11 April 2023: Clinical Research
Intraoperative Hypotension and Its Association with Postoperative Acute Kidney Injury in Patients Undergoing Pancreaticoduodenectomy: A 5-Year, Single-Center, Retrospective Cohort Study
Zbigniew Putowski1ABCDEF*, Karolina MajewskaDOI: 10.12659/MSM.938945
Med Sci Monit 2023; 29:e938945
Abstract
BACKGROUND: Intraoperative hypotension (IOH) is a common phenomenon in high-risk surgery and is often linked to postoperative acute kidney injury (AKI). Pancreaticoduodenectomy (PD), or Whipple’s procedure, is a lengthy and complex surgical procedure to remove the head of the pancreas, gallbladder and bile duct, and the first part of the duodenum. This retrospective 5-year study from a single center in Poland included 303 patients who underwent PD and evaluated IOH as a factor associated with AKI.
MATERIAL AND METHODS: We analyzed perioperative data to assess how various IOH thresholds can predict AKI (according to KDIGO criteria). Several IOH definitions were applied, including absolute and relative thresholds, based on the mean arterial pressure (MAP). Statistically significant IOH thresholds were inserted into multivariable logistic regression models with previously established independent variables.
RESULTS: We included 303 patients over a 5-year period (2016-2021). There were 58 (19.1%) cases of postoperative AKI. MAP <55 mmHg and a maximal% drop from preinduction MAP were the only IOH definitions associated with AKI. Multivariable analysis revealed that max% drop from preinduction MAP (per 10%, OR=1.65; AUROC=0.70) was the IOH definition best suited for AKI prediction in patients undergoing PD.
CONCLUSIONS: In patients undergoing PD, it is important to prevent excessive blood pressure drops in regards to preinduction blood pressure values. In this cohort, relative IOH thresholds were better suited for prediction of AKI than the absolute IOH thresholds.
Keywords: Acute Kidney Injury, Hypotension, Intraoperative Period, Pancreaticoduodenectomy, Humans, Cohort Studies, Intraoperative Complications, Postoperative Complications, Risk Factors
Background
Intraoperative hypotension (IOH) is a common phenomenon in patients undergoing surgical treatment and is a result of a combination of various factors altering blood pressure (BP). IOH can be induced by a variety of patient- and procedure-related factors, including hypovolemia, vasodilation, and myocardial depression [1,2]. One of the most distinct complications occurring in the perioperative period is acute kidney injury (AKI), which also seems to be closely related to IOH [3]. There is, therefore, a strong rationale for the perioperative hemodynamic optimization, but with no clear consensus on the best definition of clinically significant hypotension [4–6]. Numerous interpretations of IOH were proposed over the years, leading to disparity in the hypotension incidence (from 5% to 99%) and divergent conclusions [4]. Methods used to identify IOH include absolute thresholds and relative thresholds, defined as drop from preoperative/preinduction BP [3,7–9]. Most studies on IOH included patients undergoing overall noncardiac surgeries, with no specific focus on a specific subgroup [5,6,10].
Pancreaticoduodenectomy (PD) is one of the most complex and high-risk procedures in abdominal surgery. The advance in surgical technique and centralization of pancreatic cancer surgery resulted in decreased perioperative mortality after PD to 5% [11,12]. Nevertheless, perioperative morbidity remains high, affecting about 40% of patients, which is much higher than for other abdominal surgeries [13,14]. Such a long, extensive surgery, mostly performed in patients at an advanced age, and often associated with significant blood loss, may pose a considerable risk of hypotension and subsequent ischemic complications. Guidelines for perioperative care for PD were updated recently [15], but there are no specific recommendations regarding management of IOH during PD.
Therefore, the purpose of this study was to evaluate various definitions of IOH as factors associated with AKI in patients undergoing PD.
Material and Methods
ETHICS STATEMENT:
All patients gave their written informed consent for medical procedures and data management. No additional approval of the Ethics Committee was required due to the anonymous and non-interventional design of the project (PCN/0052/KB/116/22).
