14 November 2023: Clinical Research
Fertility-Sparing Treatment in Gestational Choriocarcinoma: Evaluating Oncological and Obstetrical Outcomes in Young Patients
Szymon PiątekDOI: 10.12659/MSM.942078
Med Sci Monit 2023; 29:e942078
Abstract
BACKGROUND: Gestational choriocarcinoma (GC) is an uncommon neoplasia that occurs in women who may not have completed a procreation plan. The aim of this study was to evaluate oncological and obstetrical outcomes in young patients with GC after fertility-sparing treatment.
MATERIAL AND METHODS: The eligibility criteria for the study were histopathological diagnosis of GC, age ≤40 years, and treatment with systemic chemotherapy. Patients who underwent upfront hysterectomy were excluded. The response to treatment was assessed according to beta-human chorionic gonadotropin (beta-hCG) serum measurement. Complete response and progression were considered if the beta-hCG dropped to a normal range and increased (or reached a plateau), respectively. The birth rate was calculated as the number of women who gave birth after treatment divided by the total number of patients.
RESULTS: A total of 18 patients fulfilled the study’s eligibility criteria. A complete response and progression to first-line chemotherapy were found in 13 (72.22%) and 5 (27.78%) patients, respectively. Salvage treatment was administered to patients with progression. Overall, 16 (88.88%) patients achieved complete response after treatment and 2 (11.12%) died. GC relapse was diagnosed in 1 patient 62 months after treatment. The birth rate was 22.22%, and a total of 6 children were born. All pregnancies ended in term delivery. No congenital abnormalities were detected in the newborns.
CONCLUSIONS: GC is a life-threatening form of gestational trophoblastic neoplasia, mainly due to its rapid course and resistance to chemotherapy. Most patients with GC will not be able to bear children after treatment.
Keywords: Antineoplastic Combined Chemotherapy Protocols, Birth Rate, Choriocarcinoma, fertility preservation, gestational trophoblastic disease
Background
In the female genital tract, choriocarcinoma can develop in 2 different forms. It may be one of the malignant types of gestational trophoblastic disease (gestational choriocarcinoma) that occurs after hydatidiform moles, normal pregnancy, or spontaneous abortion [1]. Alternatively, it can develop from germ cells of the ovary [2] or midline structures [3] or in association with poorly differentiated somatic carcinoma (non-gestational choriocarcinoma) [4].
The diagnosis of gestational choriocarcinoma (GC) always raises issues, as its occurrence is very rare, especially in the USA, Canada, Europe, and Oceania [5]. In addition to its low incidence, it has been shown that the number of new cases has been decreasing since the 1970s [6]. Interestingly, the incidence of GC is usually reported in different ways and varies widely between races and regions around the world [5]. It may be related to the number of pregnancies (2–200 per 100 000 pregnancies), the number of livebirths (4–19 per 100 000 live births), or the number of deliveries (8–335 per 100 000 deliveries), whereas in cancer registries, it is assessed per 100 000 women [5]. The age-adjusted incidence rate for choriocarcinoma was estimated to be 0.133 per 100 000 woman-years [6].
The clinical presentation of GC varies widely, and it can thus be a diagnostic challenge [7]. It may be confined to the uterus, and those cases may present only with vaginal bleeding. However, GC can invade blood vessels early in its development and disseminate to distant organs. Cardiopulmonary problems (20.66%) followed by gastrointestinal (18.43%) and central nervous system manifestations (17.67%) were found to be the most common concerns [7]. There may be symptoms such as cough, hemoptysis, dyspnea, chest pain, focal neurologic signs, or convulsions.
The vast majority of gestational trophoblastic neoplasia (GTN) cases are diagnosed by an elevated serum level of beta-human chorionic gonadotropin (beta-hCG) after post molar pregnancy [8], but the diagnosis of GC requires histological confirmation [9]. In Japan, when a biopsy specimen is not available, a special scoring system is created to diagnose ‘clinical choriocarcinoma’ [10]. However, this term is not widely used outside Japan [11].
Treatment of GC relies mainly on the International Federation of Gynaecology and Obstetrics (FIGO) score, which has prognostic value and predicts developing resistance to single-drug chemotherapy [12]. The variables that are assessed in the prognostic score include: a) tumor volume (beta-hCG serum level, size of metastases, and number of metastases), b) site of involvement, c) prior chemotherapy resistance, and d) duration of disease from antecedent pregnancy. A score of 0–6 and ≥7 indicates a low and high risk of resistance, respectively [12]. However, patient age, tumor localization, and histology of GTN may also influence the management of GC [1]. Since the introduction of chemotherapy to GC management, the 5-year overall survival rate has risen to 86.7% [13]. The prognosis for GC is worse than for all risk levels of GTN (5-year overall survival: 93–95%) and is comparable to high-risk GTN (5-year overall survival: 86.2%) [14–16]. Although GC is very chemosensitive, hysterectomy still plays a role in the management of GC as an upfront treatment, in chemoresistant disease, or in cases with severe vaginal hemorrhage [17]. It is reported that hysterectomy is performed in 26% of patients with GTN [18]. It was found that patients after hysterectomy had a shorter duration of hospitalization and needed less chemotherapy to achieve remission, regardless of whether or not metastases were present [19].
Quality of life has become an important issue for cancer patients [20]. In young women, post-treatment infertility is related to a diminished quality of life [21]. Fertility-sparing treatment (FST) in gynecological cancers includes various forms of therapy and mainly depends on the cancer type [22]. Apart from a few case reports on local uterine tumor excision in GC [23,24], surgery is not an FST option. Chemotherapy can be considered as an FST method in GTN [22]. As women with GC are of childbearing age, they may be interested in retaining their fertility potential after treatment, so uterine preservation is mandatory in those patients. According to FIGO, GC is associated with an increased risk of resistance to single-agent chemotherapy; therefore, the use of multiple-agent chemotherapy can be considered, even in low-risk patients [1]. However, a multi-drug chemotherapy regimen is associated with higher gonadotoxicity and a lower chance of becoming pregnant, especially if cyclophosphamide is administered [25].
