17 November 2023: Clinical Research
Hypothalamic Injury and Clinical Outcomes in Patients with Post-Traumatic Hyponatremia: A Diffusion Tensor Imaging Case-Control Study
Sung Ho Jang1ABCDEF, Min Soo Kang1ABCDEF*, Kyu Hyang Cho2ADE, Jung Hwan Park1EFDOI: 10.12659/MSM.942397
Med Sci Monit 2023; 29:e942397
Abstract
BACKGROUND: Diffusion tensor imaging (DTI) is an advanced magnetic resonance imaging (MRI) method used to identify changes in microstructures in the brain’s white matter. Severe brain injuries after trauma are associated with disorders of consciousness (DOC) and may result in hyponatremia due to damage to the hypothalamus. This case-control study aimed to use DTI to evaluate the hypothalamus in 36 patients with hyponatremia and DOC due to severe brain injuries.
MATERIAL AND METHODS: Thirty-six patients with DOC after traumatic brain injury (TBI) and 36 healthy control subjects were enrolled in this study. The diagnosis of DOC was based on the coma recovery scale-revised (CRS-R). The 36 patients were divided into 2 groups: Group A (18 with hyponatremia, serum sodium level <135 mmol/L) and group B (18 without hyponatremia). The DTI scans were conducted using a 6-channel head coil on a 1.5T Philips Gyroscan Intera scanner. Among the DTI data, fractional anisotropy (FA) and the apparent diffusion coefficient (ADC) of the hypothalamus were analyzed.
RESULTS: Patient group A had a lower FA value (P=0.044) and higher ADC value (P=0.004) of the hypothalamus and showed a longer length of hospital stay (P=0.03), lower CRS-R score at discharge (P=0.01), and less change in CRS-R score (P=0.004) compared to patient group B. The improvements in the CRS-R score revealed a moderate negative correlation (r=-0.467) with the severity of the hyponatremia (P=0.004).
CONCLUSIONS: Post-traumatic hyponatremia was associated with hypothalamic injury and the presence and severity of hyponatremia were associated with poor clinical outcomes in DOC patients.
Keywords: Brain Injuries, Traumatic, Consciousness Disorders, diffusion tensor imaging, Hyponatremia, Hypothalamus
Background
Traumatic brain injury (TBI) is a leading cause of death and disability worldwide [1]. In the US, there were approximately 223 135 TBI-related hospitalizations in 2019 and 64 362 TBI-related deaths in 2020, according to data from the Centers for Disease Control and Prevention (CDC) [2]. The prevalence of post-traumatic neuroendocrine disorders is high and has been reported to be 50–80% in TBI patients [1,3]. Hyponatremia is the most common electrolyte abnormality, reported in up to 30% of TBI patients due to post-traumatic neuroendocrine disorders [4–7] and is associated with a higher mortality and longer length of hospital stay [8,9]. Previous studies have reported that an injury to the hypothalamic-pituitary axis after TBI can cause endocrine dysfunction, including hyponatremia [10,11]. However, the precise pathophysiological mechanism of post-traumatic hyponatremia in these patients is still unclear [12,13].
The hypothalamus is an important structure of the hypothalamic-pituitary axis and functions as a control center for the autonomic nervous and endocrine systems; hormone secretion, thermoregulation, and the maintaining circadian rhythm are essential roles of the hypothalamus [14,15]. Hypothalamic injury can affect the hypothalamic-pituitary axis, and some disorders can result in hypothalamic hyposecretion, leading to pituitary hyposecretion [14,15]. When a hypothalamic inhibitory hormone is damaged, it can cause pituitary hypersecretion [15]. These hormone imbalances resulting from hypothalamic injury can lead to neuroendocrine disorders associated with hyponatremia [14,15]. However, the anatomical characteristics of the hypothalamus, namely, the very small size and location deep in the white matter, make it difficult to precisely evaluate its neuronal state using conventional brain magnetic resonance imaging (MRI) [14–16]. In contrast, with the introduction of diffusion tensor imaging (DTI), which is one of the MRI techniques that utilizes water molecular diffusion in three-dimensional space, we can indirectly assess the neuroanatomy of white matter, including the precise evaluation of the hypothalamus [17,18]. Several DTI-based studies have reported associations between hypothalamic injury and clinical features such as hypersomnia, fatigue, neurogenic fever, and depression in patients with brain injuries [19–22]. However, no DTI-based studies investigating the relationship between hyponatremia and hypothalamic injury after TBI have been reported to date.