PATIENT SELECTION AND DATA ACQUISITION:
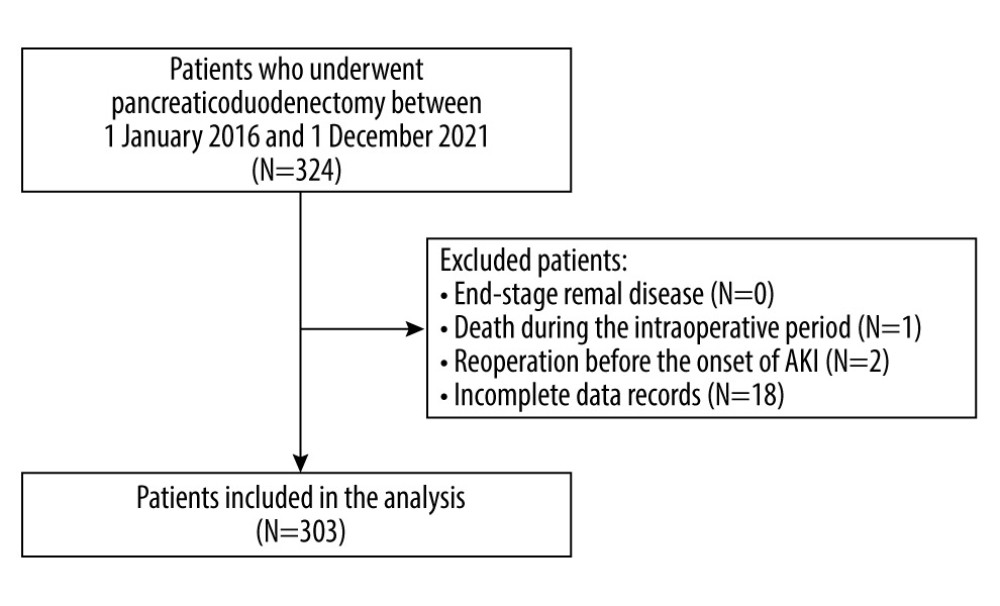
In this cohort study we screened adult patients who underwent pancreaticoduodenectomy between 1 January 2016 and 1 December 2021 in a university hospital. Patients who had end-stage renal disease (chronic kidney disease G5), died during the intraoperative period, underwent any re-operation before the onset of AKI, or had incomplete data in the medical records were excluded from the analysis [16]. Demographic and clinical data were recorded, including sex, age, body mass index (BMI), comorbidities (chronic arterial hypertension and its ongoing pharmacological therapy, chronic kidney disease CKD, coronary heart disease CHD, and diabetes mellitus DM), preoperative hemoglobin, any premedication, and any history of neoadjuvant chemotherapy. The Charlson Comorbidity Index (CCI) and Revised Cardiac Risk Index (RCRI) were subsequently calculated. The type and duration of anesthesia were recorded. Perioperative risk was assessed based on individual patient’s risk, according to the American Society of Anesthesiology (ASA) physical status (PS) classification [17]. Due to the nature of the procedure, in all cases, the procedural risk was ranked as “high” according to the European Society of Cardiology and European Society of Anesthesiology recommendations [18].
The blood pressure (BP) monitoring method used depended on the anesthesiologist’s decision. Patients had either non-invasive blood pressure measurements (via an automatic oscillometric method; Dräger Infinity Gamma XL) or invasive ones (by using an arterial catheter in the radial artery). BP was recorded before and after the induction of anesthesia and in 5-minute intervals until the patient was discharged from the operating theater. Recordings of MAP throughout the procedure were analyzed. Our study focused solely on MAP-derived IOH thresholds since these definitions seem to be the most well documented in the literature [9]. Hence, the most commonly applied IOH thresholds were distinguished: MAP <70 mmHg, <65 mmHg, <60 mmHg, and <55 mmHg [3,8,9]. Duration of such IOH events were calculated. Moreover, maximal% drops from the preinduction MAP values were analyzed. Intraoperative fluid dosing, diuresis, bleeding, and the use of norepinephrine (NE) was analyzed according to the dose and duration of infusion.