Some studies have assessed pregnancy and obstetric outcomes in patients with gestational trophoblastic disease [26,27]; however, data regarding reproductive results in GC are limited to very few reports [28,29]. The aim of the present study was to assess the survival and obstetrical outcome in patients with GC who underwent FST.
Material and Methods
ETHICS APPROVAL:
This study was conducted in accordance with ethics principles and was approved by the local bioethics committee (No: KB/02/2023). All procedures were conducted according to the Declaration of Helsinki for Medical Research involving Human Subjects. All patients provided written informed consent for the chosen treatment. Patient consent for participation in the study was waived due to its retrospective nature. Clinical decisions concerning treatment were not influenced by the study.
PATIENT SELECTION:
This retrospective study included patients with gestational choriocarcinoma treated at the Department of Gynecologic Oncology, Maria Sklodowska-Curie National Research Institute of Oncology in Warsaw (MSCNRIO) between January 1, 2000, and December 31, 2020. The inclusion criteria were as follows: 1) having a histopathological diagnosis of GC, 2) an age ≤40 years, and 3) having undergone systemic chemotherapy. Women who qualified for upfront hysterectomy were excluded from the study.
Electronic databases (MSD and Clininet) were searched using the term “gestational choriocarcinoma” and/or “gestational trophoblastic disease/neoplasia” and/or the ICD-10 code ‘C58’. Medical records were reviewed to find patients who met the inclusion criteria. The flow chart of the study is shown in Figure 1.
GESTATIONAL CHORIOCARCINOMA MANAGEMENT:
The initial diagnosis of GC was made in regional hospitals. However, every case of GC was confirmed by pathologists at MSCNRIO (Figure 2). In each case, pretreatment management included: serum measurements of beta-hCG, gynecologic examination with transvaginal ultrasonography, and computed tomography of the head, neck, chest, abdomen, and pelvis. Patients with uterine bleeding and/or uterine mass were counselled regarding their reproductive plans and methods of treatment. All women eligible for the study decided on uterine preservation management.
FIGO staging and scoring systems of gestational trophoblastic diseases (2000) were used to assess GC advancement and prognosis, respectively. The decision about the chemotherapy regimen was made according to the FIGO score. If the total number of points was <7 (low-risk disease), single-agent chemotherapy was administered. In cases with ≥7 points (high-risk disease), a multi-agent regimen was chosen. The duration of treatment depended on the serum levels of beta-hCG, which were measured before each cycle of chemotherapy. Chemotherapy was administered until the beta-hCG decreased to a normal range (<5 mIU/ml) and an additional 2 consolidation courses were given.
All serum measurements of beta-hCG during treatment were performed at the laboratory of cancer biomarkers and cytokines at MSCNRIO. During the follow-up period, patients were allowed to have blood tests in laboratories located near their place of residence, especially since March 2020 (the onset of the COVID-19 pandemic).
The follow-up included serum beta-hCG measurements every 2 weeks in the first 3–6 months, then every 1–2 months to 1 year, every 3–4 months during the second year, and every 6 months up to 5 years. Hormonal contraception for at least 12 months after treatment was recommended for all patients, except for 1 woman with pulmonary embolism, who was advised to use condoms.
RESPONSE TO TREATMENT AND OBSTETRICS ASSESSMENT:
Complete response (CR) was defined as the normalization of the beta-hCG serum level, while a rising or plateauing beta-hCG was regarded as indicative of progression of the disease.
The birth rate was defined as the number of women who gave birth to a live newborn (>22 weeks of gestation) after treatment divided by the total number of patients. Data about childbearing were gathered from patients’ medical records. In cases of women who completed the 5-year follow-up period, information regarding recurrence and childbearing was obtained via telephone.
Results
PATIENT CHARACTERISTICS:
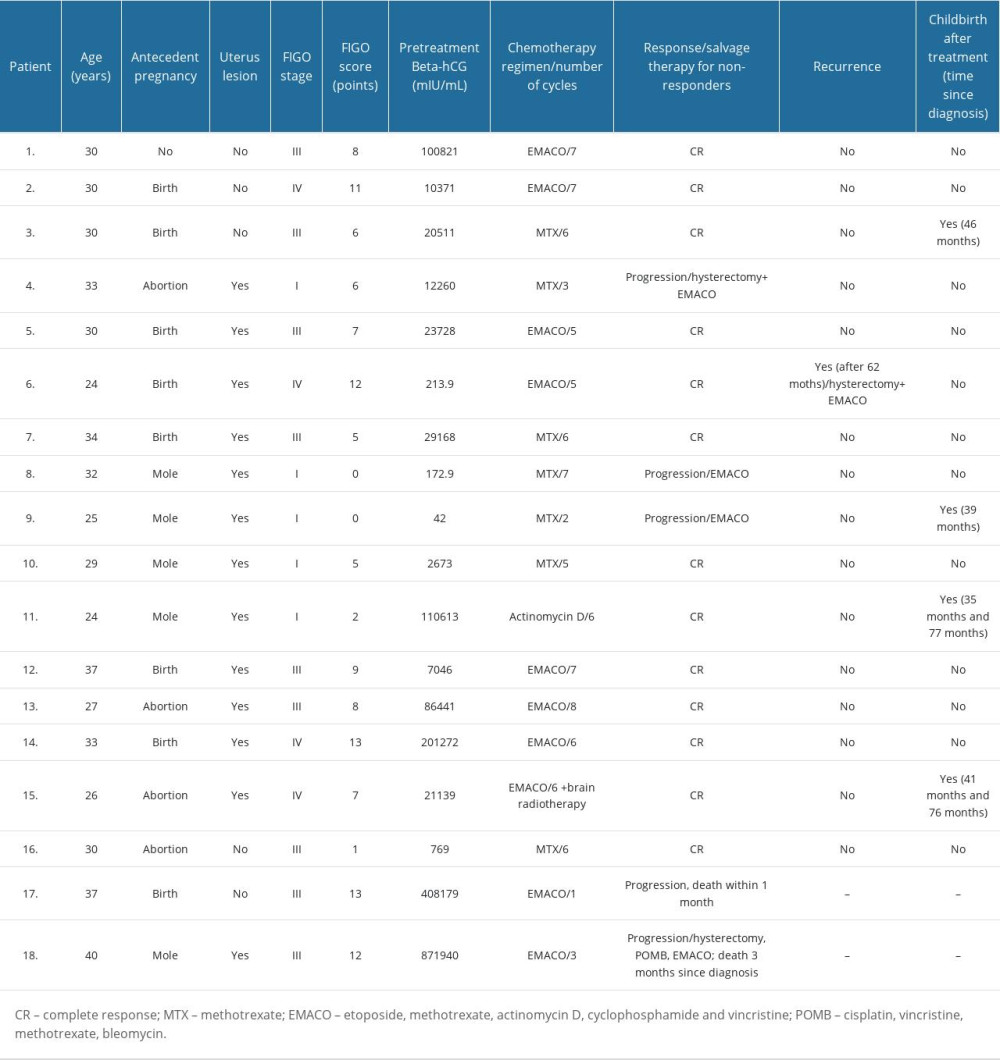
Eighteen patients met the eligibility criteria for the study. The patient’ characteristics are presented in Table 1. The average age of women upon diagnosis was 31 years (range: 24–40 years). GC was diagnosed using uterine curettage in 12 (66.66%) patients. Metastases resection/biopsy was performed in 3 (16.66%) women to confirm the diagnosis. One patient (5.55%; No. 13) was diagnosed during an emergency laparotomy due to acute hemoperitoneum. Intraoperatively, apart from blood, ruptured uterus and free parts of tumor in the abdominal cavity were found. The uterine lesion was sutured and the tumor mass was evacuated from the peritoneal cavity. The other 2 cases (11.1%) were diagnosed based on post-mortem examination: 1 (5.55%) pathologic examination was performed on the neonate, who died 28 days after delivery. The mother of that child (No. 3) was diagnosed with elevated beta-hCG and pulmonary metastases; however, no abnormalities in the uterus were found. To limit the invasive diagnostics and to immediately implement treatment, a lung biopsy was abandoned and GC was diagnosed on the basis of a post-mortem examination of the child. Another pathologic examination, which confirmed GC, was performed in a woman (5.55%, No. 17) who died 30 days following her first cycle of chemotherapy. All patients had elevated beta-hCG serum levels at the moment of diagnosis, with a mean serum concentration of 133 052 IU/ml (range: 42–871 940 IU/ml).
