11 June 2023: Clinical Research
Evaluating the One-Year Efficacy of Combined Anti-VEGF and Dexamethasone Implant Treatment for Macular Edema in Retinal Vein Occlusions
Fengmei RenB, Hui GongD, Han ZhangD, Xiaoguang ZhangE, Xiaolin LiuF, Peifeng GaoD, Junying LiG, Guisen ZhangDOI: 10.12659/MSM.939277
Med Sci Monit 2023; 29:e939277
Abstract
BACKGROUND: Retinal vein occlusion-induced macular edema (RVO-ME) is a significant global cause of vision loss, with the effectiveness of combined anti-vascular endothelial growth factor (anti-VEGF) drugs and dexamethasone implantation (DEX I) being a relevant, yet not thoroughly explored, area of interest.The aim of this study was to evaluate the 1-year clinical efficacy of combination therapy using anti-vascular endothelial growth factor (VEGF) drugs and dexamethasone implantation (DEX I) in the treatment of macular edema secondary to retinal vein occlusion (RVO-ME).
MATERIAL AND METHODS: This retrospective study analyzed data from 34 RVO-ME patients treated at the Inner Mongolia Chaoju Eye Hospital between January 2020 and December 2021. All patients underwent initial DEX I treatment, followed by the introduction of anti-VEGF drugs, and were observed for one year. Retinal structural and vascular changes were measured using spectral domain optical coherence tomography (SD-OCT) and OCT angiography (OCTA). The study also evaluated shifts in best corrected visual acuity (BCVA) throughout the observation period.
RESULTS: Following the combined therapy, patients showed significant improvements in BCVA, intraocular pressure (IOP), central retinal thickness (CRT), and retinal vessel density (VD) (all P<0.05). Upon stratifying the results by RVO type, patients with branch retinal vein occlusion (BRVO)-ME displayed more significant BCVA improvement and CRT reduction at various post-treatment intervals compared to those with central retinal vein occlusion (CRVO)-ME (all P<0.05).
CONCLUSIONS: The combined use of anti-VEGF drugs and DEX I showed promising one-year efficacy in treating RVO-ME, with greater improvements noted in patients with BRVO-ME compared to those with CRVO-ME. Despite the positive results, close monitoring of IOP elevation, a notable side effect, remains crucial.
Keywords: Estrogen Replacement Therapy, Macular edema, Retinal Vein Occlusion, Humans, Dexamethasone, Intravitreal Injections, Retrospective Studies, Tomography, Optical Coherence, Treatment Outcome, Vascular Endothelial Growth Factors, Visual Acuity
Background
Retinal vein occlusion (RVO), including central retinal vein occlusion (CRVO) and branch retinal vein occlusion (BRVO), is an obstruction of the retinal venous system, and macular edema (ME) secondary to RVO (RVO-ME) can lead to visual dysfunction [1,2]. Various cytokines such as inflammatory factors play a key role in the development of RVO-ME [3]. Therefore, inflammation has become an important target in RVO-ME therapy [4]. Since 2009, the US Food and Drug Administration (FDA) has approved intravitreal sustained-release dexamethasone implant (DEX I, OZURDEX®) as treatment for RVO-ME. In 2017, the Chinese State Food and Drug Administration (SFDA) approved OZURDEX® for the treatment of ME caused by RVO. It has been observed in domestic phase III clinical trials and clinical practices that intravitreal DEX I can improve vision acuity and reduce macular thickness in patients with RVO-ME, and has good safety [5].
In addition, a previous ex vivo study using autologous aqueous or vitreous humor indicated that inflammatory cytokine levels and vascular endothelial growth factor (VEGF) concentration are elevated and correlated with the severity of ME in eyes with RVO [6]. Therefore, we speculated that the combined use of glucocorticoids and anti-VEGF drugs can improve clinical outcomes in patients with RVO-ME. Previous case series studies in which patients receiving DEX I 2 weeks after anti-VEGF injections in eyes with ME due to RVO showed longer duration of effect, and better improvements in vision and central foveal thickness [7,8]. However, there are few reports on the effects of combination therapy using anti-VEGF drugs and steroids on RVO-ME. In this study, we summarized the real-world visual and anatomical outcomes in CRVO and BRVO patients treated with a combined treatment regimen (anti-VEGF plus DEX I).