After experiencing diffuse and focal brain injuries as a result of trauma, some patients fail to fully regain alertness or awareness, and they may enter transient or permanent states of disorders of consciousness (DOC), which include coma, the vegetative state, and the minimally conscious state [23]. The prevalence of DOC in TBI was reported to be 12% [1,3]. Hyponatremia can cause cerebral edema, leading to seizures, coma, permanent brain damage, and brain-stem herniation, all of which can affect consciousness [4]. Furthermore, DOC after TBI may be associated with injury to the hypothalamus because it is one of the major components of the ascending reticular activating system (ARAS) pathway and contains crucial arousal-promoting neurons [24–26]. However, no study on the relationship between hyponatremia with hypothalamic injury and DOC after TBI has been reported to date. Therefore, this case-control study aimed to use DTI to evaluate the hypothalamus in 36 patients with hyponatremia and DOC due to severe brain injuries after trauma.
Material and Methods
ETHICS STATEMENT:
This study protocol was reviewed and approved by the Institutional Review Board of Yeungnam University Hospital in Daegu, Korea (No. YUMC 2023-01-041) and conducted in accordance with the Helsinki Declaration of 1975.
INCLUSION AND EXCLUSION CRITERIA:
This retrospective single-center study in a tertiary university hospital from May 2014 to January 2021 enrolled 36 patients (30 males, 6 females; mean age 52.53±17.68 years [19–81 years]) and 36 age- and sex-matched healthy control subjects (28 males, 8 females; mean age 51.89±17.79 years [19–78 years]). Patient recruitment was based on the following inclusion criteria: (1) Diagnosis of TBI requiring hospital admission for management; (2) Diagnosed with DOC after TBI, specifically the vegetative state or minimally conscious state, either at the time of admission to the department of physical medicine and rehabilitation or transferred for rehabilitation after acute management; (3) Follow-up assessments of the level of consciousness and DTI scans conducted during the chronic phase (1–6 months after onset); and (4) Absence of previous medical history affecting the level of consciousness, head trauma, or neurological or psychiatric disease. The exclusion criteria were as follows: (1) Age <19 years, and (2) Previous history of endocrine disease that could cause electrolyte imbalance.
ASSESSMENTS OF HYPONATREMIA:
Hyponatremia was defined as a serum sodium level <135 mmol/L and classified as follows: Mild hyponatremia; 130–135 mmol/L, Moderate hyponatremia; 125–129 mmol/L and Severe hyponatremia <125 mmol/L [4,5,27]. Laboratory tests, including serum sodium level measurements, were conducted on all patients at least twice a week during their hospitalization period. The patients were divided into 2 groups based on their serum sodium levels: group A consisted of 18 patients with moderate to severe hyponatremia (serum sodium <130 mmol/L) in blood tests during hospitalization more than once (mean hyponatremia assessment date; 34.39±17.85 days from admission [12–69 days]), and group B consisted of 18 patients without hyponatremia during hospitalization. In this study, we excluded cases of mild hyponatremia because the mechanism by which hyponatremia affects the brain, resulting in cerebral edema with increased intracranial pressure, is mainly observed when serum sodium levels fall below 125–130 mmol/L [28,29]. A group of 36 individuals served as the healthy control group (Control).
ASSESSMENTS OF THE LEVEL OF CONSCIOUSNESS:
Assessments of the level of consciousness were conducted using the coma recovery scale-revised (CRS-R) [30]. The CRS-R consists of 6 subscales comprising auditory, visual, motor, verbal, communication, and arousal functions (range: 0~23). The total CRS-R score was used for the assessment and was assessed by rehabilitation physicians. Baseline assessments were conducted for all patients at the time of admission. All enrolled patients were in the vegetative state or minimally conscious state at the baseline assessments. Follow-up assessments were conducted when the patients were discharged from the rehabilitation center. Follow-up assessments in the chronic phase of TBI were performed for all the patients at 1–6 months (94.81±34.31 days) after onset. The change in the score between the 2 CRS-R assessments was calculated individually and considered an indicator of improvement in consciousness
