20 March 2024: Clinical Research
Serum Selenium Concentration as a Potential Diagnostic Marker for Early-Stage Colorectal Cancer: A Comparative Study
Michał ChalcarzDOI: 10.12659/MSM.942882
Med Sci Monit 2024; 30:e942882
Abstract
BACKGROUND: Selenium deficiency is an established risk factor for colorectal cancer. The aim of the present study was to determine selenium levels in blood samples obtained from colorectal cancer patients compared with the levels of this element in the blood of patients who had undergone hernia repair and cholecystectomy.
MATERIAL AND METHODS: The study group consisted of 49 patients diagnosed with colorectal cancer at our institution. The comparison group consisted of 29 and 26 patients undergoing hernia repair and cholecystectomy, respectively. The histological staging level was evaluated on a 4-grade scale. Serum selenium concentration was quantified by inductively coupled mass spectrometry using methane to reduce polyatomic interference.
RESULTS: Colorectal cancer patients had significantly lower serum selenium concentration than the comparison patients (67.24±15.55 μg/L vs 78.81±12.93 μg/L; P<0.001), and selenium concentration was below the reference range in a high percentage of colorectal cancer patients. However, among the colorectal cancer patients, no significant difference in cancer grading was observed according to selenium concentration (P=0.235). Serum selenium concentration in the patients was evaluated on the basis of 5 independent variables (R=0.6250): age (P=0.011), number of leukocytes (P=0.010), family history of cancer (P=0.045), dietary supplements (P=0.023), and exposure to chemical factors (P=0.057).
CONCLUSIONS: This study supports findings from previous studies that low serum selenium levels are associated with colorectal cancer and that selenium deficiency may be a risk factor for colorectal cancer.
Keywords: Colorectal Neoplasms, Neoplasms, Selenium, serum
Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide and the fourth most common cause of cancer-related deaths. The regions with the highest rates of CRC are Europe, Africa, and South America, with the lowest estimated rates in India and South America [1]. While CRC affects both men and women, it is more prevalent in men; the male lifetime risk for developing CRC is 5–6% and increases in those above 50 years of age [1].
Along with age and sex, genetic predisposition may also increase the risk of developing CRC. In addition, proto-oncogenes, such as Kirsten rat sarcoma virus (KRAS), v-raf murine sarcoma viral oncogene homolog B1 (BRAF), or epidermal growth factor receptor (EGFR); mismatched repair genes (hMSH2 and hMLH1); or mutations of oncogenes leading to cancer-promoting proteins, such as adenomatous polyposis coli (APC), dopa decarboxylase (DCC), and tumor protein p53 (tp53), are seen in approximately 85% of sporadic CRCs [2]. Ulcerative colitis, Crohn’s disease (particularly over a long-term course of >10 years), ureterosigmoidostomy, human papillomavirus, and human immunodeficiency virus infection increase the risk of developing CRC [3]. Other significant factors that increase the risk of CRC are poor nutrition, cigarette smoking, lack of physical activity, obesity, diabetes, and alcohol abuse [3]. Selenium deficiency may also increase the risk of all cancers as well as cardiovascular diseases and hypothyroidism [4].
Narod et al conducted a study on a Polish population of women with a history of cancer (n=97) and those without cancer at the time of entry and observation (n=184) and indicated that the optimal serum selenium concentration in women should be in the range of 70–90 μg/L [5]. Concentrations above 90 μg/L were associated with a higher risk of cancer, but the changes were not statistically significant [5]. However, Lenar et al indicated that the risk of developing lung cancer increases with decreasing serum selenium concentrations, taking a concentration of 70 μg/L as the cutoff value [6]. The authors also pointed out that determining the optimal serum selenium concentration is geographically and populationally variable [6]. Peters et al reported that the aforementioned variability is dependent on sex as well as the presence of polymorphic variants of selenoenzymes [7].
In China, as well as in some other Asian regions where selenium deficiency is common, Keshan disease (a congestive cardiomyopathy) and Kashin-Beck disease (articular cartilage dystrophy) are observed. In other regions with selenium deficiencies, higher mortality rates due to cancer and cardiovascular diseases are also observed [8]. Low selenium concentration in blood serum has been observed in patients with acquired immunodeficiency syndrome [9], immune-mediated [10] and vascular system diseases [11], phenylketonuria [12], fibrocystic disease [13], renal failure [14], acute pancreatitis [15], retinopathy [16], rheumatoid arthritis [17], various types of cancer [18], thyroid pathology, and some neurodegenerative diseases [19–21]. Nonetheless, selenium concentrations are relatively high in patients with Parkinson’s or Alzheimer’s disease [20].
