30 October 2023: Clinical Research
Serum Inflammatory Cytokine Levels in Herpes Zoster Patients and Their Association with Postherpetic Neuralgia: A Prospective Study
Jing Gu1ABEF, Yanrong Yuan1BD, Jun Wang1CF, Huili Liu1BD, Zuyong Zhang1G*, Yongxing Yan1CDDOI: 10.12659/MSM.941878
Med Sci Monit 2023; 29:e941878
Abstract
BACKGROUND: This study aimed to investigate the serum levels of inflammatory cytokines in patients with herpes zoster (HZ) and to assess their correlation with the development of postherpetic neuralgia (PHN). Understanding this relationship may offer insight into the mechanisms of PHN and provide avenues for targeted treatment.
MATERIAL AND METHODS: We selected 169 patients diagnosed with HZ and 43 healthy controls (HCs) for the study. Serum levels of inflammatory cytokines were measured in all participants. Pain severity was evaluated using the visual analog scale (VAS). Based on follow-up data, the 169 HZ patients were categorized into 2 groups: those who developed PHN (HZ-PHN) and those who did not (HZ-Con). We then analyzed the differences in cytokine levels and their correlation with PHN development.
RESULTS: Compared to the HCs group, HZ patients exhibited a significant decrease in TNF-a levels and an increase in IL-10 levels (P<0.05, P<0.01). The VAS score was negatively correlated with TNF-α levels and positively correlated with IL-10 levels in HZ patients (r=-0.3081, P<0.01; r=0.5619, P<0.01). Distinctive levels of TNF-α, IL-6, IL-8, and IL-10 were observed among different pain groups (P<0.05, P<0.01). The HZ-PHN group showed lower TNF-α and higher IL-10 levels compared to the HZ-Con group (P<0.05, P<0.01). IL-10 level was identified as an independent risk factor for PHN, with a sensitivity and specificity of 76.4% and 54.3%, respectively.
CONCLUSIONS: Abnormal levels of inflammatory cytokines are present in HZ patients, and the IL-10 level may serve as a valuable indicator for predicting the risk of developing PHN.
Keywords: Cytokines, Herpes Zoster, Neuralgia, Postherpetic
Background
Herpes zoster (HZ) is a local herpes outbreak caused by the reactivation of varicella-zoster virus (VZV) latent in the sensory ganglia [1], with frequent pain in the herpes distribution area [2]. Epidemiological data show that the prevalence rate of HZ in China was 7.7%. Generally, scar and/or pigmentation are left on the epidermis after the disappearance of skin herpes, and 9% to 34% of HZ patients will develop intractable postherpetic neuralgia (PHN) [3,4]. Neuralgia is mainly manifested as pain of different nature, such as burning, cutting, electric shock, and/or tearing, as well as pain with different characteristics, such as spontaneous pain and hyperalgesia, causing serious negative effects on the physical and mental health of patients [5–7].
Both the incidence and prevalence of HZ and PHN tend to increase with age [8], but the pathogenesis of PHN is still unclear. Once PHN occurs, the pain can be prolonged for several years or even decades, and the patient may suffer great pain, or even lose the ability to work or even live normally, which brings great medical care costs and economic burden to individuals and society [5]. Although there are many clinical methods for the treatment of PHN, most of the therapeutic effects are not satisfactory. In addition, there are no clinical indicators that can predict whether patients with HZ will be complicated with PHN. Therefore, the prevention of PHN is the focus of clinicians’ attention.
Recent studies have shown that the onset of HZ causes a series of immune reactions in the body and causes significant changes in the levels of various inflammatory cytokines [9–13]. The occurrence of PHN is closely related to the human immune response. Cytokines, as an important component of the immune system, participate in various important immune responses in the body; they can play an immune role through direct anti-inflammatory or pro-inflammatory action and participate in the occurrence of PHN. Khazan et al [14] found that the levels of cytokines such as IL-18 and IL-6 in HZ patients were significantly higher than those in the healthy control group, and were positively correlated with the severity of rash and neuralgia. Zhu et al [15] conducted a prospective cohort study on 49 HZ patients in the acute phase, showing that 10 patients who developed PHN had significantly higher levels of IL-6 in their peripheral blood than those who did not develop PHN, and that the inflammatory response in the acute phase of shingles, especially IL-6 levels, may be closely related to the occurrence of hyperalgesia and PHN. Fukuyasu et al [16] found that the level of IL-10 in the serum of HZ patients was significantly increased and positively correlated with the duration of neuralgia and the degree of pain, and the level of IL-10 was significantly lower after treatment. In summary, there are different opinions on the level of serum cytokine expression for predicting the occurrence of PHN at present [14–19]. The study of immune response to PHN is of great clinical significance to explore its pathogenesis and potential therapeutic targets. Therefore, the purpose of this study was to investigate the levels of serum inflammatory cytokines in patients with acute herpes zoster, and analyze the correlation between them and PHN, hoping to provide some basis for clinical prediction and treatment of patients with PHN.
Material and Methods
PATIENTS AND GROUPING:
A total of 169 patients with shingles hospitalized in our hospital from April 2021 to May 2023 were selected and divided into 4 groups – without pain, mild pain, moderate pain, and severe pain – according to pain scores. PHN occurrence was recorded by telephone or outpatient follow-up, and all patients were divided into 2 groups based on the occurrence of PHN: a herpes zoster postherpetic neuralgia group (HZ-PHN group) and a herpes zoster control group (HZ-Con group). Forty-three healthy volunteers who underwent physical examination in our hospital during the same period were selected as a healthy controls (HCs) group. This study was approved by the Medical Ethics Committee of our hospital (approval no. 2021KA013).