Material and Methods
STATISTICAL ANALYSIS:
All data are presented as mean±standard division (SD) or percentages, as appropriate. Normal distribution was tested by the Kolmogorov-Smirnov test. Normally distributed data were analyzed with the
Results
Overall, 40 patients were initially enrolled, and 6 patients were excluded because they were lost to follow-up. Finally, 34 eyes from 34 participants (15 males, mean age 55.12±6.28 years) identified with RVO-ME were included, and all participants underwent a mean of 3.15 ± 1.02 injections. Table 1 shows the characteristics of included participants with RVO-ME.
According to the fundus examinations, 17 eyes from 17 patients had CRVO, and 17 eyes from 17 patients had BRVO. There were no significant differences in demographics, medical history, and ophthalmic examinations between eyes with CRVO and those with BRVO (Table 2).
As shown in Table 3, BCVA, IOP, central retinal thickness (CRT), retinal vessel density (VD) of the SCP, and DCP levels were significantly different before and after combination therapy (all
Overall, at 12-month follow-up, adverse events (AEs) were observed in 10 eyes, of which most cases (n=5, 50%) were IOP elevation and 3 of them received IOP-lowering medications to manage IOP levels. Furthermore, 2 cases with subconjunctival hemorrhage were reported during the study period. No serious AEs were reported.
Discussion
We comprehensively investigated functional anatomic indicators such as structural and vascular retinal changes in eyes affected by RVO before and after anti-VEGF plus DEX I combination intravitreal injections therapy.
During the development of RVO-ME, VEGF plays a key role in vascular permeability, resulting in the appearance of ME [11–13]. Therefore, anti-VEGF drugs have become the first-line treatment for ME, and have been found to be effective in improving vision [14]. However, in some cases, the inflammatory factors might also play crucial roles in the pathogenesis of RVO occurrence [15]. Anti-inflammatory drugs may be another treatment for RVO-ME, and DEX I was approved for treatment of ME associated with RVO. The platelet-to-lymphocyte ratio (PLR), which is an inexpensive marker calculated from routine complete blood count test, is a new biomarker that shows the existence of thrombosis and inflammation. PLR are elevated in RVO, suggesting that PLR may be a useful marker for RVO [16]. We performed this retrospective study to evaluate the effects of combination therapy using anti-VEGF drugs and steroids for ME in eyes with RVO.
In this real-world retrospective study, we found that anti-VEGF plus DEX I treatment improved BCVA, CRT, and retinal vessel density in patients with BRVO-ME and CRVO-ME, after an average of 3 injections. Previous studies have shown that intravitreal injection of anti-VEGF drugs (5.5 injections in 1 year) or DEX I can relieve RVO-ME [17,18]. Additionally, our findings indicated that after combination therapy RVO-ME patients had functional effects, and the curative effect peaked at 2 months after injection. Anatomically, the curative effect can occur at 1 month and last up to 12 months after treatment. Small increases in IOP that occur after combination therapy should be noted but can usually be managed with topical medication. Similarly, IOP increases were also found in another real-world study with DEX I treatments [17]. No glaucoma incisional surgeries were required during combination therapy treatment.
Patients with BRVO-ME had larger gains in BCVA and reduction in CRT over 12 months of follow-up, confirming the benefit of combination therapy. Recently, a prospective, observational, post-marketing surveillance study revealed that RVO-ME eyes administered a first DEX I acquired stable/improved BCVA tended to decrease after 2 months through 6 months of follow-up, but the decline was greater in the CRVO subgroup [17].
Notably, we also found that our combined therapy could improve the retinal VD including SCP, and DCP. To date, few reports have been published on the effects of this combination therapy on vessel density in eyes with RVO. Previously, both anti-VEGF and steroid monotherapy had statistically significant effects on the improvement of visual acuity and increased VD of the SCP as well as the DCP [19].