Selenium deficiency is also associated with thyroid pathology, which may be due to its antioxidant activity [21]. Both deficiency and excess of selenium in the human body are detrimental, and the safe range between high and low selenium intake is small; therefore, improved nutritional knowledge of selenium is required [21].
The aim of the present study was to compare the selenium levels in blood samples obtained from CRC patients with the levels of this element in the blood of patients who had undergone hernia repair and cholecystectomy in the same medical center.
Material and Methods
ETHICS:
This retrospective, single-center study was conducted following the guidelines of the Helsinki Declaration and approved by the Bioethics Committee of the Medical University of Karol Marcinkowski in Poznań (no. 39/13; 17 December 2021). Data confidentiality and patient anonymity were maintained at all times. Patient-identifying information was deleted before the database was analyzed. Identifying patients individually is impossible, either from this article or in the database. Written informed consent was obtained from all subjects involved in the study.
STUDY DESIGN:
The study group consisted of 49 patients diagnosed with CRC at our institution (36 women and 19 men; 67.04±10.89 years). The diagnosis of CRC was made based on the presence of characteristic symptoms, such as unintentional weight loss, blood in the stool, iron deficiency anemia, and increasing abdominal pain (often at night), as well as a per rectum proctological examination and an endoscopic examination of the lower gastrointestinal tract (colonoscopy), taking a sample for histopathological examination. Since 2000, a screening program for the early detection of colorectal cancer has been implemented in Poland, under which free preventive colonoscopies are performed [22].
As part of secondary prevention, fecal occult blood tests are also recommended for people over 50 years of age. To preoperatively assess the stage of cancer and detect metastases, ultrasound examination and computed tomography (CT) of the abdominal cavity, dual-contrast radiological examination, radiological chest X-ray examination, endoscopic ultrasound, magnetic resonance imaging, and positron emission tomography (PET) were also used [22]
The comparison group consisted of 55 patients: 29 patients had undergone hernia repair and 26 patients had undergone cholecystectomy (34 women and 15 men; 68.29±13.70 years). Before joining the study, members of the comparison group were asked about their current or past illnesses, including cancer and inflammatory diseases. The inclusion and exclusion criteria for both groups are presented in Table 1.
EVALUATION OF THE SELENIUM CONCENTRATION IN SERUM SAMPLES OBTAINED FROM CRC PATIENTS AND COMPARISON GROUPS:
Serum selenium concentration was quantified by inductively coupled mass spectrometry (ICP-MS NexION 350D, Perkin Elmer, Waltham, Massachusetts, USA) using methane to reduce polyatomic interference. Calibration standards were prepared by diluting 10 mg/L Multi-Element Calibration Standard 3 (PerkinElmer Pure Plus, PerkinElmer Life and Analytical Sciences, Waltham, Massachusetts, USA) with 0.65% nitric acid solution (Suprapur, Merck, Germany) and 0.002% Triton X-100 (PerkinElmer, Tokyo, Japan). Calibration curves were created using 4 different concentrations: 0.1 g/L, 0.5 g/L, 1 g/L, 2 g/L. Germanium (PerkinElmer Pure, PerkinElmer Life and Analytical Sciences, Waltham, Massachusetts, USA) was used as the internal standard, and ClinChek Plasma Control Level I (Recipe, Germany) was used as the reference material [23].
Reference values for selenium concentrations, taking sex into account, were obtained from the “Innovative Medicine” diagnostic laboratory belonging to READ-GENE S.A. in Szczecin, Poland. These values were 75–85 μg/L for women and 85–115 μg/L for men. For each biological test, 3 technical repeats were performed.
CANCER PATHOHISTOLOGICAL GRADING SYSTEM:
Tissue material was prepared according to standard histological procedures. Preparations for histopathological examination were stained with hematoxylin and eosin according to the recommendations of Feldman et al [24].
In the histopathological examination detecting adenocarcinoma in patients, the following microscopic evaluation system was used: G1 – a high degree of histological differentiation and containing over 95% glandular structures; G2 – an average degree of histological differentiation and containing from 50% to 95% glandular structures; G3 – a low degree of differentiation and containing 5% to 50% glandular structures; G4 – undifferentiated, containing less than 5% glandular structures. Of the 49 patients, stage G1 CRC was diagnosed in 4 patients, stage G2 in 18 patients, stage G3 in 21 patients, and stage G4 in 6 patients.
