10 June 2024: Clinical Research
Enhancement of Motor Learning and Corticospinal Excitability: The Role of Electroacupuncture and Motor Training in Healthy Volunteers
Jiahui LinDOI: 10.12659/MSM.943748
Med Sci Monit 2024; 30:e943748
Abstract
BACKGROUND: This study embarked on an innovative exploration to elucidate the effects of integrating electroacupuncture (EA) with motor training (MT) on enhancing corticospinal excitability and motor learning. Central to this investigation is the interplay between homeostatic and non-homeostatic metaplasticity processes, providing insights into how these combined interventions may influence neural plasticity and motor skill acquisition.
MATERIAL AND METHODS: The investigation enrolled 20 healthy volunteers, subjecting them to 4 distinct interventions to parse out the individual and combined effects of EA and MT. These interventions were EA alone, MT alone, EA-priming followed by MT, and MT-priming followed by EA. The assessment of changes in primary motor cortex (M1) excitability was conducted through motor-evoked potentials (MEPs), while the grooved pegboard test (GPT) was used to evaluate alterations in motor performance.
RESULTS: The findings revealed that EA and MT independently contributed to enhanced M1 excitability and motor performance. However, the additional priming with EA or MT did not yield further modulation in MEPs amplitudes. Notably, EA-priming was associated with improved GPT completion times, underscoring its potential in facilitating motor learning.
CONCLUSIONS: The study underscores that while EA and MT individually augment motor cortex excitability and performance, their synergistic application does not further enhance or inhibit cortical excitability. This points to the involvement of non-homeostatic metaplasticity mechanisms. Nonetheless, EA emerges as a critical tool in preventing M1 overstimulation, thereby continuously fostering motor learning. The findings call for further research into the strategic application of EA, whether in isolation or with MT, within clinical settings to optimize rehabilitation outcomes.
Keywords: Electroacupuncture, Motor Cortex, Motor Skills, Neuronal Plasticity
Introduction
Cortical neural plasticity, fundamental to the synaptic mechanisms underpinning learning and memory, manifests in distinct long-term potentiation (LTP) and long-term depression (LTD) characteristics, governed by Hebbian-type synapses [1]. Metaplasticity, the neural plasticity’s tunability in response to temporally separated stimuli, comprises either a dynamic capability for modulating plasticity (homeostatic metaplasticity) or a static response unaffected by subsequent experiences (non-homeostatic metaplasticity) [2]. The prevalent Bienenstock-Cooper-Munro model postulates a nonlinear interplay between priming events and subsequent treatments, elucidating the evolution of homeostatic metaplasticity. This model posits that the threshold for eliciting LTP/LTD varies within a sliding physiological spectrum, contingent upon prior synaptic activity [3]. Most synaptic excitation responses in humans align with the Bienenstock-Cooper-Munro model’s homeostatic metaplasticity regulation framework, while a minority exhibit non-homeostatic characteristics [4]. Notably, non-homeostatic metaplasticity has been linked to alterations in neural excitability in the primary motor cortex (M1) and the enhancement of motor learning [5].
In the clinical context, motor training (MT) is integral to rehabilitation therapies for motor impairments, playing a pivotal role in enhancing voluntary movement [6,7]. MT is known to bolster synaptic strength and motor cortex excitability, leading to improved motor performance [8,9]. Specifically, voluntary movements of the first dorsal interosseous muscle alters motor output, demonstrating the direct impact of physical exercise on motor function [10]. Also, electrical stimulation of the first dorsal interosseous muscle leads to observable changes in motor-evoked potentials (MEPs), pointing to its ability to modulate motor cortex excitability in response to peripheral nerve stimulation [11]. Similarly, electroacupuncture (EA) has been shown to yield positive effects on motor dysfunction by modulating neural plasticity via passive somatosensory stimulation [12,13]. Furthermore, acupuncture at the
The distinct mechanisms of EA and peripheral nerve stimulation highlight the importance of further examining the unique contributions of EA. Peripheral nerve stimulation predominantly targets group I and II afferent fibers, influencing the motor cortex through the direct stimulation of deep proprioceptors and cutaneous receptors [18–20]. Conversely, EA activates alternative neural pathways, primarily involving A-δ and C fibers, thereby modulating cerebral activity via the spinal-thalamic or spino-limbic-cortical pathways [21–24]. Investigating the specific mechanisms and effects of EA not only deepens our understanding of neural modulation strategies but may also broaden the spectrum of therapeutic options for treating motor dysfunction.
In this study, we investigated EA combined with MT, as well as MT followed by EA, on the excitability of M1 and overall motor function. This included assessing the amplitude of MEPs and performance in the grooved pegboard test (GPT) to ascertain whether homeostatic or non-homeostatic metaplasticity is the underlying mechanism driving the outcomes of these interventions. It is our hope to provide valuable insights into the application of EA and MT in therapeutic approaches for motor dysfunction, potentially offering novel and effective treatment modalities.
Material and Methods
PARTICIPANTS:
Twenty healthy, right-handed volunteers aged 18 to 50 years were recruited and assigned to 2 experiments. All participants were evaluated to ensure no contraindications for undergoing transcranial magnetic stimulation (TMS) [25]. This trial was registered in the Chinese Clinical Trial Registry (NO. ChiCTR2000039910), and the register time was November 11, 2020.
The trial received ethical approval from the Ethics Committee of Guangdong Provincial Hospital of Chinese Medicine (Approval No: BF2020-228-01) and was performed in accordance with the Declaration of Helsinki. Before starting the trials, written informed consent was secured from each participant.
EXPERIMENTAL DESIGN:
This investigation was an exploratory study conducted with healthy participants, diverging from traditional clinical efficacy trials. Consequently, in estimating the sample size, the study did not adhere to conventional clinical trial methodologies. Instead, it drew inspiration from the design approaches of similar studies, resulting in the recruitment of 20 healthy participants to undergo 4 intervention measures, with each intervention group comprising 20 participants [17,26].
The present study encompassed 2 self-comparative experiments. Experiment 1 involved 20 healthy participants who were subjected to the following 2 groups in a random order. One group received EA while the other received MT. This experiment aimed to determine whether each intervention independently enhanced cortical excitability and improved motor function. Experiment 2 involved 2 additional groups of 20 participants each: 1 group was EA primed with MT, and the other was MT primed with EA. The objective was to investigate the combined effect of EA and MT, to assess whether there was an additive impact on cortical stimulation and motor function. A 1-week washout period was instituted between each intervention group to mitigate potential carryover effects. The detailed experimental procedures and group assignments are depicted in Figure 1.
EXPERIMENT 1: In the EA group, disposable stainless steel needles, measuring 30 mm in length and 25 mm in diameter, were inserted into the left Hegu acupoint (LI4) and into an adjacent area 0.5 cm from the acupoint, to a depth of approximately 15 mm. The needles were adjusted subtly to elicit the de qi sensation, a traditional Chinese medicine term indicating effective needle placement. Subsequently, the needles were connected to a Han’s Acupoint Nerve Stimulator (model: HANS-200A), set to a frequency of 2 Hz. The current intensity was adjusted to a level sufficient to induce a minor muscle twitch [27]. This EA treatment session lasted for a duration of 30 min. The identification, codename, and exact locations of the Hegu acupoint were in accordance with the standards set by the World Health Organization [28].
