obstructive sleep apnea. A multiscale nonlinear approach: Entropy (Basel), 2020; 22(12); 1404
32. Wu H, Zhan X, Zhao M, Wei Y, Mean apnea-hypopnea duration (but not apnea-hypopnea index) is associated with worse hypertension in patients with obstructive sleep apnea: Medicine (Baltimore), 2016; 95(48); e5493
33. Alex R, Manchikatla S, Machiraju K, Effect of apnea duration on apnea induced variations in cerebral blood flow velocity and arterial blood pressure: Conf Proc IEEE Eng Med Biol Soc, 2014; 2014; 270-73
34. Kulkas A, Tiihonen P, Julkunen P, Novel parameters indicate significant differences in severity of obstructive sleep apnea with patients having similar apnea-hypopnea index: Med Biol Eng Comput, 2013; 51(6); 697-708
Tables
 Table 1. Baseline characteristics overall and in POSA vs non-POSA groups.
Table 1. Baseline characteristics overall and in POSA vs non-POSA groups. Table 2. Baseline characteristics in OSA patients with vs without e-POSA.
Table 2. Baseline characteristics in OSA patients with vs without e-POSA. Table 3. Polysomnography findings overall and in POSA vs non-POSA groups.
Table 3. Polysomnography findings overall and in POSA vs non-POSA groups. Table 4. Polysomnography findings in OSA patients with vs without e-POSA.
Table 4. Polysomnography findings in OSA patients with vs without e-POSA. Table 5. Multivariate logistic regression analysis for factors independently associated with POSA and e-POSA.
Table 5. Multivariate logistic regression analysis for factors independently associated with POSA and e-POSA. In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