In the postoperative period, incidents of AKI were recorded. Preoperative creatinine was measured 1 day before the surgery. According to the KDIGO guidelines, AKI was defined as either a 0.3 mg/dL increase in the first 48 hours after surgery or a 1.5-fold increase in the first 7 days after surgery [19].
The STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) statement was applied for appropriate reporting [20].
STATISTICAL ANALYSIS:
Statistical analysis was performed using MedCalc Statistical Software version 18.1 (MedCalc Software Ltd., Ostend, Belgium). Continuous variables were expressed as median and interquartile range (IQR). Qualitative variables were expressed as absolute values and/or percent. Between-group differences for quantitative variables were assessed using the Mann-Whitney U-test or Kruskal-Wallis test. Their distribution was verified with the Shapiro-Wilk test. The χ2 test or Fisher’s exact test were applied for qualitative variables. All tests were two-tailed. A P value <0.05 was considered statistically significant. Multivariable logistic regression was implemented for each of the thresholds that achieved P values lower than 0.1. To control the potential confounding factors, we used variables regarded as independent risk factors for postoperative AKI: age, diabetes mellitus, RCRI, and ASA status [21,22]. Each of the multivariable models were then analyzed in terms of AUROC values.
Results
SUMMARY RESULTS:
The study group consisted of 303 patients (Figure 1). The median age was 64 (range, 57–69) years. There were 169 patients (55.7%) with chronic arterial hypertension, of which 94 were treated with ACEI/ARB (angiotensin-converting enzyme inhibitors/angiotensin receptor blockers). In regards to ASA-PS class, 154 (50.8%) patients were classified as class III and 141 (46.5%) as class II. The most common indication for PD was tumor of the head of the pancreas (70.6%), followed by cancer of the ampulla of the duodenum (25.7%) and biliary tract cancer (3.6%). Invasive BP monitoring was applied in 55.4% of cases. The median duration of PD was 440 minutes. The outcome of interest was postoperative AKI, which was reported in 58 patients (19.1%). Based on KDIGO classification, 47 (81%) patients had stage I AKI, 9 (15.5%) had stage II AKI, and 2 (3.5%) had stage III AKI.
There were a number of factors associated with the postoperative AKI (Tables 1, 2). In terms of preoperative factors, age, CHD, use of ACEI/ARB, and ASA III and higher preinduction BP values were associated with increased risk of AKI. As for intraoperative factors, several hemodynamic parameters were found to be associated with AKI. Patients who experienced lower BP and greater drops in BP during the procedure were more likely to develop AKI. Lastly, patients who required more norepinephrine to maintain appropriate MAP were almost statistically significantly more likely to have AKI than the non-AKI individuals.
INTRAOPERATIVE HYPOTENSION:
In regards to absolute IOH thresholds, a MAP of <55 mmHg was statistically significantly associated with postoperative AKI (Table 2). Hence, only 2 IOH definitions were put into multivariable logistic regression models: 1) Max% drop from preinduction MAP and 2) MAP <55 mmHg. Each model consisted of an IOH definition along with other independent variables mentioned in the methods section. These 2 different multivariable models were associated with AKI: the first was the model with the maximal% drop from preinduction MAP (OR: 1.65 per 10%, AUROC for the model: 0.70) and the second was with MAP <55 mmHg (OR: 2.23, AUROC for the model: 0.65) (Table 3).
Discussion
LIMITATIONS:
In terms of limitations, this was a single-center study including 1 specific surgical procedure, which limited the size of the cohort and number of included cases of AKI, but such a design was essential to provide a fairly homogenous group. Additionally, due to the retrospective nature of the study, it contains variables that could not be controlled for. The fact that extensive hemodynamic monitoring is not routinely applied in patients after PD at surgery ward is the reason why our analysis was limited to intraoperative BP monitoring. For the best evaluation of the association between intraoperative hemodynamic parameters and AKI, the postoperative period should also be taken into account. Another limitation is that BP was recorded in a 5-minute interval, so available data may fail to fully capture the exact occurrence of hypotension. Moreover, different methods of BP monitoring were used, including non-invasive and invasive measurements, which could result in disparities in acquired values, as non-invasive monitoring is less precise [36].