DIFFUSION TENSOR IMAGING:
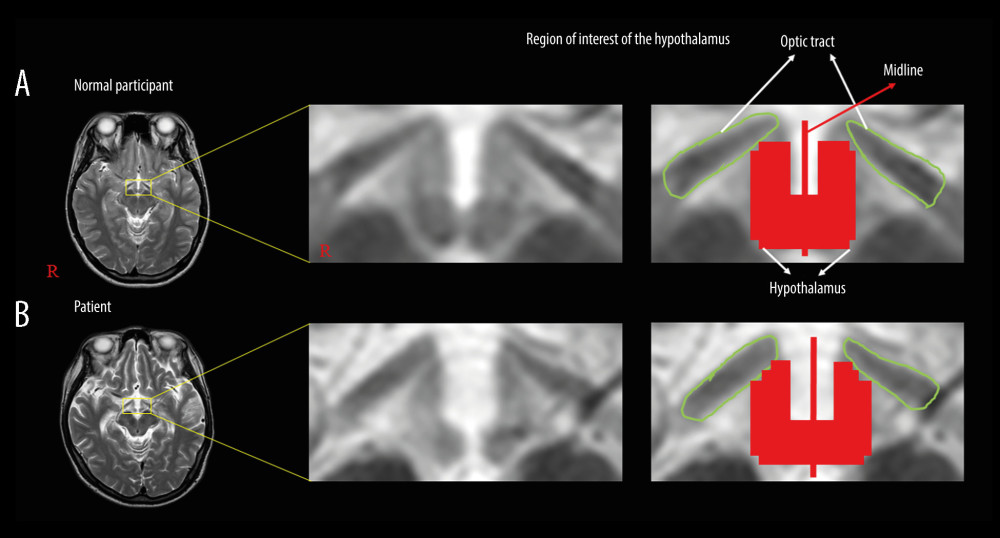
The DTI scans were conducted when the patients were admitted or transferred to the rehabilitation center (77.84±35.08 days after onset) using a 6-channel head coil on a 1.5T Philips Gyroscan Intera scanner (Hoffman-LaRoche, Best, Netherlands) by single-shot, spin-echo planar imaging. Using each of the 32 noncollinear diffusion sensitizing gradients, we acquired 67 contiguous slices that were parallel to the anterior commissure-posterior commissure line. The imaging parameters used were as follows: acquisition matrix; 96×96, reconstructed to matrix; 192×192, field of view; 240×240 mm2, repetition time; 10 398 ms, echo time; 72 ms, parallel imaging reduction factor (SENSE factor); 2, echo planar imaging factor; 59, b; 1000 s/mm2, number of excitations; 1, and slice thickness; 2.5 mm. We used the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library to analyze the acquired DTI data and correct eddy currents. In this study, the region of interest (ROI) for the hypothalamus was defined with the anterior boundary as the optic tract and the posterior boundary as the mammillary body, at the upper midbrain level on the brain magnetic resonance imaging (MRI), and it was manually traced [31] (Figure 1). DTI-Studio software (CMRM, Johns Hopkins Medical Institute, Baltimore, MD) was used for evaluation of the hypothalamus. Of the DTI parameters, fractional anisotropy (FA) and apparent diffusion coefficient (ADC) were obtained in this study.
STATISTICAL ANALYSIS:
Statistical analyses were performed using SPSS, version 22.0 (IBM Corp., Armonk, NY, USA). Average distribution data are represented as mean±SD. In this study, a normal distribution of data was not satisfied in the Kolmogorov-Smirnov test (P<0.05). The Mann-Whitney U test was used to compare continuous variables. The Spearman correlation coefficient was used to evaluate the correlations between the severity of hyponatremia, DTI parameters, and changes in consciousness. The severity of hyponatremia was classified as follows: non-hyponatremia, moderate hyponatremia, and severe hyponatremia. The statistical significance was set at P<0.05. The correlation coefficient was interpreted as follows: strong, ≤0.50; moderate, 0.30–0.49; and weak, 0.10–0.29 [32].
Results
BASELINE CHARACTERISTICS:
Demographic and clinical data of the patients are summarized in Table 1. No significant differences between the 2 patient groups were observed with respect to the demographic characteristics, clinical factors affecting the serum sodium levels, and the level of consciousness at admission (P>0.05).
CLINICAL OUTCOMES BETWEEN 2 PATIENT GROUPS:
Comparisons of the clinical outcomes, including the length of hospital stay and the changes in consciousness at discharge, between patient groups A and B are summarized in Table 1. Patient group A showed a significantly longer length of hospital stay compared to patient group B (107.06±26.98 vs 82.56±37.12, P=0.03). When the CRS-R scores were compared at discharge, patient group A had a significantly lower value than group B (15.72±5.93 vs 20.5±4.09, P=0.01), although there was no significant difference at admission (P>0.05). Moreover, the change in the CRS-R score was significantly lower in the patient group A than the patient group B (11±5.11 vs 16.06±4.56, P=0.004).
DTI PARAMETERS OF THE HYPOTHALAMUS:
Table 2 presents the comparisons of the DTI parameters of the hypothalamus (FA and ADC values) between the patient and control groups. The FA value in the patient groups was significantly lower than that of the control group (P=0.005), while the ADC value in the patient groups was significantly higher than that of the control group (P=0.001). In a comparison of the DTI parameters between the 2 patient groups, the FA value in patient group A showed a significantly lower value than that of patient group B (P=0.044). In contrast, the ADC value in patient group A was significantly higher than that of patient group B (P=0.004).