There were some limitations in our study. First, our study was a retrospective observational and auditing report on relatively small numbers without a comparator. Second, although we had acquired high-quality OCTA images, it was difficult to divide VD of parafoveal into different quadrants. Finally, our study provided “real-world” results and the evaluations were at the discretion of the treating physician and according to normal practice; thus, there was no standardization of assessments.
Conclusions
Taken together, the findings of the current study illustrate the multicenter real-world effects of combined therapy of anti-VEGF drugs and DEX I in eyes with RVO-ME, which is associated with good long-term BCVA, anatomical outcomes, and VD changes, and favorable safety profile with fewer intravitreal re-treatments.
Figures
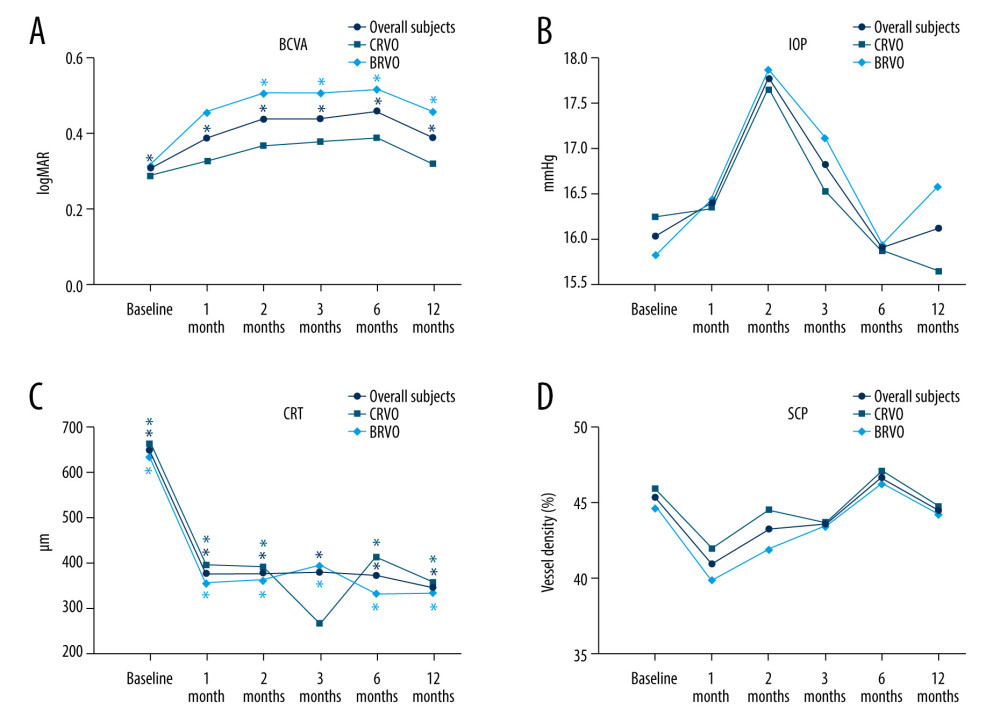 Figure 1. Changes in BCVA obtain, anatomical outcomes, VD changes within 12 months (star: compared with baseline). (A) Best corrected visual acuity (BCVA) changes. (B) Intraocular pressure (IOP) changes. (C) Central retinal thickness (CRT) changes. (D) OCTA images of the superficial capillary plexus (SCP) changes. The figure was generated using GraphPad Prism version 9.0 for Windows (La Jolla, CA, www.graphpad.com).