Conclusions
In patients undergoing pancreaticoduodenectomy, it is important to prevent excessive blood pressure drops in regards to preinduction blood pressure values. For every 10% decrease in mean arterial pressure there was approximately 1.60 increased odds of generating acute kidney injury. In this cohort, relative blood pressure thresholds were better suited for prediction of postoperative acute kidney injury.
References
1. Ke JXC, George RB, Beattie WS, Making sense of the impact of intraoperative hypotension: from populations to the individual patient: Br J Anaesth, 2018; 121(4); 689-91
2. Devereaux PJ, Yang HPOISE Study Group, Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): A randomised controlled trial: Lancet, 2008; 371(9627); 1839-47
3. Sessler DI, Bloomstone JA, Aronson S, Perioperative Quality Initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery: Br J Anaesth, 2019; 122(5); 563-74
4. Brienza N, Giglio MT, Marucci M, Fiore T, Does perioperative hemodynamic optimization protect renal function in surgical patients? A meta-analytic study: Crit Care Med, 2009; 37(6); 2079-90
5. Bijker JB, van Klei WA, Kappen TH, Incidence of intraoperative hypotension as a function of the chosen definition: Literature definitions applied to a retrospective cohort using automated data collection: Anesthesiology, 2007; 107(2); 213-20
6. van Waes JA, van Klei WA, Wijeysundera DN, Association between intraoperative hypotension and myocardial injury after vascular surgery: Anesthesiology, 2016; 124(1); 35-44
7. Ahuja S, Mascha EJ, Yang D, Associations of intraoperative radial arterial systolic, diastolic, mean, and pulse pressures with myocardial and acute kidney injury after noncardiac surgery: A retrospective cohort analysis: Anesthesiology, 2020; 132(2); 291-306
8. Salmasi V, Maheshwari K, Yang D, Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: A retrospective cohort analysis: Anesthesiology, 2017; 126(1); 47-65