CORRELATION ANALYSIS:
Correlations between the severity of hyponatremia, DTI parameters of the hypothalamus, and the CRS-R scores at admission and discharge, and the change in scores during this period are shown in Table 3. The severity of hyponatremia had a moderate positive correlation with the ADC value (r=0.475, P=0.003). There were no significant correlations observed among the CRS-R scores at admission, the severity of hyponatremia, and the DTI parameters (P>0.05). The CRS-R score at discharge revealed a moderate negative correlation with the severity of hyponatremia (r=−0.382, P=0.022) and the ADC value (r=−0.340, P=0.042). A moderate negative correlation was observed between the change in the CRS-R score and the severity of hyponatremia (r=−0.467, P=0.004).
Discussion
In this study, we evaluated the relationship between hyponatremia, hypothalamic injury, and DOC in patients with TBI. Our results are summarized as follows: (1) The DTI of the hypothalamus revealed that the patient groups had a lower FA value and higher ADC value compared to the control group. In addition, patient group A (with hyponatremia) displayed a lower FA value and a higher ADC value of the hypothalamus than patient group B (without hyponatremia). Moreover, there was a moderate positive correlation between the severity of hyponatremia and the ADC value derived from the DTI of the hypothalamus. (2) Patient group A showed a longer length of hospital stay, lower CRS-R score at discharge, and less improvement in CRS-R score compared to patient group B. The CRS-R score at discharge had a moderate negative correlation with the severity of hyponatremia and the ADC value of the hypothalamus, and the improvement in the CRS-R score revealed a moderate negative correlation with the severity of hyponatremia.
In previous DTI studies, it has been reported that decreased FA values and increased ADC values of the hypothalamus, when compared to a control group, indicate hypothalamic injuries [19,33]. Additionally, in TBI patients, injuries to the hypothalamic-pituitary axis can result in various neuroendocrine disorders, including hyponatremia [10,11], and patients who experience hypersomnia after TBI exhibit hypothalamic injuries [19,21]. Through this DTI study, we have confirmed that patients with DOC following TBI have hypothalamic injuries, and we have found an association between post-traumatic hyponatremia and hypothalamic injuries.
When assessing hypothalamic injury, signal or structural changes of the hypothalamus in conventional MRI can provide approximate information about the extent of the injury. However, the quantification of the injury is limited because the diffusion image in conventional MRI measures translational displacement of water molecules [17]. In contrast, DTI can provide more precise and quantitative information regarding damaged white matter based on several DTI parameters that are calculated from the analysis of water molecule measurements in multiple directions [17]. Among the DTI parameters, the FA value represents the motional anisotropy of water molecules, reflecting the integrity of the white matter fibers, while the ADC value represents the magnitude of water diffusion in the tissue, reflecting vasogenic edema associated with neuronal injury [17,34]. The decrease in FA value and increase in ADC value of the hypothalamus are suggestive of hypothalamic injury and these results coincided with those from previous studies that reported hypothalamic injuries [21,33]. A lower FA value and a higher ADC value in the patient group compared to the control group indicate that patients with DOC after TBI had hypothalamic injuries. Furthermore, a significantly lower FA value and a higher ADC value of the hypothalamus in patient group A compared to patient group B indicate that among the DOC patients with TBI, patients with moderate to severe hyponatremia have more severe hypothalamic injuries than those without hyponatremia. On the other hand, a moderate positive correlation between the severity of hyponatremia and the ADC value implies that the severity of the hypothalamic injury is associated with the severity of hyponatremia. Thus, our results suggest that injury to the hypothalamus could be one of the pathophysiological mechanisms of hyponatremia in DOC patients following TBI. This conclusion is supported by previous studies on hypothalamic-pituitary dysfunction after TBI [10,11].
The present results showed that the patient group with hyponatremia had a longer length of hospital stay, lower CRS-R score values, and less improvement in the scores at discharge, indicating an association between the presence of hyponatremia and poor clinical outcomes. These results appear to agree with the results of previous studies in which the presence of hyponatremia in patients with TBI was associated with a longer hospital stay [8,9]. The other results, namely, the CRS-R score at discharge and the improvement of the CRS-R score during rehabilitation, showed a moderate negative correlation with the severity of hyponatremia, indicating that more severe hyponatremia is associated with a poorer clinical outcome of DOC in TBI. However, there was no correlation between the severity of hyponatremia and the CRS-R score at admission, suggesting that the severity of hyponatremia was not directly related to the severity of DOC at the initial stage following TBI. On the other hand, our results revealed a moderate negative correlation between the CRS-R score at discharge and the ADC value of the hypothalamus, indicating an association between the severity of the hypothalamic injury and poor clinical outcome in DOC patients with TBI.