TUMOR, MODE, METASTASIS (TNM) GRADING SYSTEM:
The clinical advancement of CRC was based on the Dukes’ classification as modified by Astler and Coller [25]. The number of samples corresponding to stage I – T1N0M0 (group A according to Dukes’ original classification and as modified by Astler and Coller) was 21 samples; for stage I – T2N0M0 (group A according to Dukes’ original classification and as modified by Astler and Coller), 19 samples; for stage IIA – T3N0M0 (group B according to Dukes’ original classification and B2 in the classification as modified by Astler and Coller), 7 samples; for stage IIB – T4aN0M0 (group B according to Dukes’ original classification and B2 in the classification as modified by Astler and Coller), 5 samples; for stage IIC – 4N0M0 (group B according to Dukes’ original classification and B3A in the classification as modified by Astler and Coller), 4 samples.
STATISTICAL ANALYSIS:
Statistical analyses were performed using the Statistica 12 software package (StatSoft, Krakow, Poland). Statistical significance was defined as
SAMPLE SIZE CALCULATION:
Considering that there are about 20,000 new cases of CRC in Poland each year according to official data, the minimum number of participants required in this study was calculated to be 47 using the Kalkulator doboru próby bioinformatics tool [26].
Results
SELENIUM CONCENTRATION IN BLOOD SAMPLES FROM CRC PATIENTS AND FROM THE COMPARISON GROUPS:
Mean selenium concentration differed significantly between both groups; 67.24±15.55 μg/L in the study group vs 78.81±12.93 μg/L in the comparison group (Figure 1; P<0.001). Selenium concentrations in the CRC patients were seldom within the reference range (14% in the CRC patients vs 42% in the comparison group; Figure 2; P=0.004) and were below the reference range significantly more often than in the comparison group (82% vs 55%; Figure 2).
CONCENTRATION OF SELENIUM DEPENDING ON CANCER GRADING:
Within the CRC group, no significant difference in selenium concentration was observed between patients of different grades of cancer. In G1 patients, the selenium concentration was 64.98±12.40 μg/L; in G2 patients, the selenium concentration was 69.68±16.30 μg/L; in G3 patients, the selenium concentration was 65.99±14.52 μg/L, and in G4 patients, the selenium concentration was 66.30±14.89 μg/L (one-way ANOVA test;
CONCENTRATION OF SELENIUM DEPENDING ON TNM GRADING SYSTEM:
Significant differences between CRC patients were also not observed when TNM classification was used as a criterion for division; selenium concentrations between samples classified into different groups using the TNM grading system were not statistically significantly different (one-way ANOVA test;
MULTIPLE REGRESSION ANALYSIS OF SELENIUM CONCENTRATIONS IN PATIENTS’ BLOOD SERUM:
Selenium concentrations in the blood serum of patients with gallstones or hernias was defined as R=0.5272 based on 2 independent variables: leukocyte number (
where Le is leucocyte number (×103/μL), and S is sex.
The selenium concentration in CRC patients was defined as R=0.6250 based on 5 independent variables: age (
where A is age, Le is the leukocyte number (×103/μL), FHoC is family history of cancer, IoDS is dietary supplements, and ECHS is exposure to chemical factors.
PEARSON CORRELATION ANALYSIS BETWEEN SELENIUM CONCENTRATION AND HISTOPATHOLOGICAL DIFFERENTIATION AND CLINICAL STAGE OF CRC:
Serum selenium levels were negatively correlated with the tumor T stage, but the correlation was statistically insignificant (r=−0.052;
Discussion
Selenium has a strong antioxidant effect, although its excess causes toxic effects. The main sources of this element are meat, seafood, offal, fish, cereal products, nuts, garlic, mushrooms, and green peas [27]. It should be remembered that selenium content in food depends on its content in the soil, and the absorption of this microelement decreases after consuming large doses of ascorbic acid (vitamin C) and sweets [27]. Selenium in the form of selenocysteine is part of many biochemical pathways. In most enzymes containing selenium (called selenoenzymes), this trace element is the active center for oxidation and reduction reactions, thanks to which it has the ability to scavenge free radicals [28].