INCLUSION AND EXCLUSION CRITERIA:
Inclusion criteria were: (1) All patients’ clinical symptoms and auxiliary examinations met the HZ diagnostic criteria [20], and PHN met the diagnostic criteria [21]; (2)The diagnosis of herpes zoster was made by dermatologists; (3) Within 14 days of onset; (4) The patient can come to the hospital for re-diagnosis or telephone follow-up 30 days after the onset of the disease; and (5) Complete clinical data. Exclusion criteria were: (1) Patients with advanced malignant tumors, tuberculosis, or other serious systemic diseases; (2) Acute severe infection occurred recently; (3) Patients with senile dementia, aphasia, or language disorders who cannot express pain; and (4) Patients and their families do not agree to participate.
LEVELS OF INFLAMMATORY CYTOKINES DETECTION:
On the next day after admission, 5 ml of fasting venous blood was collected, and after self-coagulation, serum was collected by centrifugation at 3000 r/min for 15 min. The levels of TNF-α, IL-1β, IL-2R, IL-6, IL-8, IL-10, and other cytokines were detected by chemiluminescence immunoassay (CLIA) with the Siemens IMMULITE 1000 (Siemens Co. Ltd.) device according to the kit instructions.
PAIN ASSESSMENT:
The visual analog scale (VAS) was used to assess the degree of pain of HZ patients using a scale about 10 cm long marked from 0 to 10. A score of 0 means no pain, and a score of 10 means the most unbearable pain. The patient was asked to mark the corresponding position on the ruler that represents their pain level. Scores 1–3 indicate mild pain, 4–6 indicates moderate pain, and 7–10 indicates severe pain. VAS scores were assessed on the day of admission for all patients.
STATISTICAL ANALYSIS:
SPSS20.0 software (SPSS, Inc., Chicago, IL, USA) was used for data entry and statistical processing. The Kolmogorov-Smirnov test was selected for normality analysis of measurement data, and the normal distribution measurement data were expressed as mean±SD. The
Results
BASELINE ANALYSIS OF HZ PATIENTS AND HCS GROUPS:
Among the 169 patients with HZ, 79 were male and 90 were female, mean age 66.0±13.8 years. The sites of herpes zoster included 64 on the head and face, 16 on the neck, 47 on the chest and back, 33 on the waist, and 21 on the limbs. The HCs group consisted of 43 people, including 14 males and 29 females, mean age 61.6±17.4 years. There were no significant differences in age and sex between the 2 groups (P>0.05), as shown in Table 1. Compared with the HCs group, the levels of TNF-α 7.33±2.8 vs 8.6±3.6pg/ml was significantly lower in patients with herpes zoster, and the levels of IL-10 4.94±3.4 vs 3.5±1.5 were significantly higher. The differences were statistically significant (P<0.05; P<0.01), as shown Figure 1. There were no significant differences in the levels of IL-1β, IL-2R, IL-6, and IL-8 (P>0.05).
The average VAS score of 169 patients with herpes zoster was 3.6±2.3. Correlation analysis showed that VAS scores of patients with herpes zoster were negatively correlated with the levels of TNF-α (r=−0.3081, P<0.01), positively correlated with the levels of IL-10 (r=0.5619, P<0.01), and positively correlated with age (r=0.2953, P<0.05), as shown in Figures 2–4.
BASIC CONDITIONS AND CHANGES OF SERUM INFLAMMATORY CYTOKINES LEVELS IN HZ PATIENTS IN DIFFERENT PAIN GROUPS:
Among 169 patients, 9 had no pain, 86 had mild pain, 50 had moderate pain, and 24 had severe pain, and their mean ages were 53.3±19.5, 65.0±12.8, 65.2±13.2, and 76.1±10.2, respectively, with significant differences among groups (F=7.909, P<0.01). The ages of patients with mild, moderate, and severe pain were significantly higher than that of patients without pain, while the age of patients with severe pain was higher than that of patients with mild and moderate pain (P<0.05; P<0.01). There was no significant difference in sex or herpes location among patients in different pain groups (P>0.05), as shown in Table 2.
There were significant differences in the levels of TNF-α, IL-6, IL-8, and IL-10 among patients in different pain groups (F=4.14, 2.539, 2.577, 17.80, P=0.0073, 0.0384, 0.0356, 0.0000). Compared with patients in the without pain/mild pain group, patients with moderate and severe pain had significantly lower levels of TNF-α (P<0.05) and higher levels of IL-10 (P<0.01). The levels of IL-8 in patients with severe pain were significantly higher than in patients with mild to moderate pain (P<0.05). The levels of IL-6 in the severe pain group were higher than those in the moderate pain group (P<0.05). See Table 2.
CHANGES OF SERUM INFLAMMATORY CYTOKINES LEVELS IN HZ-PHN AND HZ-CON GROUPS:
According to the 1-month follow-up data of patients, there were 106 HZ patients with PHN (HZ-PHN group), with an incidence rate of 106/169 (62.7%), including 48 males and 58 females, mean age 70.3±11.7 years, with herpes on the head and face in 44 cases, on the neck in 10 cases, on the chest and back in 27 cases, on the waist in 20 cases, and on the limbs in 15 cases. The VAS score was 4.4±2.4. At 1-month follow-up, there were 63 patients (HZ-Con group) without any pain, including 31 males and 32 females, mean age 58.9±14.0 years. Herpes occurred in 20 cases on the head and face, 6 cases on the neck, 20 cases on the chest and back, 13 cases on the waist, and 6 cases on the limbs. The VAS score was 2.2±1.2. Compared with the HZ-Con group, the ages and VAS scores of patients in the HZ-PHN group were significantly higher (P<0.01), and there was no difference in sex or herpes location between the 2 groups (P>0.05). See Table 1.