In the MT group, participants were instructed to perform a 25-hole GPT. This task required them to precisely and sequentially insert 25 pegs into corresponding holes on the pegboard using their left hands [29]. The orientation of the GPT apparatus was randomly altered, resulting in 4 distinct training sets. To prevent muscle fatigue, a 2-min rest interval was implemented between each set. The entire duration of this training session was 30 min.
EXPERIMENT 2:
In the EA-priming group, participants first underwent the EA-priming session, followed by MT. The procedures for both EA and MT were identical to those used in experiment 1.
In the MT-priming group, participants first underwent the MT-priming session, followed by EA.
OUTCOME MEASUREMENTS:
In both experiments, the time taken to complete the GPT and the outcomes of TMS were recorded. For experiment 1, the measurements were taken at 4 specific time points: before the intervention (T0), immediately after the intervention (T1), 30 min after the intervention (T2), and 45 min after the intervention (T3). Similarly, in experiment 2, measurements were conducted at 4 time points: before the intervention (T0), immediately following the primer intervention (T1), immediately after the subsequent intervention (T2), and 30 min after the subsequent intervention (T3).
COMPLETION TIME OF GPT:
GPT is a well-established tool for assessing fine motor function, effectively measuring motor skill levels [30]. In this test, a stopwatch was used to record the duration taken by participants to complete the task, from the moment they picked up the first peg to the time they inserted the last one. Prior to the actual trial, participants were instructed to practice the GPT multiple times until their completion time stabilized, ensuring consistency in performance on a single day.
TMS OUTCOMES:
This assessment involved measuring various outcomes of TMS, including the amplitude of MEPs, the resting motor threshold, and the latency of MEPs. For each of these parameters, an average value was computed.
RANDOMIZATION METHODS:
The study used a straightforward randomization strategy. The “Complete Random Design” function of the PEMS 3.1 for Windows software was used to generate random sequences of numbers representing different intervention orders for experiments 1 and 2. These sequences were then transcribed onto cards and enclosed within sealed envelopes. Enrolled participants proceeded to open these envelopes in sequential order as they entered the trial, subsequently receiving the interventions as dictated by the order outlined on the cards.
STATISTICAL ANALYSIS:
The data obtained from the experiments were processed using IBM SPSS Statistics software. To ascertain the normal distribution of the data, the Shapiro-Wilk test was used while the Mauchly test was used to assess sphericity.
In experiment 1, one-way repeated measures ANOVA was conducted to analyze differences in TMS outcomes and GPT completion times between the EA group and MT group. Here, TIME served as the within-subjects factor with 4 levels: baseline (T0), immediately after intervention (T1), 30 min after intervention (T2), and 45 min after intervention (T3).
For experiment 2, two-way repeated measures ANOVA was used to investigate the interaction effects between EA and MT. This analysis used GROUP as the between-subjects factor, with 2 levels (EA-priming group and MT-priming group), and TIME as the within-subjects factor, also encompassing 4 levels: baseline (T0), immediately after primer intervention (T1), immediately after subsequent intervention (T2), and 30 min after subsequent intervention (T3). Bonferroni post hoc tests were conducted to further explore any significant interactions that emerged.
The results from these statistical analyses are presented as mean±SD, and the threshold for statistical significance was set at
Results
BASELINE CHARACTERISTICS:
All 20 participants (aged 21.05±2.72, M/F=6/14) completed the whole experiment, without reporting adverse events.
COMPLETION TIME OF GPT:
There was a significant effect of TIME in the EA group (
There was a significant effect of TIME in the MT group (F (1.88, 35.80)=46.00, P<0.01). Compared with T0, the completion time of GPT in T1, T2, and T3 was reduced by 5.10±0.60 s (95% CI: 3.35–6.82, P<0.01), 5.00±0.68 s (95% CI: 3.04–7.03, P<0.01) and 5.00±0.67 s (95% CI: 3.04–6.96, P<0.001) respectively. No statistically significant difference was found among T1, T2, and T3. (Figure 2)
MEPS AMPLITUDE:
There was a significant effect of TIME in the EA group (
There was a significant effect of TIME in the MT group (F (3, 57)=44.84, P<0.01). Increasing levels of MEPs amplitude were observed by 0.47±0.05 mV (95% CI: 0.31–0.63, P<0.01), 0.53±0.06 mV (95% CI: 0.35–0.71, P<0.01) and 0.59±0.06 mV (95% CI: 0.41–0.76, P<0.01) in T1, T2 and T3 compared with T0, respectively, while no significant difference was found among T1, T2, and T3 (Figure 3).
COMPLETION TIME OF GPT: The completion time of GPT between the EA-priming group and MT-priming group showed a significant TIME*GROUP interaction (F (3,57)=7.21, P<0.01). The simple effect of GROUP showed a significant difference in T1(F (1,19)=11.98, P<0.01) but no significant difference in T0, T2, and T3. Also, the simple effect of TIME in the EA-priming group (F (1.59, 30.25)=17.62, P<0.01) and MT-priming group (F (1.79,34.03)=20.43, P<0.01) were statistically significant. In the EA-priming group, a significant reduction was observed in T2 (6.27±1.44 s, 95% CI: 2.03–10.51, P<0.01) and T3 (7.52±1.61s, 95% CI: 2.79–12.25, P<0.01) compared with T0, and in T2 (4.06±1.04 s, 95% CI: 1.00–7.12, P<0.01) and T3 (5.31±1.16 s, 95% CI: 1.89–8.73, P<0.01) compared with T1. No significant differences were found in T1 compared with T0 and T3 compared with T2. While in the MT-priming group, a significant reduction was observed in T1 (5.87±1.17 s, 95% CI: 2.42–9.31, P<0.001), T2 (5.38±1.00 s, 95% CI: 2.45–8.31, P<0.01) and T3 (5.63±1.12 s, 95% CI: 2.34–8.92, P<0.01) compared with T0. No statistically significant difference was found among T1, T2, and T3 (Figure 4).
MEPS AMPLITUDE:
The MEPs amplitude between the EA-priming group and MT-priming group showed no TIME*GROUP interaction. The main effect of GROUP showed no statistically significant difference (
In the EA-priming group (F (3, 57)=9.66, P<0.01), the results revealed that T1 (0.39±0.88 mV, 95% CI: 0.14–0.72, P<0.01), T2 (0.40±0.11 mV, 95% CI: 0.09–0.72, P<0.01) and T3 (0.60±0.11 mV, 95% CI: 0.28–0.93, P<0.01) had ascent effects on MEPs amplitude, compared with T0. In MT-priming group (F (1.97, 37.45)=6.22, P<0.01), the results of one-way repeated measures ANOVA revealed that T1 (0.36±0.07 mV, 95% CI: 0.16–0.56, P<0.01), T2 (0.30±0.09 mV, 95% CI: 0.36–0.57, P<0.01), and T3 (0.38±0.11 mV, 95% CI: 0.07–0.69, P<0.01) had ascent effects on MEPs amplitude compared with T0. No representative changes were observed among T1, T2, and T3 in both groups (Figure 5).
RESTING MOTOR THRESHOLD:
In experiment 1, no significant change in the resting motor threshold was observed in either group. In experiment 2, no significant difference between TIME*GROUP interaction and the main effects of TIME and GROUP was observed.
LATENCY OF MEPS:
In experiment 1, no significant change in the latency of MEPs was observed in either group. In experiment 2, no significant difference between TIME*GROUP interaction and the main effects of TIME and GROUP was observed.