9. Wesselink EM, Kappen TH, Torn HM, Intraoperative hypotension and the risk of postoperative adverse outcomes: A systematic review: Br J Anaesth, 2018; 121(4); 706-21
10. Sun LY, Wijeysundera DN, Tait GA, Beattie WS, Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery: Anesthesiology, 2015; 123(3); 515-23
11. McPhee JT, Hill JS, Whalen GF, Perioperative mortality for pancreatectomy: A national perspective: Ann Surg, 2007; 246(2); 246-53
12. de Wilde RF, Besselink MG, van der Tweel I, Impact of nationwide centralization of pancreaticoduodenectomy on hospital mortality: Br J Surg, 2012; 99(3); 404-10
13. Aoki S, Miyata H, Konno H, Risk factors of serious postoperative complications after pancreaticoduodenectomy and risk calculators for predicting postoperative complications: A nationwide study of 17,564 patients in Japan: J Hepatobiliary Pancreat Sci, 2017; 24(5); 243-51
14. Kobayashi H, Miyata H, Gotoh M, Risk model for right hemicolectomy based on 19,070 Japanese patients in the National Clinical Database: J Gastroenterol, 2014; 49(6); 1047-55
15. Melloul E, Lassen K, Roulin D, Guidelines for perioperative care for pancreatoduodenectomy: Enhanced recovery after surgery (ERAS) recommendations 2019: World J Surg, 2020; 44(7); 2056-84
16. Webster AC, Nagler EV, Morton RL, Masson P, Chronic kidney disease: Lancet, 2017; 389(10075); 1238-52
17. Doyle DJ, Goyal A, Bansal P, Garmon EH: American Society of Anesthesiologists Classification (ASA Class), 2020 Available online: https://www.ncbi.nlm.nih.gov/books/NBK441940/
18. Kristensen SD, Knuuti J, Saraste A, 2014 ESC/ESA Guidelines on non-cardiac surgery: Cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: Cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA): Eur Heart J, 2014; 35(35); 2383-31
19. Khwaja A, KDIGO clinical practice guidelines for acute kidney injury: Nephron Clin Pract, 2012; 120(4); c179-c84