Antecedent gestations were documented in 17 (94.44%) patients. Pregnancies preceding the diagnosis ended in childbirth (8 of 17; 47.06%) and miscarriage (4 of 17; 23.53%). Molar pregnancy was initially diagnosed in 5 (29.41%) cases. One patient (5.55%; No. 1) had no history of previous pregnancy, but she stopped taking oral contraception 2 months prior to the diagnosis. She was diagnosed with an acute massive pulmonary embolism and underwent an emergency surgical embolectomy, which revealed metastases of GC.
Distant metastases were found in 12 (72.22%) patients. FIGO stage III was the most commonly diagnosed in the study group (n=9, 50%). FIGO I and IV were found in 5 (27.87%) and 4 (22.22%) patients, respectively. No patient was diagnosed at FIGO stage II. Most of the women were assessed as high-risk GTN, with a FIGO score ≥7 points (55.56%; n=10).
ANTI-CANCER TREATMENT OUTCOMES:
All patients were treated with chemotherapy. A single-agent regimen was used as first-line chemotherapy in 8 patients (44.44%) and a multi-drug regimen was used in 10 (55.56%) patients. Methotrexate (MTX) was administered in 7 (38.89%) cases, actinomycin D in 1 (5.55%) patient, and 10 (55.56%) patients received a regimen consisting of etoposide, methotrexate, actinomycin D, cyclophosphamide, and vincristine (EMACO). One patient with stage IV and metastases to the brain, as well as receiving multi-drug chemotherapy, was additionally treated with radiation therapy to the central nervous system. Complete response (CR) and progression to first-line chemotherapy was found in 13 (72.22%) and 5 (27.78%) patients, respectively. In 3 women with progression, methotrexate was changed to a multi-agent regimen (EMACO), and 1 of them underwent a hysterectomy. In total, 16 (88.89%) patients achieved CR after salvage treatment. Two (11.11%) patients (both FIGO III, high-risk) had unsuccessful treatment. One of them died 1 month after the first cycle of EMACO due to rapid GC progression. The second patient received salvage treatment with a hysterectomy and POMB regimen (cisplatin, vincristine, methotrexate, bleomycin) but did not respond to the administered treatment and died 3 months after the start of therapy. Treatment outcomes in patients with GC according to FIGO score and staging are presented in Figure 3.
The mean follow-up time was 69 months (range: 1–175 months). Among the 16 patients who achieved complete remission, 1 (6.25%) patient with stage FIGO IV and 12 points relapsed 62 months after treatment. She was successfully treated with an EMACO regimen and underwent a hysterectomy.
OBSTETRICAL OUTCOMES:
Four (birth rate: 22.22%) patients gave birth to a total of 6 children. Two of them delivered once (39 and 46 months after the diagnosis), while 2 patients gave birth twice. The first patient had her first childbirth 35 months and then 77 months after the diagnosis. The second patient delivered 41 months and then 76 months after the diagnosis. All pregnancies ended in term delivery, and no congenital abnormalities were detected in the newborns.
Discussion
In our study, all low-risk patients achieved complete remission and none of them relapsed. Winter et al found that the overall survival rate in low-risk GTN was also 100% [30]. Our results confirmed that patients with low-risk GC have a very good prognosis and histologic confirmation of GC did not influence survival in low-risk disease. However, in our study, 37.5% of low-risk patients did not respond to single-drug chemotherapy and required a multi-drug regimen to achieve remission. Fulop et al found that 95.1% and 93.6% of patients received CR after MTX and actinomycin D, respectively [31]. This may indicate the significant resistance of GC to single-drug chemotherapy. Lurain et al found that the diagnosis of choriocarcinoma was associated with development of resistance to single-agent methotrexate chemotherapy [32]. In their study, resistance to single-drug chemotherapy was lower (20.5%) compared to our results [32]. Ngan et al suggested lowering the threshold for the use of multiple-agent chemotherapy in patients with GC [1]. Of note, every case of GC resistance to a single-drug regimen was successfully switched to multi-drug treatment and achieved long-lasting remission. In our study, MTX was mainly used in low-risk patients and only 1 woman received actinomycin D. Similar results were presented in the review by Joneborg et al, who found that many centers preferred methotrexate as first-line chemotherapy because of its mild toxicity profile with no alopecia and with less nausea, vomiting, and myelosuppression [33]. However, a metanalysis of the Cochrane database showed that actinomycin D was more likely to achieve primary cure (risk ratio 0.65, 95% CI 0.57 to 0.75), whereas methotrexate was more likely to result in treatment failure (risk ratio 3.55, 95% CI 1.81 to 6.95) [34]. There was no difference in adverse effects between methotrexate and actinomycin D, but the latter may be associated with a greater risk of severe adverse events.