Discussion
STUDY LIMITATIONS:
The study had several limitations that must be acknowledged. First, the sample size was relatively small and may not have provided enough statistical power to generalize the findings. This limitation is crucial in interpreting the study’s results and understanding its applicability to a wider population. Second, the research only involved healthy volunteers, which means the results may not accurately reflect the responses in individuals with neurological disorders or other health conditions. This restricts the study’s relevance to clinical applications, particularly for patients with specific neuropsychiatric or motor disorders. Third, the use of MEPs as a measure shows considerable inter-individual variability. This variability can affect the consistency of the results and introduce a level of uncertainty in the data interpretation. The individual differences in MEPs can be influenced by numerous factors, such as age, neural anatomy, and physiological state, thereby affecting the reliability of MEPs as a standardized measure across different participants. Lastly, the study lacked a control group that did not receive EA or MT. This makes it challenging to clearly attribute improvements in motor activity and M1 activation to the interventions themselves, rather than placebo effects or natural fluctuations.
Conclusions
The study’s findings indicate that the phenomenon of combining MT with EA not significantly altering cortical excitability is a manifestation of non-homeostatic metaplasticity, indicating a nuanced modulation in neural plasticity mechanisms. Furthermore, EA seems to play a role in preventing plasticity saturation within motor learning circuits. These observations underscore the necessity for continued research into the applications of EA, both as a standalone intervention and in conjunction with MT, particularly for the treatment of motor dysfunctions.
Figures
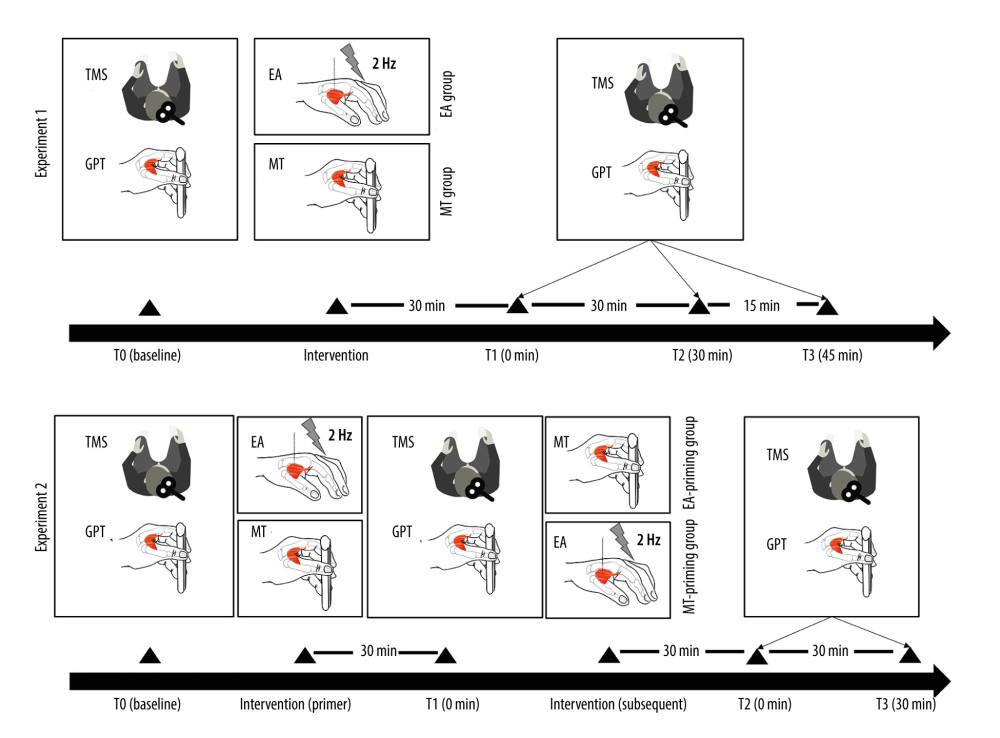 Figure 1. Experimental protocolThe experimental protocol was structured into 2 distinct experiments, each comprising outcome measurement testing phases (T0, T1, T2, and T3) and intervention phases. The outcome measurement testing phases involved acquiring transcranial magnetic stimulation (TMS) outcomes and conducting the grooved pegboard test (GPT) to evaluate the participants’ M1 excitability and motor performance, respectively. In experiment 1, the outcome measurements were taken at 4 different times: at baseline (T0), immediately after the intervention (T1), 30 min after intervention (T2), and 45 min after intervention (T3). The interventions in this experiment were electroacupuncture (EA) for the EA group and motor training (MT) for the MT group. Experiment 2 followed a similar structure for outcome measurements, with the phases conducted at baseline (T0), immediately after the primary (primer) intervention (T1), immediately following the secondary (subsequent) intervention (T2), and 30 min after the secondary intervention (T3). In this experiment, one group received EA as the primer intervention followed by MT (EA-priming group), while the other group received the interventions in the reverse order, with MT as the primer followed by EA (MT-priming group). This figure was produced using Adobe Illustrator 2023 by Adobe.
Figure 1. Experimental protocolThe experimental protocol was structured into 2 distinct experiments, each comprising outcome measurement testing phases (T0, T1, T2, and T3) and intervention phases. The outcome measurement testing phases involved acquiring transcranial magnetic stimulation (TMS) outcomes and conducting the grooved pegboard test (GPT) to evaluate the participants’ M1 excitability and motor performance, respectively. In experiment 1, the outcome measurements were taken at 4 different times: at baseline (T0), immediately after the intervention (T1), 30 min after intervention (T2), and 45 min after intervention (T3). The interventions in this experiment were electroacupuncture (EA) for the EA group and motor training (MT) for the MT group. Experiment 2 followed a similar structure for outcome measurements, with the phases conducted at baseline (T0), immediately after the primary (primer) intervention (T1), immediately following the secondary (subsequent) intervention (T2), and 30 min after the secondary intervention (T3). In this experiment, one group received EA as the primer intervention followed by MT (EA-priming group), while the other group received the interventions in the reverse order, with MT as the primer followed by EA (MT-priming group). This figure was produced using Adobe Illustrator 2023 by Adobe. 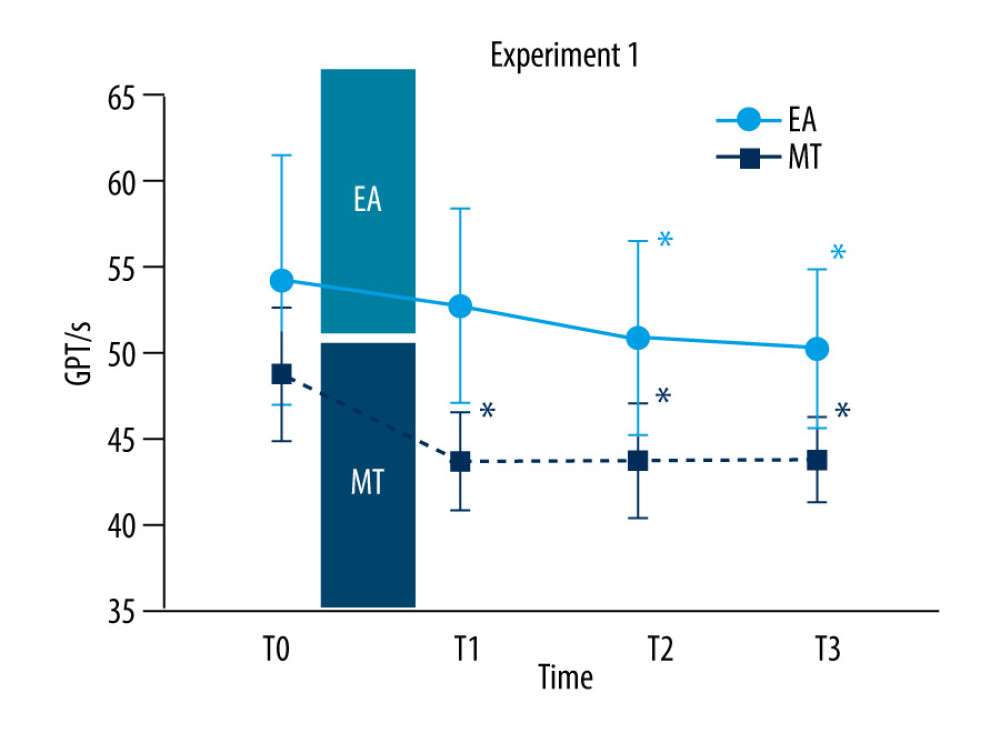 Figure 2. Completion time of the grooved pegboard test (GPT) in experiment 1Completion time of the GPT recorded at baseline (T0), immediately after intervention (T1), 30 min (T2), and 45 min (T3) after the intervention in the electroacupuncture (EA) group and motor training (MT) group. The red circles represent the EA group while the black symbols represent the MT group. The X axis means time points. The Y axis means completion time of GPT (s). Note that completion time of GPT decreased significantly when EA (T2 and T3 compared with T0) and MT (T1, T2 and T3 compared with T0) was intervened alone. Values are mean±SD. * Indicates significant differences (P<0.05) compared with baseline (T0). This figure was produced using GraphPad Prism 10 by GraphPad Software.