After the introduction of DTI as an imaging tool in TBI, a few studies have reported the association between hypothalamic injury and hypersomnia in TBI [19,21] and neurogenic fever in stroke [22]. However, no study on the relationship between a hypothalamic injury and post-traumatic neuroendocrine disorders associated with hyponatremia in TBI has been reported. The present study reveals the association between a hypothalamic injury and post-traumatic hyponatremia using DTI. Moreover, we observed that the presence and severity of hyponatremia were associated with poor clinical outcomes in DOC patients with TBI. To the best of our knowledge, this is the first study to demonstrate the relationship between post-traumatic hyponatremia and hypothalamic injuries using DTI and the impact of hyponatremia on the clinical outcomes in DOC patients with TBI.
However, this study has several limitations that deserve mention. First, the sample size was small. Second, defining the precise region of interest for the analysis of the DTI parameters of the hypothalamus is difficult due to the small size of the hypothalamus and the poor resolution of the images obtained. Third, the DTI parameters of the hypothalamus could be affected by the partial volume effect, such as eddy currents caused by cerebrospinal fluid. Fourth, the severity of the brain injury and the type of brain lesion, such as traumatic intracranial hemorrhage and diffuse axonal injury, were not considered in this study. Lastly, other post-traumatic endocrine disorders other than hyponatremia, such as growth hormone deficiency, gonadotropin deficiency, and secondary adrenal insufficiency, which could cause electrolyte imbalances, were not considered. Further prospective studies involving a larger number of subjects should be encouraged.
Conclusions
In this DTI study, we found a significant association between post-traumatic hyponatremia and hypothalamic injury in DOC patients. Moreover, we found that the presence and severity of hyponatremia were associated with poor clinical outcomes in these patients.
Tables
Table 1. Comparisons of demographic data, clinical factors, level of consciousness assessments, and clinical outcomes between the 2 patient groups.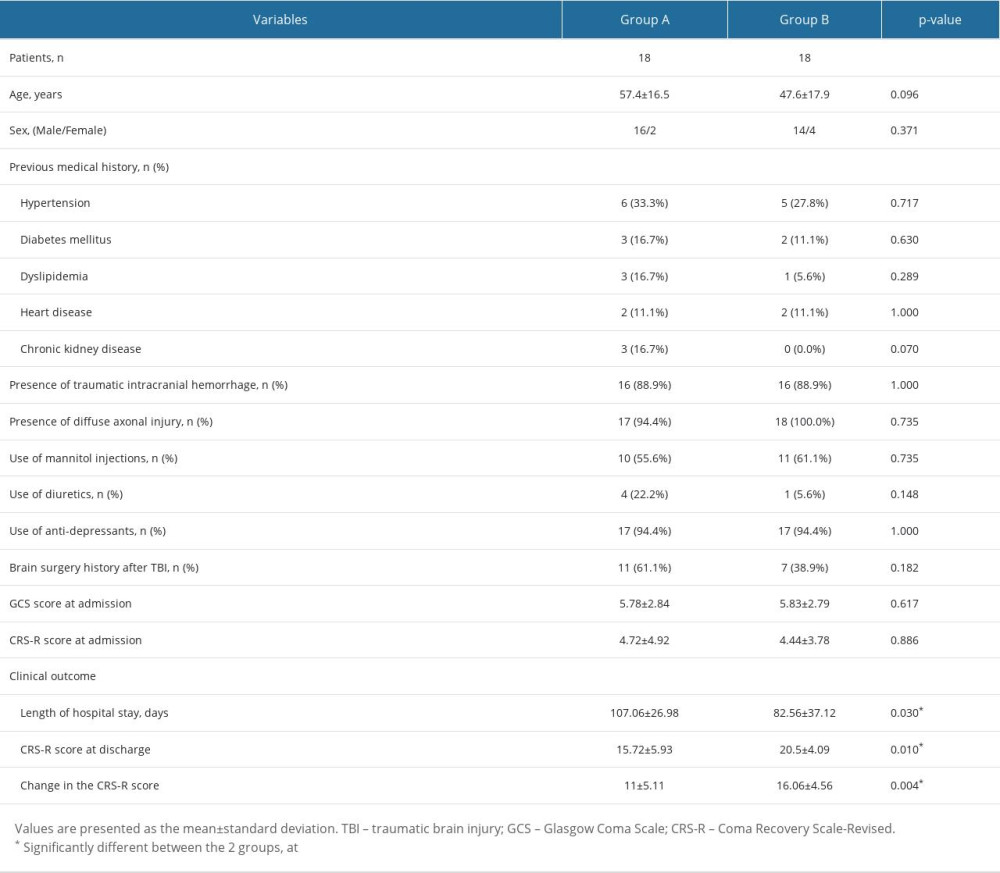 Table 2. Comparisons of diffusion tensor imaging parameters of the hypothalamus between the patient groups and the control group.