Among patients with high-risk GC, 2 women did not respond to treatment and died. The first patient died 1 month after the first cycle of the EMACO regimen due to rapid progression. The second patient also started treatment with an EMACO regimen but did not respond to this chemotherapy; therefore, she underwent a hysterectomy to decrease the tumor mass, and salvage POMB chemotherapy was given. She died due to chemoresistance 3 months after starting first-line treatment. Both patients who died were stage III, with FIGO scores of 12 and 13 points. The death rate of patients with GC scored ≥12 points was 50% (2 out of 4). In other studies of patients with all types of GTN, those who scored ≥12 or ≥13 had a 5-year survival rate of 61.6–67.9% [35,36]. Our results suggest that the worst prognosis among all GTN cases is related to the diagnosis of GC and FIGO score ≥12 points. Those patients are at high risk of resistance to standard multi-drug chemotherapy. It was demonstrated that immunotherapy may be an option for chemoresistant forms of GTN [37]. This is the result of intense immunoreactivity of programmed cell death 1 ligand (PD-L1) in choriocarcinoma syncytiotrophoblasts [38]. Pembrolizumab, a monoclonal antibody against programmed cell death (PD-1), leads to tumor-specific antigen recognition via cytotoxic T cells, triggering an increased immune response and tumor death. Recently, Mangili et al showed that among 7 patients with chemoresistant GC, all of them received CR after pembrolizumab [39]. Avelumab, another PD-L1 inhibitor, administered in patients with GTN resistant to monochemotherapy, led to hCG normalization in 53.5% of patients [40]. However, in patients with resistance to multi-agent chemotherapy, treatment with Avelumab was discontinued due to severe toxicity and disease progression [39]. The limitation of the use of immunotherapy in young women is the unknown impact on fertility and the best timing for subsequent pregnancies [39].
Apart from survival, reproduction results are a significant issue in FST. In our study, the birth rate was 22.22% (4 out of 18 women). Goto et al studied patients with GC who were treated without hysterectomy, of whom 12 (19.4%) died and 50 (80.6%) achieved complete remission after chemotherapy [28]. They compared the birth rate between patients with nonchoriocarcinoma vs choriocarcinoma and found a difference of 69.2% vs 37.09%, respectively. Jiang et al published Chinese data regarding a large cohort of 464 patients diagnosed with GTN, suggesting that the pregnancy rate after fertility preservation treatment is favorable, with the live birth rate being as high as 72.2%. Nevertheless, the authors did not distinguish choriocarcinoma separately [41]. Although the birth rate in patients with choriocarcinoma after treatment was lower than in other types of GTN, it is difficult to clearly demonstrate that the type of neoplasia affects the chance of subsequent childbearing. The low birth rate in GC patients may result from more frequent use of multi-drug chemotherapy, which is associated with a higher incidence of temporary amenorrhea and premature menopause [42]. Rustin et al reported that women with gestational trophoblastic tumors who were administered 3 or more drugs were significantly less likely to have a live birth than those who received methotrexate alone or with only 1 other agent [24]. In addition, ovarian function is more predisposed to deterioration in older women, especially if etoposide is used as part of the regimen [43]. Although the analysis of reproductive outcomes of patients from the MITO-9 study showed no differences in the pregnancy rate between single- or multi-agent chemotherapy [42], a meta-analysis by Tranoulis et al showed a statistically significantly lower pregnancy rate after multi-agent chemotherapy than after single-agent chemotherapy (OR=0.54, 95% CI 0.38 to 0.77,
Lower birth rates may also be related to the advanced age of some patients, as women >40 years are at increased risk of GC, while their reproductive potential is already diminished. It was shown that only 2 factors had a significant impact on the probability of having subsequent pregnancies after GTN treatment: patients’ age and desire for pregnancy [42]. Although many young cancer patients declare a desire for pregnancy before anti-cancer treatment, many of them are never subsequently childbearing [21]. This is not only caused by gonadotoxic treatment but may also result from the impact of treatment on the physical and psychosocial aspects of sexual function [44]. Cancer treatment is associated with fear, anxiety, and depression, which can further decrease sexual desire, function, and frequency [44]. The prevalence of sexual dysfunction in many cancer survivors is underestimated and undertreated [45]. In GTN, 70% of the women experienced no or low sexual desire, while 42% had dyspareunia and 45% had lubrication problems [46]. Moreover, 53% of patients experienced changes in their relationship with their partner within the first year after remission [46].
The assessment of reproductive results primarily focuses on the birth rate; however, perinatal outcomes are also important. Madi et al suggest that available data indicate good perinatal results in pregnancies after GTN treatment [47]. It was shown that the pooled risk of prematurity, stillbirth, and congenital malformation was rather low and was comparable to the general population [47]. No congenital anomalies were diagnosed in neonates delivered by women from our study group; it is difficult to compare pooled data for GTN with choriocarcinoma alone, as its prognosis is usually poorer, especially in high-risk cases and women requiring high-dose chemotherapy. Joneborg et al point out that detailed registration of high-risk GTN is required to obtain more data in those difficult cases and to enable better consultation of patients desiring motherhood in the future [33].
There were several limitations of our study. The ovarian reserve was not assessed before and after treatment, so the natural reproductive potential is difficult to assess. Among patients who were childbearing after treatment, it is unknown whether patients conceived naturally or with assisted reproductive technology (ART). Nevertheless, ART with the patients’ own gametes can only be employed if their fertility is preserved. In the studied time period, oncofertility procedures were not reimbursed in Poland, so most of those pregnancies must have been natural conceptions. Another unknown factor is oocyte donation programs, but there were also no reimbursements for such programs in Poland. Lastly, the number of patients who became pregnant but had a miscarriage or terminated the pregnancy is unknown.