Figure 1. Changes in BCVA obtain, anatomical outcomes, VD changes within 12 months (star: compared with baseline). (A) Best corrected visual acuity (BCVA) changes. (B) Intraocular pressure (IOP) changes. (C) Central retinal thickness (CRT) changes. (D) OCTA images of the superficial capillary plexus (SCP) changes. The figure was generated using GraphPad Prism version 9.0 for Windows (La Jolla, CA, www.graphpad.com). 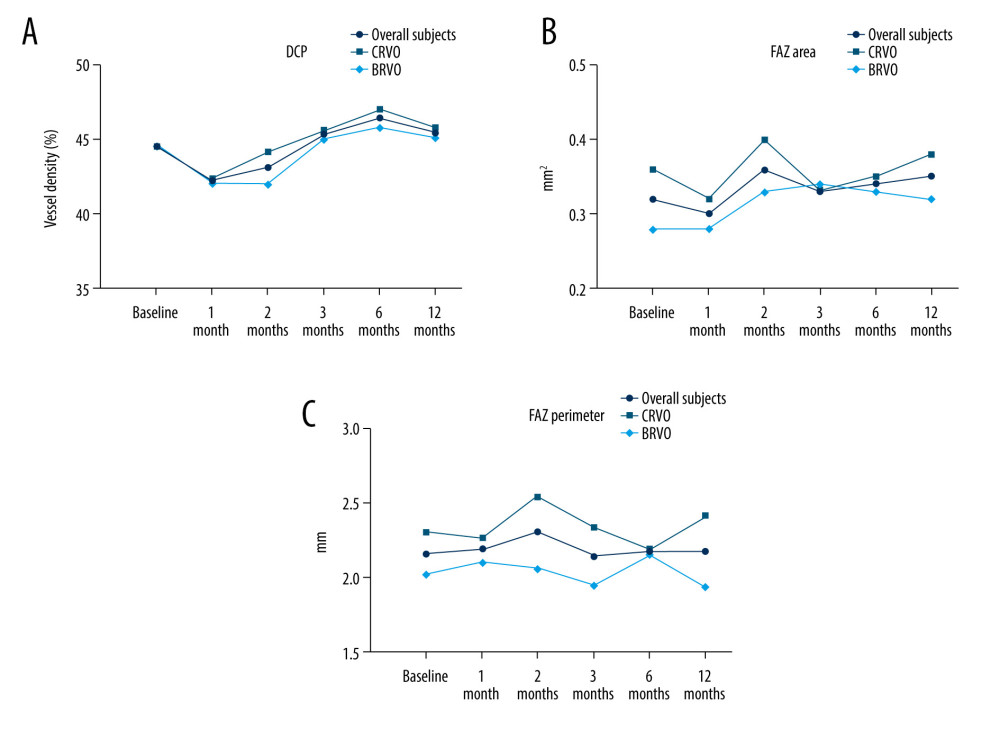 Figure 2. Changes in BCVA obtain, anatomical outcomes, VD changes within 12 months (star: compared with baseline). (A) Deep retinal capillary plexus (DCP) changes. (B) Foveal avascular zone (FAZ) area changes. (C) FAZ circumference changes. Figure was generated using GraphPad Prism version 9.0 for Windows (La Jolla, CA, www.graphpad.com).
Figure 2. Changes in BCVA obtain, anatomical outcomes, VD changes within 12 months (star: compared with baseline). (A) Deep retinal capillary plexus (DCP) changes. (B) Foveal avascular zone (FAZ) area changes. (C) FAZ circumference changes. Figure was generated using GraphPad Prism version 9.0 for Windows (La Jolla, CA, www.graphpad.com). References
1. Petrella RJ, Blouin J, Davies B, Incidence and characteristics of patients with visual impairment due to macular edema secondary to retinal vein occlusion in a representative Canadian cohort: J Ophthalmol, 2012; 2012; 723169
2. Mohamed Q, McIntosh RL, Saw SM, Interventions for central retinal vein occlusion: An evidence-based systematic review: Ophthalmology, 2007; 114(3); 507-19
3. Ehlken C, Grundel B, Michels D, Increased expression of angiogenic and inflammatory proteins in the vitreous of patients with ischemic central retinal vein occlusion: PLoS One, 2015; 10(5); e0126859
4. Garay-Aramburu G, Gomez-Moreno A, A 5-year follow-up study of the treatment of macular edema due to retinal vein occlusion using dexamethasone intravitreal implants: J Ocul Pharmacol Ther, 2018; 34(6); 436-41
5. Li X, Wang N, Liang X, Safety and efficacy of dexamethasone intravitreal implant for treatment of macular edema secondary to retinal vein occlusion in Chinese patients: Randomized, sham-controlled, multicenter study: Graefes Arch Clin Exp Ophthalmol, 2018; 256(1); 59-69
6. Hirano Y, Suzuki N, Tomiyasu T, Multimodal imaging of microvascular abnormalities in retinal vein occlusion: J Clin Med, 2021; 10(3); 405
7. Singer MA, Bell DJ, Woods P, Effect of combination therapy with bevacizumab and dexamethasone intravitreal implant in patients with retinal vein occlusion: Retina, 2012; 32(7); 1289-94
8. Singer MA, Jansen ME, Tyler L, Long-term results of combination therapy using anti-VEGF agents and dexamethasone intravitreal implant for retinal vein occlusion: An investigational case series: Clin Ophthalmol, 2017; 11; 31-38