Compared with the HZ-Con group and HCs group, the HZ-PHN group patients showed lower serum TNF-α levels and higher IL-10 levels (P<0.05; P<0.01). The levels of IL-8 and IL-1β in the HZ-PHN group were higher than those in the HCs group (P<0.05), and the levels of IL-6 in the HZ-Con group were lower than those in the HCs group (P<0.05). See Figure 5.
MULTIVARIATE BINARY LOGISTIC REGRESSION ANALYSIS:
Using complicated PHN as the dependent variable, age, VAS, levels of TNF-α, IL-10, IL-8, IL-6, and IL-1β were independent variables. Multivariate binary logistic regression analysis showed that the level of IL-10 in serum and age are the risk factors for predicting PHN as a complication (Table 3).
ROC CURVE ANALYSIS:
The present of PHN was indicated by IL-10 with an AUC of 0.635 (95% CI: 0.543–0.727). When the cutoff value was 3.67 pg/mL, the sensitivity was 76.4% and the specificity was 54.3%, respectively.
Discussion
Various causes can lead to neuralgia [22,23]. PHN, a common complication of shingles, is a typical form of neuralgia seen in the clinic. In some patients, the pain lasts for months or even years. Among patients with PHN, over 30% have pain lasting more than 1 year [24]. In severe cases, it can interfere with sleep and activities of daily living, leading to anorexia, weight loss, fatigue, depression, and anxiety, and loss of independent living ability, affecting social activities and greatly reducing quality of life. In this study, the incidence of PHN was 62.7%, which was significantly higher than in other studies [3,4]. We suggest 3 reasons for this difference. Firstly, in the previous study, the incidence of PHN in HZ patients over age 50 years was significantly higher [25], and the patients with shingles included in our study were older, with 45.0% (76/169) of patients over 70 years old. Secondly, 96.4% of the patients in our study had varying degrees of pain in the acute stage, with an average VAS of 3.6±2.3, and 43.8% of the patients had moderate to severe pain. Similarly, severe pain in the acute stage of HZ patients was closely related to the occurrence of PHN [25,26]. Thirdly, the subjects included in our study were all hospitalized patients with a wide range of rashes and severe lesions, and severe rashes are also an independent risk factor for PHN [26].
PHN is caused by inflammation of nerves affected by VZV [27]. Watson et al [28] analyzed ganglia from an early case of PHN at 2.5 months duration, suggesting that PHN is associated with chronic inflammatory responses in the ganglia. Cytokines are small molecular proteins synthesized and secreted by various cells in the body, which play an important role in stress processes such as trauma, pain, and infection, especially in the induction and maintenance of neuropathic pain [29–31]. Many types of cytokines have been identified, including interleukin (IL), interferon (IFN), tumor necrosis factor (TNF), nerve growth factor (NGF), and transforming growth factor (TGF). Cytokines can be divided into 2 categories: pro-inflammatory factors and anti-inflammatory factors. Pro-inflammatory factors include TNF and a variety of interleukins, such as IL-1, IL-2, IL-6, and IL-8, while anti-inflammatory factors include IL-4, IL-10, and transforming growth factor. Previous studies have shown that various inflammatory factors, such as interferon (IFN-γ), IL-23, IL-17, IL-22 and other cytokines, are involved in the immune response after VZV infection [14–16]. More and more studies have shown that the abnormal secretion of inflammatory cytokines caused by the disorder of cellular immune response is related to the occurrence of PHN [10,12–19,29]. Our study also found that there were abnormal cytokine levels in HZ patients. Compared with the normal population, the levels of TNF-α in HZ patients were lower and levels of IL-10 were higher, and the levels of TNF-α and IL-10 were correlated with the VAS of HZ patients. The levels of cytokines were also different in HZ patients with different degrees of pain. Compared with the HZ-CON group, the level of TNF-α in the HZ-PHN group was lower and the level of IL-10 was higher, indicating that the immune response process is disturbed in the HZ patients, and the immune response disorder is more obvious in HZ patients complicated with PHN in the later stage.
TNF-α, which is produced by mononuclear macrophages, is a pro-inflammatory factor that promotes local inflammatory response by promoting the proliferation, differentiation, and activation of a variety of immune cells. IL-10 is the main anti-inflammatory factor in the body, which can inhibit the secretion of various pro-inflammatory factors and interferon. Previous studies have shown that the incidence of HZ was significantly higher in patients treated with TFN-a inhibitors [32], which further indicates that TFN-a inhibition may be related to acute HZ infection. The pathological changes in the acute HZ phase are mainly the inflammatory response of sensory nerves. Monocytes and lymphocytes infiltrating the tissue and their release of pro-inflammatory cytokines such as IL-6 and TNF-α cause a strong immune inflammatory response. PHN is a symptom of chronic inflammation of the sensory nervous system [33]. In the acute HZ patients in our study, the level of TNF-α was lower than in the normal population, and was negatively correlated with pain in the acute phase. The lower level of the pro-inflammatory marker TNF-α may be a response of the body to the VZV infection to suppress the inflammatory response, which delay the disease course and increase the possibility of evolving into chronic inflammation. This may also be one of the reasons why the proportion of people who developed PHN later in this study was significantly higher than in other studies. Similarly, IL-10 is an anti-inflammatory factor, and high IL-10 levels can enhance the anti-inflammatory effect in vivo, weaken the inflammatory effect, enhance the replication of VZV, increase the toxic effect of VZV, prolong the duration of inflammatory disease, and make HZ patients more prone to PHN.