Conclusions
Single-drug treatment of low-risk GC is associated with a relatively high rate of treatment failure. However, considering the gonadotoxicity associated with a multi-agent regimen, treatment with a single-drug regimen should be the first choice in young patients with a pregnancy desire because initial chemotherapy resistance can be overcome by a multi-drug regimen. Patients with high-risk GC, especially those with a FIGO score ≥12, are at an increased risk of death despite treatment with a multi-drug regimen. Those patients remain a clinical challenge, and new therapeutic options, including immune checkpoint inhibitors, should be encouraged.
A minority of patients with a diagnosis of GC will be childbearing after treatment. The reasons related to the lack of subsequent childbearing are multifactorial and may depend on the diagnosis of GC itself, the methods of treatment, the patient’s desire to get pregnant, and psychoemotional factors. Therefore, each patient undergoing FST should be consulted by a multidisciplinary team consisting of a gynecologist, oncologist, reproductive medicine specialist, and psychologist/psychotherapist.
Figures
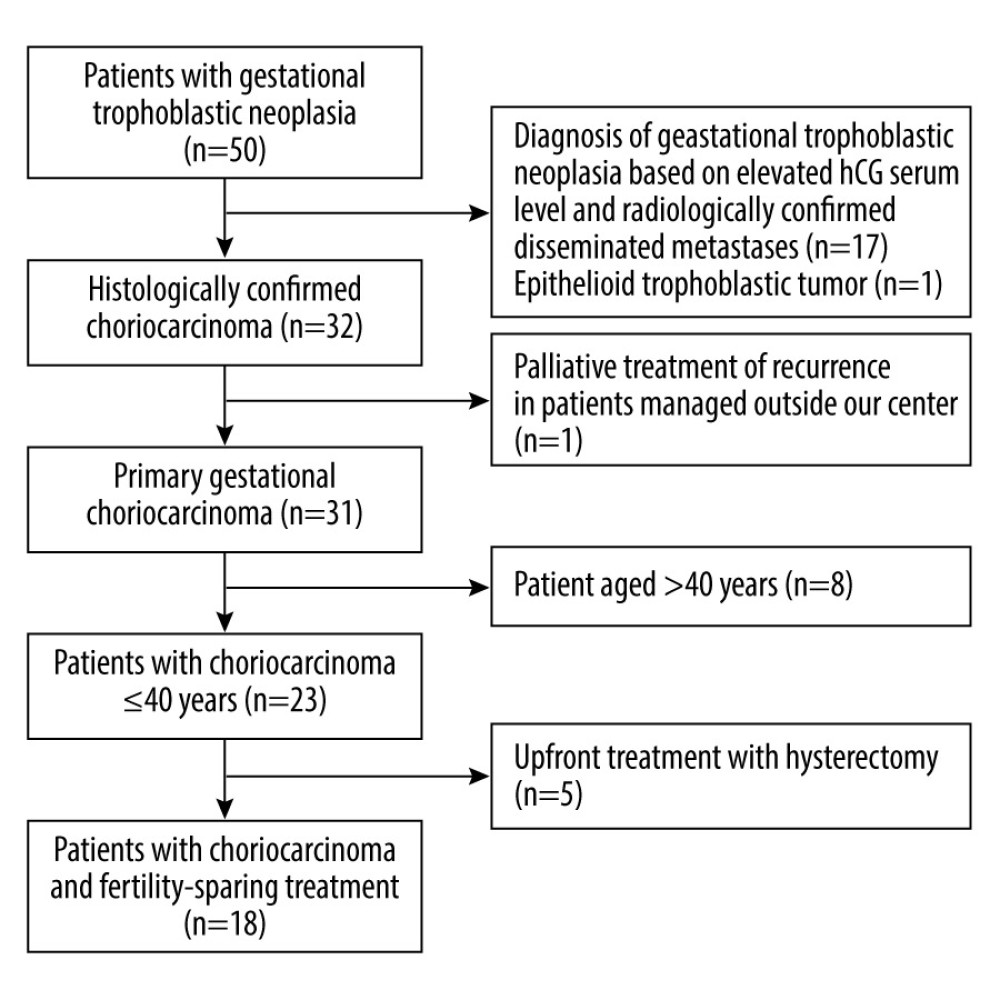 Figure 1. The study flowchart.
Figure 1. The study flowchart. 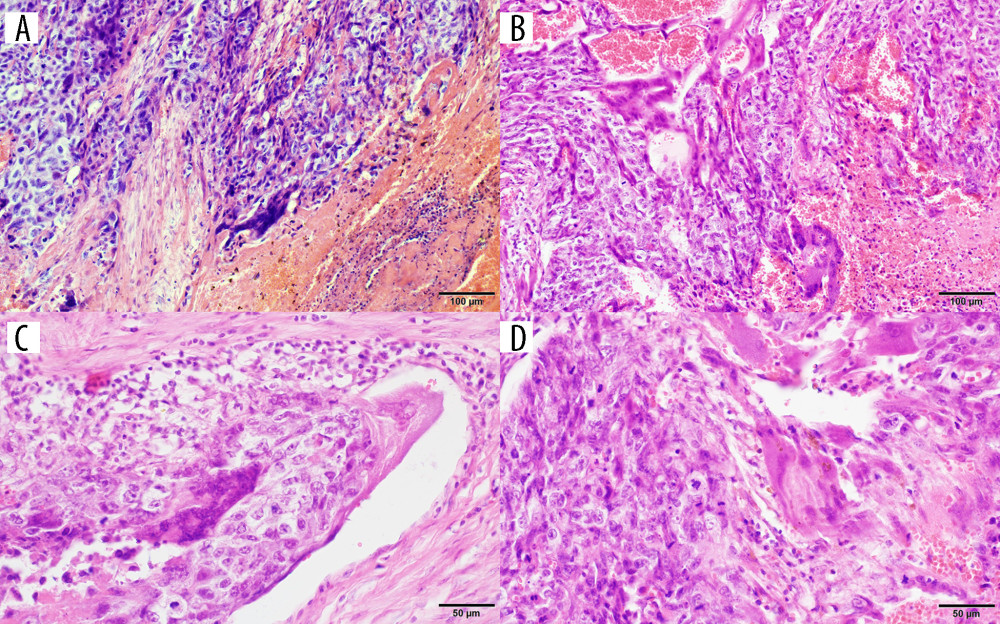 Figure 2. Photomicrographs of gestational choriocarcinoma. (A, B) Sheets of atypical cells, some large and multinucleated (syncytiotrophoblast), and smaller mononuclear cells (cytotrophoblast and intermediate trophoblast). Necrosis and hemorrhage; hematoxylin and eosin (H&E). Magnification ×200. (C) Vascular invasion of gestational choriocarcinoma; hematoxylin and eosin (H&E). Magnification ×400. (D) Marked cytological atypia, numerous mitoses; hematoxylin and eosin (H&E). Magnification ×400.