9. Wang X, Zhao Q, Tao R, Decreased retinal vascular density in Alzheimer’s disease (AD) and Mild Cognitive Impairment (MCI): An Optical Coherence Tomography Angiography (OCTA) study: Front Aging Neurosci, 2020; 12; 572484
10. Song S, Yu X, Zhang P, Changes in macular microvascular structure in macular edema secondary to branch retinal vein occlusion treated with antivascular endothelial growth factor for one year: J Ophthalmol, 2021; 2021; 6645452
11. Behar-Cohen F, Dernigoghossian M, Andrieu-Soler C, Potential antiedematous effects of intravitreous anti-VEGF, unrelated to VEGF neutralization: Drug Discov Today, 2019; 24(8); 1436-39
12. Edington M, Connolly J, Chong NV, Pharmacokinetics of intravitreal anti-VEGF drugs in vitrectomized versus non-vitrectomized eyes: Expert Opin Drug Metab Toxicol, 2017; 13(12); 1217-24
13. Liegl R, Koenig S, Siedlecki J, Temsirolimus inhibits proliferation and migration in retinal pigment epithelial and endothelial cells via mTOR inhibition and decreases VEGF and PDGF expression: PLoS One, 2014; 9(2); e88203
14. Arrigo A, Bandello F, Retinal vein occlusion: Drug targets and therapeutic implications: Expert Opin Ther Targets, 2021; 25(10); 847-64
15. Li X, Cao X, Zhao M, The changes of irisin and inflammatory cytokines in the age-related macular degeneration and retinal vein occlusion: Front Endocrinol, 2022; 13; 861757
16. Kurtul BE, Çakmak A, Elbeyli A, Assessment of platelet-to-lymphocyte ratio in patients with retinal vein occlusion: Ther Adv Ophthalmol, 2020; 12; 2515841420971949
17. Kim MS, Choi J, Lee HD, Dexamethasone intravitreal implant for the treatment of macular edema following retinal vein occlusion: Post hoc analysis of post-marketing surveillance data in the real-world setting in Korea: Clin Ophthalmol, 2021; 15; 3623-36
18. Corazza P, D’Alterio FM, Savastano MC, Long-term outcomes of anti-VEGF treatment of macular oedema due to retinal vein occlusions: Eur J Ophthalmol, 2022; 32(6); 3536-46
19. Kaya M, Ozturk T, Atas F, Effect of intravitreal dexamethasone implant versus ranibizumab on vessel density in branch retinal vein occlusion: Eur J Ophthalmol, 2021 [Online ahead of print]
Figures
 Figure 1. Changes in BCVA obtain, anatomical outcomes, VD changes within 12 months (star: compared with baseline). (A) Best corrected visual acuity (BCVA) changes. (B) Intraocular pressure (IOP) changes. (C) Central retinal thickness (CRT) changes. (D) OCTA images of the superficial capillary plexus (SCP) changes. The figure was generated using GraphPad Prism version 9.0 for Windows (La Jolla, CA, www.graphpad.com).