There are many factors influencing concurrent PHN, and it is unclear whether sex is a risk factor for PHN [25,26]. We did not find that sex is a risk factor for PHN. Similar to many previous studies [21,24–26], we found that age is an independent risk factor for PHN, and the degree of PHN is related to age. Patients with severe pain are significantly older than those with mild to moderate pain. We found that the sensitivity of IL-10 level in predicting PHN was high (76.4%), but the specificity was low (only 54.3%), which may be because there are many factors affecting PHN, such as age, herpes rash location, degree of acute pain, and other blood indicators. More prospective studies are needed to elucidate these issues. There are also some defects in this study, such as the small sample size, which may have caused bias, the single-center design, and failure to dynamically observe and compare cytokine levels in HZ patients before and after treatment.
Conclusions
PHN is a common complication of shingles, with a high incidence rate. Secretion of cytokines in HZ patients was abnormal, and the levels of TNF-α and IL-10 were related to the degree of zoster-associated pain (ZAP). TNF-α is negatively correlated with neuralgia, but IL-10 levels are positively correlated. Cytokines are involved in the occurrence and development of HZ and PHN, and the levels of IL-10 had value in predicting the occurrence of PHN.
Figures
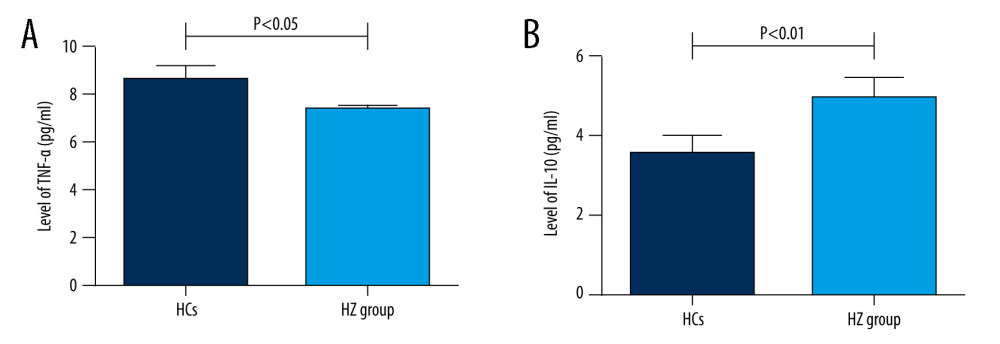 Figure 1. (A, B) Comparison of serum TNF-α and IL-10 levels between HZ and HCs groups.
Figure 1. (A, B) Comparison of serum TNF-α and IL-10 levels between HZ and HCs groups. 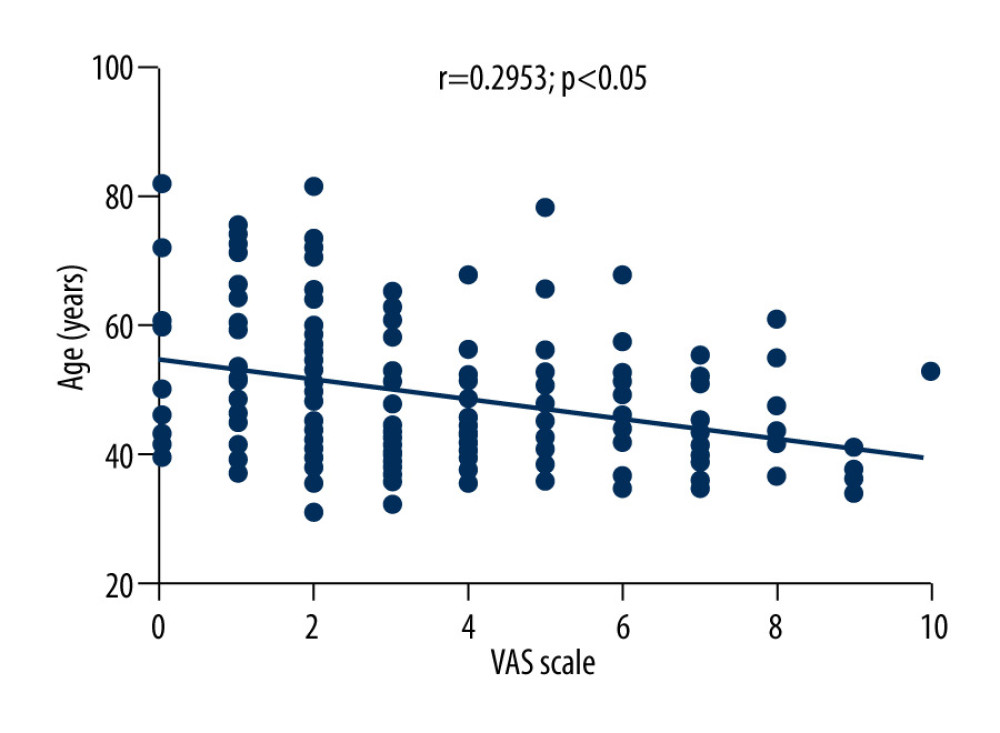 Figure 2. Correlation between VAS score and TNF-α level in HZ patients.