Figure 2. Completion time of the grooved pegboard test (GPT) in experiment 1Completion time of the GPT recorded at baseline (T0), immediately after intervention (T1), 30 min (T2), and 45 min (T3) after the intervention in the electroacupuncture (EA) group and motor training (MT) group. The red circles represent the EA group while the black symbols represent the MT group. The X axis means time points. The Y axis means completion time of GPT (s). Note that completion time of GPT decreased significantly when EA (T2 and T3 compared with T0) and MT (T1, T2 and T3 compared with T0) was intervened alone. Values are mean±SD. * Indicates significant differences (P<0.05) compared with baseline (T0). This figure was produced using GraphPad Prism 10 by GraphPad Software. 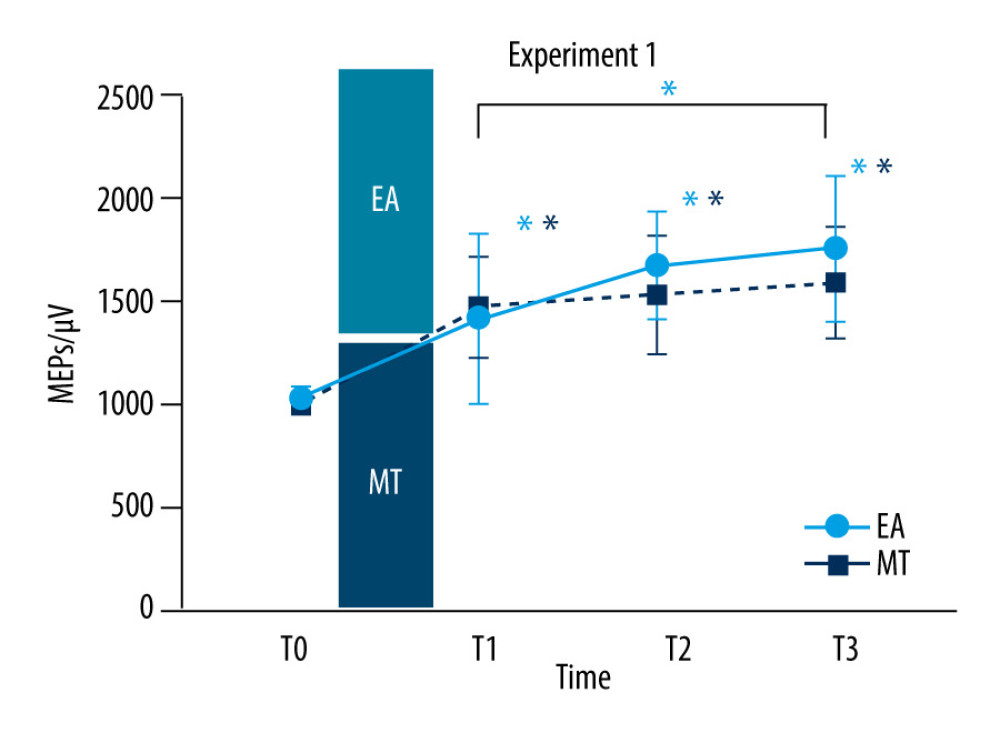 Figure 3. Motor-evoked potentials (MEPs) amplitude in experiment 1MEPs amplitude recorded at baseline (T0), immediately after intervention (T1), 30 (T2), and 45 min (T3) after the intervention in the electroacupuncture (EA) group (red circles) and electroacupuncture (MT) group (black squares). Note that MEPs amplitude increased significantly when EA (T1, T2, and T3 compared with T0 and T3 compared with T1) and MT (T1, T2, and T3 compared with T0) was intervened alone. Values are mean±SD. * Indicates significant differences (P<0.05) compared with baseline (T0) and between time points. This figure was produced using GraphPad Prism 10 by GraphPad Software.