20. Vandenbroucke JP, von Elm E, Altman DG, Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration: Int J Surg, 2014; 12(12); 1500-24
21. Goren O, Matot I, Perioperative acute kidney injury: Br J Anaesth, 2015; 115(Suppl 2); ii3-ii14
22. Biteker M, Dayan A, Tekkeşin AI, Incidence, risk factors, and outcomes of perioperative acute kidney injury in noncardiac and nonvascular surgery: Am J Surg, 2014; 207; 53-59
23. Long TE, Helgason D, Helgadottir S, Acute kidney injury after abdominal surgery: Incidence, risk factors, and outcome: Anesth Analg, 2016; 122(6); 1912-20
24. Long TE, Helgason D, Helgadottir S, Mild Stage 1 post-operative acute kidney injury: Association with chronic kidney disease and long-term survival: Clin Kidney J, 2020; 14(1); 237-44
25. Reese T, Kröger F, Makridis G, Impact of acute kidney injury after extended liver resections: HPB (Oxford), 2021; 23(7); 1000-7
26. O’Connor ME, Kirwan CJ, Pearse RM, Prowle JR, Incidence and associations of acute kidney injury after major abdominal surgery: Intensive Care Med, 2016; 42(4); 521-30
27. STARSurg Collaborative and EuroSurg Collaborative, Validation of the OAKS prognostic model for acute kidney injury after gastrointestinal surgery: BJS Open, 2022; 6(1); zrab150
28. Kim M, Brady JE, Li G, Variations in the risk of acute kidney injury across intraabdominal surgery procedures: Anesth Analg, 2014; 119(5); 1121-32
29. Walsh M, Devereaux PJ, Garg AX, Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: Toward an empirical definition of hypotension: Anesthesiology, 2013; 119(3); 507-15
30. Sun LY, Wijeysundera DN, Tait GA, Beattie WS, Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery: Anesthesiology, 2015; 123(3); 515-23
31. Czajka S, Putowski Z, Krzych ŁJ, Intraoperative hypotension and its organ-related consequences in hypertensive subjects undergoing abdominal surgery: A cohort study: Blood Press, 2021; 30(6); 348-58