Figure 1. Changes in BCVA obtain, anatomical outcomes, VD changes within 12 months (star: compared with baseline). (A) Best corrected visual acuity (BCVA) changes. (B) Intraocular pressure (IOP) changes. (C) Central retinal thickness (CRT) changes. (D) OCTA images of the superficial capillary plexus (SCP) changes. The figure was generated using GraphPad Prism version 9.0 for Windows (La Jolla, CA, www.graphpad.com). Figure 2. Changes in BCVA obtain, anatomical outcomes, VD changes within 12 months (star: compared with baseline). (A) Deep retinal capillary plexus (DCP) changes. (B) Foveal avascular zone (FAZ) area changes. (C) FAZ circumference changes. Figure was generated using GraphPad Prism version 9.0 for Windows (La Jolla, CA, www.graphpad.com).
Figure 2. Changes in BCVA obtain, anatomical outcomes, VD changes within 12 months (star: compared with baseline). (A) Deep retinal capillary plexus (DCP) changes. (B) Foveal avascular zone (FAZ) area changes. (C) FAZ circumference changes. Figure was generated using GraphPad Prism version 9.0 for Windows (La Jolla, CA, www.graphpad.com). Tables
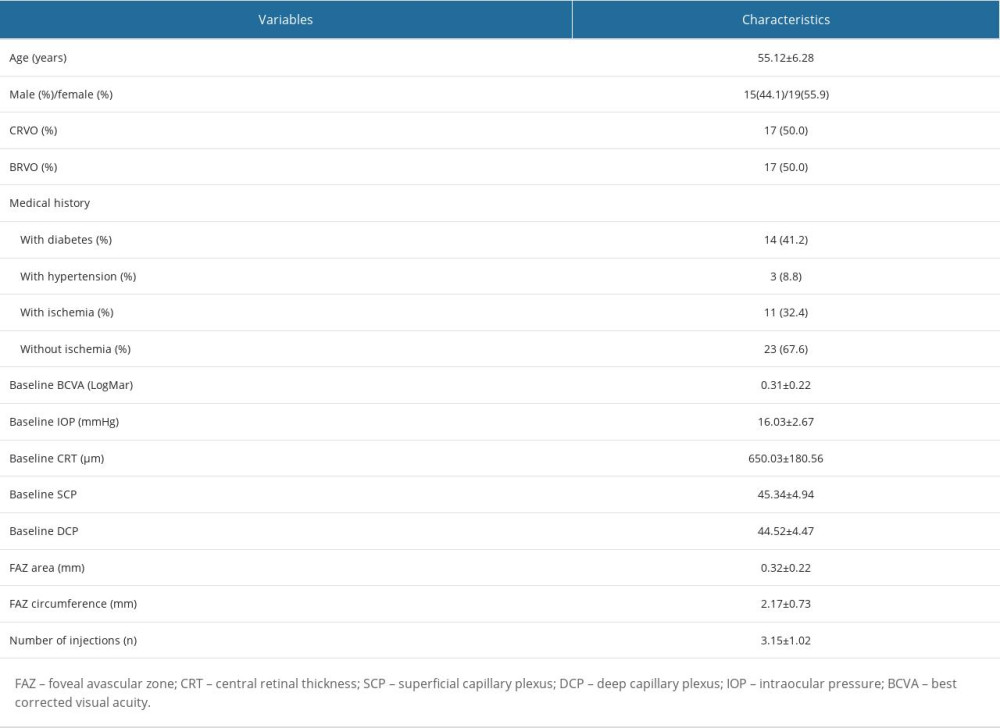 Table 1. Characteristics of all included subjects.