Figure 3. Motor-evoked potentials (MEPs) amplitude in experiment 1MEPs amplitude recorded at baseline (T0), immediately after intervention (T1), 30 (T2), and 45 min (T3) after the intervention in the electroacupuncture (EA) group (red circles) and electroacupuncture (MT) group (black squares). Note that MEPs amplitude increased significantly when EA (T1, T2, and T3 compared with T0 and T3 compared with T1) and MT (T1, T2, and T3 compared with T0) was intervened alone. Values are mean±SD. * Indicates significant differences (P<0.05) compared with baseline (T0) and between time points. This figure was produced using GraphPad Prism 10 by GraphPad Software. 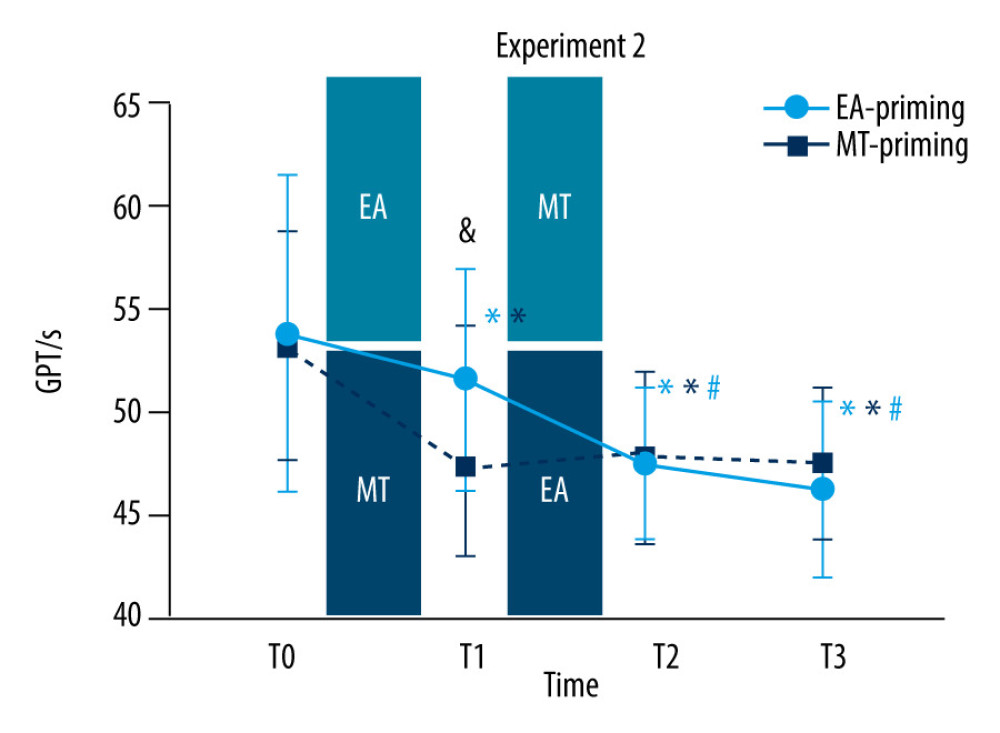 Figure 4. Completion time of grooved pegboard test (GPT) in experiment 2Completion time of GPT recorded at baseline (T0), immediately after the former intervention (T1), and the latter intervention (T2), and 30 min (T3) after the latter intervention in the electroacupuncture (EA)-priming group (red circles) and motor training (MT)-priming group (black squares). Note that in the EA-priming group, the completion time of GPT decreased significantly when the EA was intervened alone (T1 compared with T0) and when it was followed by MT (T2 compared with T0 and T1); furthermore, the completion time of GPT decreased continually at 30 min after the subsequent MT (T3 compared with T0 and T1). In MT-priming group, the completion time of GPT decreased significantly when the MT was intervened alone (T1 compared with T0); whereas, when the EA followed, the completion time of GPT remained unchanged (T2 and T3 compared with T0). Significant difference was observed in T1 between the 2 groups. Values are mean±SD. * Indicates significant differences (P<0.05) compared with baseline (T0); # indicates significant differences (P<0.05) compared with T1; & indicates significant differences (P<0.05) between the 2 groups. This figure was produced using GraphPad Prism 10 by GraphPad Software.
Figure 4. Completion time of grooved pegboard test (GPT) in experiment 2Completion time of GPT recorded at baseline (T0), immediately after the former intervention (T1), and the latter intervention (T2), and 30 min (T3) after the latter intervention in the electroacupuncture (EA)-priming group (red circles) and motor training (MT)-priming group (black squares). Note that in the EA-priming group, the completion time of GPT decreased significantly when the EA was intervened alone (T1 compared with T0) and when it was followed by MT (T2 compared with T0 and T1); furthermore, the completion time of GPT decreased continually at 30 min after the subsequent MT (T3 compared with T0 and T1). In MT-priming group, the completion time of GPT decreased significantly when the MT was intervened alone (T1 compared with T0); whereas, when the EA followed, the completion time of GPT remained unchanged (T2 and T3 compared with T0). Significant difference was observed in T1 between the 2 groups. Values are mean±SD. * Indicates significant differences (P<0.05) compared with baseline (T0); # indicates significant differences (P<0.05) compared with T1; & indicates significant differences (P<0.05) between the 2 groups. This figure was produced using GraphPad Prism 10 by GraphPad Software. 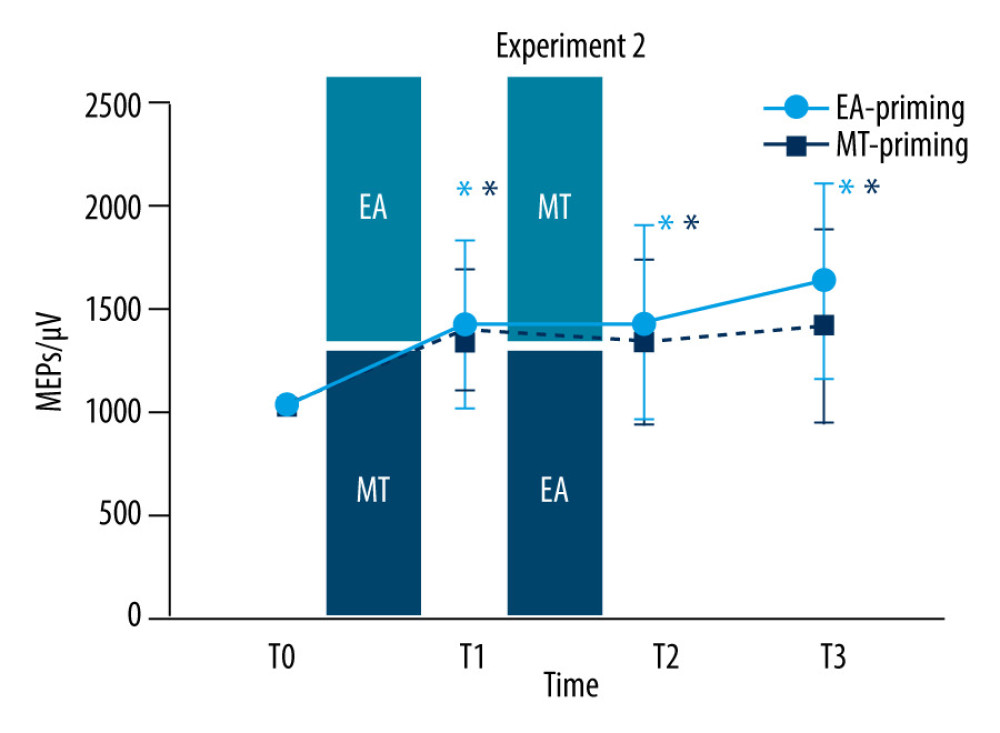 Figure 5. Motor-evoked potentials (MEPs) amplitude in experiment 2MEPs amplitude recorded at baseline (T0), immediately after the former intervention (T1), and the latter intervention (T2), 30 min (T3) after the latter intervention in the electroacupuncture (EA)-priming group (red circles) and motor training (MT)-priming group (black squares). Note that in the EA-priming group, the MEPs amplitude increased significantly when the EA was intervened alone (T1 compared with T0); whereas, when the MT followed, the MEPs amplitude remained unchanged (T2 and T3 compared with T0). In the MT-priming group, the MEPs amplitude increased significantly when the MT was intervened alone (T1 compared with T0); whereas, when the EA followed, the MEPs amplitude remained unchanged (T2 and T3 compared with T0). Values are mean±SD. * Indicates significant differences (P<0.05) compared with baseline (T0). This figure was produced using GraphPad Prism 10 by GraphPad Software.