Table 2. Comparisons of diffusion tensor imaging parameters of the hypothalamus between the patient groups and the control group.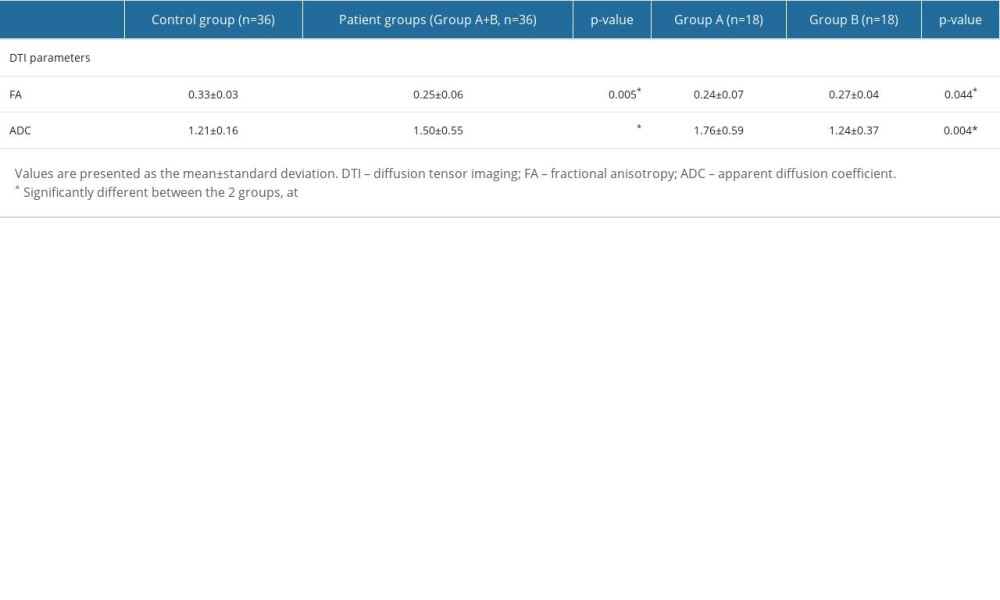 Table 3. Correlations between the severity of hyponatremia, diffusion tensor imaging parameters of the hypothalamus, and the coma recovery scale-revised scores at admission, discharge, and the changes from admission to discharge.
Table 3. Correlations between the severity of hyponatremia, diffusion tensor imaging parameters of the hypothalamus, and the coma recovery scale-revised scores at admission, discharge, and the changes from admission to discharge.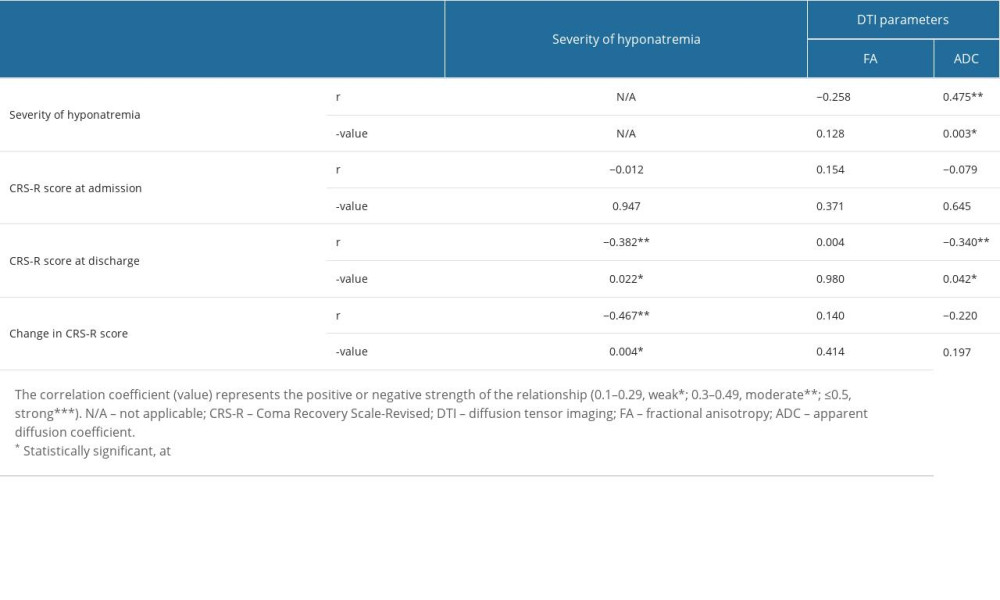
References
1. Capizzi A, Woo J, Verduzco-Gutierrez M, Traumatic brain injury: An overview of epidemiology, pathophysiology, and medical management: Med Clin North Am, 2020; 104(2); 213-38
2. Centers for Disease Control and Prevention (CDC): Traumatic brain injury & concussion, 2023 Available from: https://www.cdc.gov/traumaticbraininjury/index.html
3. Kowalski RG, Hammond FM, Weintraub AH, Recovery of consciousness and functional outcome in moderate and severe traumatic brain injury: JAMA Neurol, 2021; 78(5); 548-57
4. Adrogué HJ, Madias NE, Hyponatremia: N Engl J Med, 2000; 342(21); 1581-89
5. Upadhyay A, Jaber BL, Madias NE, Incidence and prevalence of hyponatremia: Am J Med, 2006; 119(7 Suppl 1); S30-S35
6. Lohani S, Devkota UP, Hyponatremia in patients with traumatic brain injury: Etiology, incidence, and severity correlation: World Neurosurg, 2011; 76(3–4); 355-60
7. Yumoto T, Sato K, Ugawa T, Prevalence, risk factors, and short-term consequences of traumatic brain injury-associated hyponatremia: Acta Med Okayama, 2015; 69(4); 213-18
8. Shanavas C, Noufal B, Jacob P, Rojan K, A Prospective study on hyponatremia in traumatic brain injury: Indian Journal of Neurotrauama, 2016; 13(02); 94-100
9. Smith M, Baltazar GA, Pate A, Hyponatremia on initial presentation correlates with suboptimal outcomes after traumatic brain injury: Am Surg, 2017; 83(4); e126-e28
10. Krahulik D, Zapletalova J, Frysak Z, Vaverka M, Dysfunction of hypothalamic-hypophysial axis after traumatic brain injury in adults: J Neurosurg, 2010; 113(3); 581-84
11. Powner DJ, Boccalandro C, Alp MS, Vollmer DG, Endocrine failure after traumatic brain injury in adults: Neurocrit Care, 2006; 5(1); 61-70
12. Moro N, Katayama Y, Igarashi T, Hyponatremia in patients with traumatic brain injury: Incidence, mechanism, and response to sodium supplementation or retention therapy with hydrocortisone: Surg Neurol, 2007; 68(4); 387-93
13. Kirkman MA, Albert AF, Ibrahim A, Doberenz D, Hyponatremia and brain injury: Historical and contemporary perspectives: Neurocrit Care, 2013; 18(3); 406-16
14. Settle M, The hypothalamus: Neonatal Netw, 2000; 19(6); 9-14
15. Sanchez Jimenez JG, De Jesus O, Hypothalamic dysfunction: StatPearls February 12, 2023, Treasure Island (FL), StatPearls Publishing