Table 1. Characteristics of all included subjects.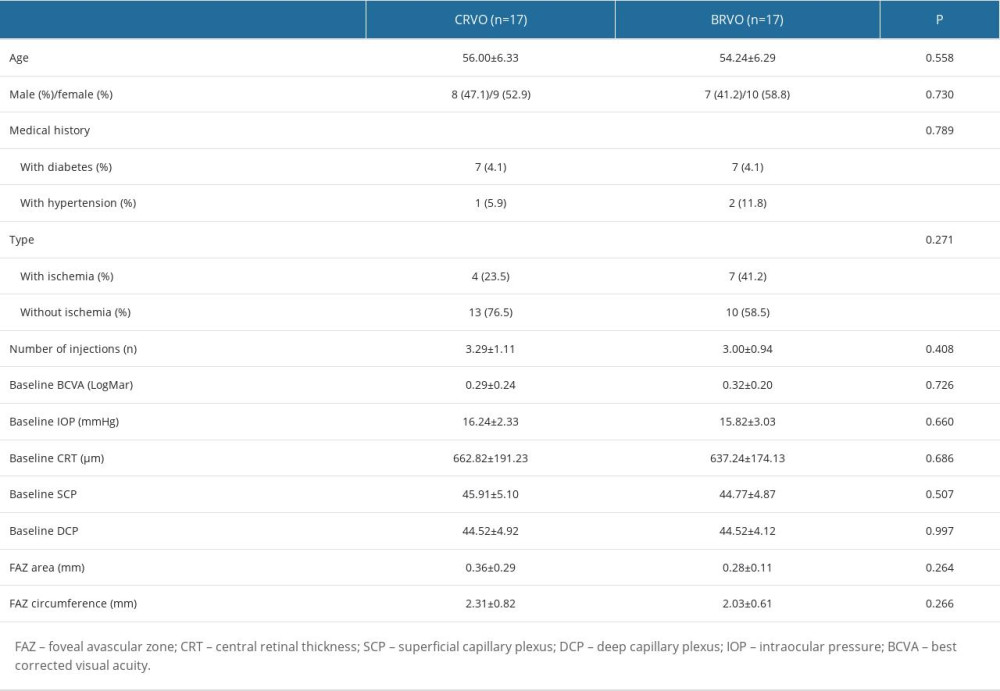 Table 2. Characteristics of all included subjects by subgroup.
Table 2. Characteristics of all included subjects by subgroup.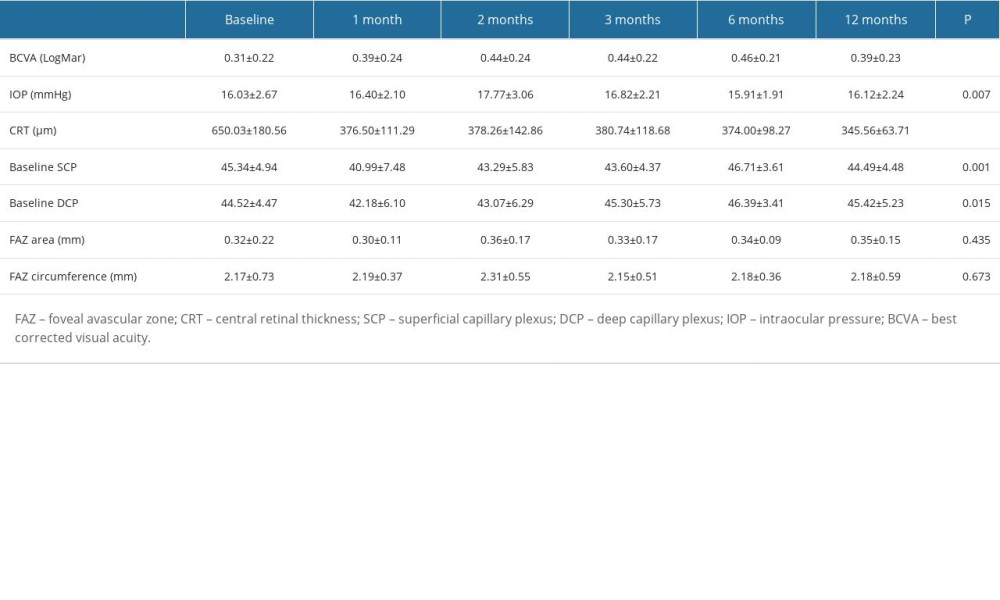 Table 3. Overall subjects’ baseline and follow-up of variables.
Table 3. Overall subjects’ baseline and follow-up of variables. Table 1. Characteristics of all included subjects.
Table 1. Characteristics of all included subjects. Table 2. Characteristics of all included subjects by subgroup.
Table 2. Characteristics of all included subjects by subgroup. Table 3. Overall subjects’ baseline and follow-up of variables.
Table 3. Overall subjects’ baseline and follow-up of variables. In Press
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