Figure 2. Correlation between VAS score and TNF-α level in HZ patients. 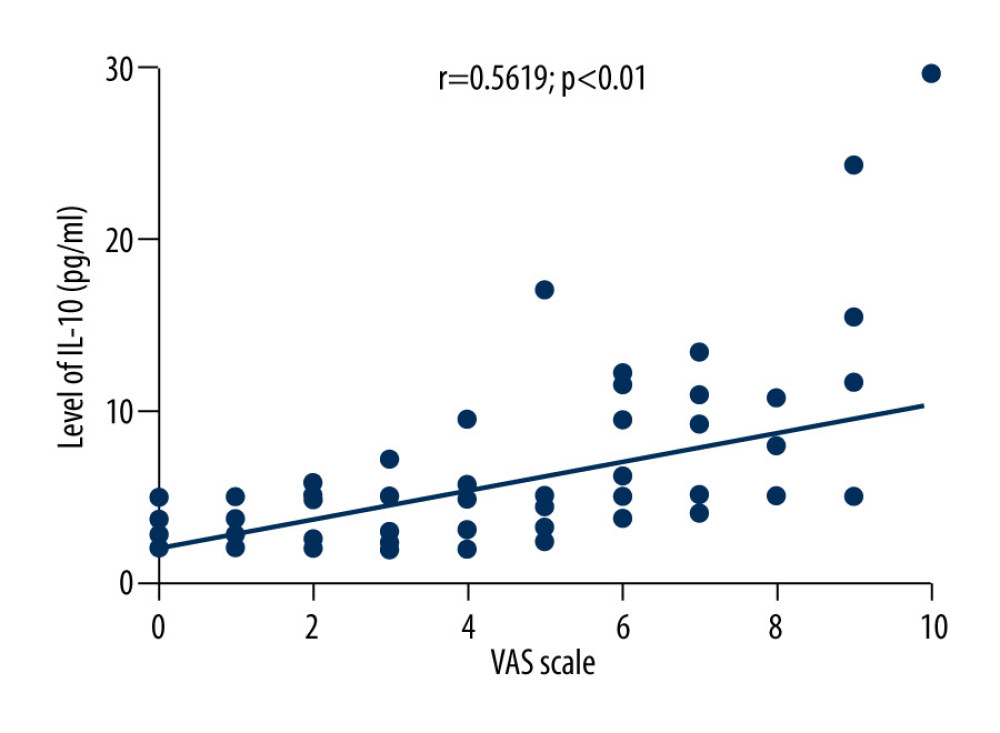 Figure 3. Correlation between VAS score and IL-10 level in patients with herpes zoster.
Figure 3. Correlation between VAS score and IL-10 level in patients with herpes zoster. 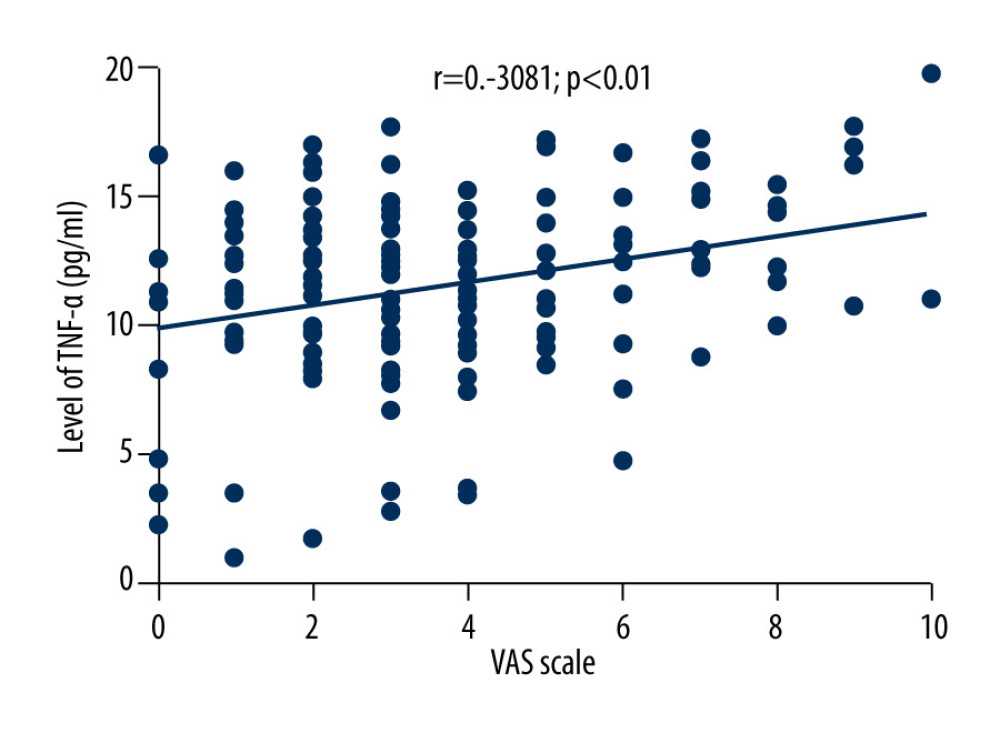 Figure 4. Correlation between VAS score and age in patients with herpes zoster.