16. Baroncini M, Jissendi P, Balland E, MRI atlas of the human hypothalamus: Neuroimage, 2012; 59(1); 168-80
17. Assaf Y, Pasternak O, Diffusion tensor imaging (DTI)-based white matter mapping in brain research: A review: J Mol Neurosci, 2008; 34(1); 51-61
18. Ranzenberger LR, Snyder T, Diffusion tensor imaging: StatPearls July 26, 2022, Treasure Island (FL), StatPearls Publishing
19. Jang SH, Kwon HG, Injury of the ascending reticular activating system in patients with fatigue and hypersomnia following mild traumatic brain injury: Two case reports: Medicine (Baltimore), 2016; 95(6); e2628
20. Jang SH, Kwon HG, Injury of the hypothalamus in patients with hypoxic-ischemic brain injury: A diffusion tensor imaging study: Am J Phys Med Rehabil, 2018; 97(3); 160-63
21. Jang SH, Yi JH, Kim SH, Kwon HG, Relation between injury of the hypothalamus and subjective excessive daytime sleepiness in patients with mild traumatic brain injury: J Neurol Neurosurg Psychiatry, 2016; 87(11); 1260-61
22. Jang SH, Seo YS, Neurogenic fever due to injury of the hypothalamus in a stroke patient: Case report: Medicine (Baltimore), 2021; 100(13); e24053
23. Schnakers C, Monti MM, Disorders of consciousness after severe brain injury: Therapeutic options: Curr Opin Neurol, 2017; 30(6); 573-79
24. Valko PO, Gavrilov YV, Yamamoto M, Damage to arousal-promoting brainstem neurons with traumatic brain injury: Sleep, 2016; 39(6); 1249-52
25. Jang SH, Chang CH, Jung YJ, Relationship between impaired consciousness and injury of ascending reticular activating system in patients with intracerebral hemorrhage: Stroke, 2019; 50(8); 2234-37
26. Jang SH, Kwon HG, The ascending reticular activating system from pontine reticular formation to the hypothalamus in the human brain: A diffusion tensor imaging study: Neurosci Lett, 2015; 590; 58-61
27. Spasovski G, Vanholder R, Allolio B, Clinical practice guideline on diagnosis and treatment of hyponatraemia [published correction appears in Eur J Endocrinol. 2014;171(1):X1]: Eur J Endocrinol, 2014; 170(3); G1-G47
28. Giuliani C, Peri A, Effects of hyponatremia on the brain: J Clin Med, 2014; 3(4); 1163-77
29. Kleindienst A, Hannon MJ, Buchfelder M, Verbalis JG, Hyponatremia in neurotrauma: The role of vasopressin: J Neurotrauma, 2016; 33(7); 615-24
30. Giacino JT, Kalmar K, Whyte J, The JFK Coma Recovery Scale-Revised: Measurement characteristics and diagnostic utility: Arch Phys Med Rehabil, 2004; 85(12); 2020-29
31. Duvernoy Henri M: The human brain: Surface, three-dimensional sectional anatomy with MRI, and blood supply, 2012; 496, Springer Science & Business Media
32. Cohen J: Statistical power analysis for the behavioral sciences, 1988, Hillsdale, NJ, Lawrence Erlbaum Associates
33. Lee SJ, Jang SH, Hypothalamic injury in spontaneous subarachnoid hemorrhage: A diffusion tensor imaging study [published correction appears in Clin Auton Res. 2021;32(2):343]: Clin Auton Res, 2021; 31(2); 321-22