Moreover, in areas where selenium deficiency is observed, increased mortality due to cardiovascular diseases and cancer has been observed. It is believed that selenium supplementation and eating fish or foods rich in selenium and folic acid are factors modifying the incidence and development of colorectal cancer [29]. This is because folate plays an important role in the synthesis, repair, and methylation of deoxyribonucleic acid (DNA) [30]. Moreover, selenium protects against oxidative stress because it quenches the reactions of free radicals and protects enzymatic systems against their actions. Deficiencies of this element weaken the enzymatic activity of proteins involved in detoxification processes, which is a factor in the development of cancer [31]. Therefore, taking into account the information from the available literature, the examination of selenium concentrations in patients with CRC is fully justified.
In our study, we showed significantly lower concentrations of selenium in serum samples obtained from patients with CRC compared with samples obtained from the comparison groups (
Importantly, when interpreting the results of selenium concentration and assessing the relationship between its concentration and the risk of cancer, factors such as geographical location and the study population should be taken into account [39–42]. Determining the concentration of selenium in serum may meet the assumptions of a biomarker in the assessment of the risk of cancer, but it is important to remember that serum level also reflects the content of this microelement in food, which is determined on the basis of the selenium content in the soil and depends on geographical location [43]. Most studies that have not demonstrated a protective effect of selenium were conducted in populations living in regions where the selenium content in the soil is high and/or there is a high rate of supplement use, including supplements containing selenium. Therefore, the lower concentration of selenium in the serum of residents of Poland or Estonia, where the selenium content is lower than in North American countries, should not be surprising [44,45]. Based on data from the National Health and Nutritional Examination Survey conducted in the United States, the average concentration of selenium in the serum of women aged 40 years and older was 134.7 μg/L [46]. Therefore, selenium supplementation can be justified in people whose microelement concentration is in the lowest tertile (≤105.2 ng/mL) [47]. Narod et al showed that selenium supplementation in the Polish population should be considered in people with serum selenium concentration below 70 μg/L, with the aim of maintaining the concentration in the range of 70–90 μg/L [48].
The fact that patients with gallstones or hernias had higher selenium concentrations than CRC patients is likely due to the malabsorption of selenium in CRC patients. It is likely that the difference in mean selenium concentrations in blood serum between CRC patients and the comparison group is due to the difference in selenium absorption between the 2 groups. The most probable reason is that the obtained differentiation in patients’ multiple regression analysis results from the etiology of the examined diseases. There may be differences in selenium absorption and use in patients with the diseases examined in our study. We could not determine why the serum selenium concentration of patients with gallstones and hernias was influenced by only 2 dependent variables (leukocyte number and sex), while in CRC patients, it was influenced by 5 dependent variables (age, leukocyte number, family history of cancer, dietary supplements, and exposure to chemical factors).
In our study, we found no significant difference in serum selenium concentration in CRC patients depending on the histopathological grade of the differentiated cancer (G1–G4) or the clinical grade of the cancer (TNM system). Statistical analyses performed showed no statistically significant correlation between patients’ blood selenium levels and either the degree of histopathological differentiation of CRC or the clinical stage of the lesion (TNM system). This indicates that determination of selenium levels can be helpful in capturing patients with CRC, but has little value in differentiating tumor grade or histopathological differentiation of CRC. Similarly, in previous studies, no correlation was found between selenium and the tumor pathological subtype in either breast cancer or papillary thyroid samples [49,50].
The first limitation of the present study is that it included only the Polish population, which in general has a relatively low selenium concentration. The second significant limitation is the study’s retrospective nature, which did not allow for the assessment of selenium concentration before the occurrence of colorectal cancer in patients living in a country with a proven low selenium content. The third limitation of the study is the number of participants in both groups. The sample size of the examined group of patients was used as the average sample size. The sample size of the comparison group was higher than that used by Shehata et al [51] and Utiyam et al [52] but lower than that in other related studies [53,54]. However, the total number of patients in CRC-related studies [54–58] was higher than the number of patients examined in the aforementioned studies, and the patient number ranged from 50 [59] to 150 patients [60].
Fourth, a limitation of our analysis is that we did not include a control group of healthy volunteers in the study, which we will take into account in future research projects. Nevertheless, Narod et al used healthy volunteers as controls and noted the same pattern of selenium concentrations in the study and comparison groups [50] as in our observation, which indicates our study’s importance in the context of understanding the role of selenium in the process of carcinogenesis.