Figure 5. Motor-evoked potentials (MEPs) amplitude in experiment 2MEPs amplitude recorded at baseline (T0), immediately after the former intervention (T1), and the latter intervention (T2), 30 min (T3) after the latter intervention in the electroacupuncture (EA)-priming group (red circles) and motor training (MT)-priming group (black squares). Note that in the EA-priming group, the MEPs amplitude increased significantly when the EA was intervened alone (T1 compared with T0); whereas, when the MT followed, the MEPs amplitude remained unchanged (T2 and T3 compared with T0). In the MT-priming group, the MEPs amplitude increased significantly when the MT was intervened alone (T1 compared with T0); whereas, when the EA followed, the MEPs amplitude remained unchanged (T2 and T3 compared with T0). Values are mean±SD. * Indicates significant differences (P<0.05) compared with baseline (T0). This figure was produced using GraphPad Prism 10 by GraphPad Software. References
1. Magee JC, Grienberger C, Synaptic plasticity forms and functions: Annu Rev Neurosci, 2020; 43; 95-117
2. Abraham WC, Bear MF, Metaplasticity: The plasticity of synaptic plasticity: Trends Neurosci, 1996; 19(4); 126-30
3. Bienenstock EL, Cooper LN, Munro PW, Theory for the development of neuron selectivity: Orientation specificity and binocular interaction in visual cortex: J Neurosci, 1982; 2(1); 32-48
4. Hulme SR, Jones OD, Abraham WC, Emerging roles of metaplasticity in behaviour and disease: Trends Neurosci, 2013; 36(6); 353-62
5. Müller-Dahlhaus F, Ziemann U, Metaplasticity in human cortex: Neuroscientist, 2015; 21(2); 185-202
6. French B, Thomas LH, Coupe J, Repetitive task training for improving functional ability after stroke: Cochrane Database Syst Rev, 2016; 11(11); CD006073
7. Israely S, Leisman G, Carmeli E, Improvement in arm and hand function after a stroke with task-oriented training: BMJ Case Rep, 2017; 2017; bcr2017219250
8. Sanes JN, Donoghue JP, Plasticity and primary motor cortex: Annu Rev Neurosci, 2000; 23; 393-415
9. Bütefisch CM, Davis BC, Wise SP, Mechanisms of use-dependent plasticity in the human motor cortex: Proc Natl Acad Sci USA, 2000; 97(7); 3661-65
10. Hortobágyi T, Richardson SP, Lomarev M, Interhemispheric plasticity in humans: Med Sci Sports Exerc, 2011; 43(7); 1188-99
11. Bonnesen MT, Fuglsang SA, Siebner HR, Christiansen L, The recent history of afferent stimulation modulates corticospinal excitability. Neuroimage: Sep, 2022; 258; 119365
12. Li SS, Hua XY, Zheng MX, Electroacupuncture treatment improves motor function and neurological outcomes after cerebral ischemia/reperfusion injury: Neural Regen Res, 2022; 17(7); 1545-55
13. Li W, Yang Y, Huang J, Electroacupuncture-induced plasticity between face and hand representations in motor cortex is associated with recovery of function after facial nerve injury: Acupunct Med, 2021; 39(1); 75-77
14. Maioli C, Falciati L, Marangon M, Short- and long-term modulation of upper limb motor-evoked potentials induced by acupuncture: Eur j Neurosci, 2006; 23(7); 1931-38
15. McCambridge AB, Zaslawski C, Bradnam LV, Investigating the mechanisms of acupuncture on neural excitability in healthy adults: Neuroreport, 2019; 30(2); 71-76
16. Li JM, Huang JP, Liu JHEffect of electroacupuncture combined with motor training on motor learning and motor cortex excitability: Zhongguo Zhen Jiu, 2021; 41(12); 1365-69 [in Chinese]
17. Bisio A, Avanzino L, Biggio M, Motor training and the combination of action observation and peripheral nerve stimulation reciprocally interfere with the plastic changes induced in primary motor cortex excitability: Neuroscience, 2017; 348; 33-40
18. Kaelin-Lang A, Luft AR, Sawaki L, Modulation of human corticomotor excitability by somatosensory input: J Physiol, 2002; 540(Pt 2); 623-33
19. Kaye AD, Ridgell S, Alpaugh ES, Peripheral nerve stimulation: A review of techniques and clinical efficacy: Pain Ther, 2021; 10(2); 961-72
20. Veldman MP, Maffiuletti NA, Hallett M, Direct and crossed effects of somatosensory stimulation on neuronal excitability and motor performance in humans: Neurosci Biobehav Rev, 2014; 47; 22-35
21. Zhao ZQ, Neural mechanism underlying acupuncture analgesia: Prog Neurobiol, 2008; 85(4); 355-75
22. Kagitani F, Uchida S, Hotta H, Afferent nerve fibers and acupuncture: Auton Neurosci, 2010; 157(1–2); 2-8
23. Han JS, Acupuncture analgesia: Areas of consensus and controversy: Pain, 2011; 152(3 Suppl); S41-S48
24. Napadow V, Liu J, Li M, Somatosensory cortical plasticity in carpal tunnel syndrome treated by acupuncture: Hum Brain Mapp, 2007; 28(3); 159-71
25. Rossi S, Antal A, Bestmann S, basis of this article began with a Consensus Statement from the IFCN Workshop on “Present, Future of TMS: Safety, Ethical Guidelines”, Siena, October 17-20, 2018, updating through April 2020. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines: Clin Neurophysiol, 2021; 132(1); 269-306
26. Petrichella S, Johnson N, He B, The influence of corticospinal activity on TMS-evoked activity and connectivity in healthy subjects: A TMS-EEG study: PLoS One, 2017; 12(4); e0174879
27. Peng W, Yang T, Yuan J, Electroacupuncture-induced plasticity between different representations in human motor cortex: Neural Plast, 2020; 2020; 8856868
28. Lim S, WHO standard acupuncture point locations: Evid Based Complement Alternat Med, 2010; 7(2); 167-68
29. Gershon RC, Cella D, Fox NA, Assessment of neurological and behavioural function: the NIH Toolbox: Lancet Neurol, 2010; 9(2); 138-39
30. Petrigna L, Pajaujiene S, Iacona GM, The execution of the Grooved Pegboard test in a Dual-Task situation: A pilot study: Heliyon, 2020; 6(8); e04678
31. Hassanzahraee M, Zoghi M, Jaberzadeh S, How different priming stimulations affect the corticospinal excitability induced by noninvasive brain stimulation techniques: A systematic review and meta-analysis: Rev Neurosci, 2018; 29(8); 883-99
32. Hamada M, Terao Y, Hanajima R, Bidirectional long-term motor cortical plasticity and metaplasticity induced by quadripulse transcranial magnetic stimulation: J Physiol, 2008; 586(16); 3927-47
33. Cosentino G, Fierro B, Paladino P, Transcranial direct current stimulation preconditioning modulates the effect of high-frequency repetitive transcranial magnetic stimulation in the human motor cortex: Eur J Neurosci, 2012; 35(1); 119-24
34. Lang N, Siebner HR, Ernst D, Preconditioning with transcranial direct current stimulation sensitizes the motor cortex to rapid-rate transcranial magnetic stimulation and controls the direction of after-effects: Biol Psychiatry, 2004; 56(9); 634-39
35. Doeltgen SH, Ridding MC, Modulation of cortical motor networks following primed θ burst transcranial magnetic stimulation: Exp Brain Res, 2011; 215(3–4); 199-206
36. Müller JF, Orekhov Y, Liu Y, Ziemann U, Homeostatic plasticity in human motor cortex demonstrated by two consecutive sessions of paired associative stimulation: Eur J Neurosci, 2007; 25(11); 3461-68 [Erratum in: Eur J Neurosci. 2007;26(4):1077]
37. Ziemann U, Ilić TV, Pauli C, Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex: J Neurosci, 2004; 24(7); 1666-72 [Erratum in: J Neurosci. 2004;24(46):1]
38. Stagg CJ, Jayaram G, Pastor D, Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning: Neuropsychologia, 2011; 49(5); 800-4
39. Trudgen A, Cirillo J, Byblow WD, Somatosensory and transcranial direct current stimulation effects on manual dexterity and motor cortex function: A metaplasticity study: Brain Stimul, 2019; 12(4); 938-47