34. Mori S, Crain BJ, Chacko VP, van Zijl PC, Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging: Ann Neurol, 1999; 45(2); 265-69
Tables
 Table 1. Comparisons of demographic data, clinical factors, level of consciousness assessments, and clinical outcomes between the 2 patient groups.
Table 1. Comparisons of demographic data, clinical factors, level of consciousness assessments, and clinical outcomes between the 2 patient groups. Table 2. Comparisons of diffusion tensor imaging parameters of the hypothalamus between the patient groups and the control group.
Table 2. Comparisons of diffusion tensor imaging parameters of the hypothalamus between the patient groups and the control group. Table 3. Correlations between the severity of hyponatremia, diffusion tensor imaging parameters of the hypothalamus, and the coma recovery scale-revised scores at admission, discharge, and the changes from admission to discharge.
Table 3. Correlations between the severity of hyponatremia, diffusion tensor imaging parameters of the hypothalamus, and the coma recovery scale-revised scores at admission, discharge, and the changes from admission to discharge. Table 1. Comparisons of demographic data, clinical factors, level of consciousness assessments, and clinical outcomes between the 2 patient groups.
Table 1. Comparisons of demographic data, clinical factors, level of consciousness assessments, and clinical outcomes between the 2 patient groups. Table 2. Comparisons of diffusion tensor imaging parameters of the hypothalamus between the patient groups and the control group.
Table 2. Comparisons of diffusion tensor imaging parameters of the hypothalamus between the patient groups and the control group. Table 3. Correlations between the severity of hyponatremia, diffusion tensor imaging parameters of the hypothalamus, and the coma recovery scale-revised scores at admission, discharge, and the changes from admission to discharge.
Table 3. Correlations between the severity of hyponatremia, diffusion tensor imaging parameters of the hypothalamus, and the coma recovery scale-revised scores at admission, discharge, and the changes from admission to discharge. In Press
12 Mar 2024 : Database Analysis
Risk Factors of Age-Related Macular Degeneration in a Population-Based Study: Results from SHIP-TREND-1 (St...Med Sci Monit In Press; DOI: 10.12659/MSM.943140
12 Mar 2024 : Clinical Research
Preoperative Blood Transfusion Requirements for Hemorrhoidal Severe Anemia: A Retrospective Study of 128 Pa...Med Sci Monit In Press; DOI: 10.12659/MSM.943126
12 Mar 2024 : Clinical Research
Tissue Inhibitors of Metalloproteinase 1 (TIMP-1) and 3 (TIMP-3) as New Markers of Acute Kidney Injury Afte...Med Sci Monit In Press; DOI: 10.12659/MSM.943500
12 Mar 2024 : Review article
Optimizing Behçet Uveitis Management: A Review of Personalized Immunosuppressive StrategiesMed Sci Monit In Press; DOI: 10.12659/MSM.943240
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952