Conclusions
This study supports findings from previous studies that low serum selenium levels are associated with CRC, and that selenium deficiency may be a risk factor for CRC. Importantly, when interpreting results related to selenium concentration and assessing the relationship between its concentration and the risk of cancer, factors such as geographical location and the study population should be considered.
Figures
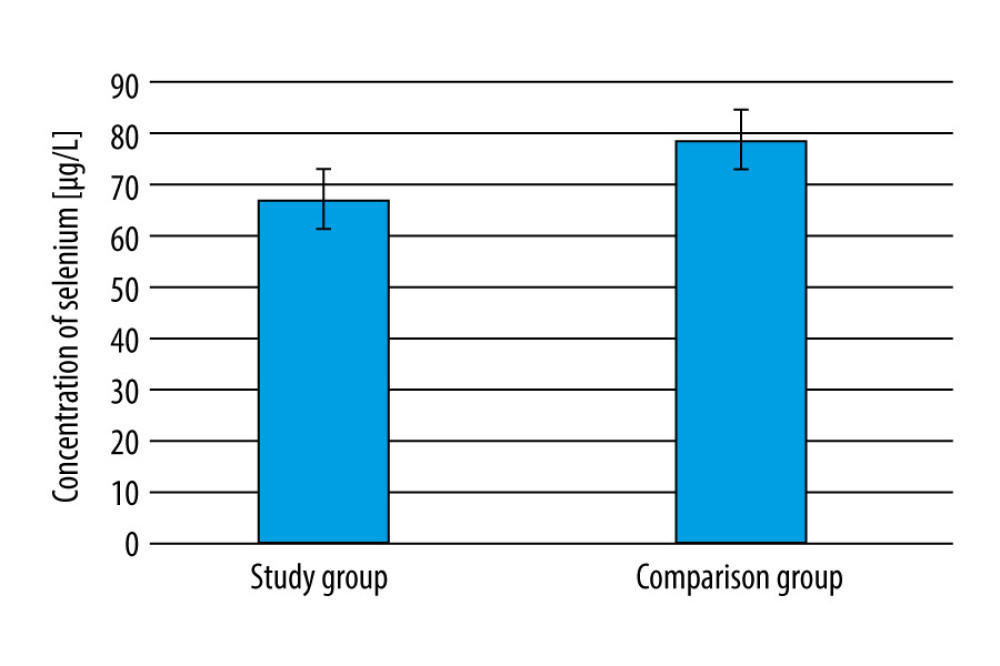 Figure 1. Differences in selenium concentration in blood samples obtained from the colorectal cancer patients and comparison groups.
Figure 1. Differences in selenium concentration in blood samples obtained from the colorectal cancer patients and comparison groups. 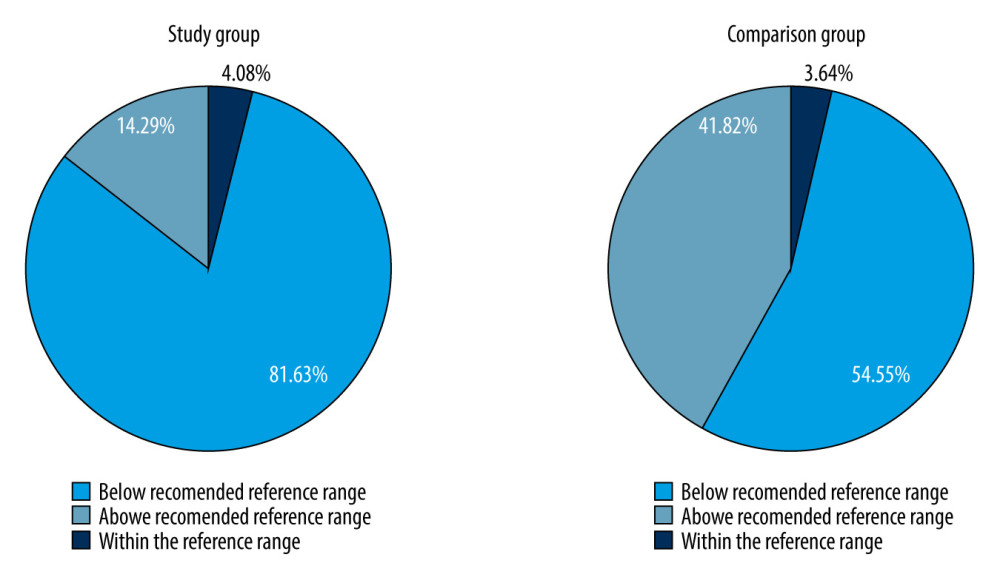 Figure 2. Percentage of patients in the colorectal cancer patients group and the comparison groups with selenium levels that were within normal, below normal, or above normal range.