40. Stöckel T, Summers JJ, Hinder MR, Reversed effects of intermittent theta burst stimulation following motor training that vary as a function of training-induced changes in corticospinal excitability: Neural Plast, 2015; 2015; 578620
41. Cantarero G, Lloyd A, Celnik P, Reversal of long-term potentiation-like plasticity processes after motor learning disrupts skill retention: J Neurosci, 2013; 33(31); 12862-69
42. Pham MV, Miyaguchi S, Watanabe H, Effect of repetitive passive movement before motor skill training on corticospinal excitability and motor learning depend on BDNF polymorphisms: Front Hum Neurosci, 2021; 15; 621358
43. Wankerl K, Weise D, Gentner R, L-type voltage-gated Ca2+ channels: A single molecular switch for long-term potentiation/long-term depression-like plasticity and activity-dependent metaplasticity in humans: J Neurosci, 2010; 30(18); 6197-204
44. Lee HK, Metaplasticity framework for cross-modal synaptic plasticity in adults: Front Synaptic Neurosci, 2023; 14; 1087042
45. Bockaert J, Perroy J, Ango F, The complex formed by group I metabotropic glutamate receptor (mGluR) and Homer1a plays a central role in metaplasticity and homeostatic synaptic scaling: J Neurosci, 2021; 41(26); 5567-78
46. Steele PM, Mauk MD, Inhibitory control of LTP and LTD: Stability of synapse strength: J Neurophysiol, 1999; 81(4); 1559-66
47. Miehl C, Gjorgjieva J, Stability and learning in excitatory synapses by nonlinear inhibitory plasticity: PLoS Comput Biol, 2022; 18(12); e1010682
48. Ziemann U, Siebner HR, Modifying motor learning through gating and homeostatic metaplasticity: Brain Stimul, 2008; 1(1); 60-66
49. Stefan K, Wycislo M, Gentner R, Temporary occlusion of associative motor cortical plasticity by prior dynamic motor training: Cereb Cortex, 2006; 16(3); 376-85
50. Frazer AK, Howatson G, Ahtiainen JP, Priming the motor cortex with anodal transcranial direct current stimulation affects the acute inhibitory corticospinal responses to strength training: J Strength Cond Res, 2019; 33(2); 307-17
51. Peineau S, Taghibiglou C, Bradley C, LTP inhibits LTD in the hippocampus via regulation of GSK3beta: Neuron, 2007; 53(5); 703-17
52. Iezzi E, Suppa A, Conte A, Short-term and long-term plasticity interaction in human primary motor cortex: Eur J Neurosci, 2011; 33(10); 1908-15
53. Nitsche MA, Roth A, Kuo MF, Timing-dependent modulation of associative plasticity by general network excitability in the human motor cortex: J Neurosci, 2007; 27(14); 3807-12
54. Gamboa OL, Antal A, Laczo B, Impact of repetitive theta burst stimulation on motor cortex excitability: Brain Stimul, 2011; 4(3); 145-51
55. Monte-Silva K, Kuo MF, Liebetanz D, Shaping the optimal repetition interval for cathodal transcranial direct current stimulation (tDCS): J Neurophysiol, 2010; 103(4); 1735-40
56. Jung P, Ziemann U, Homeostatic and nonhomeostatic modulation of learning in human motor cortex: J Neurosci, 2009; 29(17); 5597-604
57. Chen L, Li X, Tjia M, Thapliyal S, Homeostatic plasticity and excitation-inhibition balance: The good, the bad, and the ugly: Curr Opin Neurobiol, 2022; 75; 102553
58. Li J, Park E, Zhong LR, Chen L, Homeostatic synaptic plasticity as a metaplasticity mechanism – a molecular and cellular perspective: Curr Opin Neurobiol, 2019; 54; 44-53
59. Nguyen-Vu TB, Zhao GQ, Lahiri S, A saturation hypothesis to explain both enhanced and impaired learning with enhanced plasticity: Elife, 2017; 6; e20147
60. França FJR, Callegari B, Ramos LAV, Motor control training compared with transcutaneous electrical nerve stimulation in patients with disc herniation with associated radiculopathy: A randomized controlled trial: Am J Phys Med Rehabil, 2019; 98(3); 207-14
61. Gottlieb GL, Corcos DM, Jaric S, Agarwal GC, Practice improves even the simplest movements: Exp Brain Res, 1988; 73(2); 436-40
62. Vallence AM, Kurylowicz L, Ridding MC, A comparison of neuroplastic responses to non-invasive brain stimulation protocols and motor learning in healthy adults: Neurosci Lett, 2013; 549; 151-56
63. Tater P, Pandey S, Post-stroke movement disorders: clinical spectrum, pathogenesis, and management: Neurol India, 2021; 69(2); 272-83
Figures
 Figure 1. Experimental protocolThe experimental protocol was structured into 2 distinct experiments, each comprising outcome measurement testing phases (T0, T1, T2, and T3) and intervention phases. The outcome measurement testing phases involved acquiring transcranial magnetic stimulation (TMS) outcomes and conducting the grooved pegboard test (GPT) to evaluate the participants’ M1 excitability and motor performance, respectively. In experiment 1, the outcome measurements were taken at 4 different times: at baseline (T0), immediately after the intervention (T1), 30 min after intervention (T2), and 45 min after intervention (T3). The interventions in this experiment were electroacupuncture (EA) for the EA group and motor training (MT) for the MT group. Experiment 2 followed a similar structure for outcome measurements, with the phases conducted at baseline (T0), immediately after the primary (primer) intervention (T1), immediately following the secondary (subsequent) intervention (T2), and 30 min after the secondary intervention (T3). In this experiment, one group received EA as the primer intervention followed by MT (EA-priming group), while the other group received the interventions in the reverse order, with MT as the primer followed by EA (MT-priming group). This figure was produced using Adobe Illustrator 2023 by Adobe.
Figure 1. Experimental protocolThe experimental protocol was structured into 2 distinct experiments, each comprising outcome measurement testing phases (T0, T1, T2, and T3) and intervention phases. The outcome measurement testing phases involved acquiring transcranial magnetic stimulation (TMS) outcomes and conducting the grooved pegboard test (GPT) to evaluate the participants’ M1 excitability and motor performance, respectively. In experiment 1, the outcome measurements were taken at 4 different times: at baseline (T0), immediately after the intervention (T1), 30 min after intervention (T2), and 45 min after intervention (T3). The interventions in this experiment were electroacupuncture (EA) for the EA group and motor training (MT) for the MT group. Experiment 2 followed a similar structure for outcome measurements, with the phases conducted at baseline (T0), immediately after the primary (primer) intervention (T1), immediately following the secondary (subsequent) intervention (T2), and 30 min after the secondary intervention (T3). In this experiment, one group received EA as the primer intervention followed by MT (EA-priming group), while the other group received the interventions in the reverse order, with MT as the primer followed by EA (MT-priming group). This figure was produced using Adobe Illustrator 2023 by Adobe. Figure 2. Completion time of the grooved pegboard test (GPT) in experiment 1Completion time of the GPT recorded at baseline (T0), immediately after intervention (T1), 30 min (T2), and 45 min (T3) after the intervention in the electroacupuncture (EA) group and motor training (MT) group. The red circles represent the EA group while the black symbols represent the MT group. The X axis means time points. The Y axis means completion time of GPT (s). Note that completion time of GPT decreased significantly when EA (T2 and T3 compared with T0) and MT (T1, T2 and T3 compared with T0) was intervened alone. Values are mean±SD. * Indicates significant differences (P<0.05) compared with baseline (T0). This figure was produced using GraphPad Prism 10 by GraphPad Software.