Figure 2. Photomicrographs of gestational choriocarcinoma. (A, B) Sheets of atypical cells, some large and multinucleated (syncytiotrophoblast), and smaller mononuclear cells (cytotrophoblast and intermediate trophoblast). Necrosis and hemorrhage; hematoxylin and eosin (H&E). Magnification ×200. (C) Vascular invasion of gestational choriocarcinoma; hematoxylin and eosin (H&E). Magnification ×400. (D) Marked cytological atypia, numerous mitoses; hematoxylin and eosin (H&E). Magnification ×400. 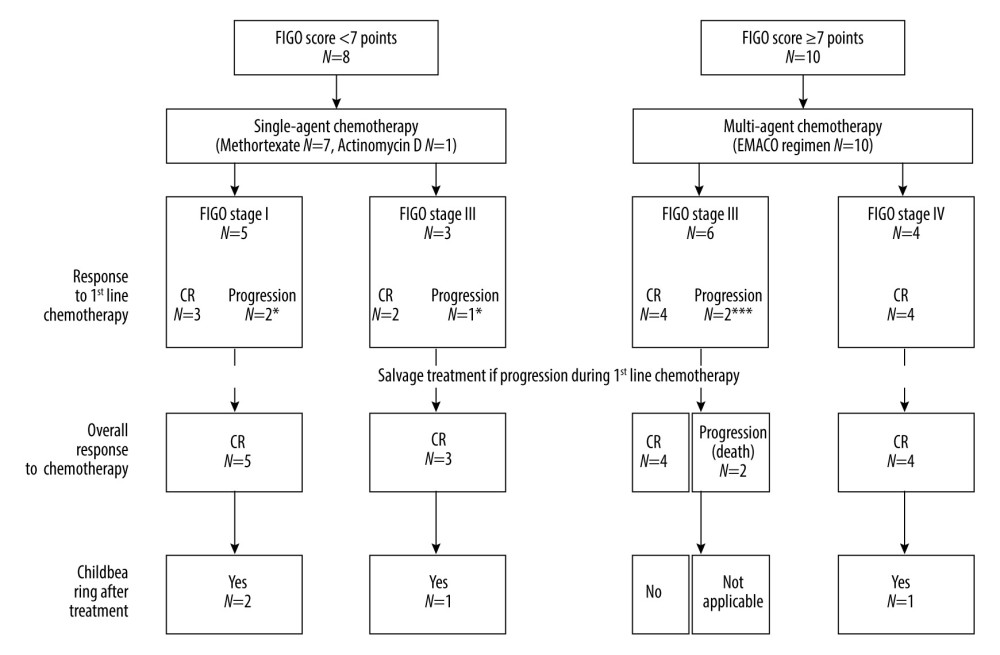 Figure 3. Treatment results and obstetrical outcomes in patients with gestational choriocarcinoma according to International Federation of Gynecology and Obstetrics (FIGO) score and FIGO stage. Response to treatment was assessed according to beta-human chorionic gonadotropin (beta-hCG) serum measurement. Complete response (CR) and progression were considered if beta-hCG dropped to a normal range and increased (or reached plateau), respectively. Patients with progression to first-line chemotherapy underwent salvage treatment: * etoposide, methotrexate, actinomycin D, cyclophosphamide and vincristine (EMACO) regimen to both patients and hysterectomy in one patient; ** EMACO regimen; *** cisplatin, vincristine, methotrexate, and bleomycin (POMB) regimen and hysterectomy were used in 1 patient, while another patient had rapid progression and died without salvage treatment.