Figure 2. Percentage of patients in the colorectal cancer patients group and the comparison groups with selenium levels that were within normal, below normal, or above normal range. References
1. Haggar FA, Boushey RP, Colorectal cancer epidemiology: Incidence, mortality, survival, and risk factors: Clin Colon Rectal Surg, 2009; 22(4); 191-97
2. Armaghany T, Wilson JD, Chu Q, Mills G, Genetic alterations in colorectal cancer: Gastrointest Cancer Res, 2012; 5(1); 19-27
3. Tan J, Chen YX, Dietary and lifestyle factors associated with colorectal cancer risk and interactions with microbiota: Fiber, red or processed meat and alcoholic drinks [published correction appears in Gastrointest Tumors. 2018;4(3–4):104]: Gastrointest Tumors, 2016; 3(1); 17-24
4. Ala M, Kheyri Z, The rationale for selenium supplementation in inflammatory bowel disease: A mechanism-based point of view: Nutrition, 2021; 85; 111153
5. Narod SA, Huzarski T, Jakubowska A, Serum selenium level and cancer risk: A nested case-control study: Hered Cancer Clin Pract, 2019; 17; 33
6. Lener M, Muszyńska M, Jakubowska A, Selenium as a marker of cancer risk and of selection for control examinations in surveillance: Contemp Oncol (Pozn), 2015; 19(1A); A60-A61
7. Peters U, Takata Y, Selenium and the prevention of prostate and colorectal cancer: Mol Nutr Food Res, 2008; 52(11); 1261-72
8. Ullah H, Liu G, Yousaf B, Developmental selenium exposure and health risk in daily foodstuffs: A systematic review and meta-analysis: Ecotoxicol Environ Saf, 2018; 149; 291-306
9. Ubesie AC, Ibe BC, Emodi IJ, Iloh KK, Serum selenium status of HIV-infected children on care and treatment in Enugu, Nigeria: S Afr J Child Health, 2017; 11; 21-25
10. Sahebari M, Rezaieyazdi Z, Khodashahi M, Selenium and autoimmune diseases: A review article: Curr Rheumatol Rev, 2019; 15(2); 123-34
11. Hu XF, Sharin T, Chan HM, Dietary and blood selenium are inversely associated with the prevalence of stroke among Inuit in Canada: J Trace Elem Med Biol, 2017; 44; 322-30
12. Okano Y, Hattori T, Fujimoto H, Nutritional status of patients with phenylketonuria in Japan: Mol Genet Metab Rep, 2016; 8; 103-10
13. Wallach JD, Lan M, Yu WH, Common denominators in the etiology and pathology of visceral lesions of cystic fibrosis and Keshan disease: Biol Trace Elem Res, 1990; 24(3); 189-205
14. Joyce T, Court Brown F, Wallace D, Trace element and vitamin concentrations in paediatric dialysis patients: Pediatr Nephrol, 2018; 33(1); 159-65
15. Musil F, Zadák Z, Solichová D, Dynamics of antioxidants in patients with acute pancreatitis and in patients operated for colorectal cancer: A clinical study: Nutrition, 2005; 21(2); 118-24
16. Longo-Mbenza B, Mvitu Muaka M, Masamba W, Retinopathy in non diabetics, diabetic retinopathy and oxidative stress: A new phenotype in Central Africa?: Int J Ophthalmol, 2014; 7(2); 293-301
17. Yu N, Han F, Lin X, The association between serum selenium levels with rheumatoid arthritis: Biol Trace Elem Res, 2016; 172(1); 46-52
18. Lener MR, Gupta S, Scott RJ, Can selenium levels act as a marker of colorectal cancer risk?: BMC Cancer, 2013; 13; 214
19. Helveston W, Cibula JE, Hurd R, Abnormalities of antioxidant metabolism in a case of Friedreich’s disease: Clin Neuropharmacol, 1996; 19(3); 271-75
20. Hemmati-Dinarvand M, Taher-Aghdam AA, Mota A, Dysregulation of serum NADPH oxidase1 and ferritin levels provides insights into diagnosis of Parkinson’s disease: Clin Biochem, 2017; 50(18); 1087-92
21. Squadrone S, Brizio P, Mancini C, Blood metal levels and related antioxidant enzyme activities in patients with ataxia telangiectasia: Neurobiol Dis, 2015; 81; 162-67