Figure 2. Completion time of the grooved pegboard test (GPT) in experiment 1Completion time of the GPT recorded at baseline (T0), immediately after intervention (T1), 30 min (T2), and 45 min (T3) after the intervention in the electroacupuncture (EA) group and motor training (MT) group. The red circles represent the EA group while the black symbols represent the MT group. The X axis means time points. The Y axis means completion time of GPT (s). Note that completion time of GPT decreased significantly when EA (T2 and T3 compared with T0) and MT (T1, T2 and T3 compared with T0) was intervened alone. Values are mean±SD. * Indicates significant differences (P<0.05) compared with baseline (T0). This figure was produced using GraphPad Prism 10 by GraphPad Software. Figure 3. Motor-evoked potentials (MEPs) amplitude in experiment 1MEPs amplitude recorded at baseline (T0), immediately after intervention (T1), 30 (T2), and 45 min (T3) after the intervention in the electroacupuncture (EA) group (red circles) and electroacupuncture (MT) group (black squares). Note that MEPs amplitude increased significantly when EA (T1, T2, and T3 compared with T0 and T3 compared with T1) and MT (T1, T2, and T3 compared with T0) was intervened alone. Values are mean±SD. * Indicates significant differences (P<0.05) compared with baseline (T0) and between time points. This figure was produced using GraphPad Prism 10 by GraphPad Software.
Figure 3. Motor-evoked potentials (MEPs) amplitude in experiment 1MEPs amplitude recorded at baseline (T0), immediately after intervention (T1), 30 (T2), and 45 min (T3) after the intervention in the electroacupuncture (EA) group (red circles) and electroacupuncture (MT) group (black squares). Note that MEPs amplitude increased significantly when EA (T1, T2, and T3 compared with T0 and T3 compared with T1) and MT (T1, T2, and T3 compared with T0) was intervened alone. Values are mean±SD. * Indicates significant differences (P<0.05) compared with baseline (T0) and between time points. This figure was produced using GraphPad Prism 10 by GraphPad Software. Figure 4. Completion time of grooved pegboard test (GPT) in experiment 2Completion time of GPT recorded at baseline (T0), immediately after the former intervention (T1), and the latter intervention (T2), and 30 min (T3) after the latter intervention in the electroacupuncture (EA)-priming group (red circles) and motor training (MT)-priming group (black squares). Note that in the EA-priming group, the completion time of GPT decreased significantly when the EA was intervened alone (T1 compared with T0) and when it was followed by MT (T2 compared with T0 and T1); furthermore, the completion time of GPT decreased continually at 30 min after the subsequent MT (T3 compared with T0 and T1). In MT-priming group, the completion time of GPT decreased significantly when the MT was intervened alone (T1 compared with T0); whereas, when the EA followed, the completion time of GPT remained unchanged (T2 and T3 compared with T0). Significant difference was observed in T1 between the 2 groups. Values are mean±SD. * Indicates significant differences (P<0.05) compared with baseline (T0); # indicates significant differences (P<0.05) compared with T1; & indicates significant differences (P<0.05) between the 2 groups. This figure was produced using GraphPad Prism 10 by GraphPad Software.
Figure 4. Completion time of grooved pegboard test (GPT) in experiment 2Completion time of GPT recorded at baseline (T0), immediately after the former intervention (T1), and the latter intervention (T2), and 30 min (T3) after the latter intervention in the electroacupuncture (EA)-priming group (red circles) and motor training (MT)-priming group (black squares). Note that in the EA-priming group, the completion time of GPT decreased significantly when the EA was intervened alone (T1 compared with T0) and when it was followed by MT (T2 compared with T0 and T1); furthermore, the completion time of GPT decreased continually at 30 min after the subsequent MT (T3 compared with T0 and T1). In MT-priming group, the completion time of GPT decreased significantly when the MT was intervened alone (T1 compared with T0); whereas, when the EA followed, the completion time of GPT remained unchanged (T2 and T3 compared with T0). Significant difference was observed in T1 between the 2 groups. Values are mean±SD. * Indicates significant differences (P<0.05) compared with baseline (T0); # indicates significant differences (P<0.05) compared with T1; & indicates significant differences (P<0.05) between the 2 groups. This figure was produced using GraphPad Prism 10 by GraphPad Software. Figure 5. Motor-evoked potentials (MEPs) amplitude in experiment 2MEPs amplitude recorded at baseline (T0), immediately after the former intervention (T1), and the latter intervention (T2), 30 min (T3) after the latter intervention in the electroacupuncture (EA)-priming group (red circles) and motor training (MT)-priming group (black squares). Note that in the EA-priming group, the MEPs amplitude increased significantly when the EA was intervened alone (T1 compared with T0); whereas, when the MT followed, the MEPs amplitude remained unchanged (T2 and T3 compared with T0). In the MT-priming group, the MEPs amplitude increased significantly when the MT was intervened alone (T1 compared with T0); whereas, when the EA followed, the MEPs amplitude remained unchanged (T2 and T3 compared with T0). Values are mean±SD. * Indicates significant differences (P<0.05) compared with baseline (T0). This figure was produced using GraphPad Prism 10 by GraphPad Software.
Figure 5. Motor-evoked potentials (MEPs) amplitude in experiment 2MEPs amplitude recorded at baseline (T0), immediately after the former intervention (T1), and the latter intervention (T2), 30 min (T3) after the latter intervention in the electroacupuncture (EA)-priming group (red circles) and motor training (MT)-priming group (black squares). Note that in the EA-priming group, the MEPs amplitude increased significantly when the EA was intervened alone (T1 compared with T0); whereas, when the MT followed, the MEPs amplitude remained unchanged (T2 and T3 compared with T0). In the MT-priming group, the MEPs amplitude increased significantly when the MT was intervened alone (T1 compared with T0); whereas, when the EA followed, the MEPs amplitude remained unchanged (T2 and T3 compared with T0). Values are mean±SD. * Indicates significant differences (P<0.05) compared with baseline (T0). This figure was produced using GraphPad Prism 10 by GraphPad Software. In Press
Clinical Research
Improving Function and Quality of Life in Patients with Chronic Neck Pain, Tension-Type Headache, and Forwa...Med Sci Monit In Press; DOI: 10.12659/MSM.944315
Clinical Research
Assessment of Undergraduate Nursing Students' Understanding of Herbal Medicines and Herb-Drug Interactions ...Med Sci Monit In Press; DOI: 10.12659/MSM.944352
Animal Research
Curcumin as a Potential Therapeutic Agent for Mitigating Carbon Monoxide Poisoning: Evidence from an Experi...Med Sci Monit In Press; DOI: 10.12659/MSM.943739
Animal Research
Targeting the Gut-Kidney Axis in Diarrhea with Kidney-Yang Deficiency Syndrome: The Role of Sishen Pills in...Med Sci Monit In Press; DOI: 10.12659/MSM.944185
Most Viewed Current Articles
17 Jan 2024 : Review article 1,586,125
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research 1,513,621
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research 690,252
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial 50,200
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