Figure 4. Correlation between VAS score and age in patients with herpes zoster. 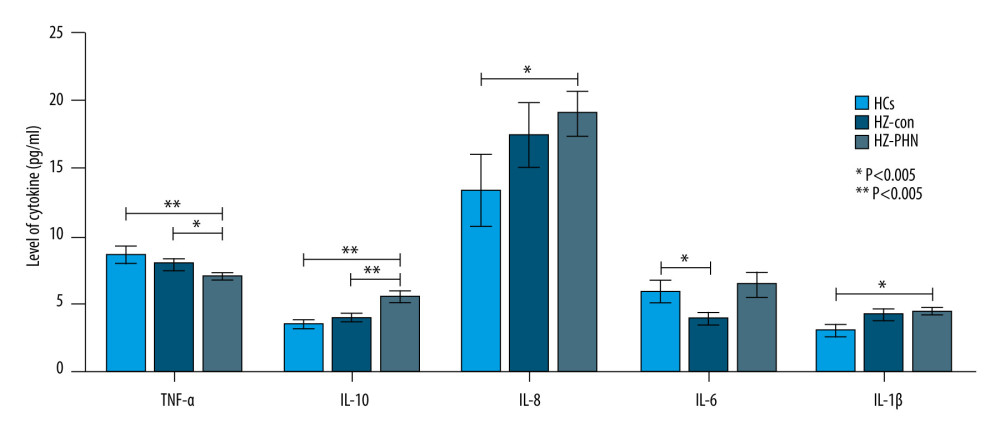 Figure 5. Comparison of serum TNF-α, IL-10, IL-8, IL-6, and IL-1β levels among HCs group, HZ-Con group, and HZ-PHN group patients.
Figure 5. Comparison of serum TNF-α, IL-10, IL-8, IL-6, and IL-1β levels among HCs group, HZ-Con group, and HZ-PHN group patients. References
1. Cohen JI, Clinical practice: Herpes zoster: N Engl J Med, 2013; 369(3); 255-63
2. Schmader K, Herpes zoster: Ann Intern Med, 2018; 169(3); ITC19-ITC31
3. Kost RG, Straus SE, Postherpetic neuralgia – pathogenesis, treatment, and prevention: N Engl J Med, 1996; 335(1); 32-42
4. Yang F, Yu S, Fan B, The epidemiology of herpes zoster and postherpetic neuralgia in China: Results from a cross-sectional study: Pain Ther, 2019; 8(2); 249-59
5. Harvey M, Prosser LA, Rose AM, Aggregate health and economic burden of herpes zoster in the United States: Illustrative example of a pain condition: Pain, 2020; 161(2); 361-38
6. Scholz J, Finnerup NB, Attal N, The IASP classification of chronic pain for ICD-11: chronic neuropathic pain: Pain, 2019; 160(1); 53-59
7. Berra LV, Armocida D, Pesce A, Rita AD, Santoro A, Herpes simplex reactivation after surgical treatment of trigeminal neuralgia: A retrospective cohort study: World Neurosurg, 2019; 127; e16-e21
8. Hecke Ov, Austin SK, Khan RA, Neuropathic pain in the general population: A systematic review of epidemiological studies: Pain, 2014; 155(4); 654-62
9. Cao S, Zhang D, Yuan J, Inflammatory cytokine expression in the skin of patients with postherpetic neuralgia: J Int Med Res, 2020; 48(8); 300060520929582
10. Zajkowska A, Garkowski A, Świerzbińska R, Evaluation of chosen cytokine levels among patients with herpes zoster as ability to provide immune response: PLoS One, 2016; 11(3); e0150301
11. Sen N, Sung P, Panda A, Arvin AM, Distinctive roles for type I and type II interferons and interferon regulatory factors in the host cell defense against varicella-zoster virus: J Virol, 2018; 92(21); e1151-18
12. Zak-Prelich M, McKenzie RC, Sysa-Jedrzejowska A, Norval M, Local immune responses and systemic cytokine responses in zoster: Relationship to the development of postherpetic neuralgia: Clin Exp Immunol, 2003; 131(2); 318-23
13. Kim MS, Kim DJ, Na CH, Shin BS, A study of the changes of T helper 17 cells and regulatory T cells in herpes zoster: Ann Dermatol, 2017; 29(5); 578-85
14. Khazan M, Nasiri S, Riahi SM, Measurement of melatonin, indole-dioxygenase, IL-6, IL-18, ferritin, CRP, and total homocysteine levels during herpes zoster: J Med Virol, 2020; 92(8); 1253-59
15. Zhu S-m, Liu Y-m, An E-d, Chen Q-l, Influence of systemic immune and cytokine responses during the acute phase of zoster on the development of postherpetic neuralgia: J Zhejiang Univ Sci B, 2009; 10(8); 625-30
16. Fukuyasu A, Kamata M, Hau CS, Serum interleukin-10 level increases in patients with severe signs or symptoms of herpes zoster and predicts the duration of neuralgia: J Dermatol, 2021; 48(4); 511-18
17. Peng Q, Guo X, Luo Y, Dynamic immune landscape and VZV-specific T cell responses in patients with herpes zoster and postherpetic neuralgia: Front Immunol, 2022; 13; 887892
18. Yu X, Li L, Chan MTV, Wu WKK, Bioinformatic analyses suggest augmented interleukin-17 signaling as the mechanism of COVID-19-associated herpes zoster: Environ Sci Pollut Res Intl, 2021; 28(46); 65769-75