22. Jaroszyńska Z, Wiśniewska K, Epidemiologia raka jelita grubego (C18–C21) w Polsce: J Educ Health Sport, 2021; 11(10); 143-56 [in Polish]
23. Rogoża-Janiszewska E, Malińska K, Baszuk P, Serum seleniumlevel and 10-year survival after melanoma: Biomedicines, 2021; 9(8); 991
24. Feldman AT, Wolfe D, Tissue processing and hematoxylin and eosin staining: Methods Mol Biol, 2014; 1180; 31-43
25. Banias L, Jung I, Chiciudean R, Gurzu S, From Dukes-MAC Staging System to Molecular Classification: Evolving concepts in colorectal cancer: Int J Mol Sci, 2022; 23(16); 9455
26. : Kalkulator doboru próby [in Polish]https://www.naukowiec.org/dobor.html
27. Zofkova I, Davis M, Blahos J, Trace elements have beneficial, as well as detrimental effects on bone homeostasis: Physiol Res, 2017; 66(3); 391-402
28. Zhao H, Xu C, Wang T, Liu J, Biomimetic construction of artificial selenoenzymes: Biomimetics (Basel), 2023; 8(1); 54
29. Nawi AM, Chin SF, Azhar Shah S, Jamal R, Tissue and serum trace elements concentration among colorectal patients: A systematic review of case-control studies: Iran J Public Health, 2019; 48(4); 632-43
30. Duthie SJ, Narayanan S, Sharp L, Folate, DNA stability and colorectal neoplasia: Proc Nutr Soc, 2004; 63(4); 571-78
31. Avery JC, Hoffmann PR, Selenium, selenoproteins, and immunity: Nutrients, 2018; 10(9); 1203
32. Connelly-Frost A, Poole C, Satia JA, Selenium, apoptosis, and colorectal adenomas: Cancer Epidemiol Biomarkers Prev, 2006; 15(3); 486-93
33. Reid ME, Duffield-Lillico AJ, Sunga A, Selenium supplementation and colorectal adenomas: An analysis of the nutritional prevention of cancer trial: Int J Cancer, 2006; 118(7); 1777-81
34. Clark LC, Hixson LJ, Combs GF, Plasma selenium concentration predicts the prevalence of colorectal adenomatous polyps: Cancer Epidemiol Biomarkers Prev, 1993; 2(1); 41-46
35. Juloski JT, Rakic A, Ćuk VV, Colorectal cancer and trace elements alteration: J Trace Elem Med Biol, 2020; 59; 126451
36. Lavilla I, Costas M, Miguel PS, Elemental fingerprinting of tumorous and adjacent non-tumorous tissues from patients with colorectal cancer using ICP-MS, ICP-OES and chemometric analysis: Biometals, 2009; 22(6); 863-75
37. Peters KM, Carlson BA, Gladyshev VN, Tsuji PA, Selenoproteins in colon cancer: Free Radic Biol Med, 2018; 127; 14-25
38. Hughes DJ, Kunická T, Schomburg L, Expression of selenoprotein genes and association with selenium status in colorectal adenoma and colorectal cancer: Nutrients, 2018; 10(11); 1812
39. Gómez-Tomás Á, Pumarega J, Alguacil J, Concentrations of trace elements and KRAS mutations in pancreatic ductal adenocarcinoma: Environ Mol Mutagen, 2019; 60(8); 693-703
40. Hughes DJ, Duarte-Salles T, Hybsier S, Prediagnostic selenium status and hepatobiliary cancer risk in the European Prospective Investigation into Cancer and Nutrition cohort: Am J Clin Nutr, 2016; 104(2); 406-14
41. Matthews NH, Koh M, Li WQ, A prospective study of toenail trace element levels and risk of skin cancer: Cancer Epidemiol Biomarkers Prev, 2019; 28(9); 1534-43
42. Vinceti M, Filippini T, Del Giovane C, Selenium for preventing cancer: Cochrane Database Syst Rev, 2018; 1(1); CD005195
43. Rayman MP, Food-chain selenium and human health: Emphasis on intake: Br J Nutr, 2008; 100(2); 254-68
44. Rayman MP, Selenium in cancer prevention: A review of the evidence and mechanism of action: Proc Nutr Soc, 2005; 64(4); 527-42
45. Jaworska K, Gupta S, Durda K, A low selenium level is associated with lung and laryngeal cancers [published correction appears in PLoS One. 2013;8(8)]: PLoS One, 2013; 8(3); e59051
46. Laclaustra M, Stranges S, Navas-Acien A, Serum selenium and serum lipids in US adults: National Health and Nutrition Examination Survey (NHANES) 2003–2004: Atherosclerosis, 2010; 210(2); 643-48