Figure 3. Treatment results and obstetrical outcomes in patients with gestational choriocarcinoma according to International Federation of Gynecology and Obstetrics (FIGO) score and FIGO stage. Response to treatment was assessed according to beta-human chorionic gonadotropin (beta-hCG) serum measurement. Complete response (CR) and progression were considered if beta-hCG dropped to a normal range and increased (or reached plateau), respectively. Patients with progression to first-line chemotherapy underwent salvage treatment: * etoposide, methotrexate, actinomycin D, cyclophosphamide and vincristine (EMACO) regimen to both patients and hysterectomy in one patient; ** EMACO regimen; *** cisplatin, vincristine, methotrexate, and bleomycin (POMB) regimen and hysterectomy were used in 1 patient, while another patient had rapid progression and died without salvage treatment. References
1. Ngan HYS, Seckl MJ, Berkowitz RS, Diagnosis and management of gestational trophoblastic disease: 2021 update: Int J Gynecol Obstet, 2021; 155(S1); 86-93
2. Nishino K, Yamamoto E, Ikeda Y, A poor prognostic metastatic nongestational choriocarcinoma of the ovary: A case report and the literature review: J Ovarian Res, 2021; 14(1); 56
3. Weiss S, Amit A, Schwartz MR, Kaplan AL, Primary choriocarcinoma of the vulva: Int J Gynecol Cancer, 2001; 11(3); 251-54
4. Martins VF, Moreno F, Vizcaíno JR, Santos J, Primary gastric choriocarcinoma: A rare case: Int J Surg Case Rep, 2015; 14; 44-47
5. Altieri A, Franceschi S, Ferlay J, Epidemiology and aetiology of gestational trophoblastic diseases: Lancet Oncol, 2003; 4(11); 670-78
6. Smith H, Trends in gestational choriocarcinoma: A 27-year perspective: Obstet Gynecol, 2003; 102(5); 978-87
7. Mangla M, Singla D, Kaur H, Sharma S, Unusual clinical presentations of choriocarcinoma: A systematic review of case reports: Taiwan J Obstet Gynecol, 2017; 56(1); 1-8
8. Soper JT, Gestational trophoblastic disease: Current evaluation and management: Obstet Gynecol, 2021; 137(2); 355-70
9. Cole LA, 28.5 Positive hCG tests: Causes other than pregnancy: Human Chorionic Gonadotropin (hCG), 2015; 297, Elsevier
10. Sasaki S, Management of gestational trophoblastic diseases in Japan – a review: Placenta, 2003; 24(Suppl A); S28-S32
11. Seckl MJ, Sebire NJ, Fisher RA, Gestational trophoblastic disease: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up: Ann Oncol, 2013; 24(Suppl 6); vi39-50
12. Parker VL, Seckl MJ, Hancock BW, Global differences in management and treatment: A critical appraisal from a UK perspective: Gestational Trophoblastic Disease, 2022 Available from: https://isstd.org/gtd-book.html
13. Li J, Yang J, Liu P, Clinical characteristics and prognosis of 272 postterm choriocarcinoma patients at Peking Union Medical College Hospital: A retrospective cohort study: BMC Cancer, 2016; 16(1); 347
14. Powles T, Savage PM, Stebbing J, A comparison of patients with relapsed and chemo-refractory gestational trophoblastic neoplasia: Br J Cancer, 2007; 96(5); 732-37
15. Alifrangis C, Agarwal R, Short D, EMA/CO for high-risk gestational trophoblastic neoplasia: Good outcomes with induction low-dose etoposide-cisplatin and genetic analysis: J Clin Oncol, 2013; 31(2); 280-86
16. Bower M, Newlands ES, Holden L, EMA/CO for high-risk gestational trophoblastic tumors: Results from a cohort of 272 patients: J Clin Oncol, 1997; 15(7); 2636-43
17. Clark RM, Nevadunsky NS, Ghosh S, The evolving role of hysterectomy in gestational trophoblastic neoplasia at the New England Trophoblastic Disease Center: J Reprod Med, 2010; 55(5–6); 194-98
18. Ramesan CK, Thomas DS, Sebastian A, Role of hysterectomy in gestational trophoblastic neoplasia: Indian J Surg Oncol, 2021; 12(2); 386-90
19. Hammond CB, Weed JC, Currie JL, The role of operation in the current therapy of gestational trophoblastic disease: Am J Obstet Gynecol, 1980; 136(7); 844-58
20. Kaal SEJ, Lidington EK, Prins JB, Health-related quality of life issues in adolescents and young adults with cancer: Discrepancies with the perceptions of health care professionals: J Clin Med, 2021; 10(9); 1833
21. Smith KL, Gracia C, Sokalska A, Moore H, Advances in fertility preservation for young women with cancer: Am Soc Clin Oncol Educ Book, 2018; 38; 27-37
22. Piątek S, Szymusik I, Bidziński M, Reproductive results in cancer survivors after fertility sparing management: The need for the standardization of definitions: Cancers (Basel), 2023; 15(14); 3569
23. Tjalma WA, Vermorken JB, The role of hysterotomy in the management of gestational trophoblastic neoplasia: Int J Gynecol Cancer, 2006; 16(2); 882-83
24. Case AM, Wilson S, Colgan TJ, Greenblatt EM, Fertility-sparing surgery, with subsequent pregnancy, in persistent gestational trophoblastic neoplasia: Case report: Hum Reprod, 2001; 16(2); 360-64
25. Rustin GJ, Booth M, Dent J, Pregnancy after cytotoxic chemotherapy for gestational trophoblastic tumours: BMJ, 1984; 288(6411); 103-6
26. Tranoulis A, Georgiou D, Sayasneh A, Tidy J, Gestational trophoblastic neoplasia: A meta-analysis evaluating reproductive and obstetrical outcomes after administration of chemotherapy: Int J Gynecol Cancer, 2019; 29(6); 1021-31
27. Capozzi VA, Butera D, Armano G, Obstetrics outcomes after complete and partial molar pregnancy: Review of the literature and meta-analysis: Eur J Obstet Gynecol Reprod Biol, 2021; 259; 18-25
28. Song H, Wu P, Wang Y, Pregnancy outcomes after successful chemotherapy for choriocarcinoma and invasive mole: Long-term follow-up: Am J Obstet Gynecol, 1988; 158(3); 538-45
29. Goto S, Ino K, Mitsui T, Survival rates of patients with choriocarcinoma treated with chemotherapy without hysterectomy: Effects of anticancer agents on subsequent births: Gynecol Oncol, 2004; 93(2); 529-35
30. Winter MC, Treatment of low-risk gestational trophoblastic neoplasia: Best Pract Res Clin Obstet Gynaecol, 2021; 74; 67-80
31. Fülöp V, Szigetvári I, Szepesi J, The diagnostics and treatment of low-risk gestational trophoblastic neoplasia (GTN): 42-year experience: Eur J Gynaecol Oncol, 2021; 42(6); 1159-65
32. Lurain JR, Elfstrand EP, Single-agent methotrexate chemotherapy for the treatment of nonmetastatic gestational trophoblastic tumors: Am J Obstet Gynecol, 1995; 172(2 Pt 1); 574-79