19. Liang X, Fan Y, Bidirectional two-sample Mendelian randomization analysis reveals a causal effect of interleukin-18 levels on postherpetic neuralgia risk: Front Immunol, 2023; 14; 1183378
20. Koshy E, Mengting L, Kumar H, Jianbo W, Epidemiology, treatment and prevention of herpes zoster: A comprehensive review: Indian J Dermatol Venereol Leprol, 2018; 84(3); 251-62
21. Chinese expert consensus on the diagnosis and treatment of postherpetic neuralgia: Chinese Journal of Pain Medicine, 2016; 22(3); 161-167 [in Chinese]
22. Rosenberger DC, Blechschmidt V, Timmerman H, Challenges of neuropathic pain: Focus on diabetic neuropathy: J Neural Transm (Vienna, Austria: 1996), 2020; 127(4); 589-624
23. Berra LV, Armocida D, Mastino L, Trigeminal neuralgia secondary to intracranial neoplastic lesions: A case series and comprehensive review: J Neurol Surg A Cent Eur Neurosurg, 2021; 82(2); 118-24
24. Kawai K, Gebremeskel BG, Acosta CJ, Systematic review of incidence and complications of herpes zoster: Towards a global perspective: BMJ Open, 2014; 4(6); e004833
25. Kawai K, Rampakakis E, Tsai T-F, Predictors of postherpetic neuralgia in patients with herpes zoster: A pooled analysis of prospective cohort studies from North and Latin America and Asia: Int J Infect Dis, 2015; 34; 126-31
26. Opstelten W, Zuithoff N, Essen G, Predicting postherpetic neuralgia in elderly primary care patients with herpes zoster: Prospective prognostic study: Pain, 2007; 132(Suppl 1); S52-S59
27. Patil A, Goldust M, Wollina U, Herpes zoster: A review of clinical manifestations and management: Viruses, 2022; 14(2); 192
28. Watson CPN, Deck JH, Morshead C, Post-herpetic neuralgia: Further post-mortem studies of cases with and without pain: Pain, 1991; 44(2); 105-17
29. Üçeyler N, Valet M, Kafke W, Local and systemic cytokine expression in patients with postherpetic neuralgia: PLoS One, 2014; 9(8); e105269
30. Kuffler DP, Mechanisms for reducing neuropathic pain: Mol Neurobiol, 2020; 57(1); 67-87
31. Kuffler DP, Injury-induced effectors of neuropathic pain: Mol Neurobiol, 2020; 57(1); 51-66
32. Santella C, Bitton A, Filliter C, Anti-TNF therapy and the risk of herpes zoster among patients with inflammatory bowel disease: Inflamm Bowel Dis, 2022; 28(2); 176-82
33. Nagel MA, Gilden D, Neurological complications of varicella zoster virus reactivation: Curr Opin Neurol, 2014; 27(3); 356-60
Figures
 Figure 1. (A, B) Comparison of serum TNF-α and IL-10 levels between HZ and HCs groups.
Figure 1. (A, B) Comparison of serum TNF-α and IL-10 levels between HZ and HCs groups. Figure 2. Correlation between VAS score and TNF-α level in HZ patients.
Figure 2. Correlation between VAS score and TNF-α level in HZ patients. Figure 3. Correlation between VAS score and IL-10 level in patients with herpes zoster.
Figure 3. Correlation between VAS score and IL-10 level in patients with herpes zoster. Figure 4. Correlation between VAS score and age in patients with herpes zoster.
Figure 4. Correlation between VAS score and age in patients with herpes zoster. Figure 5. Comparison of serum TNF-α, IL-10, IL-8, IL-6, and IL-1β levels among HCs group, HZ-Con group, and HZ-PHN group patients.
Figure 5. Comparison of serum TNF-α, IL-10, IL-8, IL-6, and IL-1β levels among HCs group, HZ-Con group, and HZ-PHN group patients. Tables
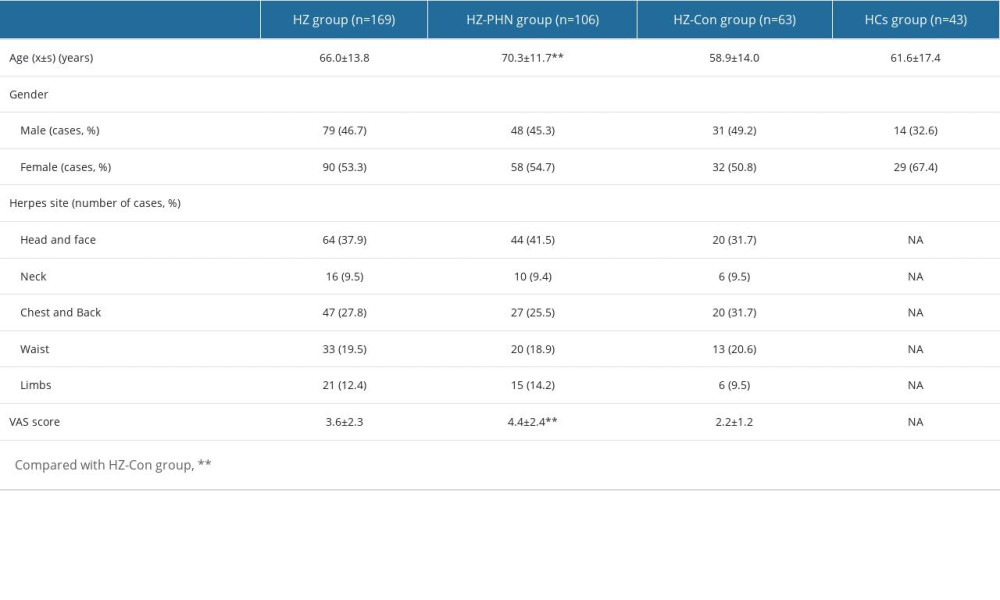 Table 1. Baseline characteristics analysis of HZ-PHN group, HZ-Con group, and HCs group.