47. Duffield-Lillico AJ, Reid ME, Turnbull BW, Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: A summary report of the Nutritional Prevention of Cancer Trial: Cancer Epidemiol Biomarkers Prev, 2002; 11(7); 630-39
48. Ge S, Zhao J, Yao J, The association between serum selenium levels and pathological features of papillary thyroid cancer in 284 patients: Front Endocrinol (Lausanne), 2023; 14; 1242250
49. Sandsveden M, Nilsson E, Borgquist S, Prediagnostic serum selenium levels in relation to breast cancer survival and tumor characteristics [published correction appears in Int J Cancer. 2021;149(1):E1–E2]: Int J Cancer, 2020; 147(9); 2424-36
50. Narod SA, Huzarski T, Jakubowska A, Serum selenium level and cancer risk: A nested case-control study: Hered Cancer Clin Pract, 2019; 17; 33
51. Shehata MS, Elkordy M, Nafe MA, Radwan M, Cyanoacrylate glue mesh fixation versus suture mesh fixation in open inguinal hernia repair: Egypt J Hosp Med, 2017; 69; 2502-11
52. Utiyama EM, Damous SR, Tanaka EY, Early assessment of bilateral inguinal hernia repair: A comparison between the laparoscopic total extraperitoneal and Stoppa approaches: J Minim Access Surg, 2016; 12(3); 271-77
53. Bulbuller N, Kirkil C, Godekmerdan A, The comparison of inflammatory responses and clinical results after groin hernia repair using polypropylene or polyester meshes: Indian J Surg, 2015; 77(Suppl 2); 283-87
54. Helvaci MR, Ozkan OV, Akkucuk S, Inguinal hernia may not have a chronic low-grade inflammatory background on vascular endothelium: ME-JFM, 2018; 16; 32-36
55. Denić K, Tarabar D, Obradović S, Biochemical liver function test parameters do not indicate any difference in the degree of hepatotoxicity in patients with metastatic colorectal carcinoma treated with conventional anticancer drugs regardless the use of bevacizumab: Vojnosanit Pregl, 2017; 74; 757-62
56. Huang C, Huang R, Jiang T, Laparoscopic and open resection for colorectal cancer: An evaluation of cellular immunity: BMC Gastroenterol, 2010; 10; 127
57. Jakobs TF, Paprottka KJ, Raeßler F, Robust evidence for long-term survival with 90Y radioembolization in chemorefractory liver-predominant metastatic colorectal cancer: Eur Radiol, 2017; 27(1); 113-19
58. Shimura T, Shibata M, Gonda K, Association between circulating galectin-3 levels and the immunological, inflammatory and nutritional parameters in patients with colorectal cancer: Biomed Rep, 2016; 5(2); 203-7
59. Liu D, Jiang XY, Zhou LS, Effects of probiotics on intestinal mucosa barrier in patients with colorectal cancer after operation: Meta-analysis of randomized controlled trials: Medicine (Baltimore), 2016; 95(15); e3342
60. Liu ZH, Huang MJ, Zhang XW, The effects of perioperative probiotic treatment on serum zonulin concentration and subsequent postoperative infectious complications after colorectal cancer surgery: A double-center and double-blind randomized clinical trial: Am J Clin Nutr, 2013; 97(1); 117-26
Figures
 Figure 1. Differences in selenium concentration in blood samples obtained from the colorectal cancer patients and comparison groups.
Figure 1. Differences in selenium concentration in blood samples obtained from the colorectal cancer patients and comparison groups. Figure 2. Percentage of patients in the colorectal cancer patients group and the comparison groups with selenium levels that were within normal, below normal, or above normal range.
Figure 2. Percentage of patients in the colorectal cancer patients group and the comparison groups with selenium levels that were within normal, below normal, or above normal range. In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952