32. Futier E, Lefrant JY, Guinot PG, Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: A randomized clinical trial: JAMA, 2017; 318(14); 1346-57
33. Ida M, Sumida M, Naito Y, Tachiiri Y, Kawaguchi MImpact of intraoperative hypotension and blood loss on acute kidney injury after pancreas surgery: Braz J Anesthesiol, 2020; 70(4); 343-48 [in Portuguese]
34. Prowle JR, Forni LG, Bell M, Postoperative acute kidney injury in adult non-cardiac surgery: Joint consensus report of the Acute Disease Quality Initiative and PeriOperative Quality Initiative: Nat Rev Nephrol, 2021; 17(9); 605-18
35. Shen J, Chu Y, Wang C, Yan S, Risk factors for acute kidney injury after major abdominal surgery in the elderly aged 75 years and above: BMC Nephrol, 2022; 23(1); 224
36. Waxman S, Fuensalida S, Sánchez F: Vet Anaesth Analg, 2021; 48(2); 252-55
Tables
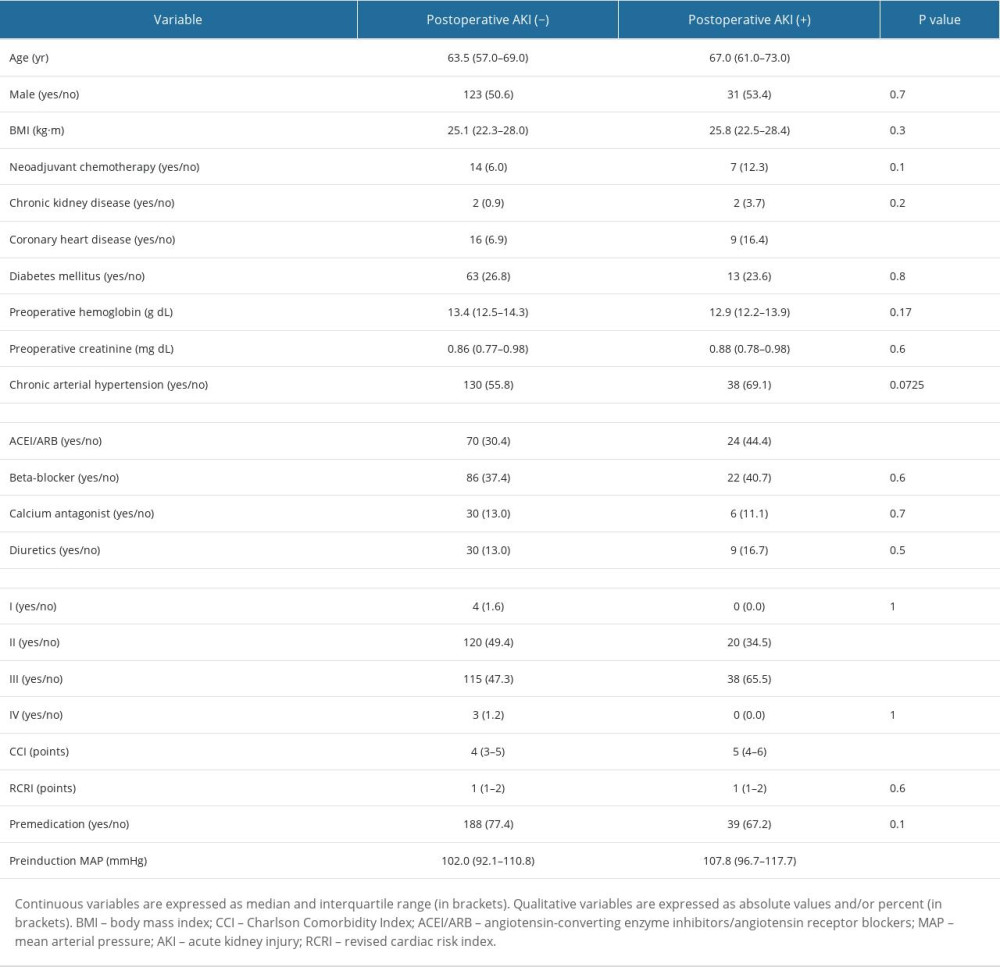 Table 1. Patient-related factors associated with postoperative AKI.
Table 1. Patient-related factors associated with postoperative AKI.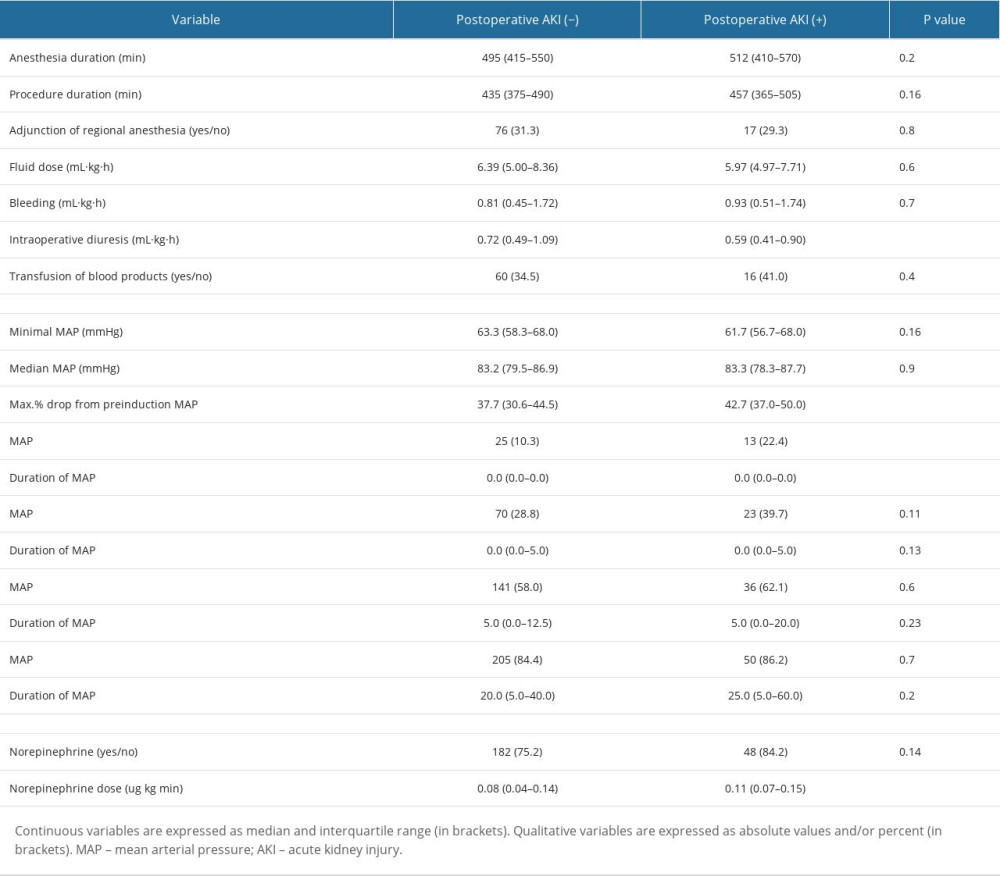 Table 2. Procedure-related factors associated with postoperative AKI.
Table 2. Procedure-related factors associated with postoperative AKI.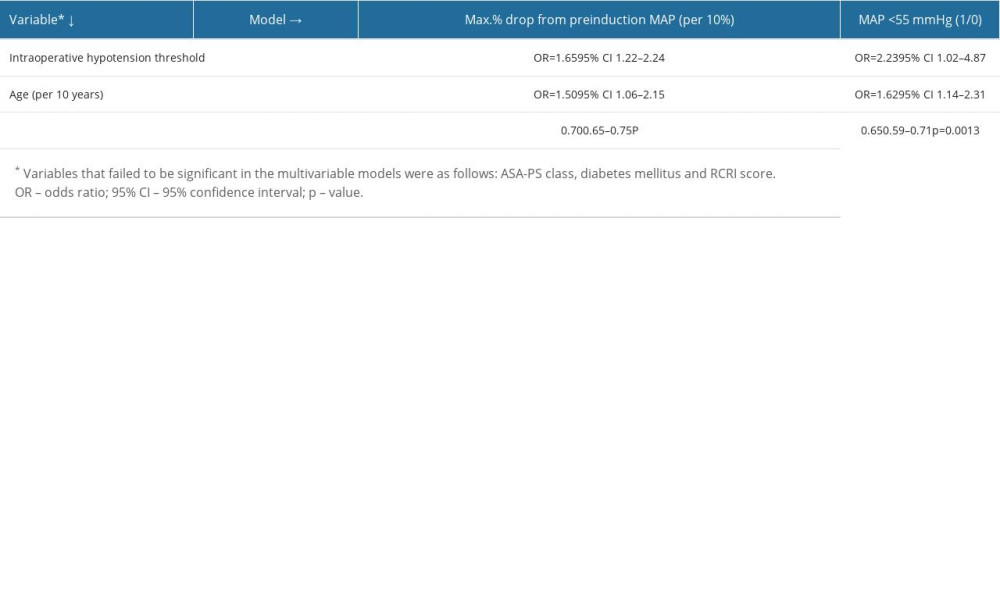 Table 3. Multivariate logistic regression models in predicting the occurrence of AKI.
Table 3. Multivariate logistic regression models in predicting the occurrence of AKI. Table 1. Patient-related factors associated with postoperative AKI.
Table 1. Patient-related factors associated with postoperative AKI. Table 2. Procedure-related factors associated with postoperative AKI.
Table 2. Procedure-related factors associated with postoperative AKI. Table 3. Multivariate logistic regression models in predicting the occurrence of AKI.
Table 3. Multivariate logistic regression models in predicting the occurrence of AKI. In Press
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952