Table 1. Baseline characteristics analysis of HZ-PHN group, HZ-Con group, and HCs group.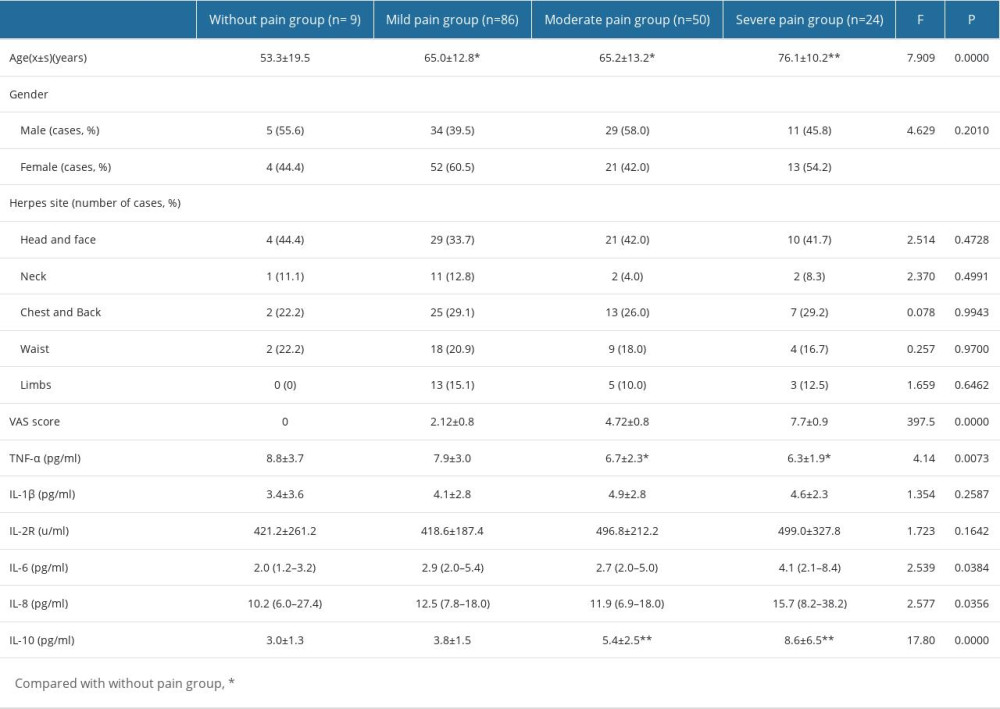 Table 2. Comparison of serum inflammatory cytokines levels in HZ patients in different pain groups.
Table 2. Comparison of serum inflammatory cytokines levels in HZ patients in different pain groups.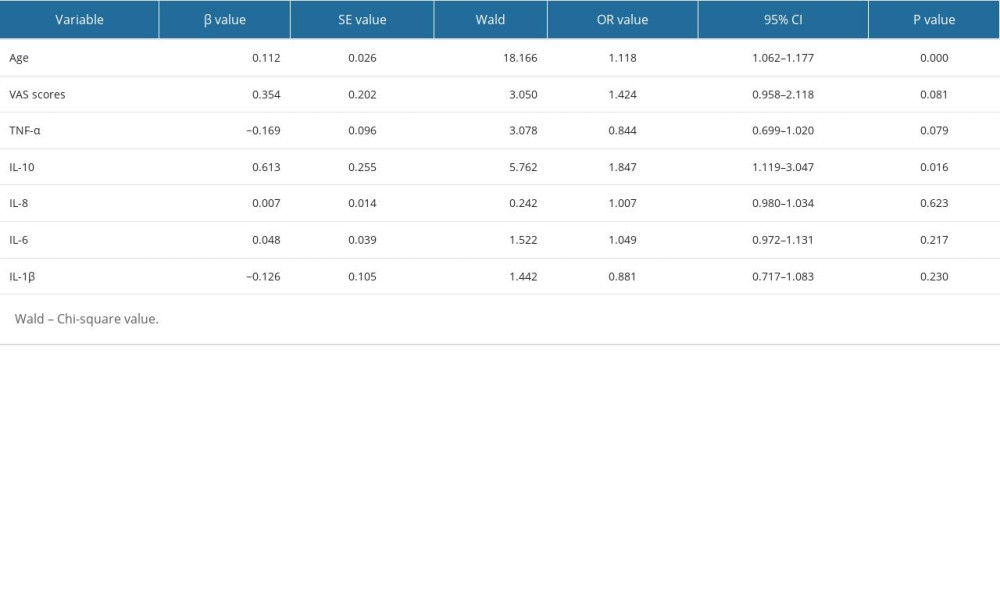 Table 3. Multivariate binary logistic regression analysis of complicate PHN in patients with shingles.
Table 3. Multivariate binary logistic regression analysis of complicate PHN in patients with shingles. Table 1. Baseline characteristics analysis of HZ-PHN group, HZ-Con group, and HCs group.
Table 1. Baseline characteristics analysis of HZ-PHN group, HZ-Con group, and HCs group. Table 2. Comparison of serum inflammatory cytokines levels in HZ patients in different pain groups.
Table 2. Comparison of serum inflammatory cytokines levels in HZ patients in different pain groups. Table 3. Multivariate binary logistic regression analysis of complicate PHN in patients with shingles.
Table 3. Multivariate binary logistic regression analysis of complicate PHN in patients with shingles. In Press
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
12 Mar 2024 : Clinical Research
Comparing Neuromuscular Blockade Measurement Between Upper Arm (TOF Cuff®) and Eyelid (TOF Scan®) Using Miv...Med Sci Monit In Press; DOI: 10.12659/MSM.943630
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