33. Joneborg U, Coopmans L, Van Trommel N, Fertility and pregnancy outcome in gestational trophoblastic disease: Int J Gynecol Cancer, 2021; 31(3); 399-411
34. Lawrie TA, Alazzam M, Tidy J, First-line chemotherapy in low-risk gestational trophoblastic neoplasia: Cochrane Database Syst Rev, 2016; 2016(6); CD007102
35. Bolze PA, Riedl C, Massardier J, Mortality rate of gestational trophoblastic neoplasia with a FIGO score of ≥13: Am J Obstet Gynecol, 2016; 214(3); 390e1-e8
36. Li J, Yue H, Wang X, Chemotherapy for gestational trophoblastic neoplasia patients with a FIGO score of 12 or greater: A multistudy analysis: Eur J Obstet Gynecol Reprod Biol, 2019; 238; 164-69
37. Huang M, Pinto A, Castillo RP, Slomovitz BM, Complete serologic response to pembrolizumab in a woman with chemoresistant metastatic choriocarcinoma: J Clin Oncol, 2017; 35(27); 3172-74
38. Veras E, Kurman RJ, Wang TL, Shih IM, PD-L1 expression in human placentas and gestational trophoblastic diseases: Int J Gynecol Pathol, 2017; 36(2); 146-53
39. Mangili G, Sabetta G, Cioffi R, Current evidence on immunotherapy for gestational trophoblastic neoplasia (GTN): Cancers (Basel), 2022; 14(11); 2782
40. You B, Bolze PA, Lotz JP, Avelumab in patients with gestational trophoblastic tumors with resistance to single-agent chemotherapy: Cohort A of the TROPHIMMUN phase II trial: J Clin Oncol, 2020; 38(27); 3129-37
41. Jiang F, Yang K, Wan XR, Reproductive outcomes after floxuridine-based regimens for gestational trophoblastic neoplasia: A retrospective cohort study in a national referral center in China: Gynecol Oncol, 2020; 159(2); 464-69
42. Cioffi R, Bergamini A, Gadducci A, Reproductive outcomes after gestational trophoblastic neoplasia. A comparison between single-agent and multiagent chemotherapy: Retrospective analysis from the MITO-9 group: Int J Gynecol Cancer, 2018; 28(2); 332-37
43. Joel SP, Shah R, Clark PI, Slevin ML, Predicting etoposide toxicity: Relationship to organ function and protein binding: J Clin Oncol, 1996; 14(1); 257-67
44. Pereira N, Schattman GL, Fertility preservation and sexual health after cancer therapy: J Oncol Pract, 2017; 13(10); 643-51
45. Hill EK, Sandbo S, Abramsohn E, Assessing gynecologic and breast cancer survivors’ sexual health care needs: Cancer, 2011; 117(12); 2643-51
46. Cagayan MS, Sexual dysfunction as a complication of treatment of gestational trophoblastic neoplasia: J Reprod Med, 2008; 53(8); 595-99
47. Madi JM, Paganella MP, Litvin IE, Perinatal outcomes of first pregnancy after chemotherapy for gestational trophoblastic neoplasia: A systematic review of observational studies and meta-analysis: Am J Obstet Gynecol, 2022; 226(5); 633-45e8
Figures
 Figure 1. The study flowchart.
Figure 1. The study flowchart. Figure 2. Photomicrographs of gestational choriocarcinoma. (A, B) Sheets of atypical cells, some large and multinucleated (syncytiotrophoblast), and smaller mononuclear cells (cytotrophoblast and intermediate trophoblast). Necrosis and hemorrhage; hematoxylin and eosin (H&E). Magnification ×200. (C) Vascular invasion of gestational choriocarcinoma; hematoxylin and eosin (H&E). Magnification ×400. (D) Marked cytological atypia, numerous mitoses; hematoxylin and eosin (H&E). Magnification ×400.
Figure 2. Photomicrographs of gestational choriocarcinoma. (A, B) Sheets of atypical cells, some large and multinucleated (syncytiotrophoblast), and smaller mononuclear cells (cytotrophoblast and intermediate trophoblast). Necrosis and hemorrhage; hematoxylin and eosin (H&E). Magnification ×200. (C) Vascular invasion of gestational choriocarcinoma; hematoxylin and eosin (H&E). Magnification ×400. (D) Marked cytological atypia, numerous mitoses; hematoxylin and eosin (H&E). Magnification ×400. Figure 3. Treatment results and obstetrical outcomes in patients with gestational choriocarcinoma according to International Federation of Gynecology and Obstetrics (FIGO) score and FIGO stage. Response to treatment was assessed according to beta-human chorionic gonadotropin (beta-hCG) serum measurement. Complete response (CR) and progression were considered if beta-hCG dropped to a normal range and increased (or reached plateau), respectively. Patients with progression to first-line chemotherapy underwent salvage treatment: * etoposide, methotrexate, actinomycin D, cyclophosphamide and vincristine (EMACO) regimen to both patients and hysterectomy in one patient; ** EMACO regimen; *** cisplatin, vincristine, methotrexate, and bleomycin (POMB) regimen and hysterectomy were used in 1 patient, while another patient had rapid progression and died without salvage treatment.
Figure 3. Treatment results and obstetrical outcomes in patients with gestational choriocarcinoma according to International Federation of Gynecology and Obstetrics (FIGO) score and FIGO stage. Response to treatment was assessed according to beta-human chorionic gonadotropin (beta-hCG) serum measurement. Complete response (CR) and progression were considered if beta-hCG dropped to a normal range and increased (or reached plateau), respectively. Patients with progression to first-line chemotherapy underwent salvage treatment: * etoposide, methotrexate, actinomycin D, cyclophosphamide and vincristine (EMACO) regimen to both patients and hysterectomy in one patient; ** EMACO regimen; *** cisplatin, vincristine, methotrexate, and bleomycin (POMB) regimen and hysterectomy were used in 1 patient, while another patient had rapid progression and died without salvage treatment. In Press
12 Mar 2024 : Clinical Research
Tissue Inhibitors of Metalloproteinase 1 (TIMP-1) and 3 (TIMP-3) as New Markers of Acute Kidney Injury Afte...Med Sci Monit In Press; DOI: 10.12659/MSM.943500
12 Mar 2024 : Review article
Optimizing Behçet Uveitis Management: A Review of Personalized Immunosuppressive StrategiesMed Sci Monit In Press; DOI: 10.12659/MSM.943240
12 Mar 2024 : Clinical Research
Metabolomic Alterations in Methotrexate Treatment of Moderate-to-Severe PsoriasisMed Sci Monit In Press; DOI: 10.12659/MSM.943360
14 Mar 2024 : Clinical Research
Renal Dysfunction Increases Risk of Adverse Cardiovascular Events in 5-Year Follow-Up Study of Intermediate...Med Sci Monit In Press; DOI: 10.12659/MSM.943956
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952